Abstract
Introduction
Optical diagnosis is necessary when selecting the resection modality for large superficial colorectal lesions. The COlorectal NEoplasia Endoscopic Classification to Choose the Treatment (CONECCT) encompasses overt (irregular pit or vascular pattern) and covert (macroscopic features) signs of carcinoma in an all‐in‐one classification using validated criteria. The CONECCT IIC subtype corresponds to adenomas with a high risk of superficial carcinoma that should be resected en bloc with free margins.
Methods
This prospective multicentre study investigated the diagnostic accuracy of the CONECCT classification for predicting submucosal invasion in colorectal lesions >20 mm. Optical diagnosis before en bloc resection by endoscopic submucosal dissection (ESD) was compared with the final histological diagnosis. Diagnostic accuracy for the CONECCT IIC subtype was compared with literature‐validated features of concern considered to be risk factors for submucosal invasion (non‐granular large spreading tumour [NG LST], macronodule >1 cm, SANO IIIA area, and Paris 0‐IIC area).
Results
Six hundred 63 lesions removed by ESD were assessed. The en bloc, R0, and curative resection rates were respectively 96%, 85%, and 81%. The CONECCT classification had a sensitivity (Se) of 100%, specificity (Sp) of 26.2%, positive predictive value of 11.6%, and negative predictive value (NPV) of 100% for predicting at least submucosal adenocarcinoma. The sensitivity of CONECCT IIC (100%) to predict submucosal cancer was superior to all other criteria evaluated. COlorectal NEoplasia Endoscopic Classification to Choose the Treatment IIC lesions constituted 11.5% of all submucosal carcinomas.
Conclusion
The CONECCT classification, which combines covert and overt signs of carcinoma, identifies with very perfect sensitivity (Se 100%, NPV 100%) the 30% of low‐risk adenomas in large laterally spreading lesions treatable by piecemeal endoscopic mucosal resection or ESD according to expertise without undertreatment. However, the low specificity of CONECCT leads to a large number of potentially not indicated ESDs for suspected high‐risk lesions.
Keywords: colonoscopy, EMR, endoscopy, ESD, optical diagnosis, submucosal carcinoma
INTRODUCTION
Real‐time optical diagnosis of polyps enables the prediction of histology and selection of an optimal therapeutic approach. Several classification systems have been developed for colorectal polyps. The macroscopic aspect is included in the Paris 1 and LST 2 classifications, via white light imaging. An invasive carcinoma can be present despite the absence of an irregular pattern 3 in non‐granular Laterally spreading tumour (LST) (NG LST) or under a macronodule or an area of depression (Paris 0‐IIc). Closed analysis (chromoendoscopy with/without magnification) yields useful details, including the pit pattern of the Kudo classification 4 and the vascular patterns of the Sano, 5 NBI International Colorectal Endoscopic (NICE), 6 and JNET classifications. 7 , 8 A national evaluation confirmed the poor knowledge of the usual classifications, and many benign lesions are wrongly treated by surgery. 9 Therefore, physicians from the research and development committee of the Société Française d'Endoscopie Digestive (SFED) wanted to simplify optical diagnosis by combining macroscopic, pit, and vascular patterns with the validated criteria used in the Paris, LST, NICE, Kudo, JNET, and Sano classifications based on the latest European guidelines. 10 This CONECCT for COlorectal Neoplasia Endoscopic Classification to Choose the Treatmen (Figure 1) classification was first validated in a teaching program, 11 then validated by experts for optical diagnosis. The CONECCT classification had higher inter‐observer agreement and reproducibility than other classifications. 12
Key summary.
What is known?
Endoscopic characterisation is an essential precursor to colorectal lesion resection. Several classifications have been validated for lesions <2 cm, using chromoendoscopy under magnification. For lesions >2 cm, the risk of carcinoma is related to macroscopic features, but this has not been incorporated in the current SANO and Japan NBI Expert Team (JNET) classifications.
What is new here?
The CONECCT classification, which can be make use of any non‐magnifying endoscope, combines covert (macroscopic features) and overt signs of carcinoma (irregular pit or vascular patterns). It enables determination of the risk of submucosal carcinoma and selection of an endoscopic treatment for large superficial lesions with high sensitivity and a negative predictive value (NPV) of 100%. The CONECCT classification would decrease unnecessary surgery and non‐curative piecemeal endoscopic mucosal resection (EMR) in cases of submucosal cancer but increase the rate of endoscopic submucosal dissection (ESD) for benign lesions.
FIGURE 1.
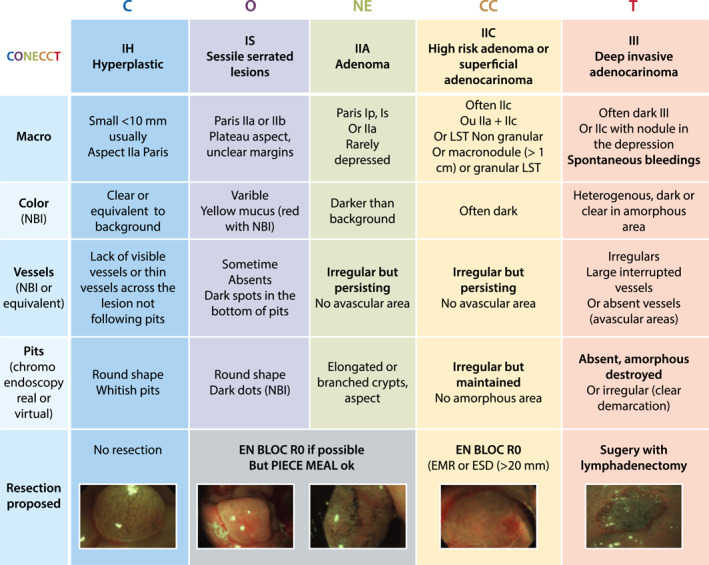
The COlorectal NEoplasia Endoscopic Classification to Choose the Treatment (CONECCT) classification
The European guidelines, 13 , 14 , 15 state that it is important to differentiate between lesions with a very low risk of undetected carcinoma, which should be resected by low‐risk endoscopic procedures, and lesions with a high risk of at least superficial carcinoma, which should be resected en bloc with free margins. En bloc resection allows correct pathological examination and avoids underestimation of the invasion depth. The CONECCT IIC subtype corresponds to lesions at high risk of submucosal carcinoma based on macroscopic aspects—such as NG LST or granular LST (G‐LST)—with a macronodule >10 mm or a depressed area (Paris O‐IIc) and because of an irregular mucosal/vascular pattern (Kudo Vi and Sano's IIIA; also type 2B of the JNET classification).
