Abstract
Angiotensin-converting enzyme-2, or ACE2, is primarily a zinc-dependent peptidase and ectoenzyme expressed in numerous cell types and functioning as a counterbalance to ACE in the renin-angiotensin system. It was discovered 21 years ago more than 40 years after the discovery of ACE itself. Its primary physiological activity is believed to be in the conversion of angiotensin II to the vasodilatory angiotensin-(1−7) acting through the Mas receptor. As such it has been implicated in numerous pathological conditions, largely in a protective mode which has led to the search for ACE2 activatory mechanisms. ACE2 has a diverse substrate specificity allowing its participation in multiple peptide pathways. It also regulates aspects of amino acid transport through its homology with a membrane protein, collectrin. It also serves as a viral receptor for the SARS virus, and subsequently SARS-CoV2, driving the current COVID-19 pandemic. ACE2 therefore provides a therapeutic target for the treatment of COVID and understanding the biological events following viral binding can provide insight into the multiple pathologies caused by the virus, particularly inflammatory and vascular. In part this may relate to the ability of ACE2, like ACE, to be shed from the cell membrane. The shed form of ACE2 (sACE2) may be a factor in determining susceptibility to certain COVID pathologies. Hence, for just over 20 years, ACE2 has provided numerous surprises in the field of vasoactive peptides with, no doubt, more to come but it is its central role in COVID pathology that is producing the current intense interest in its biology.
List of abbreviations: Aβ, amyloid β-peptide; ACE, angiotensin-converting enzyme; AD, Alzheimer disease; ADAM, a disintegrin and metalloproteinase; AICD, APP intracellular domain; Ang, angiotensin; APP, amyloid precursor protein; CoV, coronavirus; COVID, coronavirus disease; DIZE, diminazene aceturate; ECE, endothelin-converting enzyme; ICD, intracellular domain; NEP, neprilysin; RAS, renin-angiotensin system; RBD, receptor-binding domain; sACE2, soluble or “shed” ACE2; SARS, severe acute respiratory syndrome; SIRT1, silent information regulator T1
Keywords: Amyloid, Angiotensin, Angiotensin-converting enzyme, Cardiovascular, Coronavirus, COVID, Neprilysin, Renin-angiotensin system, SARS, Vasopeptidase
Graphical Abstract
1. Introduction
As a prelude to the celebration of 30 years of vasoactive peptides symposia, this review highlights, in particular, the discovery and diverse biology of angiotensin-converting enzyme 2 (ACE2) over two decades [1], [2] in which it has emerged as a key vasopeptidase providing a missing link in the renin-angiotensin system (RAS) cascade, a regulator of amino acid transport and a key target in our understanding of the underlying pathologies arising from infection by the human coronoavirus SARS-CoV2 [3], [4]. This has led to ACE2 being one of the most published-on proteins in 2020 following the emergence of COVID-19, with the annual number of publications increasing from single figures in the early 2000s up to several thousand in 2020–2021 [4], [5].
It is remarkable that ACE2 remained unknown and unpredicted for almost 50 years following the discovery of ACE. The unearthing of ACE2 in 2000, however, was built on studies over decades of the families of membrane vasopeptidases that regulate the RAS and metabolism of other circulating regulatory peptides. These include the neprilysin (NEP) family and the evolutionary-related endothelin-converting enzymes (ECE-1 and ECE-2) [6]. In addition to the RAS, NEP plays a key role in metabolism of atrial natriuretic peptide (ANP) and the Alzheimer disease-related amyloid β-peptide (Aβ) [7], [8]. That these key vasopeptidases occurred as members of a related family left ACE as something of an anomaly and stimulated us to search for additional ACE-like genes in the emerging human genome sequence in the 1990s, particularly since, in other species, ACE homologues were already known to occur. In particular, in Drosophila melanogaster, six ACE genes are found of which two, Ance and Acer, produce functional zinc metallopeptidases, both essential for normal development, although with major differences in peptide substrate specificity [9], [10]. The mosquito genome, Anopheles gambiae, contains 9 ACE-like genes.
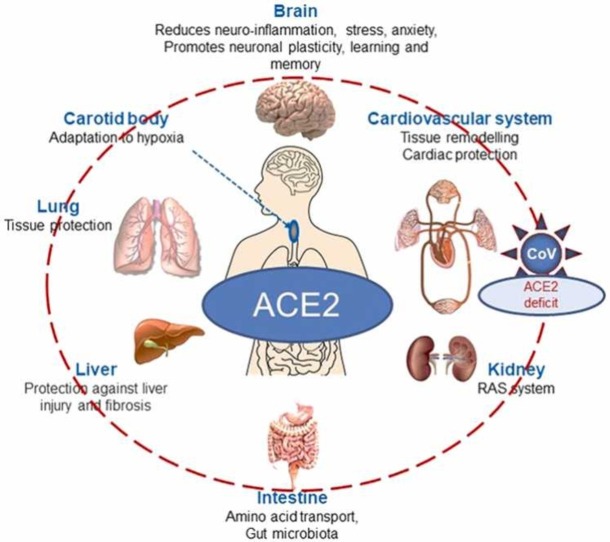
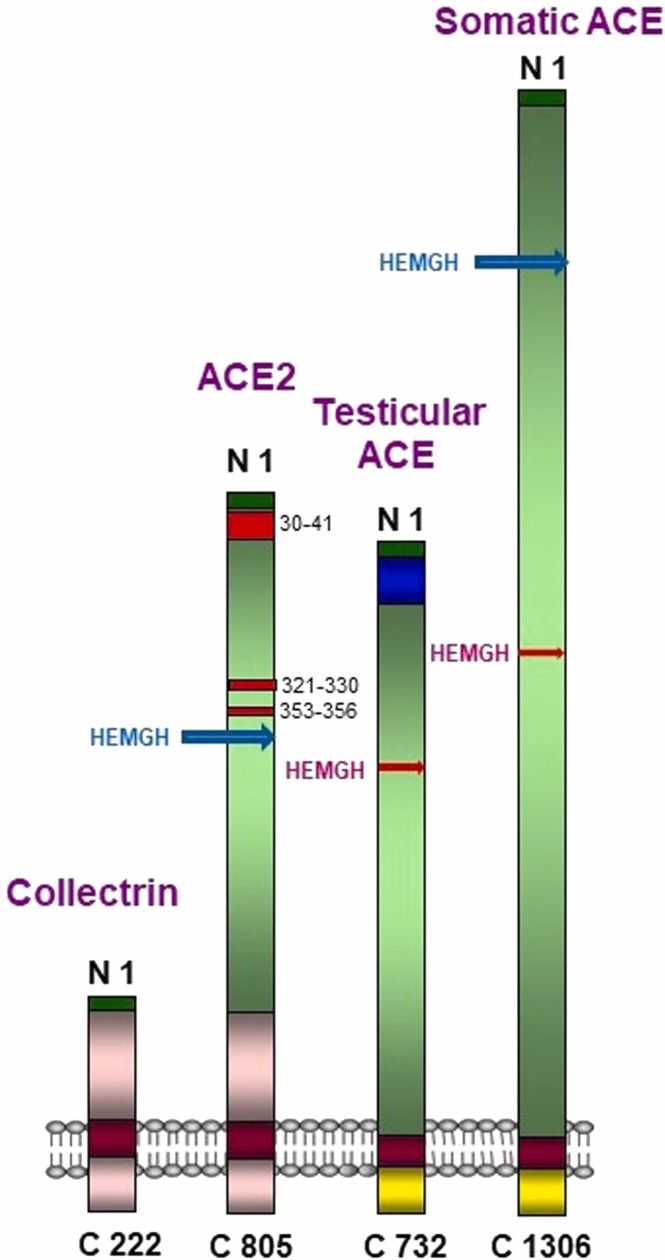
Two independent genomic screens led to the discovery, expression and characterization of human ACE2 [1], [2]. The predicted ACE2 protein sequence revealed characteristic features of a plasma membrane-bound zinc metallopeptidase with close resemblance to ACE, particularly in the catalytic region. Like ACE, ACE2 is a single-span transmembrane protein with an extracellular facing N-terminal catalytic site available to hydrolyse circulating vasoactive and other regulatory peptides ( Fig. 1). However, unlike the vascular or somatic form of ACE, which exists as a membrane protein with duplicated extracellular catalytic site regions (N- and C-domains), ACE2, like the Drosophila ACE proteins, is a single active site protein with its catalytic site resembling the N domain of ACE. An alternative truncated human ACE transcript that is expressed in testis also occurs as a single active site protein resembling the C-domain of ACE and is essential for male fertility. The C-terminal region of ACE2, comprising its short cytoplasmic region, transmembrane anchor and a part of the extracellular domain, resembles a quite distinct protein, collectrin, involved in amino acid transport. Hence ACE2 was revealed as more than just an enzyme being a fusion of two distinct physiological activities. Expression of the ACE2 gene was shown to be high in heart, as expected, kidney and testis [1] with presence also in lung, liver and the gut but many subsequent studies have shown a much broader distribution consistent with the known routes of infection and widespread tissue damage and inflammation seen in severe cases of COVID-19 [3], [11].
