Multiple cases of cardiac complications and sudden death have been reported following vaccination with COVID-19 mRNA vaccines. The underlying pathophysiology is unclear. Herein, we report the case of a previously healthy 26-year-old man who developed myocarditis and pericarditis resulting in prominent J waves and ventricular fibrillation (VF) after receiving the BNT162b2 (Pfizer-BioNTech, New York, NY) vaccine. Histopathology of endomyocardial biopsy specimens showed inflammatory infiltrates with a histiocyte predominant. This may provide a clue to the mechanism of adverse cardiac events after the BNT162b2 vaccination.
A previously healthy 26-year-old man was brought to the emergency department following an out-of-hospital cardiac arrest. He had received a first dose of the BNT162b2 mRNA coronavirus disease vaccine 5 days previously and had developed fatigue and headache the following day. On the day of admission, he had lost consciousness while eating. On arrival of the emergency medical services, the electrocardiogram (ECG) showed VF. Two defibrillations, epinephrine, and cardiopulmonary resuscitation successfully converted the rhythm to sinus rhythm. He had no history of illicit drug use and no family history of sudden death.
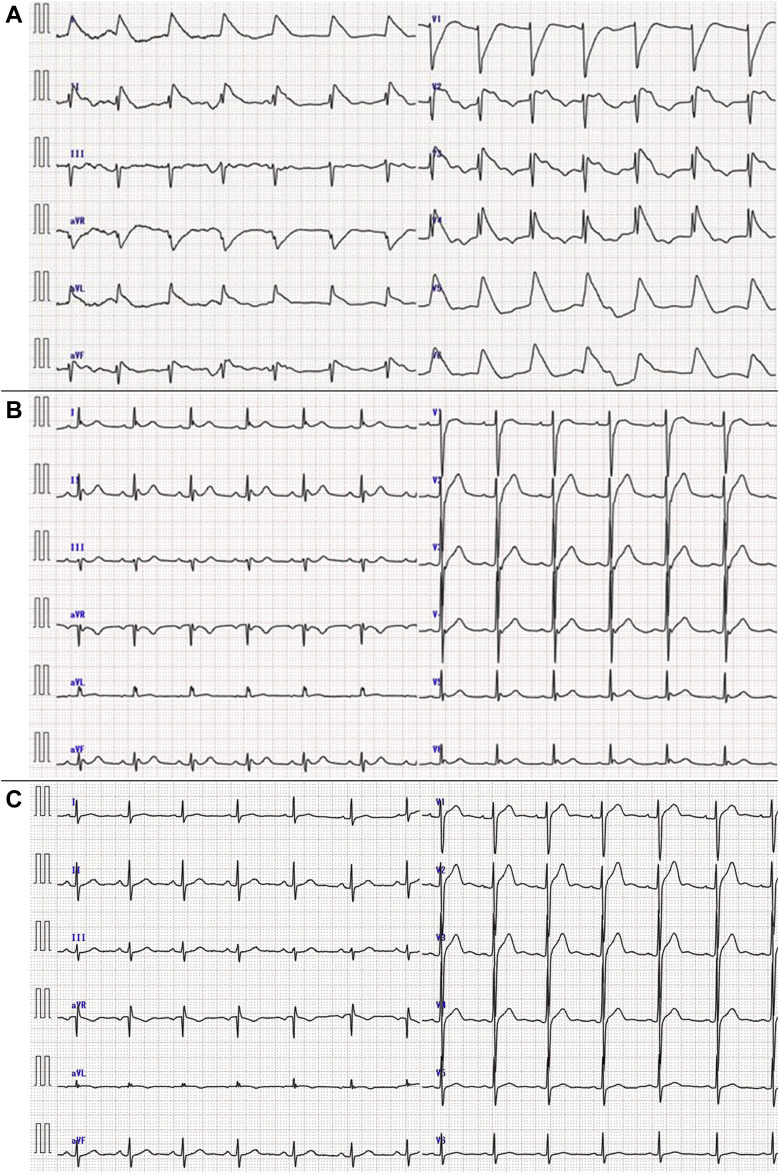
Physical examination showed no findings of note. Laboratory tests showed lymphocytosis (7.6 [normal: 1.1-3.2] × 109 cells/L) with normal lymphocyte morphology and a normal total white blood cell count (8.51 [normal: 3.5-9.1] × 109 cells/L). His troponin-I level was elevated (0.095 [normal: ≤ 0.030] ng/mL). Biochemical tests of liver and renal function were within normal limits. Other laboratory investigations revealed no evidence of systemic infection or rheumatic disease. On arrival, the ECG showed a combined J wave and ST-T segment wave in leads I, II, aVL, aVF, and V2–6 (Fig. 1 A, Supplemental Table S1). The ST elevation improved within minutes. A notch-type J wave was observed in the inferior and lateral leads, with a mirror image in the anterior leads (Fig. 1B, Supplemental Table S1). Cardiac ultrasound revealed diffuse mild hypokinesis of the left ventricle. Emergency coronary angiography showed no significant stenosis.
