Abstract
Reactivation of varicella-zoster virus (VZV) has been reported after the administration of different vaccine platforms against SARS-CoV-2, also among individuals without known immunosuppressive states. Herein, we describe for the first time a case of herpes zoster after mRNA vaccination against SARS-CoV-2 in a 53-year-old immunocompetent adult without any known comorbidities, who was previously vaccinated with a live attenuated zoster vaccine. The fact that the patient had no history of varicella and had been tested seronegative for VZV prior to immunization with the live attenuated zoster vaccine further contribute to the challenge of this unusual case. This advocates for a high level of vigilance on the part of clinicians regarding this rare complication among receivers of COVID-19 vaccines.
Keywords: COVID-19, mRNA, Herpes zoster, Live attenuated
1. Introduction
As mass vaccination programs against COVID-19 are ongoing worldwide, physicians are witnessing a wide range of adverse events [1,2]. Recently, reactivation of varicella-zoster virus (VZV) has been reported after administration of different vaccines against SARS-CoV-2, although causality is still under investigation. VZV reactivation is principally attributed to perturbations of the immune status and age of the patients, with altered immunocompromised state and senescence being the most significant risk factors. The development of herpes zoster (HZ) has been reported as a side effect following vaccination with mRNA vaccines in patients, with or without known immunosuppression [1,3,4]. Herein, we describe for the first time an interesting case of herpes zoster after mRNA vaccination against SARS-CoV-2 in a 53-year-old immunocompetent adult without any known comorbidities who was previously vaccinated with a live attenuated zoster vaccine (Zostavax).
2. Case report
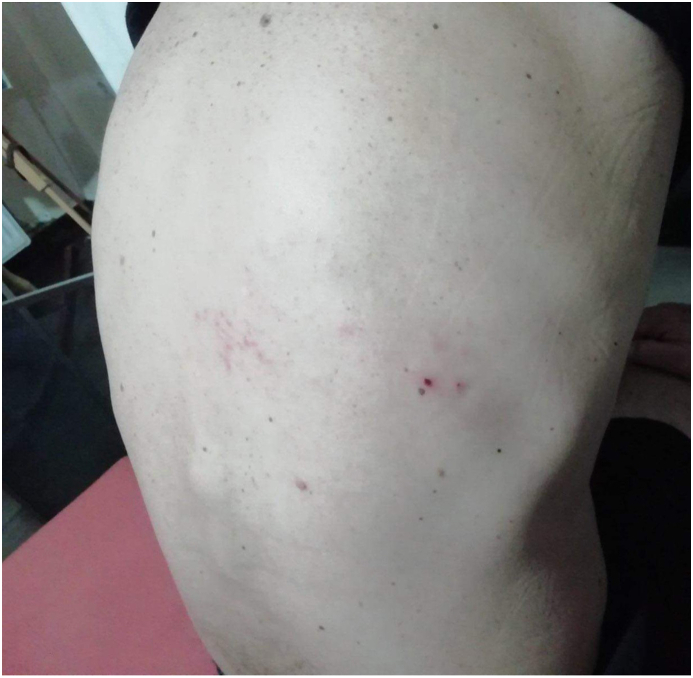
A 53-year-old male patient with a body mass index of 24.2 kg/m2, presented to the Outpatient Clinic of the hospital due to a painful erythematous rash on his right upper back which appeared eleven days after receiving the first dose of BNT162b2 mRNA COVID-19 (Pfizer-BioNTech) vaccine (Fig. 1). His medical history was negative with respect to chronic illnesses, and he was currently not receiving any medication. On further anamnestic questioning, the patient reported never having been ill with chickenpox. Regarding his vaccination history apart from the recent COVID-19 vaccine, the patient had received a full live attenuated herpes zoster vaccination two years before on the basis of negative VZV antibody serologic testing. He reported no contacts with patients with chickenpox or shingles thereafter. Other than a herpetiform, vesicular and erythematous rash with a zosteriform pattern on the distribution of the 6th thoracic dermatome of his right hemithorax, further physical examination was unremarkable.
Fig. 1.
Τhe improved vesicular rash of the patient in a resolution course after completion of valacyclovir treatment.
Based on his past medical history, his clinical presentation with the typical HZ rash morphology and distribution as well as burning pain, a provisional diagnosis of vaccine-strain related herpes zoster was made. The patient received valacyclovir 1000 mg thrice daily for seven days with subsequent resolution of the rash. His clinical course was favorable, although he reported postherpetic neuralgia for over a month. After a 10-day delay in the vaccination schedule, he received the second dose BNT162b2 which was well tolerated, while no further HZ recurrences were observed after both the second and third (booster) doses.