Fig. 1.
Schematic representation of the ACE/ACE2 protein family. The active sites (containing the zinc-binding amino acid motif HEMGH) of the N domains in ACE and ACE2 are shown in blue and of the C domains in the somatic and testicular ACE are shown in red. The numbers in the ACE2 extracellular domain correspond to the SARS-CoV-2 binding sites [99]. The ACE2 C-terminal region resembles that of the protein collectrin (shown in pink in both proteins). Collectrin shares no similarity with testicular or somatic ACE. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The crystal structure for the catalytic domain of ACE2 in complex with an inhibitor, MLN-4760 [12], followed relatively soon after that for ACE [13] and comparisons with ACE revealed the structural basis for the distinct catalytic differences between the two enzymes and also aided in the screening for novel ACE2 inhibitors (e.g. [14]). A major functional catalytic difference between ACE and ACE2 is in their substrate specificities and hence in their respective physiological roles [15]. In essence, ACE2 is a zinc monocarboxypeptidase removing a single C-terminal amino acid from a susceptible peptide whereas ACE removes a C-terminal dipeptide from its various substrates (peptidyl dipeptidase activity). Hence, the two enzymes play quite distinct roles in their principal metabolic pathway, the RAS, and in metabolism of other regulatory peptides. ACE2 is unique among mammalian carboxypeptidases in containing the zinc motif, HEXXH, but in this respect it resembles the bacterial M32 family Taq carboxypeptidase [16]. Bacterial (M32) carboxypeptidases, like ACE2, are also able to catalyse conversion of Ang II to Ang-(1−7) [17], [18] and could possibly be engineered to treat hypertension and cardiac dysfunction [18].
2. ACE2 and the RAS
The concept of the RAS as a simple linear pathway in which the decapeptide angiotensin I is cleaved from the precursor protein angiotensinogen and is, in turn, hydrolysed by ACE to the vasoconstrictor octapeptide, Ang II, had to be modified in the light of the discovery in the 1980 s of Ang-(1−7), which counterbalances the actions of Ang II via a receptor distinct from the AT1 and AT2 receptors that mediate the actions of Ang II (reviewed in [19]). Two major questions in the field remained unanswered through the 1990s but were subsequently resolved with the discovery of ACE2 as the Ang-(1−7) generating activity [1], [2] and the identification of the Mas oncogene, first reported in 1986 [20], as the endogenous Ang-(1−7) receptor [21].
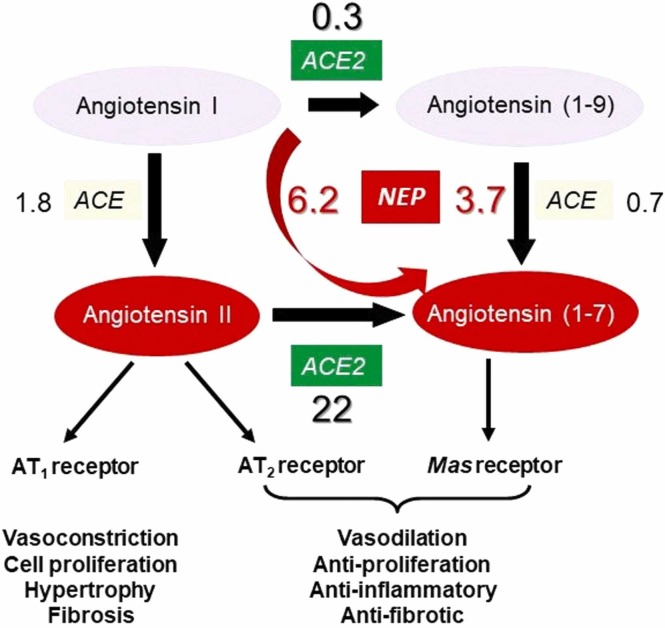
Rather than reinforcing each other in their actions, ACE2 counterbalances the vasoconstrictor role of ACE in the RAS through its production of the vasodilator Ang-(1−7) from Ang II and, as such, is cardioprotective ( Fig. 2). It also converts Ang I to Ang-(1−9) although two orders of magnitude less efficiently [15], [22]. NEP is also able to form Ang-(1−7) directly from Ang I [23]. A key role for ACE2 in cardiovascular regulation was confirmed following generation of ACE2-null mice, which showed major cardiac defects [24].
Fig. 2.
Major components of the RAS pathway. ACE and ACE2 play counterbalancing roles in metabolism of the angiotensin family peptides. The major role of ACE2 is to convert the vasoconstrictor peptide Ang II to a protective peptide Ang-(1−7). NEP can also produce Ang-(1−7) from Ang I as such contributing to its protective role. The binding of SARS-CoV-2 to ACE2 will reduce its ability to produce Ang-(1−7) leading to the pathology of COVID-19. In this condition NEP will play a compensatory role in partially maintaining the RAS balance. The numbers in the figures represent the relative efficiency of hydrolysis of the peptides by the respective enzymes (kcat/Km) [15].
Apart from involvement in the RAS, ACE2 hydrolyses a number of other regulatory peptides, in particular apelins, des-Arg9-bradykinin (but not bradykinin itself), neurotensin metabolites, dynorphin and ghrelin [4]. Apelin is also a positive regulator of ACE2 and can stimulate ACE2 transcription and enzyme activity [25], reviewed in [26]. In all cases ACE2 functions as a strict carboxypeptidase and the enzyme is not inhibited by classical anti-hypertensive ACE inhibitors such as captopril [1]. The physiological significance of ACE2 in metabolism of peptides other than in the RAS is much less explored although involvement in the kallikrein-kinin system has been reviewed elsewhere [27]. The physiological actions of ACE2 do not just impact on the cardiovascular system but are more widespread influencing, for example, liver, gut and skeletal muscle function [28], [29], [30].