Here, we evaluated the diagnostic accuracy of the CONECCT classification (optical diagnosis) compared to pathological examination, in particular for predicting at least submucosal carcinoma in large laterally spreading colorectal lesions (>20 mm) removed by en bloc ESD.
MATERIALS AND METHODS
Study design
This prospective study included lesions (>20 mm) that were consecutively treated by ESD in two referral centres. The study was approved by the Institutional review board and the Ethics Committee of Limoges University Hospital (n° 87RI20‐0021_FECCo; NCT04592003). All patients were informed of their right to object to use of their data, which were collected and managed using Microsoft Excel (Microsoft).
Endoscopists and equipment
Four experienced operators performed optical diagnosis of lesions before resection by ESD. All operators had at least 5 years of experience in characterisation and were trainers in a teaching program for endoscopic characterisation. Before starting the study, each operator had experience comprising 50–100 colorectal ESDs in humans and animal models. The endoscopy suites were equipped with Olympus Exera III CV 190 units (190‐generation colonoscopes) or Fujifilm 700 Eluxeo units (760‐generation colonoscopes). All lesions were assessed by white light imaging and virtual chromoendoscopy without magnification.
Participants
We included all patients who were referred for endoscopic resection of a large lesion (>20 mm), regardless of its characteristics. In the participating centres, beginning in January 2016, we performed ESD for lesions >20 mm, for both benign lesions and lesions at risk of superficial cancer; this approach followed Japanese guidelines. 16
ESD allows endoscopic en bloc resection with negative margins (R0 resection). This facilitates pathological examination and prevents local recurrence, although it is associated with a higher risk of complications when performed by inexperienced endoscopists. 17
The exclusion criteria were adenocarcinoma associated with chronic inflammatory bowel disease, a recurrent or residual lesion after EMR, or prior piecemeal resection that created histological uncertainties and/or rendered characterisation incomplete because one or more of the LST, Paris, CONECCT, KUDO, SANO, and JNET classifications could not be applied.
Lesions associated with obvious deeply invasive adenocarcinomas (CONECCT III and SANO IIIB lesions) were analysed separately.
Lesion characterisation and data collection
All lesions were cleaned and subjected to both an overview and a close‐up examination under white light imaging. We performed virtual chromoendoscopy without magnification under optimal insufflation conditions. All lesions were described in real‐time using the Paris, LST, Sano, and CONECCT classifications. Patient data (age, sex), the type of endoscope used, and complete lesional data (size, location, type, presence of a macronodule >10 mm in diameter, Paris morphological analysis, Sano vascular pattern analysis, Kudo pit pattern analysis, and CONECCT type) were recorded immediately after the procedure by the operators performing the ESD.
Diagnostic gold standard
Diagnosis employed the CONECCT classification. A presumptive diagnosis was made in real‐time (before resection). The lesions were pinned on corks and sent to the pathologists for histological diagnosis (the gold standard) using 2 mm sections. The pathologists received information concerning lesion morphology, location, and size; they were not informed of the optical diagnoses. Histological diagnoses were performed within 10 days of resection, in line with the revised Vienna classification. 18 Serrated polyps were analysed using the criteria of the World Health Organization. 19 Each report included the histological diagnosis, resection margin details, the extent of malignant differentiation, the invasion depth, and any evidence of lymphatic/vascular emboli or budding. Submucosal invasion was measured as described by the Japanese guidelines 16 commencing from the lower edge of the muscularis mucosa. When the muscularis mucosa could not be identified, the depth of submucosal invasion was measured commencing from the mucosal layer. Lesions lacking deep invasion included those histologically diagnosed as hyperplastic polyps, sessile serrated lesions without dysplasia, low‐grade dysplasia (LGD, Vienna 3), carcinoma in situ (pTIS, Vienna 4), or adenocarcinomas with submucosal invasion ≤1000 μm (Vienna 5, pT1a). Deep invasion was diagnosed if the submucosal invasion depth was >1000 μm (Vienna 5, pT1b) or if the lesion invaded the muscularis propria (≥pT2).
Definitions
Laterally spreading tumours were defined in accordance with the Japanese classification as lesions >10 mm that were wider than high. NG‐LSTs featured smooth (non‐granular) surfaces and were further divided into slightly elevated LSTs (0‐IIa NG‐LST) or pseudo‐depressed (0‐IIc NG‐LST) LSTs. G‐LSTs featured granular surfaces that were otherwise homogenous (GH‐LSTs) or exhibited macronodules (granular mixed‐type LSTs [GM‐LSTs]). A protruding lesion was >2 cm and was more high than wide (Appendix A).
Outcomes
The principal outcome was the diagnostic accuracy of the CONECCT classification in terms of predicting invasive adenocarcinoma of the submucosa (or deeper) compared to histology.
The secondary outcomes were comparisons with other risk factors considered independently (macronodule >1 cm, Paris 0‐IIc area, NG‐LST, and SANO IIIA), comparisons of the endoscopes used (Olympus/NBI or Fujifilm/Blue laser imaging [BLI]), and the development of risk tables.