3. Discussion
Zostavax, the live-attenuated VZV vaccine is highly effective in the prevention of HZ and postherpetic neuralgia. In a study among 38,546 adults aged over 60, vaccination reduced the 3.1-year incidence of HZ and post-herpetic neuralgia vs. placebo by 51.3% and 66.5%, respectively [5,6]. Efficacy against VZV has also been shown to be high among individuals aged between 50 and 59 years (approximately 70% over a median 1.3 years vs. placebo [7]). Overall, the clinical course of a typical Herpes-Zoster appearance on our previously live-attenuated VZV-vaccinated patient who was anamnestically and serologically negative for a previous chickenpox infection raises the question of a possible reactivation of the latent VZV-vaccine viral strain. It has been established that a latent infection with the live-attenuated viral strain (vOka) can occur after vaccination, and subsequent Herpes Zoster thereafter may occur through reactivation of the wild-type virus or due to vOka, although the latter appears to be extremely rare [8,9]. An alternative possibility would include the unlikely assumption that our patient acquired a wild-type VZV after vaccination with Zostavax, without having exhibited clinical signs of overt chickenpox. A past, currently not recalled chickenpox illness could be a third plausible explanation, although the negative anti VZV-IgG antibodies prior to vaccination present a solid counterargument against this scenario.
Furthermore, in our patient, the occurrence after eleven days of the COVID-19 vaccine support the possibility of a relationship with the recent COVID-19 vaccination. Although the timely association with the mRNA-platform vaccination does not prove causality between the two events, there is accumulating evidence highlighting the increased incidence of VZV reactivation following COVID-19 vaccination, not only from the mRNA-based vaccines, but also from the adenovirus-vector and inactivated vaccines, as well [[1], [2], [3],10]. A total of 2527 cases of VZV infection following vaccination against SARS-CoV-2 have been reported in the United Kingdom until today [1]. Most cases have been linked to the immunosuppression of the host-recipient of the vaccine. However, there are also cases in immunocompetent individuals [[1], [2], [3]]. It is widely known that the immune system responds with a significant T-cell activation after COVID-19 vaccination [1,2]. More specifically, increased CD8+ T cell and T helper type 1 CD4+ T cell counts have been demonstrated to occur after administration of mRNA-based vaccines for SARS-COV-2 [2]. A plausible explanation is that VZV-specific CD8+ cells are not able to preserve the VZV latent state after the massive shift of naïve CD8+ cells in the context of SARS-CoV-2 vaccination [2]. Another hypothesis suggests that alterations in Toll-like receptor expression among vaccinated people are associated with enhancement in type I interferon and pro-inflammatory cytokines leading to immunogenicity for the COVID-19 vaccine at the cost of reactivation of VZV [1,3] 3].
A major limitation of our report concerns the lack of identification of the VZV strain implicated in this clinical case due to the lack of availability of a corresponding real-time polymerase chain reaction assay, so that the dominant working hypothesis of an vOka-strain related zoster cannot be definitely proven. In either case, the occurrence of herpes zoster in an immunocompetent and otherwise healthy patient, previously vaccinated against VZV is certainly an unexpected event, which warrants for a high level of vigilance on the part of clinicians regarding this rare complication among recipients of COVID-19 vaccines.
Financial support
None.
CRediT authorship contribution statement
Natalia G. Vallianou: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Dimitrios Tsilingiris: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Irene Karampela: Investigation, Writing – review & editing. Junli Liu: Investigation, Writing – review & editing. Maria Dalamaga: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, All authors have had access to the data and results and have reviewed and approved the latest version of the manuscript.
Declaration of competing interest
None declared.
Contributor Information
Dimitrios Tsilingiris, Email: tsilingirisd@gmail.com.
Maria Dalamaga, Email: madalamaga@med.uoa.gr.
References
- 1.Katsikas Triantafyllidis K., Giannos P., Mian I.T., Kyrtsonis G., Kechagias K.S. Varicella Zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines. 2021;9 doi: 10.3390/vaccines9091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 4.Psichogiou M., Samarkos M., Mikos N., Hatzakis A. Reactivation of varicella Zoster virus after vaccination for SARS-CoV-2. Vaccines. 2021;9 doi: 10.3390/vaccines9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxman M.N., Levin M.J., Johnson G.R., Schmader K.E., Straus S.E., Gelb L.D., et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 6.Oxman M.N., Levin M.J., Shingles Prevention Study G. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J. Infect. Dis. 2008;197(Suppl 2):S228–S236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmader K.E., Levin M.J., Gnann J.W., Jr., McNeil S.A., Vesikari T., Betts R.F., et al. vol. 54. an official publication of the Infectious Diseases Society of America; 2012. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years; pp. 922–928. (Clinical infectious diseases). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng H.F., Schmid D.S., Harpaz R., LaRussa P., Jensen N.J., Rivailler P., et al. vol. 58. an official publication of the Infectious Diseases Society of America; 2014. Herpes zoster caused by vaccine-strain varicella zoster virus in an immunocompetent recipient of zoster vaccine; pp. 1125–1128. (Clinical infectious diseases). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinmann S., Chun C., Schmid D.S., Roberts M., Vandermeer M., Riedlinger K., et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J. Infect. Dis. 2013;208:1859–1868. doi: 10.1093/infdis/jit405. [DOI] [PubMed] [Google Scholar]
- 10.Arora P., Sardana K., Mathachan S.R., Malhotra P. Herpes zoster after inactivated COVID-19 vaccine: a cutaneous adverse effect of the vaccine. J Cosmet Dermatol. 2021;20:3389–3390. doi: 10.1111/jocd.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]