3. ACE2, collectrin and amino acid transport
The cloning and sequencing of ACE2 confirmed its identity as the main enzyme in the RAS involved in the biosynthesis of Ang-(1−7) but also revealed that it plays another key physiological function. The C-terminal domain of ACE2, including the cytoplasmic, transmembrane and part of its extracellular regions, bears no similarity with ACE but shares 48% identity with a kidney protein, collectrin (also known as Tmem27), whose gene is located next to ACE2 on chromosome Xp22 whereas ACE is present on human chromosome 17. Collectrin functions in renal amino acid transport acting as an essential chaperone of neutral amino acid transporters such as B0AT1 and B0AT3 to the cell surface controlling their protein expression [31]. Similarly, for the transport of B0AT1 to the intestinal plasma membrane, ACE2 plays an analogous role [32]. Hence, ACE2 is important for maintaining intestinal amino acid homeostasis and the gut microbiome [33], [34]. Furthermore, defects in ACE2 lead to deficiencies in tryptophan transport and hence serotonin biosynthesis and neurogenesis [35].
4. ACE2 as coronaviral receptor
A third key action of ACE2 is its ability to interact with some coronaviruses. The first evidence that ACE2 may serve as a cell surface receptor for the SARS virus, mediating viral RNA entry, was published soon after the outbreak of the epidemic of severe acute respiratory syndrome (hence, SARS) in 2003 [36] and confirmed functionally as a genuine SARS-CoV receptor [37]. Later, it was shown that ACE2 also binds another prevalent human respiratory coronavirus, CoV-NL63 [38]. ACE2 also binds the most recent COVID-19 pandemic causative virus SARS-CoV-2 with higher affinity than it binds the SARS-CoV [39].
Interaction between ACE2 and the virus occurs via a viral spike (S) protein which has a receptor-binding domain (RBD) interacting with ACE2. Mutations in the spike protein of SARS-CoV-2 can result in appearance of new variants of the virus [40], [41]. The structure and amino acid specificity in the binding site of the spike protein determines receptor tropism and also cell entry via membrane fusion followed by cleavage of the S protein into S1 and S2 subunits by a serine protease, TMPRSS2 [42]. The S2 subunit is required for fusion of the virus with the host cell followed by the cell entry of viral RNA. Apart from proteolytic cleavage of the viral S protein by TMPRSS2 along with another serine protease, HAT, it can also cleave ACE2 between arginine and lysine residues within the ACE2 amino acid sequence 697–716, which is essential for viral entry, as shown for the SARS virus [43]. Binding of SARS viruses downregulates presentation of ACE2 on the cell surface and this loss through endocytosis results in significant changes in cellular functions leading to a cascade of pathological reactions typical of the viral pathology such as the prevalent cardiovascular, lung and general inflammatory responses [44]. Disease severity in COVID-19 cases shows a sex bias with aged males tending to suffer worse disease progression. This correlates with the elevated ACE2 levels seen in aged males in various organs and with the diversity of tissues affected in the disease [45].
The ACE2-null mouse serves as an excellent animal model for studying the cytokine storm-causing inflammation in severe lung injury as seen in SARS-CoV-2 infection [46], [47]. Reduced ACE2 levels in the tissues caused by SARS-CoV-2 virus binding also result in deficit of Ang-(1−7) synthesis, shifting the balance towards NEP-driven Ang-(1−7) production. This allows us to consider NEP as a possible protective player in COVID-19 pathology [48]. However, as a consequence, caution may be needed in the treatment of heart failure with the combined NEP inhibitor/angiotensin receptor blocker, Entresto (sacubitril/valsartan), in COVID situations. However, it has been argued that there may be benefits to administration of Entresto in severe COVID cases during disease treatment [49].
5. ACE2 in the brain
ACE2 was shown to be expressed at mRNA levels in a variety of brain structures [11] with detectable amounts at the protein level originally observed mainly in brain endothelial and smooth muscle cells [50] as well as in glia [51] and subsequently in neurons [52]. The role of ACE2 in the brain RAS system has been reviewed in [53]. Apart from its role in regulation of blood pressure via acting on the sympathetic nervous system, ACE2 is also involved in regulation of various cerebral functions, including neuro-inflammation, reaction to stress and anxiety-like behaviour, neuronal plasticity, learning and memory [54], [55]. ACE2 overexpression was shown to reduce anxiety-like behaviour in mice via activation of Mas receptors and facilitating GABA release in the basal-lateral amygdala [56]. ACE2 expression was also found in nociceptors suggesting that it can participate in pain perception [57].
Importantly, in humans significant ACE2 expression was reported in the neuroepithelium of olfactory bulbs which was higher than in the cells of the upper airway epithelial cells [58]. This might explain the loss of olfaction upon binding of the SARS-CoV-2 virus in COVID-19 patients [59]. In mouse, single cell sequencing demonstrated that ACE2 is mainly expressed in support cells, stem cells, and perivascular cells rather than in neurons suggesting that binding of the virus to these cells might also impair olfaction [60]. Although it is still not certain how SARS-Co-V2 can enter the brain [61] its binding to ACE2 in the nervous system will have a significant impact on numerous physiological systems.
Apart from the central role in the brain RAS which is considered a possible therapeutic target in neurodegeneration and AD [62], another important aspect of ACE2 enzymology derives from its participation in Aβ catabolism. ACE2, as a carboxypeptidase, can convert a longer form of amyloid peptide Aβ43 to the more toxic Aβ42 which, in turn, can be shortened by ACE to even less toxic Aβ40 [63] which is further cleaved by a major brain amyloid-degrading enzyme NEP (for review see [8]). ACE2 activity was shown to be reduced in human AD brain inversely correlating with accumulation of Aβ [64] which suggests that AD pathology not only affects the brain RAS system but also amyloid metabolism. Activation of ACE2 by i.p. administration of a reported ACE2 activator, diminazene aceturate (DIZE) [65] to aged Tg2576 mice modelling AD demonstrated a significant reduction in Aβ levels, especially in the hippocampus, and reversion of cognitive deficit of the animals [66]. ACE2 and Ang-(1−7), may also be protective in Parkinson’s disease by reducing α-synuclein aggregation through alleviating dysfunctional autophagy [67]. Again, ACE2 activation may provide a therapeutic approach.
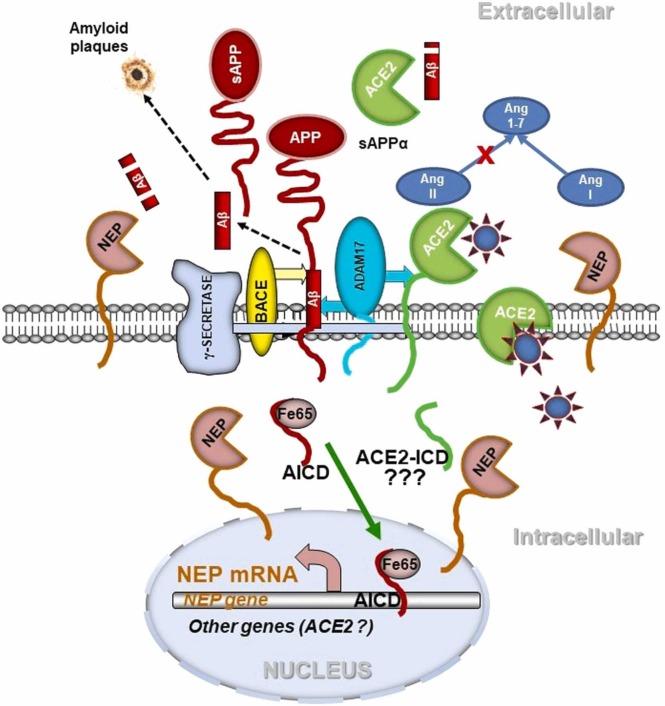
With regard to Aβ metabolism, it is important to note here that ACE2 works again in cohort with NEP resulting in more efficient removal of toxic amyloid species. NEP expression in neuronal cells is regulated by a feed-back mechanism whereby amyloidogenic processing of the amyloid precursor protein (APP) by the consecutive action of β-secretase (BACE) and γ-secretase, which produces Aβ, also releases the APP intracellular domain (AICD) ( Fig. 3). AICD, together with a stabilising protein Fe65, translocates to the nucleus and binds to the NEP promoter activating gene expression [68], [69]. AICD can also regulate expression of numerous other genes including the Aβ transporter protein transthyretin [70]. Whether ACE2 can be regulated by this mechanism, is still unknown but merits further investigation. However, in astrocytes ACE2 can be transcriptionally regulated by Ang II and Ang-(1−7) [51] suggesting existence of a feedback mechanism.
