Abstract
Parathyroid carcinoma is very rare in pregnancy. Clinical features are similar to primary hyperparathyroidism. A 38-year-old pregnant woman had repeated hospital admissions for palpitations, headaches, dizziness and polydipsia. Blood investigations showed severe hypercalcaemia with raised parathyroid hormone and 24-hour ECG showed ventricular bigeminy and premature ventricular contractions. Neck ultrasound showed a lesion in the right thyroid lobe. Consequently, she underwent an en bloc resection of the right parathyroid and thyroid lobe at 23 weeks gestation. Histology results confirmed parathyroid cancer. This case highlights the complexities of identifying hypercalcaemia in pregnancy due to the overlapping features with common disorders of pregnancy. Early recognition and timely surgical management can prevent maternal and fetal complications. Also, the case demonstrates the value of interprofessional collaboration between different specialities in providing quality care and improving outcomes. An abridged version of this case was presented at European Congress of Endocrinology 2021.
Keywords: calcium and bone, endocrinology, pregnancy, endocrine cancer
Background
Parathyroid carcinoma is a rare entity with an estimated prevalence of 0.005% of all cancers types.1 It accounts for 0.5%–5% of all cases of primary hyperparathyroidism (PHPT).1–4 In contrast to PHPT which is more common in women, parathyroid carcinoma occurs equally in men and women and can present a decade earlier than benign causes of PHPT.5 The aetiology is largely unknown with prior neck radiation and rare genetic syndromes such as familial hyperparathyroidism, multiple endocrine neoplasia type 1 and type 2A, and hereditary hyperparathyroidism-jaw tumour syndrome associated with an increased risk of the disease.2 6
Parathyroid carcinoma in pregnancy is extremely rare with fewer than ten cases previously reported in literature.7–9 Patients can present in a similar manner to PHPT with symptoms of hypercalcaemia such as fatigue, abdominal pain, depression, thirst, constipation and depression. Long-term effects of significant hypercalcaemia include cardiac arrhythmias, bone disease and nephrolithiasis.10 Although PHPT is one of the main causes of hypercalcaemia in pregnancy, other factors such as renal failure, vitamin A/D toxicity, hyperparathyroidism, granulomatous diseases and malignancy can also cause hypercalcaemia.11
Diagnosis is challenging as it requires a high index of clinical suspicion and most cases are only confirmed on histology postoperatively.12 The vague nature of symptoms, entwined with the overlapping features of common disorders of pregnancy, makes recognition of parathyroid carcinoma in pregnancy challenging. A delay in treatment can significantly increase the risk of maternal and fetal complications.8 We present a unique case of parathyroid carcinoma in pregnancy where ventricular bigeminy was the presenting feature.
Case presentation
A 38-year-old multiparous woman presented with severe palpitations at the start of her second trimester of pregnancy (15 weeks gestation). This was on a background of a 3-week history of increasing thirst, headaches, dizziness and fatigue. She had also visited her general practitioner prior to this on several occasions due to persistent vomiting where she was diagnosed with nausea and vomiting of pregnancy (NVP) and managed with antiemetics. The patient had no significant personal history and there was no family history of endocrine disease. Her obstetric history consisted of four caesarean sections with no complications. On clinical assessment, her physical observations and examination were unremarkable. A 12-lead ECG demonstrated ventricular bigeminy with a normal heart rate. Admission blood investigations revealed hypercalcaemia (adjusted serum calcium 3.07 mmol/L (normal range 2.2–2.6 mmol/L)). The patient was given a provisional diagnosis of hypercalcaemia secondary to dehydration and managed with intravenous fluids. She responded well and thus was discharged the same day. However, the patient represented 1 week later with worsening symptoms.