Sample size and statistical analysis
Statistical analyses were performed with the aid of Stata ver. Twelve software (Stata Corporation, College Station). A p‐value <0.05 was considered to indicate statistical significance. All analyses followed the standards for Reporting Diagnostic accuracy studies recommendations of 2015. 20 Quantitative variables are described as means (±standard deviations, minima and maxima). Qualitative data are presented as counts with percentages. Diagnostic accuracy parameters (Se, Sp, positive predictive value (PPV), and NPV) are shown in percentages with 95% confidence intervals. The Pearson chi‐squared test was used to compare the diagnostic accuracies of CONECCT IIC and SANO IIIA (compared to histology) in terms of predicting invasive adenocarcinomas in the submucosa or deeper. The number of subjects required was based on the hypothesis that CONECCT IIc would exhibit a Se of 84% when predicting adenocarcinomas involving the submucosa or deeper layers. Assuming a significance level of 0.05 and a precision of 3%, the number of subjects required was 574. The diagnostic accuracy of CONECCT and other classifications was compared by calculating areas under the receiver operating characteristic curves with the aid of the Pearson chi‐squared test, as was the comparison of CONECCT diagnostic accuracies when different endoscopes were used. Multivariate analysis employed a descending, stepwise, logistic regression model. The explanatory variables were those yielding p‐values <0.20 on univariate analysis.
RESULTS
Flow chart
Between January 2016 and December 2019, 993 colorectal lesions were removed en‐bloc using ESD; 330 lesions met the exclusion criteria. We thus assessed 663 lesions of 623 patients, including 467 colonic and 196 rectal lesions (Figure 2).
FIGURE 2.
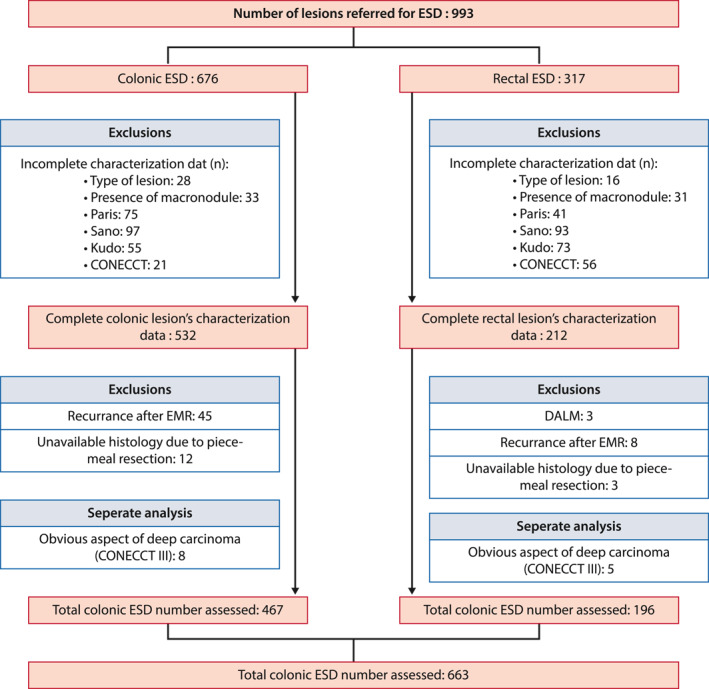
Flow chart
Cohort characteristics
The mean large diameter was 57.9 mm; 80.2% of lesions were >40 mm and 56% of lesions were >50 mm. Of all lesions, 43.7% (290) were in the recto‐sigmoid region, 8.5% (56) were in the left‐side colon, 6.8% (45) were in the transverse colon, and 40.9% (271) were in various regions of the right‐side colon. Morphologically, 12.8% (85) were protruded lesions, 66.4% (440) were G‐LST lesions (26% GH‐LST, 40.4% GM‐LST), and 19% (126) were NG‐LST lesions (11.9% flat and 7.1% pseudo‐depressed). Features of concern included macronodules >10 mm (53.4% of the lesions [354]), depressed areas (Paris 0‐IIc; 13.9%), and SANO IIIA or Kudo Vi areas (40% [265]). Lesions exhibiting submucosal invasion risk factors (macronodules >10 mm, SANO IIIA areas, or NG‐LST or depressed areas [0‐IIc]) were classified as CONECCT IIC in 76% of cases (504).
The en bloc resection rate was 96%, the R0 resection rate was 85%, and the curative resection rate was 81%. Only 0.9% of the patients required surgery for a complication (Table 1).
TABLE 1.
Cohort characteristics
| Patients characteristicslc | |
| Number, n | 623 |
| Sex male : Female, n (%) | 360 (57.8) : 263 (42.2) |
| Mean age, years (min‐max) | 69.3 (34–96) |
| Lesions characteristics | |
| Number, n | 663 |
| Mean size, mm (min‐max) | 57.9 (20–210) |
| >40 mm, n (%) | 532 (80.2) |
| >50 mm, n (%) | 371 (56%) |
| Location, n (%) | |
| Recto‐sigmoid | 290 (43.7) |
| Descending colon | 56 (8.5) |
| Transverse colon | 45 (6.5) |
| Ascending colon | 271 (40.9) |
| Characterization data, n (%) | |
| Macroscopic type | |
| Protruding lesion | 85 (12.8) |
| G‐LST | 440 (66.4) |
| NG‐LST | 126 (19) |
| Macronodule >1 cm | 354 (53.4) |
| Depressed area (Paris 0‐IIC) | 92 (13.9) |
| Sano IIIA/Kudo Vi | 265 (40) |
| CONECCT IIA | 146 (22) |
| CONECCT IIC | 504 (76) |
| Histology, n (%) | |
| V3 – LGD | 216 (32.6) |
| V4 – pTis | 376 (57) |
| V5 – pT1a <1000 um | 29 (4.4) |
| V5 – pT1a >1000 um | 26 (3.9) |
| ≥pT2 | 4 (0.6) |
| Details of sumucosal cancer | |
| Low risk submucosal cancer (<1000, LV‐, budding‐) | 15 (2.3%) |
| High risk submucosal cancer (>1000 or LV + or budding +) | 39 (5.9%) |
| “Intermediate risk” (>1000 and <2000, LV‐ and budding ‐) | 9 (1.4%) |
| ESD procedure, n (%) | |
| Duration of procedure (min) | 49 (IQR 30–92) |
| En bloc resection | 640 (96.4%) |
| R0 resection | 565 (85.1%) |
| Curative | 538 (81.1%) |
| Intra‐operative perforation | 48 (7%) |
| lesions <5 cm | 9 (3%) |
| lesions >5 cm | 34 (9.1%) |
| Secondary surgery | 46 (7%) |
| Secondary surgery for complications | 6 (0.9%) |
Abbreviations: CONECCT, COlorectal NEoplasia Endoscopic Classification to Choose the Treatment; LV, lymphovascular.