Fig. 3.
Possible mechanisms of involvement of the RAS system in angiotensin and amyloid metabolism in the brain upon SARS-CoV-2 virus infection. ACE2 in neuronal cells is involved in conversion of Ang II to Ang-(1−7) and degradation of Aβ43 peptide to Aβ42 which is further degraded by NEP. NEP also produces Ang-(1−7) from Ang I. Upon virus binding to ACE2, production of the protective Ang-(1−7) or cleavage of Aβ43 is diminished. ACE2 can be shed from the cell surface by ADAM-17. ADAM-17 also initiates non-amyloidogenic processing of APP preventing Aβ formation. The amyloidogenic pathway of APP processing, involving β-secretase (BACE) and γ-secretase, produces Aβ which can aggregate into amyloid plaques. It also releases the APP C-terminal fragment AICD, which is stabilized by Fe65 and translocated to the nucleus where it regulates gene expression, in particular of the amyloid-degrading enzyme NEP [56], [57], [58]. An ACE2 intracellular domain (ACE2-ICD) formed by γ-secretase might act similarly [110], [111].
6. Regulation of ACE2 expression, localisation and activity in relation to COVID-19
In polarized cells such as in kidney, ACE2 is primarily localized to the apical surface whereas ACE is equally distributed between apical and basolateral domains [71]. As an ectoenzyme ACE2 is susceptible to post-translational proteolytic cleavage from the cell-surface releasing a soluble and catalytically active form of the protein (sACE2) into the extracellular space, a process known as “shedding” catalysed by a membrane protein secretase or “sheddase”. ACE, in particular, has been known to exist as a soluble form in plasma since its original discovery and characterization in 1956 in horse plasma [72] and hence plasma ACE activity can be used to monitor the efficacy of ACE inhibitor anti-hypertensive therapy. Other cell-surface vasopeptidases can also be regulated by shedding, for example ECE-1 [73] and NEP [74]. In the case of ACE2, its principal sheddase has been identified as a zinc metalloprotease, a disintegrin and metalloproteinase-17 (ADAM-17) (also known as α-secretase (Fig. 3)) by using overexpression, siRNA and selective ADAM inhibitor treatments [75]. The corresponding sheddase for ACE has still not been unequivocally determined. The ACE2 shedding process is regulated by calmodulin which binds to the cytoplasmic domain of the enzyme inhibiting shedding; this interaction is reduced by calmodulin inhibitors, hence stimulating shedding [76]. Ang II also induces ADAM-17-mediated shedding of ACE2 providing a positive feed-back mechanism in the RAS [77]. The normal plasma level of sACE2 is extremely low with a mean value in human subjects of 33pM compared with sACE levels of around 7 nM and sNEP levels of 0.3 nM [22]. A trend to increasing sACE2 levels seen in COVID-19 disease progression appears to be associated with increased acute cardiac injury and risk of death suggesting that reduction of ADAM-17 activity may be therapeutically useful [78]. Coronavirus binding to its receptor, ACE2, can also trigger ACE2 internalisation as first shown in the case of the original SARS-CoV virus [37], [79] and confirmed also with SARS-Co-V-2 [80] contributing to the cardiovascular consequences. As with ACE2 shedding, internalisation, as induced for example through virus-induced endocytosis, provides an alternative and important process removing ACE2 from the cell-surface which will, in turn, trigger dysbalance in vasoactive peptide metabolism.
SARS-CoV-2 stimulation of ACE2 shedding in the lung may be a contributory factor to the COVID-induced acute respiratory distress syndrome. The shed, or soluble, form of ACE2 likely plays a role in response to COVID infection (see below). sACE2, through its RGD motif, also binds integrins and hence may influence cell-cell interactions [81]. Likewise, SARS-CoV-2 spike protein possesses an RGD motif and activates integrins, which is an essential part of the infection process [82]. The integrin-binding motif is present at the surface of the spike protein, close to the ACE2 receptor-binding region [83]. This motif is absent from other known coronaviruses.
Epigenetics may play an important role in COVID-19 pathogenic mechanisms [84]. In particular, expression of the ACE2 transcript is controlled by the activity of cytokines via SIRT1 (silent information regulator T1) under conditions of cell energy stress such as hypoxia when ACE2 is upregulated [85]. The epigenetic regulation of ACE2 is recently reviewed in [86]. ACE2 has also been reported to be regulated post-transcriptionally by microRNAs, being down-regulated, for example, by miR-421 in human cardiac myofibroblasts [87] and in patients with chronic kidney disease [88]. Importantly, elevated levels of miRNA-200c-3p down-regulating ACE2 have been shown to be crucial in acute respiratory distress syndrome [89]. Hence, miRNA species may represent potential biomarkers and therapeutic targets for severe respiratory infections such as COVID-19 [90]. Analysing the ACE2-SARS-CoV-2 interactome in human induced pluripotent stem cell-derived cardiomyocytes, Wicik and colleagues have identified several miRNAs which are common regulators in ACE2 and virus-related protein networks [91]. This suggests that SARS-CoV-2 infection can affect epigenetically expression of a number of proteins. Among the most affected genes were EGFR, HSP90AA1, fibronectin 1, TP53, and APP suggesting a link not only to heart failure, cancer and diabetes but also to AD pathology.
With regard to hypoxia which accompanies COVID-19 pathology it is important to note that ACE2 in addition to NEP plays an important role in sensing low oxygen levels in the carotid body which is the main oxygen-sensing organ [92]. Any decrease in ACE2 activity in this organ following viral infection can switch the AngII/Ang-(1−7) balance affecting the process of remodelling in this organ in response to hypoxia and impair respiratory regulation and hypoxia sensing. This disturbance in carotid body function might provide an alternative explanation why COVID-19 patients experience so called “happy” or “silent“ hypoxia and do not develop ventilatory response to very low oxygen levels in the body [93].