Investigations
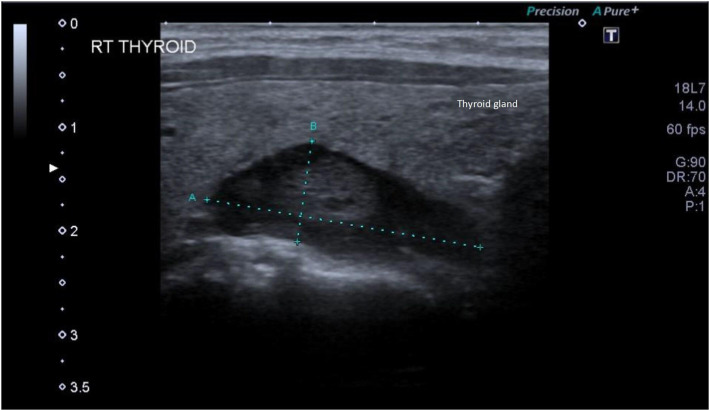
Blood investigations from the second admission showed hypercalcaemia 2.97 mmol/L, raised parathyroid hormone (PTH) 87 ng/L, low phosphate 0.79 mmol/L and mildly low vitamin D as shown in table 1. Magnesium, thyroid stimulating hormone (TSH), urea and creatinine levels were within normal limits. Her 24-hour urinary calcium (6.1 mmol/24 hours) and creatinine (7.8 mmol/24 hours) levels were normal. A 24-hour ECG recording was performed to further explore the cause of palpitations and showed ventricular bigeminy and high frequency premature ventricular contractions (PVCs) amounting to a burden of 26%. Echocardiography showed a mildly dilated right atrium with good biventricular systolic function and there was no evidence of valvular abnormalities. An ultrasound of the neck was performed to evaluate the cause of severe hypercalcaemia. It revealed a hypoechoic lesion measuring 15×11 mm in the posterior aspect of the right lobe of the thyroid gland with no thyroid enlargement, retrosternal extension, tracheal deviation or pathological lymph nodes. Additionally, it was difficult to determine if the lesion was intrathyroidal or extrathyroidal (figure 1). She also had a fetal growth scan which did not show any abnormalities.
Table 1.
Blood investigations throughout the pregnancy
| Weeks of pregnancy | ||||||||||||
| 15/40 | 16/40 | 18/40 | 19/40 | 20/40 | 21/40 | 22/40 | 23/40 | 24/40 | 26/40 | 30/40 | 35/40 | |
| Calcium (2.1–2.6 mmol/L) |
3.07 | 2.97 | 3.03 | 2.87 | 3.06 | 3.09 | 2.95 | 2.90 | 2.55 | 2.35 | 2.45 | |
| PTH (1.6–6.9 pmol/L) |
87.00 | 68.00 | 18.00 | 5.90 | ||||||||
| Phosphate (0.74–1.4 mmol/L) |
0.79 | 0.86 | 0.68 | 0.83 | 0.85 | 0.79 | ||||||
| TSH* | 1.30 | 2.10 | 2.40 | 6.80 | 7.20 | |||||||
| Vitamin D (≥30 ng/mL) |
43.00 | 58.80 | ||||||||||
*Trimester-specific normal range of TSH is: trimester 1: 0.04–3.77 μIU/mL; trimester 2: 0.30–3.21 μIU/mL and trimester 3: 0.60–4.50 μIU/mL.
PTH, parathyroid hormone; TSH, thyroid stimulating hormone.
Figure 1.
Ultrasound of the thyroid gland showing a well-defined hypoechoic lesion measuring 15 mm by 11 mm.
Differential diagnosis
There is a plethora of differential diagnoses that can result in persistent vomiting during pregnancy including NVP, hyperemesis gravidarum, acute fatty liver of pregnancy and infection.13 In the context of her clinical presentation, blood investigations and ECG changes, PHPT secondary to atypical parathyroid adenoma or parathyroid carcinoma were the main differentials and were supported by the ultrasound findings.
Treatment
A multidisciplinary approach was adopted with input from the cardiology, endocrine and obstetric teams. She was initially managed with intravenous fluids for hypercalcaemia and bisoprolol 2.5 mg for PVCs. She was also booked for serial growth scans to ensure her pregnancy was monitored more closely. Despite drinking copious amount of fluids at home, she continued to have severe hypercalcaemia needing multiple hospital attendances for intravenous rehydration. Her case was discussed in the endocrine multidisciplinary team meeting as the ultrasound results were inconclusive (intrathyroidal vs extrathyroidal). Surgical management was deemed to be the most suitable option given her recurrent hospital admissions and potential risks to fetal well-being. At 23 weeks gestation, the patient underwent an urgent right unilateral neck exploration. During the procedure, there was a high suspicion of cancer as the right parathyroid gland was enlarged and appeared to be invading the right thyroid gland. Hence an en bloc resection of the right parathyroid along with the right thyroid lobe was performed and sent for histology and immunohistochemistry.
