Abstract
The emergence of coagulase-negative staphylococci not only as human pathogens but also as reservoirs of antibiotic resistance determinants requires the deployment and development of methods for their rapid and reliable identification. Internal transcribed spacer-PCR (ITS-PCR) was used to identify a collection of 617 clinical staphylococcal isolates. The amplicons were resolved in high-resolution agarose gels and visually compared with the patterns obtained for the control strains of 29 staphylococcal species. Of the 617 isolates studied, 592 (95.95%) were identified by ITS-PCR and included 11 species: 302 isolates of Staphylococcus epidermidis, 157 of S. haemolyticus, 79 of S. aureus, 21 of S. hominis, 14 of S. saprophyticus, 8 of S. warneri, 6 of S. simulans, 2 of S. lugdunensis, and 1 each of S. caprae, S. carnosus, and S. cohnii. All species analyzed had unique ITS-PCR patterns, although some were very similar, namely, the group S. saprophyticus, S. cohnii, S. gallinarum, S. xylosus, S. lentus, S. equorum, and S. chromogenes, the pair S. schleiferi and S. vitulus, and the pair S. piscifermentans and S. carnosus. Four species, S. aureus, S. caprae, S. haemolyticus, and S. lugdunensis, showed polymorphisms on their ITS-PCR patterns. ITS-PCR proved to be a valuable alternative for the identification of staphylococci, offering, within the same response time and at lower cost, higher reliability than the currently available commercial systems.
Staphylococci are nosocomial pathogens associated with multiple antimicrobial resistance mechanisms. For many years Staphylococcus aureus was the only species recognized as an important human pathogen, whereas the coagulase-negative staphylococci (CNS) were viewed mostly as clinically nonrelevant contaminants. Only recently, the importance of CNS strains as a major cause of nosocomial infections, mainly associated with the use of prosthetic and indwelling devices and immunocompromised patients, began to be ascertained (15). Although Staphylococcus epidermidis accounts for the majority of infections caused by CNS, many other species have been identified in association with human infections (12, 15, 17).
The emergence of CNS as human pathogens and reservoirs of antimicrobial resistance determinants requires their rapid and reliable identification in order to have an early prediction of the potential pathogenicity or antibiotic susceptibility of each clinical isolate (12, 15, 17) and to clarify the clinical significance of each species.
In recent years, several commercial systems for the rapid identification of staphylococci have been developed as an alternative to the classical identification protocols (13, 14), which are too laborious and time-consuming to be used in most clinical laboratories. The commercial systems, based on miniaturized biochemical or immunologic reactions, are widely used today for both clinical and research purposes (12). However, these diagnostic systems present problems, such as cost and response time, but more importantly, they often provide unreliable results. Several of the problems associated with these systems result from the variable expression of phenotypic characters that are used as diagnostic parameters. Additionally, many of these kits are based on colorimetric results, and subjectivity in their interpretation may lead to ambiguity.
For these reasons, significant efforts have been made in order to develop alternative identification methods combining speed, reliability, and low cost. These criteria are met by methods based on molecular rather than phenotypic characters. One of these methods is ribosome spacer PCR or internal transcribed spacer-PCR (ITS-PCR), the rationale for which is described briefly below.
In prokaryotes, the rRNA genetic loci contain the genes for 16S, 23S, and 5S rRNAs. These genes are separated by spacer regions which show a high degree of variability in both sequence and size at the genus and species level (2, 8). The diversity of the intergenic spacer regions is due in part to variations in the number and type of tRNA sequences found among these spacers. In staphylococci, there are several copies of the rrn operon. Gürtler and Barrie (7) characterized the spacer sequences of S. aureus strains, including methicillin-resistant S. aureus (MRSA) isolates, and identified nine rrn operons whose 16S-23S spacer region varied from 303 to 551 bp. According to these authors, three of these spacers contain the tRNAIle gene and two contain both the tRNAIle and the tRNAAla genes, while the remaining four 16S-23S spacers have no tRNA gene (7, 8). Forsman et al. (5) sequenced the 16S-23S spacer of five staphylococcal species (S. aureus, S. epidermidis, S. hyicus, S. simulans, and S. xylosus) and found that in addition to S. aureus, S. hyicus and S. simulans also had a tRNAIle gene in some of their rrn operons.