7. An evolutionary puzzle: ACE3
The occurrence of multiple ACE genes and protein products in various insect species led us to search for further ACE-like genes in the human genome which allowed us to identify a third gene (ACE3) which is located on chromosome 17 (17q23) just 30 kb downstream from the ACE gene itself [94]. However, although the gene encodes a predicted zinc-binding sequence, there are numerous gene deletions or insertions compared with the ACE gene, and there has been no report of an expressed ACE3 protein leading to the conclusion it is likely a pseudogene in humans. In contrast, in some other species (mouse, rat, dog, cow) the gene is expressed although key catalytic residues in the protein are changed, in particular the catalytic Glu residue is changed to Gln, and there is no evidence that any expressed protein has catalytic activity. It is possible, however, that the expressed protein could act as a peptide receptor. In the mouse genome, the ACE3 gene is located just 9 kb downstream from the ACE gene on chromosome 11 (11E1). Functional activity has been ascribed to the mouse ACE3 protein providing protection against pressure overload-induced cardiac hypertrophy by blocking the MEK-ERK1/2 signalling pathway [95] and as a partner to the sperm protein IZUMO1 at the acrosomal cap area and hence contributing to sperm-egg fusion [96].
8. Advances and unanswered questions
While vaccine development has provided the first line of anti-microbial defence against SARS-CoV-2 infection, more targeted interventions would provide alternative options. With the discovery that the receptor for both the SARS and SARS-CoV-2 viruses was ACE2, the acute lung injury seen in COVID could be attributed to the dysregulation of the RAS due to loss of ACE2 activity and consequent enhancement of the pro-inflammatory arm of the pathway. Rebalancing the arms of the RAS could hence mediate a protective strategy [97]. In this regard two approaches have emerged: the use of recombinant human, soluble ACE2 as a “decoy” receptor for the virus [98] or the design and application of inhibitors of ACE2-spike protein binding based on the known structural biology of virus-ACE2 interactions [99]. In particular, clinical trials of sACE2 show promise and even the possibility of developing an aerosol-administered form of the recombinant protein is currently in progress. Such an approach appears to have promise in protecting against a range of viral variants in the future [41], [100], [101]. While the application of ACE2 inhibitors may have limited use in cardiovascular therapeutics because of diminishing the cardioprotective role of ACE2, there may be scope for further development of reported ACE2 activators such as DIZE. Indeed, several small molecule activators have been proposed which have a beneficial effect on blood pressure and myocardial function [102] and might well pave the path to designing potent drugs to combat COVID-19. However, the precise mechanism of action of some of the reported ACE2 activators has been questioned [103].
Recent reports have highlighted the possibility of coronaviral infection triggering an autoimmunity reaction to soluble ACE2 [104], [105] whose plasma levels increase with age [22] and are particularly elevated in critically ill COVID-19 patients [106]. Such an autoimmune reaction could, at least in part, explain the severity of the infection and tissue inflammatory response in some individuals. Such a hypothesis merits further investigation.
Another new development in ACE2 biology has been the detection of an isoform of ACE2 which is a truncated variant whose expression is upregulated in response to interferon treatment [107], [108]. This so-called delta isoform lacks a functional catalytic site and coronavirus spike protein binding site. The variant is particularly localised to lung airway epithelia and liver bile duct epithelia [109]. The physiological role of this isoform and whether its expression has any direct relevance to COVID infection or protection against disease remains to be elucidated.
Another aspect of ACE2 biology requiring further investigation is the relevance, if any, of a cleaved intracellular domain of ACE2 which might act as a transcriptional regulator analogous to the intracellular domain of APP (AICD) or the notch receptor following ectodomain cleavage by a “sheddase” such as ADAM-17. Such a metabolic path could be a contributor to COVID-19 pathogenesis. A recent report [110] has demonstrated that, following ectodomain cleavage, ACE2 is cleaved by a γ-secretase-like activity releasing a soluble C-terminal fragment (endodomain) of the protein. Whether this intracellular fragment (ACE2-ICD) translocates to the nucleus possibly regulating transcription like AICD (Fig. 3) or is just subject to proteasomal degradation requires elucidation but adds to the ever-increasing complex biology of ACE2. A The possible relevance of such a regulatory pathway is summarized in [111]. The possibility of epigenetic regulation of ACE2 transcription by AICD or related ICDs, analogous to the regulation of the vasopeptidase NEP [68], [70], also merits investigation.
9. Conclusions
Thirty years ago, at the time of the first of the vasoactive peptide symposia, the occurrence and physiological relevance of Ang-(1−7) to the RAS was well established but the two key unknowns were the pathway to its biosynthesis and the cell receptor through which it acted. It was more than a decade before these two issues were unequivocally resolved with the identification and characterization of both ACE2 [1], [2] and the involvement of the Mas receptor [21]. Both have produced significant biological challenges and advances: in the case of ACE2 in particular, its multiple biological roles as enzyme, amino acid transporter, and serendipitously as viral receptor. Most importantly, in the vasoactive peptide field, ACE2 functions as a cardioprotective agent counterbalancing the actions of ACE in the RAS. Furthermore, it plays key roles in the metabolism of other biologically active peptides, notably apelin. The advances in our understanding of the structural and cell biology of ACE2 have been enormous but no doubt there will be more surprises in store with regard to the functions of this protein and its roles in health and disease, particularly in relation to COVID-19 and its treatment.
Author’s contribution
Both authors contributed equally to conceptualization, writing and editing of the manuscript, and designing the figures.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
No funding sources involved.
References
- 1.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 2.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 3.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper N.M., Lambert D.W., Turner A.J. Discovery and characterization of ACE2 - a 20-year journey of surprises from vasopeptidase to COVID-19. Clin. Sci. 2020;134(18):2489–2501. doi: 10.1042/CS20200476. [DOI] [PubMed] [Google Scholar]
- 5.Bader M., Turner A.J., Alenina N. ACE2, a multifunctional protein - from cardiovascular regulation to COVID-19. Clin. Sci. 2020;134(23):3229–3232. doi: 10.1042/CS20201493. [DOI] [PubMed] [Google Scholar]
- 6.Bland N.D., Pinney J.W., Thomas J.E., Turner A.J., Isaac R.E. Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships. BMC Evol. Biol. 2008;8:16. doi: 10.1186/1471-2148-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalivaeva N.N., Zhuravin I.A., Turner A.J. Neprilysin expression and functions in development, ageing and disease. Mech. Ageing Dev. 2020;192 doi: 10.1016/j.mad.2020.111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nalivaeva N.N., Turner A.J. Targeting amyloid clearance in Alzheimer's disease as a therapeutic strategy. Br. J. Pharmacol. 2019;176(18):3447–3463. doi: 10.1111/bph.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaac R.E., Siviter R.J., Stancombe P., Coates D., Shirras A.D. Conserved roles for peptidases in the processing of invertebrate neuropeptides. Biochem. Soc. Trans. 2000;28(4):460–464. PMID: 10961940. [PubMed] [Google Scholar]