Figure 1.
Serial electrocardiograms (ECGs) of the patient. (A) The ECG performed on arrival at the hospital shows a combined J wave and ST-T segment in leads I, II, aVL, aVF, and V2-6. (B) An ECG performed several minutes after arrival shows notch-type J waves in the inferior and lateral leads. (C) In an ECG performed 3 months later, the ST-T segment is normal in all leads.
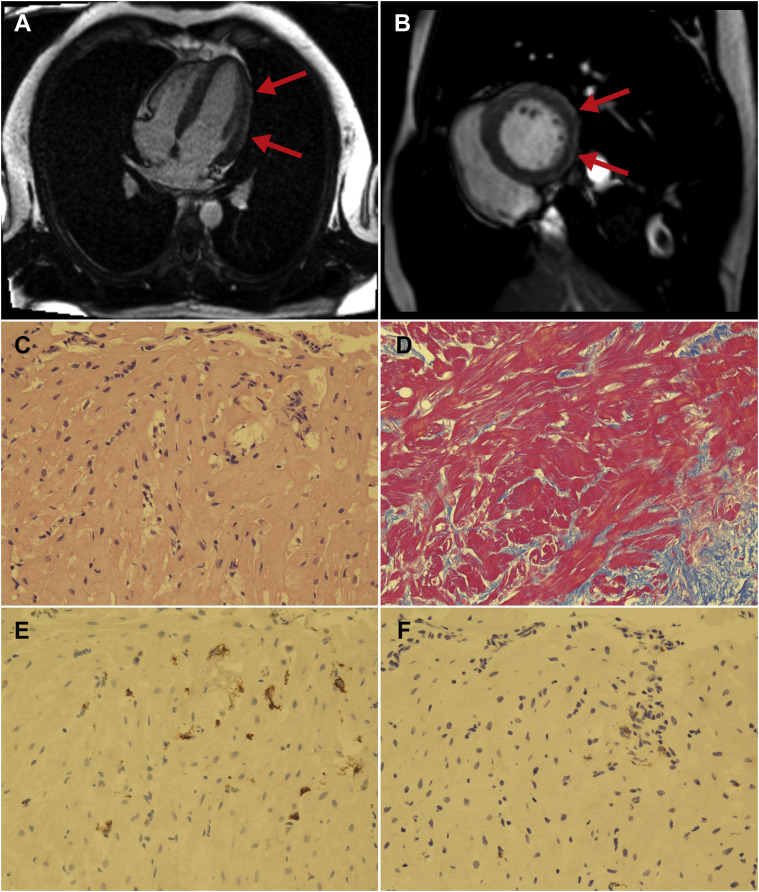
Cardiac magnetic resonance imaging (MRI) displayed late gadolinium enhancement (LGE) of the epicardial side of the inferior and lateral walls, indicating the presence of myocarditis and pericarditis (Fig. 2 A,B). An endomyocardial biopsy of the septal side of the right ventricle performed on day 8 showed myocardial fibrosis on histology, suggestive of subacute myocarditis (Fig. 2C,D). Immunohistochemistry revealed CD68-positive histiocyte and CD3-positive lymphocyte infiltrates, with histiocyte predominance (Fig. 2E,F). Other investigations, including left ventricular angiography, an ergonovine coronary spasm induction test, and a pilsicainide challenge test, showed no remarkable findings. Because his cardiac event occurred 5 days after the vaccination with no other triggers identified, we suspected that the myocarditis and pericarditis that had led to J-wave syndrome and consequent VF was an adverse effect of the BNT162b2 vaccine. We reported it to the Japanese Pharmaceuticals and Medical Devices Agency.
Figure 2.