The highly polymorphic nature of the 16S-23S spacer sequences may be analyzed by PCR, using as primers conserved sequences from the adjacent 16S and 23S genes. This method, originally described by Barry et al. (2), is known as ITS-PCR. Identification of staphylococci by ITS-PCR was first studied by Jensen et al. (10), who successfully applied this technique to differentiate strains of four staphylococcal species, S. aureus, S. epidermidis, S. saprophyticus, and S. warneri. Some authors tested ITS-PCR for the identification of staphylococci from diverse origins, using different protocols (3, 18), whereas others designed PCR primers based on species-specific sequences of 16S-23S spacers for the detection of particular staphylococcal species (5, 20).
In this work, ITS-PCR was applied to the identification of over 600 staphylococcal samples received from different hospitals, using as controls reference strains of 29 of the 32 species that are presently recognized in the genus Staphylococcus (15). The experimental conditions used were those described by Jensen et al. (10), introducing a high-resolution agarose that allowed rapid resolution of the amplification products.
Part of this work was presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy (I. Couto, S. Pereira, M. Miragaia, I. Sanches, and H. de Lencastre, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 900, 2000).
MATERIALS AND METHODS
Bacterial strains. (i) Control strains.
Thirty-three control strains were used, representing 29 staphylococcal species and 7 subspecies. These controls included the type strains of Staphylococcus arlettae (ATCC43957T), S. auricularis (ATCC33753T), S. capitis (ATCC27840T), S. caprae (ATCC35538T), S. carnosus (ATCC51365T), S. chromogenes (ATCC43764T), S. cohnii subsp. cohnii (ATCC29974T), S. cohnii subsp. urealyticum (ATCC49330T), S. delphini (ATCC49171T), S. epidermidis (ATCC14990T), S. equorum (ATCC43958T), S. felis (ATCC49168T), S. gallinarum (ATCC35539T), S. haemolyticus (ATCC29970T), S. hominis (ATCC27844T), S. hyicus subsp. hyicus (ATCC11249T), S. intermedius (ATCC29663T), S. kloosii (ATCC43959T), S. lentus (ATCC29070T), S. lugdunensis (ATCC43809T), S. pasteuri (ATCC51129T), S. piscifermentans (ATCC51136T), S. saprophyticus (ATCC15305T), S. schleiferi subsp. coagulans (ATCC49545T), S. schleiferi subsp. schleiferi (ATCC43808T), S. sciuri subsp. carnaticus (ATCC700058T), S. sciuri subsp. rodentium (ATCC700061T), S. sciuri subsp. sciuri (ATCC29062T), S. simulans (ATCC27848T), S. vitulus (ATCC51145T), S. xylosus (ATCC29971T), S. warneri (ATCC27836T), and a well-characterized strain of S. aureus (NCTC8325) (19).
(ii) Clinical strains.
A total of 617 clinical staphylococcal samples of human origin from the Molecular Genetics Laboratory of the Instituto de Tecnologia Química e Biológica da Universidade Nova de Lisboa, Oeiras, Portugal, culture collection were studied. The majority of the samples were isolated during 1997 and 1998 from colonization and infection sites of hospitalized patients.
Strain purification procedure.
CNS samples received from clinical laboratories often contained mixed cultures of staphylococci. These mixtures were more easily detected after a prolonged incubation for 48 h at 37°C followed by an additional 48 h at room temperature. This procedure, previously suggested by other authors (11), was followed for all isolates prior to their characterization. All strains were grown in tryptic soy agar (Difco Laboratories, Detroit, Mich.).
Identification criteria. (i) Preliminary identification.
Upon reception and purification, all isolates were tested in our laboratory for catalase and oxidase assays, mannitol fermentation, and coagulase production.
(ii) Final identification.
The ITS-PCR amplification patterns of the clinical isolates were visually compared with those of the reference strains. Samples showing similar patterns distantly positioned in the working gels were run side by side in new gels in order to confirm their similarity. A final identification was assigned to those isolates with ITS-PCR patterns that matched any of the control strains, considering the results on the mannitol and coagulase tests. When additional characterization of isolates was needed, in order to complete or confirm ITS-PCR results, two commercial identification systems were used, API STAPH and ID32 STAPH (BioMérieux, Marcy l'Etoile, France) according to the manufacturer's instructions.