- 10.Siviter R.J., Nachman R.J., Dani M.P., Keen J.N., Shirras A.D., Isaac R.E. Peptidyl dipeptidases (Ance and Acer) of Drosophila melanogaster: major differences in the substrate specificity of two homologs of human angiotensin I-converting enzyme. Peptides. 2002;23(11):2025–2034. doi: 10.1016/s0196-9781(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 11.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 532(1-2) 2002:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 12.Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., Patane M.A., Pantoliano M.W. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279(17):17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natesh R., Schwager S.L., Sturrock E.D., Acharya K.R. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature. 2003;421(6922):551–554. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- 14.Rella M., Rushworth C.A., Guy J.L., Turner A.J., Langer T., Jackson R.M. Structure-based pharmacophore design and virtual screening for novel angiotensin converting enzyme 2 inhibitors. J. Chem. Inf. Model. 2006;46(2):708–716. doi: 10.1021/ci0503614. [DOI] [PubMed] [Google Scholar]
- 15.Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem. J. 2004;383(1):45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner A.J., Tipnis S.R., Guy J.L., Rice G., Hooper N.M. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can. J. Physiol. Pharmacol. 2002;80(4):346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 17.Lee M.M., Isaza C.E., White J.D., Chen R.P., Liang G.F., He H.T., Chan S.I., Chan M.K. Insight into the substrate length restriction of M32 carboxypeptidases: characterization of two distinct subfamilies. Proteins. 2009;77(3):647–657. doi: 10.1002/prot.22478. [DOI] [PubMed] [Google Scholar]
- 18.Minato T., Nirasawa S., Sato T., Yamaguchi T., Hoshizaki M., Inagaki T., Nakahara K., Yoshihashi T., Ozawa R., Yokota S., Natsui M., Koyota S., Yoshiya T., Yoshizawa-Kumagaye K., Motoyama S., Gotoh T., Nakaoka Y., Penninger J.M., Watanabe H., Imai Y., Takahashi S., Kuba K. B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction. Nat. Commun. 2020;11(1):1058. doi: 10.1038/s41467-020-14867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos R.A.S., Sampaio W.O., Alzamora A.C., Motta-Santos D., Alenina N., Bader M., Campagnole-Santos M.J. The ACE2/Angiotensin-(1–7)/MAS Axis of the renin-angiotensin system: focus on angiotensin-(1–7) Physiol. Rev. 2018;98(1):505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young D., Waitches G., Birchmeier C., Fasano O., Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45:711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 21.Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V., Lopes M.T., Bader M., Mendes E.P., Lemos V.S., Campagnole-Santos M.J., Schultheiss H.P., Speth R., Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA. 2003;100(14):8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice G.I., Jones A.L., Grant P.J., Carter A.M., Turner A.J., Hooper N.M. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48(5):914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- 23.Vaghy P.L., Russell J.S., Lantry L.E., Stephens R.E., Ward P.E. Angiotensin and bradykinin metabolism by peptidases identified in cultured human skeletal muscle myocytes and fibroblasts. Peptides. 1995;16(8):1367–1373. doi: 10.1016/0196-9781(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 24.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 25.Sato T., Suzuki T., Watanabe H., Kadowaki A., Fukamizu A., Liu P.P., Kimura A., Ito H., Penninger J.M., Imai Y., Kuba K. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Invest. 2013;123(12):5203–5211. doi: 10.1172/JCI69608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee P., Gheblawi M., Wang K., Vu J., Kondaiah P., Oudit G.Y. Interaction between the apelinergic system and ACE2 in the cardiovascular system: therapeutic implications. Clin. Sci. 2020;134(17):2319–2336. doi: 10.1042/CS20200479. [DOI] [PubMed] [Google Scholar]
- 27.Abassi Z., Skorecki K., Hamo-Giladi D.B., Kruzel-Davila E E., Heyman S.N. Kinins and chymase: the forgotten components of the renin-angiotensin system and their implications in COVID-19 disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021;320(3):L422–L429. doi: 10.1152/ajplung.00548.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner F.J., Rajapaksha H., Shackel N., Herath C.B. ACE2: from protection of liver disease to propagation of COVID-19. Clin. Sci. 2020;134(23):3137–3158. doi: 10.1042/CS20201268. [DOI] [PubMed] [Google Scholar]
- 29.Perlot T., Penninger J.M. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15(13):866–873. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto K., Takeshita H., Rakugi H. ACE2, angiotensin 1-7 and skeletal muscle: review in the era of COVID-19. Clin. Sci. 2020;134(22):3047–3062. doi: 10.1042/CS20200486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danilczyk U., Sarao R., Remy C., Benabbas C., Stange G., Richter A., Arya S S., Pospisilik J.A., Singer D D., Camargo S.M., Makrides V., Ramadan T., Verrey F., Wagner C.A., Penninger J.M. Essential role for collectrin in renal amino acid transport. Nature. 2006;444(7122):1088–1091. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 32.Singer D., Camargo S.M. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels ((Austin)) 2011;5(5):410–423. doi: 10.4161/chan.5.5.16470. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., Wild B., Camargo S.M., Singer D., Richter A., Kuba K., Fukamizu A., Schreiber S., Clevers H., Verrey F., Rosenstiel P., Penninger J.M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penninger J.M., Grant M.B., Sung J.J.Y. The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Gastroenterology. 2021;160(1):39–46. doi: 10.1053/j.gastro.2020.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klempin F., Mosienko V., Matthes S., Villela D.C., Todiras M., Penninger J.M., Bader M., Santos R.A.S., Alenina N. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell. Mol. Life Sci. 2018;75(19):3625–3634. doi: 10.1007/s00018-018-2815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu K., Li W., Peng G., Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc. Natl. Acad. Sci. U. S. A. 2009;106(47):19970–19974. doi: 10.1073/pnas.0908837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L., Jackson C.B., Mou H., Ojha A., Peng H., Quinlan B.D., Rangarajan E.S., Pan A., Vanderheiden A., Suthar M.S., Li W., Izard T., Rader C., Farzan M., Choe H. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020;11(1):6013. doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann M., Kleine-Wever H., Kruger N., Muller M., Drotsten C., Pholhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry in target cells. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.05. [DOI] [Google Scholar]