Histological analysis
Histology revealed sessile serrated lesions in 12 cases, LGD (Vienna 3) in 216 cases (32.6%), pTis (V4) in 376 cases (57%), adenocarcinomas with submucosal invasion ≤1000 μm (Vienna 5–pT1a) in 29 cases (4. 4%), adenocarcinomas with submucosal invasion >1000 μm (Vienna 5–pT1b) in 26 cases (3.9%), and ≥ pT2 adenocarcinomas in four cases (Tables 1 and 2).
TABLE 2.
Lesion's characteristics according to histology
| Histology data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Other | V3 | V4 | V5–pT1a | V5–pT1b | ≥pT2 | Adenocarcinoma | Adenocarcinoma | |
| (LGD) | (pTis) | (≤1,000 µm) | (>1,000 µm) | ≥V4.4 | ≥V5 | |||
| Characterization data, n (%) | ||||||||
| Type | ||||||||
| Protruding lesion | ‐ | 20 (23.5) | 51 (60) | 5 (5.9) | 7 (8.2) | 2 (2.3) | 34 (40) | 14 (16.5) |
| G ‐LST | ‐ | 153 (34.8) | 253 (57.5) | 15 (3.4) | 17 (3.9) | 2 (0.5) | 134 (30.5) | 34 (7.7) |
| GH‐LST | ‐ | 91 (52.9) | 77 (44.8) | 1 (0.6) | 1 (0.6) | ‐ | 19 (11) | 2 (1.2) |
| GN‐LST | ‐ | 61 (22.7) | 174 (64.9) | 15 (5.6) | 16 (6) | 2 (0.8) | 115 (42.9) | 32 (11.9) |
| NG‐LST | ‐ | 43 (34.1) | 72 (57.1) | 9 (7.1) | 2 (1.6) | ‐ | 28 (22.2) | 11 (8.7) |
| Flat | ‐ | 30 (38) | 45 (59.5) | 3 (3.8) | 1 (1.3) | ‐ | 15 (19) | 4 (5.1) |
| Pseudo‐depressed | ‐ | 13 (27.7) | 27 (57.5 | 6 (12.8) | 5 (10.6) | ‐ | 13 (27.7) | 7 (14.9) |
| Macronodule >1cm | ‐ | 82 (23.2) | 227 (64.1) | 18 (5.1) | 23 (6.5) | 4 (1,2) | 150 (42,4) | 45 (12,7) |
| Depressed area (0‐IIc) | ‐ | 24 (26.1) | 52 (56.5) | 9 (9.8) | 7 (7.6) | ‐ | 36 (39.1) | 16 (17.4) |
| SANO IIIA area | ‐ | 56 (21.1) | 170 (64.2) | 19 (7.2) | 18 (6.8) | 2 (0.8) | 111 (41.9) | 39 (14.7) |
| CONECCT IIA | ‐ | 80 (54.8) | 65 (44.5) | ‐ | ‐ | ‐ | 10 (6.8) | ‐ |
| CONECCT IIC | ‐ | 137 (27.2) | 313 (62.1) | 29 (5.8) | 26 (5.2) | 4 (0.8) | 187 (36.9) | 59 (11.7) |
| ALL LESION n/663, (%) | 12 (1.8) | 216 (32.6) | 376 (56.7) | 29 (4.4) | 26 (3.9) | 4 (0.6) | 196 (29.6) | 59 (8.9) |
Primary outcome
The CONECCT IIC classification exhibited a sensitivity (Se) of 100.0%, a specificity (Sp) of 26.2%, a PPV of 11.9%, and a NPV of 100.0% (95% confidence interval 0.59–0.64) for predicting invasion of the submucosa or beyond. The accuracy was 32.6%. Of CONECCT IIA lesions lacking features of concern, none exhibited invasion of the submucosa or beyond (Table 3).
TABLE 3.
Diagnostic accuracy of the CONECCT classification compared to each poor prognostic factor
| CONECCT IIc | NG LST | p value | |
|---|---|---|---|
| Se (%) | 100,0 | 18,6 | <0,0001 |
| Sp (%) | 26,2 | 81,0 | <0,0001 |
| PPV (%) | 11,9 | 8,7 | 0,119 |
| NPV (%) | 100,0 | 91,1 | 0,127 |
| AUC | 0,622 | 0,498 | <0,0001 |
| CONECCT IIc | Sano IIIA | p value | |
|---|---|---|---|
| Se (%) | 100,0 | 66,1 | 0,018 |
| Sp (%) | 26,2 | 62,6 | 0,024 |
| PPV (%) | 11,9 | 14,7 | 0,353 |
| NPV (%) | 100,0 | 95,0 | 0,191 |
| AUC | 0,622 | 0,643 | 0,623 |
| CONECCT IIc | Macronodule | p value | |
|---|---|---|---|
| Se (%) | 100,0 | 76,3 | 0,026 |
| Sp (%) | 26,2 | 48,8 | 0,033 |
| PPV (%) | 11,9 | 12,7 | 0,741 |
| NPV (%) | 100,0 | 95,5 | 0,203 |
| AUC | 0,622 | 0,625 | 0,914 |
| CONECCT IIc | Paris 0‐IIc | p value | |
|---|---|---|---|
| Se (%) | 100,0 | 27,1 | <0,0001 |
| Sp (%) | 26,7 | 87,4 | 0,003 |
| PPV (%) | 11,7 | 17,4 | 0,189 |
| NPV (%) | 100,0 | 92,5 | 0,145 |
| AUC | 0,622 | 0,572 | 0,296 |
Abbreviation: CONECCT, COlorectal NEoplasia Endoscopic Classification to Choose the Treatment.
Performance of endoscopic predictive factors in terms of predicting submucosal carcinoma
The CONECCT IIc classification detected submucosal invasion significantly more sensitively (100%) than the other risk factors (Se values 66.1% for a Sano IIIA area, 76.3% for a macronodule >1 cm; and 27.1% for a depressed area [Paris 0‐II]; all p < 0.05)(Table 3).