ITS-PCR patterns similar but not identical to that of a given control strain were considered polymorphisms of that species only if the putative identification was corroborated by the results obtained with the commercial systems referred to above (with an identification of excellent according to the manufacturer's criteria).
ITS-PCR.
DNA was isolated by the guanidine isothiocyanate extraction method as described before (1). The ITS-PCR was performed as previously described (10), using primers G1 (5′-GAAGTCGTAACAAGG) and L1 (5′-CAAGGCATCCACCGT) (Gibco-BRL, Life Technology Ltd., Paisley, Scotland). Amplification reaction was performed on a Perkin Elmer Gene Amp PCR system 9600 apparatus (Perkin Elmer Applied Biosystems, Cheshire, England). The program consisted of an initial denaturation step at 94°C for 4 min and 25 amplification cycles, each with 1 min at 94°C, 2-min ramp to 55°C, 7 min at 55°C, 2-min ramp to 72°C, and 2 min at 72°C, followed by an additional extension step of 7 min at 72°C (10).
Amplification products were resolved in high-resolution gels (3% Metaphor agarose [FMC BioProducts, Rockland, Maine]) in 1× TAE buffer (0.04 M Tris-acetate, 0.001 M EDTA [pH 8]) supplemented with 0.25 μg of ethidium bromide per ml for 6 h, using 100-bp ladder molecular size markers (USB, Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) as standards. After photography by standard procedures, DNA patterns were visually analyzed.
RESULTS
ITS-PCR patterns of control strains.
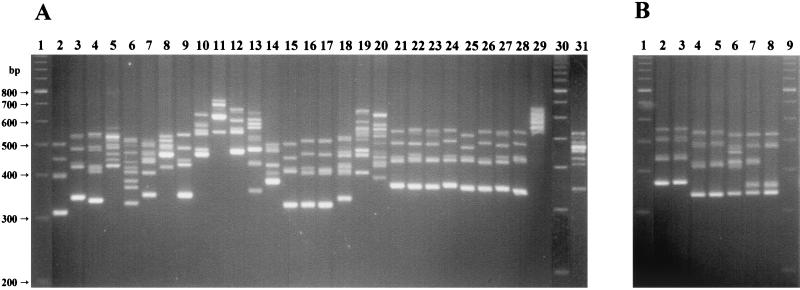
The usefulness of ITS-PCR for the identification of staphylococcal species was first tested by analyzing 33 reference strains, representing 29 staphylococcal species and 7 subspecies (Fig. 1A and 1B). The ITS-PCR patterns obtained for the 29 species tested consisted of four to nine bands, ranging from 320 to 750 bp; each species produced a stronger band, usually the smallest one (Fig. 1).
FIG. 1.
ITS-PCR amplification patterns for staphylococcal control strains. (A) Lanes 1 and 30, 100-bp ladder molecular size markers. Lane 2, S. auricularis; lane 3, S. vitulus; lane 4, S. schleiferi subsp. schleiferi; lane 5, S. haemolyticus; lane 6, S. capitis; lane 7, S. caprae; lane 8, S. warneri; lane 9, S. epidermidis; lane 10, S. hyicus subsp. hyicus; lane 11, S. delphini; lane 12, S. felis; lane 13, S. kloosii; lane 14, S. hominis; lane 15, S. simulans; lane 16, S. carnosus; lane 17, S. piscifermentans; lane 18, S. sciuri subsp. sciuri; lane 19, S. intermedius; lane 20, S. aureus; lane 21, S. xylosus; lane 22, S. lentus; lane 23, S. saprophyticus; lane 24, S. equorum; lane 25, S. chromogenes; lane 26, S. cohnii subsp. cohnii; lane 27, S. gallinarum; lane 28, S. arlettae; lane 29, S. lugdunensis; lane 31, S. pasteuri. (B) Lanes 1 and 9, 100-bp ladder molecular size markers. Lane 2, S. cohnii subsp. cohnii; lane 3, S. cohnii subsp. urealyticum; lane 4, S. schleiferi subsp. schleiferi; lane 5, S. schleiferi subsp. coagulans; lane 6, S. sciuri subsp. sciuri; lane 7, S. sciuri subsp. rodentium; lane 8, S. sciuri subsp. carnaticus.