- 45.Viveiros A., Gheblawi M., Aujla P.K., Sosnowski D.K., Seubert J.M., Kassiri Z., Oudit G.Y. Sex- and age-specific regulation of ACE2: insights into severe COVID-19 susceptibility. J. Mol. Cell. Cardiol. 2021;164:13–16. doi: 10.1016/j.yjmcc.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S., McCray P.B., Jr, Chappell M., Hackam D.J., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314(1):L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohammed El Tabaa M., Mohammed El Tabaa M. Targeting neprilysin (NEP) pathways: a potential new hope to defeat COVID-19 ghost. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellis A., Mauro C., Barbato E., Trimarco B., Morisco C. The rationale for angiotensin receptor neprilysin inhibitors in a multi-targeted therapeutic approach to COVID-19. Int. J. Mol. Sci. 2020;21(22):8612. doi: 10.3390/ijms21228612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallagher P.E., Chappell M.C., Ferrario C.M., Tallant E.A. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am. J. Physiol. Cell. Physiol. 2006;290(2):C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 52.Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008;107(6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohammed M., Berdasco C., Lazartigues E. Brain angiotensin converting enzyme-2 in central cardiovascular regulation. Clin. Sci. 2020;134(19):2535–2547. doi: 10.1042/CS20200483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Bohlen Und Halbach O. The angiotensin converting enzyme 2 (ACE2) system in the brain: possible involvement in Neuro-Covid. Histol. Histopathol. 2021:18356. doi: 10.14670/HH-18-356. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., de Kloet A.D., Pati D., Hiller H., Smith J.A., Pioquinto D.J., Ludin J.A., Oh S.P., Katovich M.J., Frazier C.J., Raizada M.K., Krause E.G. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology. 2016;105:114–123. doi: 10.1016/j.neuropharm.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiers S., Ray P.R., Wangzhou A., Sankaranarayanan I., Esteves Tatsui C., Rhines L.D., Li Y., Uhelski M.L., Dougherty P.M., Price T.J. ACE2 and SCARF expression in human dorsal root ganglion nociceptors: implications for SARS-CoV-2 virus neurological effects. Pain. 2020;161(11):2494–2501. doi: 10.1097/j.pain.0000000000002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen M., Shen W., Rowan N.R., Kulaga H., Hillel A., Ramanathan M., Jr., Lane A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schweitzer F., Kleineberg N.N., Göreci Y., Onur O.A., Franke C., Warnke C. Neuro-COVID-19 is more than anosmia: clinical presentation, neurodiagnostics, therapies, and prognosis. Curr. Opin. Neurol. 2021;34(3):423–431. doi: 10.1097/WCO.0000000000000930. [DOI] [PubMed] [Google Scholar]
- 60.Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.-J., Fletcher R.B., Das D., Street K., Roux de Bezieux H., Choi Y.-G., Risso D., Dudoit S., Purdom E., Mill J., Abi Hachem R., Matsunami H., Logan D.W., Goldstein B.J., Grubb M.S., Ngai J., Datta S.R. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6(31):eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burks S.M., Rosas-Hernandez H., Ramirez-Lee M.A., Cuevas E., Talpos J.C. Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain Behav. Immun. 2021;95:7–14. doi: 10.1016/j.bbi.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loera-Valencia R., Eroli F., Garcia-Ptacek S., Maioli S. Brain renin-angiotensin system as novel and potential therapeutic target for Alzheimer’s disease. Int. J. Mol. Sci. 2021;22(18):10139. doi: 10.3390/ijms221810139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu S., Liu J., Miura Y., Tanabe C., Maeda T., Terayama Y., Turner A.J., Zou K., Komano H. Conversion of Aβ43 to Aβ40 by the successive action of angiotensin-converting enzyme 2 and angiotensin-converting enzyme. J. Neurosci. Res. 2014;92(9):1178–1186. doi: 10.1002/jnr.23404. [DOI] [PubMed] [Google Scholar]
- 64.Kehoe P.G., Wong S., Al Mulhim N., Palmer L.E., Miners J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer's disease in association with increasing amyloid-β and tau pathology. Alzheimers Res. Ther. 2016;8(1):50. doi: 10.1186/s13195-016-0217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulemina L.V., Ostrov D.A. Prediction of off-target effects on angiotensin-converting enzyme 2. J. Biomol. Screen. 2011;16(8):878–885. doi: 10.1177/1087057111413919. [DOI] [PubMed] [Google Scholar]
- 66.Evans C.E., Miners J.S., Piva G., Willis C.L., Heard D.M., Kidd E.J., Good M.A., Kehoe P.G. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020;139(3):485–502. doi: 10.1007/s00401-019-02098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Q., Chen R., Wu L., Huang Q., Wang X.X., Tian Y.Y., Zhang Y.D. Angiotensin-(1-7) reduces α-synuclein aggregation by enhancing autophagic activity in Parkinson's disease. Neural. Regen. Res. 2022;17(5):1138–1145. doi: 10.4103/1673-5374.324854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belyaev N.D., Nalivaeva N.N., Makova N.Z., Turner A.J. Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep. 2009;10(1):94–100. doi: 10.1038/embor.2008.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belyaev N.D., Kellett K.A.B., Beckett C., Makova N.Z., Revett T.J., Nalivaeva N.N., Hooper N.M., Turner A.J. The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a β-secretase-dependent pathway. J. Biol. Chem. 2010;285(53):41443–41454. doi: 10.1074/jbc.M110.141390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beckett C., Nalivaeva N.N., Belyaev N.D., Turner A.J. Nuclear signalling by membrane protein intracellular domains: the AICD enigma. Cell Signal. 2012;24(2):402–409. doi: 10.1016/j.cellsig.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Warner F.J., Lew R.A., Smith A.I., Lambert D.W., Hooper N.M., Turner A.J. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J. Biol. Chem. 2005;280(47):39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- 72.Skeggs L.T., Jr, Kahn J.R., Shumway N.P. The preparation and function of the hypertensin-converting enzyme. J. Exp. Med. 1956;103(3):295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuruppu S., Reeve S., Smith A.I. Characterisation of endothelin converting enzyme-1 shedding from endothelial cells. FEBS Lett. 2007;581(23):4501–4506. doi: 10.1016/j.febslet.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 74.Kuruppu S., Rajapakse N.W., Minond D., Smith A.I. Production of soluble Neprilysin by endothelial cells. Biochem. Biophys. Res. Commun. 2014;446(2):423–427. doi: 10.1016/j.bbrc.2014.01.158. [DOI] [PubMed] [Google Scholar]
- 75.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280(34):30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert D.W., Clarke N.E., Hooper N.M., Turner A.J. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008;582(2):385–390. doi: 10.1016/j.febslet.2007.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 78.Wang K., Gheblawi M., Nikhanj A., Munan M., MacIntyre E., O’Neil C., Poglitsch M., Colombo D., Del Nonno F., Kassiri Z., Sligl W., Oudit G.Y. Dysregulation of ACE (Angiotensin-converting enzyme)-2 and renin-angiotensin peptides in SARS-CoV-2 mediated mortality and end-organ injuries. Hypertension. 2022;79(2):365–378. doi: 10.1161/HYPERTENSIONAHA.121.18295. [DOI] [PubMed] [Google Scholar]
- 79.Wang S., Guo F., Liu K., Wang H., Rao S., Yang P., Jiang C. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008;136(1):8–15. doi: 10.1016/j.virusres.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamaguchi T., Hoshizaki M., Minato T., Nirasawa S., Asaka M.N., Niiyama M., Imai M., Uda A., Chan J.F., Takahashi S., An J., Saku A., Nukiwa R., Utsumi D., Kiso M., Yasuhara A., Poon V.K., Chan C.C., Fujino Y., Motoyama S., Nagata S., Penninger J.M., Kamada H., Yuen K.Y., Kamitani W., Maeda K., Kawaoka Y., Yasutomi Y., Imai Y., Kuba K. ACE2-like carboxypeptidase B38-CAP protects from SARS-CoV-2-induced lung injury. Nat. Commun. 2021;12(1):6791. doi: 10.1038/s41467-021-27097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clarke N.E., Fisher M.J., Porter K.E., Lambert D.W., Turner A.J. Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simons P P., Rinaldi D.A., Bondu V., Kell A.M., Bradfute S., Lidke D.S., Buranda T. Integrin activation is an essential component of SARS-CoV-2 infection. Sci. Rep. 2021;11(1):20398. doi: 10.1038/s41598-021-99893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sigrist C.J., Bridge A., Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res. 2020;177 doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaneko S., Takasawa K., Asada K., Shinkai N., Bolatkan A., Yamada M., Takahashi S., Machino H., Kobayashi K., Komatsu M., Hamamoto R. Epigenetic mechanisms underlying COVID-19 pathogenesis. Biomedicines. 2021;9(9):1142. doi: 10.3390/biomedicines9091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarke N.E., Belyaev N.D., Lambert D.W., Turner A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin. Sci. 2014;126(7):507–516. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- 86.Beacon T.H., Delcuve G.P., Davie J.R. Epigenetic regulation of ACE2, the receptor of the SARS-CoV-2 virus. Genome. 2021;64(4):386–399. doi: 10.1139/gen-2020-0124. [DOI] [PubMed] [Google Scholar]