Outcome and follow-up
The patient recovered well postoperatively. Her histology showed a thick fibrous capsule composed mainly of follicle-like structures and sheets of cells (predominantly chief cells with minimum oxyphil cells). Three separate nodules were identified outside the main nodule with similar architectural and cytological features which were infiltrating the surrounding connective, adipose and thyroid tissue. Perineural invasion was also seen. On immunohistochemistry, most of the tumour cells had positive staining for parafibromin and focal PGP9.5 staining. Ki67 showed a proliferative index in the busiest area of 2%. Although the morphological appearance was consistent with parathyroid carcinoma with capsular, perineural and thyroid tissue invasion, immunohistochemistry was not entirely supportive. After further discussion between the medical, surgical and pathology teams a diagnosis of parathyroid carcinoma was confirmed.
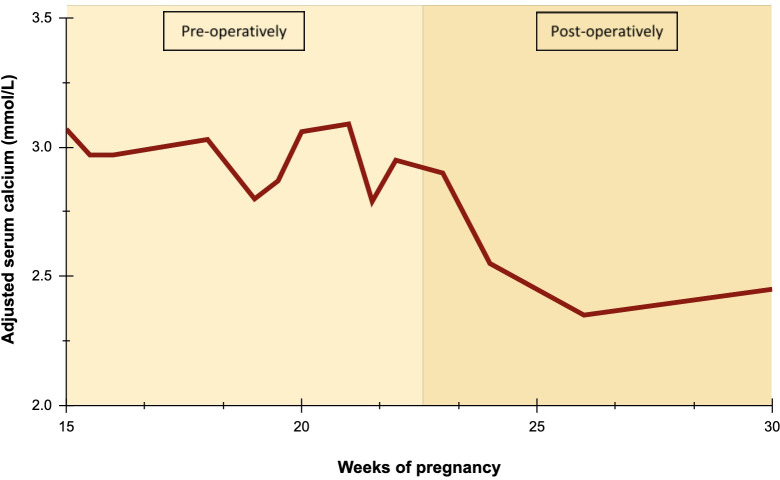
On follow-up 2 weeks after the surgery, her repeat blood investigations showed a mildly raised TSH (6.8 mU/L) with normal calcium and PTH. She was started on Levothyroxine for biochemical hypothyroidism. Figure 2 shows her serial calcium levels throughout the pregnancy. Given the rarity of her diagnosis, she also underwent genetic testing to check for germline mutations in MEN1, CDC73, CASR, GNA11, CDKN1B, RET, GCM2 or AP2S1 which can cause hereditary tumour syndromes in line with guidance.14 15 There was no evidence of germline mutations found in her case. Her calcium and thyroid levels were routinely monitored for the remainder of her pregnancy. She underwent an elective caesarean section at 39 weeks gestation based on personal preference with good maternal and fetal outcomes.
Figure 2.
Serial adjusted serum calcium levels during pregnancy.
Discussion
Our case highlights some key learning points in recognising and managing parathyroid carcinoma in pregnancy. First, it demonstrates the challenges around symptom recognition particularly in patients presenting with cardiac abnormalities which can lead to underdiagnosis and delays in treatment. The most common ECG findings of hypercalcaemia are a shortened ST segment, reduced QT interval, varying degrees of heart block and ventricular conduction defects.16 17 However, arrhythmias are uncommon with only one case of ventricular bigeminy secondary to a parathyroid adenoma previously reported.14 Serious maternal complications of hypercalcaemia may occur in cases of delayed or no treatment and include bone fractures, pancreatitis, parathyroid crisis, pre-eclampsia and hyperemesis gravidarum. Fetal complications such as premature birth, intrauterine growth restriction, neonatal hypocalcaemia, stillbirth and hypoparathyroidism have also been reported.18
Neck ultrasound is the investigation of choice in pregnancy for localisation. Parathyroid carcinoma is distinguishable from typical parathyroid adenoma on ultrasound based on the following features: larger size, heterogeneous echotexture, irregular shape, intranodular calcifications and suspicious lymph nodes.19 In cases where ultrasound results are inconclusive, a Technetium-99m sestamibi scitingraphy can be considered.20 However, differentiating between parathyroid carcinoma and atypical parathyroid adenoma remains a challenge as many of the intraoperative and histological features overlap. In our case, capsular invasion and a steep drop in PTH levels from 87 pmol/L to 18 pmol/L were observed post-surgery which were similar to previous cases of parathyroid carcinoma in pregnancy.8 Furthermore, reduced parafibromin expression on immunohistochemistry usually suggests parathyroid carcinoma7 21 but this was not seen in our case. In addition, capsular and/or vascular invasion can also suggest parathyroid carcinoma over atypical adenoma.22
It is well established that parathyroid carcinoma is associated with significantly elevated preoperative PTH levels.23–25 However, as a normal physiological response to pregnancy, PTH levels decrease to the low-normal range (10%–30% of the non-pregnant value) during the first and second trimester and normalise by term26–28 while ionised calcium levels remain relatively constant throughout pregnancy.26 These physiological changes could account for the levels observed in our case.