Prevalence and predictive factors of submucosal invasion
Of GH‐LST tumours (without macronodules), 1.2% contained adenocarcinomas exhibiting submucosal invasion; the corresponding values were 11.9% for GM‐LST tumours (with macronodules >1 cm), 5.1% for flat NG‐LST tumours, 14.9% for pseudo‐depressed NG‐LST tumours, and 16.5% for protruding lesions. The submucosal carcinoma rates were 17.3% for lesions with depressed (Paris 0‐IIc) areas, 14.7% for lesions with SANO IIIA areas, and 8.7% for NG lesions. All GH‐LST tumours with submucosal invasion exhibited depressed areas (Paris 0‐IIc) (Table 2).
NG‐LST tumours with submucosal invasion showed a SANO IIIA vascular pattern. Even in cases of LST‐NG‐PD, no submucosal cancer was found in the absence of SANO IIIA area (Table 4).
TABLE 4.
Submucosal cancer risk according to macroscopic type and vascular pattern
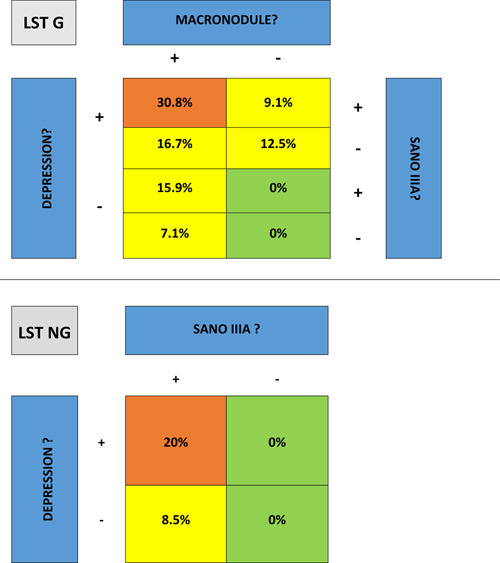
|
All G‐LST tumours with submucosal invasion had a macroscopic morphologic risk factor of carcinoma (macronodule or depressed area); vascular abnormality (SANO IIIA) did not influence this risk of submucosal carcinoma (Table 4).
Differences between the colon and rectosigmoid region
The rate of submucosal cancer was two fold higher in the rectum than in the colon, irrespective of the morphological risk factor:
Macronodules (16.6% in the rectosigmoid region vs. 7.7% elsewhere; p = 0.013).
SANO IIIA areas (20.9 vs. 8.8%; p = 0.003).
CONECCT IIc ranking (16 vs. 7.7%; p = 0.006; Appendix B).
Endoscope model
The diagnostic accuracies of all predictive factors did not vary according to the endoscope used (Fujifilm/BLI or Olympus/NBI)(Appendix C).
Submucosal cancer prediction based on the number of endoscopic predictive factors
When the number of features of concern increased (thus an NG‐LST tumour, macronodule >1 cm in diameter, depressed area [Paris 0‐IIC], and SANO IIIA area) the percentages of adenocarcinomas exhibiting submucosal invasion or beyond also increased: no feature of concern (n = 154) 0% submucosal invasion; one feature (n = 236) 8.5%; two features (n = 218) 11.9%; and three features (n = 55) 23.6% (p < 0.0001).
Prediction of adenocarcinomas exhibiting deep submucosal invasion
Thirteen lesions subjected to ESD were finally classified as CONECCT III and analysed separately. All were either resected via ESD or underwent surgery. Seven (53.8%) exhibited adenocarcinomas with submucosal invasion beyond 1000 μm, four exhibited superficial submucosal cancers (30.8%), and two (15.4%) exhibited intramucosal carcinomas. The Se, Sp, PPV, and NPV of the CONECCT III classification in terms of predicting deep submucosal invasion were respectively 18.9%, 99.1%, 53.8%, and 95.5%.
DISCUSSION
Endoscopic characterisation is recommended prior to resection of any superficial lesion. 10 , 14 , 15 , 16 , 23 An effort to predict the final histology is necessary when selecting the optimal resection modality (piecemeal endoscopic mucosal resection [PM‐EMR], ESD, or surgery) for large spreading lesions. The existing classifications (Kudo, 4 SANO, 5 and JNET 8 ) are based principally on overt carcinoma signs; they have been validated principally on lesions <2 cm using an NBI endoscope under magnification. For lesions >2 cm, the risk of covert carcinoma 3 is related to macroscopic features (NG‐LST status, macronodule >1 cm in diameter, O‐IIC depressed area), but this has not been integrated into the classifications. The CONECCT classification combines the macroscopic aspect with the pit/vascular pattern and allows any lesion to be described using any endoscope with a virtual chromoendoscopy function.
The CONECCT IIc subtype combines overt signs of carcinoma validated by Japanese classifications (Sano IIIA, Kudo Vi, and JNET IIB) with covert signs significantly associated with macroscopic aspects (NG‐LST status, a macronodule >1 cm in diameter, and a depressed area). The combination of these risk factors was essential because it is difficult to analyse the whole glandular and vascular patterns of large lesions (>2 cm).
The sensitivity of CONECCT IIC (100%) for predicting submucosal cancer was superior to all other criteria; all classifications, including the CONECCT, had a very low PPV and accuracy, which led to difficulty identify submucosal cancer. Indeed, CONECCT IIA lesions >2 cm have no risk of submucosal cancer. Such lesions may always be curatively treated via PM‐EMR; there is no need for ESD. However, these lesions represent only 25% of lesions referred for endoscopic resection. NG‐LST with a regular pit and vascular pattern could be considered low‐risk according to our results; they could be curatively removed by PM‐EMR from a carcinologic perspective. This result increases the proportion of low‐risk lesions to 30%.
For granular lesions, macroscopic features are necessary to evaluate the risk of submucosal carcinoma according to our results. Indeed, all granular lesions with submucosal cancer had a macroscopic morphologic risk factor—macronodule or depressed area or both (Figure 3). This is unsurprising because cancer can develop in the deepest part of the nodule without any superficial visible abnormalities.
FIGURE 3.