All 29 staphylococcal species showed unique ITS-PCR patterns, although some were more difficult to distinguish than others. The species showing more related ITS-PCR patterns could be clustered in three groups: (i) the so-called S. saprophyticus group, which comprises, besides S. saprophyticus, six additional species (S. chromogenes, S. cohnii, S. equorum, S. gallinarum, S. lentus, and S. xylosus); (ii) the pair S. schleiferi and S. vitulus; and finally (iii) the pair S. carnosus and S. piscifermentans, which showed almost identical ITS-PCR patterns (Fig. 1A).
The reference strains studied also included the type strains from the subspecies of S. cohnii, S. schleiferi, and S. sciuri. The ITS-PCR patterns obtained for the type strains of the two S. cohnii subspecies were indistinguishable, and the same was verified for the two S. schleiferi subspecies. On the other hand, the type strains of the three S. sciuri subspecies showed different amplification patterns (Fig. 1B).
Testing of different ITS-PCR conditions.
In an attempt to improve the differentiation of the staphylococcal control strains, different ITS-PCR conditions were tested. For this purpose, other ITS-PCR primers were used, namely, the ones proposed by Gürtler and Stanish (8). The primers tested corresponded to region 2 of the 16S ribosomal DNA (rDNA) (5′-TTGTACACACCGCCCGTC), combined with primers for region 7 (5′-GGTACTTAGATGTTT) or 10 (5′-CCTTTCCCTCACGGTACTG) of the 23S rDNA (8). These pairs of primers did not improve the discrimination among the species analyzed; primers 2 and 10 originated compact ITS-PCR patterns, with high-molecular-weight bands of difficult resolution, while primers 2 and 7 produced patterns similar to the ones obtained with G1 and L1, although with larger ITS fragments that needed longer electrophoresis runs in order to be resolved and visually differentiated (results not shown).
We also tested another ITS-PCR protocol described earlier by Mendoza and colleagues (18). These authors used a lower annealing temperature and no ramping in the amplification reaction, as well as a different protocol for DNA preparation. The ITS-PCR patterns obtained following this alternative protocol had lower resolution (data not shown) due to poorer quality of the template DNA and the lower annealing temperature.
Identification of clinical staphylococcal isolates.
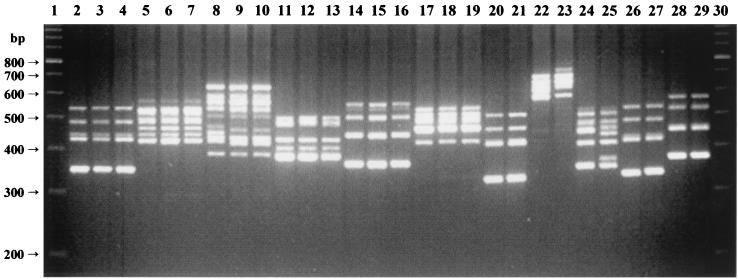
The ITS-PCR amplification patterns of the clinical staphylococcal isolates analyzed were visually compared with those obtained for the reference strains (Fig. 2).
FIG. 2.
ITS-PCR amplification patterns of clinical strains. Lanes 1 and 30, 100-bp ladder molecular size markers. Lane 2, S. epidermidis type strain; lanes 3 and 4, S. epidermidis clinical isolates; lane 5, S. haemolyticus type strain; lanes 6 and 7, S. haemolyticus clinical isolates; lane 8, S. aureus control strain; lanes 9 and 10, S. aureus clinical isolates; lane 11, S. hominis type strain; lanes 12 and 13, S. hominis clinical isolates; lane 14, S. saprophyticus type strain; lanes 15 and 16, S. saprophyticus clinical isolates; lane 17, S. warneri type strain; lanes 18 and 19, S. warneri clinical isolates; lane 20, S. simulans type strain; lane 21, S. simulans clinical isolate; lane 22, S. lugdunensis type strain; lane 23, S. lugdunensis clinical isolate; lane 24, S. caprae type strain; lane 25, S. caprae clinical isolate; lane 26, S. carnosus type strain; lane 27, S. carnosus clinical isolate; lane 28, S. cohnii subsp. cohnii type strain; lane 29, S. cohnii clinical isolate.