Due to the paucity of guidelines, management of parathyroid carcinoma in pregnancy needs to be individualised to optimise maternal and fetal well-being using a multidisciplinary approach. In our case, early involvement of the endocrine and obstetric team after confirmation of the biochemical diagnosis enabled adjustments to be made in order to provide optimal antenatal care. Surgery remains the definitive treatment of choice with the second trimester being the safest time to proceed; although cases have also been reported during the first and third. Surgical options comprise of minimally invasive parathyroidectomy for high-risk pregnancies or a four gland exploration.29
Patient’s perspective.
I have to thank everyone who took care of me when I had been going through the process. I was already apprehensive with my pregnancy, but this high calcium problem made things even worse for me. The doctors didn’t give up on me and did everything they could to make sure me and my baby were okay.
Learning points.
The main cause of hypercalcaemia in pregnancy is PHPT but other causes include hyperthyroidism, vitamin A/D toxicity, renal failure and malignancy.
Symptoms of hypercalcaemia can present in a similar way to other features of pregnancy and hence calcium levels should be checked in all women presenting with common disorders of pregnancy.
Prompt diagnosis and treatment, along with interprofessional collaboration between different specialities, play an important role in preventing maternal and fetal complications.
Surgery is the best treatment option with second trimester being the safest time to proceed for better outcomes.
Footnotes
Contributors: SMSK identified and consented the patient. KS analysed the data and LG drafted the manuscript. All authors reviewed and approved the final manuscript prior to submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Sharretts JM, Kebebew E, Simonds WF. Parathyroid cancer. Semin Oncol 2010;37:580–90. 10.1053/j.seminoncol.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Givi B, Shah JP. Parathyroid carcinoma. Clin Oncol 2010;22:498–507. 10.1016/j.clon.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraro V, Sgaramella LI, Di Meo G, et al. Current concepts in parathyroid carcinoma: a single centre experience. BMC Endocr Disord 2019;19:46. 10.1186/s12902-019-0368-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes EC, Morton DL, Ketcham AS. Parathyroid carcinoma: a collective review. Ann Surg 1969;169:631–40. 10.1097/00000658-196904000-00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawat N, Khetan N, Williams DW, et al. Parathyroid carcinoma. Br J Surg 2005;92:1345–53. 10.1002/bjs.5182 [DOI] [PubMed] [Google Scholar]
- 6.Hundahl SA, Fleming ID, Fremgen AM, et al. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: a national cancer data base report. the American College of surgeons Commission on cancer and the American cancer Society. Cancer 1999;86:538–44. [DOI] [PubMed] [Google Scholar]
- 7.Paul RG, Elston MS, Gill AJ. Hypercalcaemia due to parathyroid carcinoma presenting in the third trimester of pregnancy: primary hyperparathyroidism in pregnancy. Aust N Z J Obstet Gynaecol 2012;52:204–7. [DOI] [PubMed] [Google Scholar]
- 8.Panchani R, Varma T, Goyal A, et al. Parathyroid carcinoma masquerading as morning sickness in pregnancy. Indian J Endocrinol Metab 2013;17:198–200. 10.4103/2230-8210.119570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baretić M, Tomić Brzac H, Dobrenić M, et al. Parathyroid carcinoma in pregnancy. World J Clin Cases 2014;2:151–6. 10.12998/wjcc.v2.i5.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graal MB, Wolffenbuttel BH. Consequences of long-term hyperparathyroidism. Neth J Med 1998;53:37–42. 10.1016/S0300-2977(98)00010-2 [DOI] [PubMed] [Google Scholar]