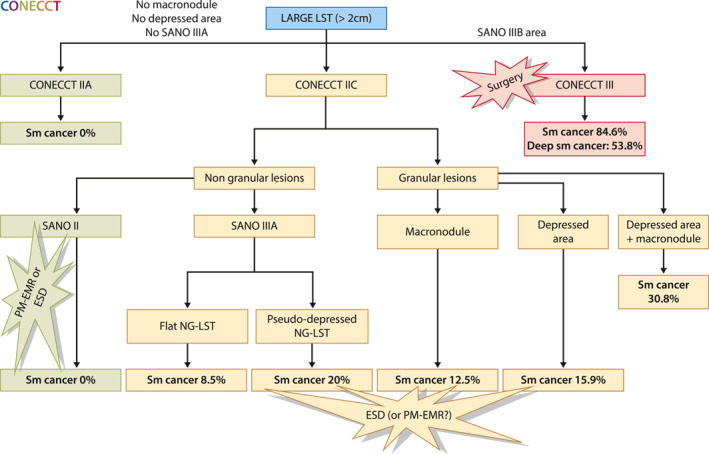
Algorithm for a stratified approach
Moreover, PM‐EMR is often challenging in NG‐LST or GM‐LST with large macronodules because of frequent fibrosis. ESD may have a technical advantage for resection of such lesions.
A CONECCT IIC status was associated with a non‐negligible risk of adenocarcinoma with submucosal invasion. For example, Sano IIIA area, depressed area, or macronodule >1 cm in diameter was associated with a >10% risk of submucosal invasive cancers, of which 50% were superficial and curatively treated via ESD. Using the CONECCT IIC status, no adenocarcinoma with submucosal invasion was missed, but only 5.8% of lesions with shallow submucosal invasion benefitted from ESD. In contrast, 6% of CONECCT IIC lesions were not curatively resected via ESD; further surgery was necessary. However, ESD could treat curatively 47% of CONECCT III lesions with small SANO IIIB areas, demonstrating the potential for overestimation by endoscopic characterisation. New criteria are needed to differentiate deep and shallow submucosal tumours and propose endoscopic or surgical resections. However, because primary ESD followed by salvage surgery has no carcinologic impact, it is difficult to propose primary surgery even for CONECCT IIc lesions with three features of concern, because 80% of them are curatively treated by ESD.
Choosing a classification with high sensitivity decreases the rate of undertreated lesions (T1 with PM‐EMR) but leads to a high number of ESDs. PM‐EMR would have been a curative option for 322 adenomas (63.9% of CONECCT IIC lesions) and an acceptable option for 128 intramucosal adenocarcinomas (25.4%). Several authors consider these ESDs to be unnecessary from a carcinologic perspective. 21 However, ESD decreases the recurrence rate, as well as the need for uncomfortable and costly control colonoscopy. Indeed, a recent retrospective study of more than 500 patients from Korea reported that the 3‐year cumulative total costs of patients treated via ESD or PM‐EMR were similar. 22 ESD was more expensive in year 1, but such patients required fewer control colonoscopies than the EMR group, despite the low recurrence rate (7.5%) in the EMR group. New ESD devices and strategies must be evaluated in comparative medico‐economic trials (e.g., the ongoing French trial comparing ESD to PM‐EMR [National Clinical Trials 03962868]).
Increasing specificity and PPV would improve the CONECCT classification accuracy. In terms of the increasing risk of submucosal invasion as the number of features of concern increases, further studies are needed to define the minimum number of such features to trigger selection of ESD instead of PM‐EMR. When three such factors were present, approximately 25% of lesions exhibited submucosal invasion; we suggest that piecemeal resection is unacceptable in such cases.
Although we believe that this approach should not be considered as overtreatment for a benign lesion, the low specificity of CONECCT leads to a large number of potentially not indicated ESDs for suspected high‐risk lesions. In fact, classification directly depends on the endoscopic resection technique used. If PM‐EMR is used, a classification with high specificity to predict submucosal cancer is needed (e.g., the O‐IIc criteria) to select ESD for these lesions. If ESD is used, as in the present study and our previous study, a classification with a high sensitivity (e.g., CONECCT) is favourable because it prevents undertreatment.
The ESD results 23 , 24 are very good, as in the present study: 96% en bloc resection rate, 85% R0 resection rate, and 49 min median procedure time for 57 mm lesions. The perforation rate was high in the present study, identical to the rates in the largest Asian studies for lesions >5 cm, which can be explained by the greater proportion of large lesions in the present study (10.6% in the study by Hong et al. 25 and 8% in the study by Saito et al. 26 for lesions >50 mm). Only 0.9% of patients had surgery for a complication.
Surgery is overtreatment in all cases, PM‐EMR is undertreatment if there is a risk of submucosal cancer, and ESD with poor technical and carcinologic results is overtreatment of benign lesions. Moreover, the prevalence (pre‐test likelihood) of submucosal cancer in large LST is low (10%), thus statistically justifying a classification with high sensitivity.
Finally, at a time when extensive criteria are being evoked, our strategy allows adapted treatment by ESD of nine supplementary intermediate‐risk lesions (submucosal infiltration 1000–2000 μm without budding or lymphovascular involvement).
The risk of submucosal invasion by lesions in the rectosigmoid region was two fold greater than the risk of lesions in the proximal colon, irrespective of the lesional features, as reported previously. 21 This higher distal risk is poorly understood but should be considered; ESD should be prioritised earlier for distal lesions.
The strengths of our study are its real‐world design and the high numbers of lesions treated. This is one of the few studies to consider only lesions resected en bloc (ESD), reducing the risk of missing pathological information compared to studies based on PM‐EMR. All lesions referred for endoscopic resection that lacked any sign of a deep submucosal feature were included, whatever their size; there was no selection bias. Moreover, this study is the first to compare two scopes from different suppliers with similar accuracies when applying the CONECCT classification.