(A, B) Cardiac magnetic resonance image showing late gadolinium enhancement on the epicardial side of the inferior and lateral walls (red arrows). (A) Four-chamber view; (B) short-axis view. (C-F) Histology of myocardium from the septal side of the right ventricle. (C) Hematoxylin and eosin stain shows infiltration of inflammatory cells. There are no giant cells or eosinophils (× 200). (D) Azan stain reveals moderate fibrosis (× 200). (E, F) Immunohistochemistry reveals CD68-positive histiocytes (E) and CD3-positive T lymphocytes (F), with histiocyte predominance (× 200).
The patient’s subsequent clinical course was uneventful. No J waves were observed in any of the leads after the acute phase (Fig. 1C, Supplemental Table S1). After performing various tests, we administered bepridil to prevent VF. For secondary prevention, on the 22nd day of hospitalization, he was transferred to another hospital for implantation of a subcutaneous implantable cardioverter defibrillator (S-ICD). After completion of S-ICD implantation, bepridil was discontinued, and the patient is doing well on follow-up
Discussion
J-wave syndromes, including Brugada syndrome and early repolarization syndrome, are characterized by the appearance of prominent J waves, which can cause VF.1 Particular attention should be paid to the presence of J waves in global leads (ie, inferior, lateral, and right precordial leads), as this is associated with a higher incidence of VF.2 , 3
J-wave syndromes may be caused by genetic predisposition, acute myocardial ischemia, electrolyte imbalance, or hypothermia. Pericarditis is a rare cause of J-wave syndrome.4 In this case, the results of pilsicainide challenge were negative, which is not consistent with Brugada syndrome,3 and the site of LGE on cardiac MRI coincided with the region represented by the leads where prominent J waves were seen on ECG. Therefore, the J-wave syndrome appears to have been caused by myocarditis and pericarditis. In previous studies, the ECG findings in postvaccine myocarditis and pericarditis included ST-segment elevation, nonspecific ST-T changes, and T-wave inversions. On the other hand, to our knowledge, there were no cases with prominent J waves, making this case very unusual.
Despite multiple reports of cardiac adverse events and sudden death following the BNT162b2 vaccine, the mechanism remains unclear. In this case, based on the findings of the cardiac MRI and endomyocardial biopsy, clinical history, and the exclusion of other causes of myocarditis and pericarditis, the patient was diagnosed with myocarditis and pericarditis possibly associated with the COVID-19 vaccine. The histopathology of the endomyocardial biopsy specimen showed inflammatory infiltrates with a histiocyte predominance. This differs from the typical pathology of viral myocarditis or immune-mediated myocarditis. Previous reports of biopsy-confirmed post-COVID-19 vaccination myocarditis also had infiltrates with histiocyte predominance, suggesting that the pathogenic mechanism may involve histiocyte-linked immunologic injury to the myocardium.5
The optimal treatment of life-threatening arrhythmias after mRNA COVID-19 vaccination has not been established. In this case, we decided to perform S-ICD implantation for secondary prevention.
Although a direct causal relationship to the BNT162b2 vaccine and the myocarditis and pericarditis could not be confirmed, no other cause was identified. Further investigation is necessary to clarify the causality, although it should be noted that the COVID-19 vaccine is the most promising tool to achieve herd immunity with the possibility to stop this global crisis and that cardiac complications after vaccination are rare events.
Acknowledgements
The authors thank Dr Maruyama, Department of Hematology, Oncology and Cardiovascular Medicine, Kyushu University Hospital, for his contribution to patient care. The authors also thank Dr Asano, Department of Diagnostic Pathology, Suwa Central Hospital, for his interpretation of the pathology and preparation of the figures.
Funding Sources
No funding was received for this article.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 847 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2022.02.005.
Supplementary Material
References
- 1.Wilde A.A., Antzelevitch C., Borggrefe M., et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M., Sacher F., Derval N., et al. Early repolarization in the inferolateral leads: a new syndrome associated with sudden cardiac death. J Interv Card Electrophysiol. 2007;18:281. [Google Scholar]
- 3.Antzelevitch C., Yan G.X. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura T., Takatsuki S., Aizawa Y., et al. Ventricular fibrillation associated with J-wave manifestation following pericarditis after catheter ablation for paroxysmal atrial fibrillation. Can J Cardiol. 2013;29:1330.e1–1330.e3. doi: 10.1016/j.cjca.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen T.D., Mall G., Westphal J.G., Weingärtner O., Möbius-Winkler S., Schulze P.C. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. ESC Heart Fail. 2021;8:4710–4714. doi: 10.1002/ehf2.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.