- 11.Rey E, Jacob C-E, Koolian M, et al. Hypercalcemia in pregnancy - a multifaceted challenge: case reports and literature review. Clin Case Rep 2016;4:1001–8. 10.1002/ccr3.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long KL, Sippel RS. Current and future treatments for parathyroid carcinoma. International Journal of Endocrine Oncology 2018;5:IJE06. 10.2217/ije-2017-0011 [DOI] [Google Scholar]
- 13.Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am 2011;40:309–34. 10.1016/j.gtc.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonds WF. Genetics of hyperparathyroidism, including parathyroid cancer. Endocrinol Metab Clin North Am 2017;46:405–18. 10.1016/j.ecl.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharretts JM, Simonds WF. Clinical and molecular genetics of parathyroid neoplasms. Best Pract Res Clin Endocrinol Metab 2010;24:491–502. 10.1016/j.beem.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John T-J, John K, Jansen van Rensburg R, et al. Hypercalcaemia and a short QT interval. QJM Int J Med 2020;113:55–6. 10.1093/qjmed/hcz109 [DOI] [PubMed] [Google Scholar]
- 17.Patnaik S, Lai YK. Just hypercalcaemia or acute ST elevation myocardial infarction? A review of hypercalcaemia-related electrocardiographic changes. BMJ Case Rep 2015;2015:bcr2015211177. 10.1136/bcr-2015-211177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chadli MC, Chaieb L, Jemni L, et al. Bigeminal arrhythmia associated with hyperparathyroid crisis. CMAJ 1988;138:1115–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Dochez V, Ducarme G. Primary hyperparathyroidism during pregnancy. Arch Gynecol Obstet 2015;291:259–63. 10.1007/s00404-014-3526-8 [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Zhan WW, Zhou JQ, et al. Role of ultrasound in the differentiation of parathyroid carcinoma and benign parathyroid lesions. Clin Radiol 2020;75:179–84. 10.1016/j.crad.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Juhlin CC, Höög A. Parafibromin as a diagnostic instrument for parathyroid carcinoma-lone ranger or part of the posse? Int J Endocrinol 2010;2010:1–5. 10.1155/2010/324964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn CE, Healy J, Lebastchi AH, et al. Modern experience with aggressive parathyroid tumors in a high-volume new England referral center. J Am Coll Surg 2015;220:1054–62. 10.1016/j.jamcollsurg.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 23.Mohebati A, Shaha A, Shah J. Parathyroid carcinoma. Hematol Oncol Clin North Am 2012;26:1221–38. 10.1016/j.hoc.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Villar-del-Moral J, Jiménez-García A, Salvador-Egea P, et al. Prognostic factors and staging systems in parathyroid cancer: a multicenter cohort study. Surgery 2014;156:1132–44. 10.1016/j.surg.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 25.Christakis I, Bussaidy N, Clarke C, et al. Differentiating atypical parathyroid neoplasm from parathyroid cancer. Ann Surg Oncol 2016;23:2889–97. 10.1245/s10434-016-5248-6 [DOI] [PubMed] [Google Scholar]
- 26.Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am 2011;40:795–826. 10.1016/j.ecl.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Togashi K, Ishida O, Yamazaki M, et al. [Changes in serum parathyroid hormone levels during pregnancy]. Rinsho Byori 1989;37:1269–73. [PubMed] [Google Scholar]
- 28.Sharma JB, Sharma S, Usha BR, et al. Cross-Sectional study of serum parathyroid hormone level in high-risk pregnancies as compared to nonpregnant control. Indian J Endocrinol Metab 2016;20:92–6. 10.4103/2230-8210.172288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMullen TPW, Learoyd DL, Williams DC, et al. Hyperparathyroidism in pregnancy: options for localization and surgical therapy. World J Surg 2010;34:1811–6. 10.1007/s00268-010-0569-2 [DOI] [PubMed] [Google Scholar]