The first limitation of this study was the high endoscopic expertise of the four operators. The results should be confirmed by other teams. Moreover, because this was a real‐time optical diagnosis study, inter‐ and intra‐observer agreement could not be evaluated. However, we recently published a study base on pictures for which the inter‐ and intra‐observer agreement were significantly higher than the agreements of other classifications. 12
The non‐use of magnification was also a limitation of this study. However, therapeutic colonoscopes dedicated to ESD do not have a zoom function. Moreover, the combination of chromoendoscopy and zoom increases diagnostic accuracy for superficial submucosal adenocarcinoma and deep submucosal carcinoma, but it does not affect diagnostic accuracy for low‐ and high‐risk lesions. 27 Importantly, the use of endoscopes lacking a zoom function could be considered a strength that enables our results to be extrapolated throughout the West.
In conclusion, our optical diagnosis strategy (Figure 3), the CONECCT classification, combines covert and overt signs of carcinoma to identify—with very high sensitivity (Se 100%, NPV 100%)—the 30% of low‐risk adenomas in large laterally spreading lesions treatable by PM‐EMR or ESD according to expertise without undertreatment (PM‐EMR for T1 cancer). For the remaining 70% of lesions, ESD prevents unnecessary surgery for superficial sm‐cancer, whereas PM‐EMR prevents ESD for benign lesions at the cost of more surgery for carcinologic reasons and more colonoscopies to treat recurrences. A comparative trial incorporating technical and medico‐economic endpoints is needed to clarify the optimal treatment modality in this situation; patients should be informed of the results and included in the decision‐making process.
CONFLICT OF INTERESTS
Jeremie Jacques: Consultant for Boston Scientific, Olympus, Fujifilm and Erbe; Romain Legros, Mathieu Pioche, Jerome Rivory and Thierry Ponchon: Consultants for Boston Scientific and Olympus. No disclosures for the others.
ACKNOWLEDGEMENT
No funding for this work.
APPENDIX A. Definitions of LST
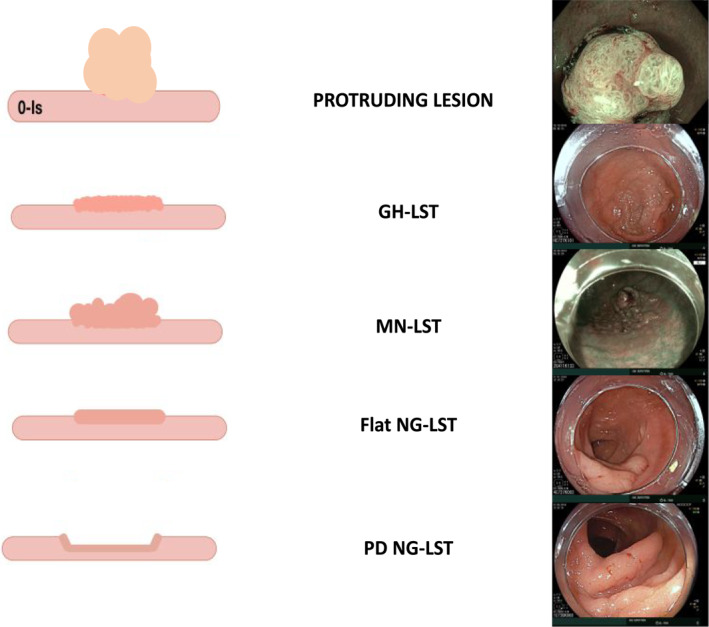
APPENDIX B. Differences between the colon and rectosigmoid region
| Recto‐sigmoïd (n = 291) | Colon (n = 372) | p value | |
|---|---|---|---|
| Size (mean +/− SD) | 61.48 +/− 29.17 | 54.50 +/− 22.84 | 0.0007 |
| Type of lesion (n, %) | |||
| Protruding | 54 (18.6%) | 31 (8.3%) | <0.0001 |
| Sm invasion | 10 (18.5%) | 4 (12.9%) | |
| G LST | 208 (71.7%) | 231 (62.1%) | |
| Sm invasion | 25 (12%) | 9 (3.9%) | |
| NG LST (n, %) | |||
| Yes | 28 (9.7%) | 98 (26.3%) | <0.0001 |
| Sm invasion | 4 (14.3%) | 7 (7.1%) | 0.23 |
| Macronodule (n, %) | |||
| Yes | 199 (68.6%) | 155 (41.7%) | <0.0001 |
| Sm invasion | 33 (16.6%) | 12 (7.7%) | 0.0013 |
| Depressed area (0‐IIc) (n, %) | |||
| Yes | 37 (12.8%) | 55 (14.8%) | 0.455 |
| Sm invasion | 9 (24.3%) | 7 (12.7%) | 0.15 |
| SANO IIIA area (n, %) | |||
| Yes | 129 (44.5%) | 136 (36.6%) | 0.039 |
| Sm invasion | 27 (20.9%) | 12 (8.8%) | 0.003 |
| CONECCT IIc (n, %) | |||
| Yes | 244 (84.1%) | 260 (69.9%) | <0.0001 |
| Sm invasion | 39 (16%) | 20 (7.7%) | 0.006 |
| Adenocarcinoma > V4 (n, %) | |||
| Yes | 119 (41%) | 77 (20.7%) | <0.0001 |
| Adenocarcinoma > V5 (n, %) | |||
| Yes | 39 (13.5%) | 20 (5.4%) | <0.0001 |
APPENDIX C. Comparison of the endoscopes used in terms of predicting invasion of the submucosa or beyond
| FUJIFILM 700 | OLYMPUS 190 | p value | |
|---|---|---|---|
| Se (%) | |||
| SANO IIIA | 64 | 80 | 0.574 |
| CONECCT Iic | 100 | 100 | 1 |
| Spe (%) | |||
| SANO IIIA | 62.5 | 58.7 | 0.641 |
| CONECCT Iic | 25.3 | 28.9 | 0.588 |
| PPV (%) | |||
| SANO IIIA | 14 | 13.8 | 0.988 |
| CONECCT Iic | 11.4 | 10.4 | 0.941 |
| NPV (%) | |||
| SANO IIIA | 94.8 | 97.3 | 0.668 |
| CONECCT Iic | 100 | 100 | 1 |
Abbreviation: CONECCT, COlorectal NEoplasia Endoscopic Classification to Choose the Treatment.