- 87.Lambert D.W., Lambert L.A., Clarke N.E., Hooper N.M., Porter K.E., Turner A.J. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin. Sci. 2014;2127(4):243–249. doi: 10.1042/CS20130420. [DOI] [PubMed] [Google Scholar]
- 88.Trojanowicz B., Imdahl T., Ulrich C., Fiedler R., Girndt M. Circulating miR-421 targeting leucocytic angiotensin converting enzyme 2 is elevated in patients with chronic kidney disease. Nephron. 2019;141(1):61–74. doi: 10.1159/000493805. [DOI] [PubMed] [Google Scholar]
- 89.Liu Q., Du J., Yu X., Xu J., Huang F., Li X., Zhang C., Li X., Chang J., Shang D., Zhao Y., Tian M., Lu H., Xu J., Li C., Zhu H., Jin N., Jiang C. miRNA-200c-3p is crucial in acute respiratory distress syndrome. Cell Discov. 2017 doi: 10.1038/celldisc.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paul S., Vázquez L.A.B., Reyes-Pérez P.R., Estrada-Meza C C., Alburquerque R.A.A., Pathak S., Banerjee A., Bandyopadhyay A., Chakraborty S S., Srivastava A. The role of microRNAs in solving COVID-19 puzzle from infection to therapeutics: a mini-review. Virus Res. 2021;14 doi: 10.1016/j.virusres.2021.198631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wicik Z., Eyileten C., Jakubik D., Simões S.N., Martins D.C., Pavão R., Siller-Matula J.M., Postula M. ACE2 interaction networks in COVID-19: A physiological framework for prediction of outcome in patients with cardiovascular risk factors. J. Clin. Med. 2020;9(11):3743. doi: 10.3390/jcm9113743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porzionato A., Emmi A., Stocco E., Barbon S., Boscolo-Berto R., Macchi V., De Caro R. The potential role of the carotid body in COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;319(4):L620–L626. doi: 10.1152/ajplung.00309.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nouri-Vaskeh M., Sharifi A., Khalili N., Zand R., Sharifi A. Dyspneic and non-dyspneic (silent) hypoxemia in COVID-19: Possible neurological mechanism. Clin. Neurol. Neurosurg. 2020;198 doi: 10.1016/j.clineuro.2020.106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rella M., Elliot J.L., Revett T.J., Lanfear J., Phelan A., Jackson R.M., Turner A.J., Hooper N.M. Identification and characterisation of the angiotensin converting enzyme-3 (ACE3) gene: a novel mammalian homologue of ACE. BMC Genomics. 2007;8:194. doi: 10.1186/1471-2164-8-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu C.J., Tang L.L., Liang C., Chen X., Song S.Y., Ding X.Q., Zhang K.Y., Song B.L., Zhao D., Zhu X.Y., Li H.L., Zhang Z.R. Angiotensin-converting enzyme 3 (ACE3) protects against pressure overload-induced cardiac hypertrophy. J. Am. Heart Assoc. 2016;5(2) doi: 10.1161/JAHA.115.002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inoue N., Kasahara T., Ikawa M., Okabe M. Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarzani R., Giulietti F., Di Pentima C., Giordano P., Spannella F. Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am. J. 2020;319(2):L325–L336. doi: 10.1152/ajplung.00189.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Monteil V., Kwon Y., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lubbe L., Cozier G.E., Oosthuizen D., Acharya K.R., Sturrock E.D. ACE2 and ACE: structure-based insights into mechanism, regulation and receptor recognition by SARS-CoV. Clin. Sci. 2020;134(21):2851–2871. doi: 10.1042/CS20200899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wirnsberger G., Monteil V., Eaton B., Postnikova E., Murphy M., Braunsfeld B., Crozier I., Kricek F., Niederhöfer J., Schwarzböck A., Breid H., Sanchez Jimenez A., Bugajska-Schretter A., Dohnal A., Ruf C., Gugenberger R., Hagelkruys A., Montserrat N., Holbrook M.R., Oostenbrink C., Shoemaker R.H., Mirazimi A A., Penninger J.M. Clinical grade ACE2 as a universal agent to block SARS-CoV-2 variants. bioRxiv. 2021;10 doi: 10.1101/2021.09.10.459744. 2021.09.10.459744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shoemaker R.H., Panettieri R.A., Jr, Libutti S.K., Hochster H.S., Watts N.R., Wingfield P.T., Starkl P., Pimenov L., Gawish R., Hladik A., Knapp S., Boring D., White J.M., Lawrence Q., Boone J., Marshall J.D., Matthews R.L., Cholewa B.D., Richig J.W., Chen B.T., McCormick D.L., Gugensberger R., Höller S., Penninger J.M., Wirnsberger G. Development of a novel, pan-variant aerosol intervention for COVID-19. bioRxiv. 2021;14 doi: 10.1101/2021.09.14.459961. 2021.09.14.459961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hernández Prada J.A., Ferreira A.J., Katovich M.J., Shenoy V., Qi Y., Santos R.A., Castellano R.K., Lampkins A.J., Gubala V., Ostrov D.A., Raizada M.K. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- 103.Haber P.K., Ye M., Wysocki J., Maier C., Haque S.K., Batlle D. ACE2-independent action of presumed ACE2 activators: studies in vivo, ex vivo and in vitro. Hypertension. 2014;63(4):774–782. doi: 10.1161/HYPERTENSIONAHA.113.02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McMillan P., Uhal B.D. COVID-19-A theory of autoimmunity to ACE-2. MOJ Immunol. 2020;7(1):17–19. PMID: 32656314. [PMC free article] [PubMed] [Google Scholar]
- 105.McMillan P., Dexhiemer T., Neubig R.R., Uhal B.D. COVID-19-A theory of autoimmunity against ACE-2 explained. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.582166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Lier D., Kox M., Santos K., van der Hoeven H., Pillay J., Pickkers P P. Increased blood angiotensin converting enzyme 2 activity in critically ill COVID-19 patients. ERJ Open Res. 2021;7(1):00848–02020. doi: 10.1183/23120541.00848-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Onabajo O.O., Banday A.R., Stanifer M.L., Yan W., Obajemu A., Santer D.M., Florez-Vargas O., Piontkivska H., Vargas J.M., Ring T.J., Kee C., Doldan P., Tyrrell D.L., Mendoza J.L., Boulant S., Prokunina-Olsson L. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat. Genet. 2020;52(12):1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blume C., Jackson C.L., Spalluto C.M., Legebeke J., Nazlamova L., Conforti F., Perotin M.J., Frank M., Butler J., Crispin M., Coles J., Thompson J., Ridley R.A., Dean L.S.N., Loxham M., Reikine S., Azim A., Tariq K., Johnston D.A., Skipp P.J., Djukanovic R., Baralle D., McCormick C.J., Davies D.E., Lucas J.S., Wheway G., Mennella V. A novel ACE2 isoform is expressed in human respiratory epithelia and is upregulated in response to interferons and RNA respiratory virus infection. Nat Genet. 2021;53(2):205–214. doi: 10.1038/s41588-020-00759-x. [DOI] [PubMed] [Google Scholar]
- 109.Williams T.L., Strachan G., Macrae R.G.C., Kuc R.E., Nyimanu D., Paterson A.L., Sinha S., Maguire J.J., Davenport A.P. Differential expression in humans of the viral entry receptor ACE2 compared with the short deltaACE2 isoform lacking SARS-CoV-2 binding sites. Sci. Rep. 2021;11(1):24336. doi: 10.1038/s41598-021-03731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartolomé A., Liang J., Wang P., Ho D.D., Pajvani U.B. Angiotensin converting enzyme 2 is a novel target of the gamma-secretase complex. Sci. Rep. 2021;11(1):9803. doi: 10.1038/s41598-021-89379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gonzalez S.M., Siddik A.B., Su R.C. Regulated intramembrane proteolysis of ACE2: a potential mechanism contributing to COVID-19 pathogenesis? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.612807. [DOI] [PMC free article] [PubMed] [Google Scholar]