Brule C, Pioche M, Albouys J, Rivory J, Geyl S, Legros R, et al. The COlorectal NEoplasia Endoscopic Classification to Choose the Treatment classification for identification of large laterally spreading lesions lacking submucosal carcinomas: a prospective study of 663 lesions. United European Gastroenterol J. 2022;10(1):80–92. 10.1002/ueg2.12194
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Participants in the Paris Workshop . The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc. 2003;58:S3–S43. [DOI] [PubMed] [Google Scholar]
- 2. Oka S, Tanaka S, Kanao H, Oba S, Chayama K. Therapeutic strategy for colorectal laterally spreading tumor. Dig Endosc. 2009;21:S43–6. [DOI] [PubMed] [Google Scholar]
- 3. Yamada M, Saito Y, Sakamoto T, Nakajima T, Kushima R, Parra‐Blanco A, et al. Endoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumors. Endoscopy. 2016;48:456–64. [DOI] [PubMed] [Google Scholar]
- 4. Kudo S, Rubino C, Teixeira C, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2004;33:367–73. [DOI] [PubMed] [Google Scholar]
- 5. Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. SANO’S capillary pattern classification for narrow‐band imaging OF early colorectal lesions: SANO’S classification OF NBI. Dig Endosc. 2011;23:112–15. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the Narrow‐Band Imaging International Colorectal Endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625–32. [DOI] [PubMed] [Google Scholar]
- 7. Sumimoto K, Tanaka S, Shigita K, Hirano D, Tamaru Y, Ninomiya Y, et al. Clinical impact and characteristics of the narrow‐band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017;85:816–21. [DOI] [PubMed] [Google Scholar]
- 8. Sano Y, Tanaka S, Kudo S, Saito S, Matsuda T, Wada Y, et al. Narrow‐band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team: Japan NBI Expert Team classification. Dig Endosc. 2016;28:526–33. [DOI] [PubMed] [Google Scholar]
- 9. Le Roy F, Manfredi S, Hamonic S, Piette C, Bouguen G, Riou F, et al. Frequency of and risk factors for the surgical resection of nonmalignant colorectal polyps: a population‐based study. Endoscopy. 2015;48:263–70. [DOI] [PubMed] [Google Scholar]
- 10. Pimentel‐Nunes P, Dinis‐Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, et al. Endoscopic submucosal dissection: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2015;47:829–54. [DOI] [PubMed] [Google Scholar]
- 11. Fabritius M, Gonzalez J‐M, Becq A, Dray X, Coron E, Brenet‐Defour L, et al. A simplified table using validated diagnostic criteria is effective to improve characterization of colorectal polyps: the CONECCT teaching program. Endosc Int Open. 2019;07:E1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonniaud P, Jacques J, Gonzalez JM, Dray X, Coron E, Leblanc S, et al. Endoscopic characterisation of colorectal neoplasia with the different published classifications: comparative study involving Conecct classification. Endosc Int Open. 2022 Jan 14;10(1)E145–E153 10.1055/a-1613-5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pimentel‐Nunes P, Pioche M, Albéniz E, Berr F, Deprez P, Ebigbo A, et al. Curriculum for endoscopic submucosal dissection training in europe: European society of gastrointestinal endoscopy (ESGE) position statement. Endoscopy. 2019;51:980–92. [DOI] [PubMed] [Google Scholar]
- 14. Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau J‐M, Paspatis G, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European society of gastrointestinal endoscopy (ESGE) clinical guideline. Endoscopy. 2017;49:270–97. [DOI] [PubMed] [Google Scholar]
- 15. Kamiński M, Hassan C, Bisschops R, Pohl J, Pellisé M, Dekker E, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2014;46:435–57. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417–34. [DOI] [PubMed] [Google Scholar]
- 17. Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, et al. Endoscopic submucosal dissection in a European setting. A multi‐institutional report of a technique in development. Endoscopy. 2011;43:664–70. [DOI] [PubMed] [Google Scholar]
- 18. Schlemper RJ, Kato Y, Stolte M. Diagnostic criteria for gastrointestinal carcinomas in Japan and Western countries: proposal for a new classification system of gastrointestinal epithelial neoplasia. J Gastroenterol Hepatol. 2000;15:G49–57. [DOI] [PubMed] [Google Scholar]
- 19. Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. Stard 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiol. 2015;277:826–32. [DOI] [PubMed] [Google Scholar]
- 21. Sidhu M, Tate D, Desomer L, Brown G, Hourigan L, Lee E, et al. The size, morphology, site, and access score predicts critical outcomes of endoscopic mucosal resection in the colon. Endoscopy. 2018;50:684–92. [DOI] [PubMed] [Google Scholar]
- 22. Ham NS, Kim J, Oh EH, Hwang SW, Park SH, Yang D‐H, et al. Cost of endoscopic submucosal dissection versus endoscopic piecemeal mucosal resection in the colorectum. Dig Dis Sci. 2020;65:969–77. [DOI] [PubMed] [Google Scholar]
- 23. Bordillon P, Pioche M, Wallenhorst T, Rivory J, Legros R, Albouys J, et al. Double‐clip traction for colonic endoscopic submucosal dissection: a multicenter study of 599 consecutive cases (with video). Gastrointest Endosc. 2021;94:333–43. [DOI] [PubMed] [Google Scholar]
- 24. Jacques J, Charissoux A, Bordillon P, Legros R, Rivory J, Hervieu V, et al. High proficiency of colonic endoscopic submucosal dissection in Europe thanks to countertraction strategy using a double clip and rubber band. Endosc Int Open. 2019;07:E1166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hong SN, Byeon JS, Lee B‐I, Yang D‐H, Kim J, Cho KB, et al. Prediction model and risk score for perforation in patients undergoing colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2016;84:98–108. [DOI] [PubMed] [Google Scholar]
- 26. Saito Y, Yamada M, So E, Abe S, Sakamoto T, Nakajima T, et al. Colorectal endoscopic submucosal dissection: technical advantages compared to endoscopic mucosal resection and minimally invasive surgery. Dig Endosc. 2014;26(Suppl 1):52–61. [DOI] [PubMed] [Google Scholar]
- 27. Iwatate M, Sano Y, Tanaka S, Kudo Se, Saito S, Matsuda T, et al. Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