Of the 617 isolates tested, 592 (95.95%) were identified by ITS-PCR and included 11 species: 302 isolates of S. epidermidis, 157 of S. haemolyticus, 79 of S. aureus, 21 of S. hominis, 14 of S. saprophyticus, 8 of S. warneri, 6 of S. simulans, 2 of S. lugdunensis, and 1 each of S. caprae, S. carnosus, and S. cohnii. The remaining 25 isolates not identified by ITS-PCR were all gram-positive cocci that produced catalase and gave negative results in the modified oxidase assay. All these isolates were resistant to bacitracin and susceptible to lysostaphin and were identified by the commercial systems only at the genus level as Staphylococcus. Their ITS-PCR patterns differed from those of the staphylococcal type strains tested but were within the range of the ITS-PCR profiles obtained for the staphylococci tested in terms of number and molecular weight of amplification products.
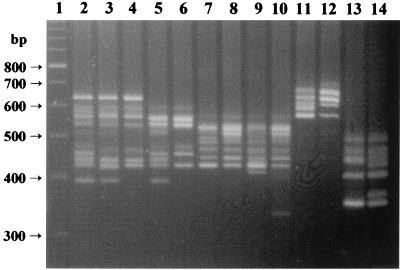
The characterization of this large collection of isolates allowed us to detect the existence of intraspecific polymorphisms among the ITS-PCR patterns of four species, S. aureus, S. haemolyticus, S. caprae, and S. lugdunensis, shown in Fig. 3. S. aureus was the species for which the largest number of different ITS-PCR polymorphisms were detected.
FIG. 3.
Examples of intraspecific ITS-PCR polymorphisms detected in this work. Lane 1, 100-bp ladder molecular size marker. Lanes 2 to 6, S. aureus strains; lanes 7 to 10, S. haemolyticus strains; lanes 11 and 12, S. lugdunensis strains; lanes 13 and 14, S. caprae strains. For each species, the leftmost sample is the control strain.
Of the 617 isolates characterized in this study, 192 were previously identified by the clinical laboratories where they were isolated by their in-house protocols. Species identification was redetermined in our laboratory by ITS-PCR, and 85 (44.3%) discrepancies were found between our identification results and those provided by the hospitals. Most (70.6%) of these discrepancies occurred with CNS isolates, particularly S. haemolyticus and S. epidermidis strains that were misidentified by the clinical laboratories as S. aureus.
We also found three S. aureus isolates that failed to produce coagulase in the test tube. These isolates fermented mannitol and were identified as S. aureus by ITS-PCR, although with different patterns (polymorphisms) from each other. Another group of 10 S. aureus isolates produced coagulase but failed to ferment mannitol. All these isolates were identified as S. aureus by ITS-PCR, showing patterns identical to those of typical S. aureus strains.
DISCUSSION
In this work we tested different ITS-PCR protocols described in the literature in order to apply this method to the identification of staphylococci of human clinical origin and found that of the several conditions assayed, the combination of primers G1 and L1 with the amplification procedure described by Jensen et al. (10) provided the best discrimination among the several species tested. In these conditions, ITS-PCR proved to be a reliable method for the identification of staphylococci. Each of the 29 control strains tested showed distinct amplification patterns, which subsequently allowed the identification of nearly 600 clinical isolates, corresponding to 95.95% of the samples tested. Only 25 isolates could not be identified either by ITS-PCR or by the commercial systems used.
The collection of isolates that were analyzed included the three staphylococcal species recognized as clinically more relevant, S. aureus, S. epidermidis, and S. haemolyticus. It also included other species commonly associated with human infections, such as S. saprophyticus, S. hominis, S. lugdunensis, and S. warneri, as well as species less frequently isolated, namely, S. simulans, S. cohnii, and S. caprae. Finally, we identified an isolate of S. carnosus, a species rarely isolated from clinical sources (15, 17).
The ITS-PCR method was simple and met our needs for rapid identification of large numbers of clinical isolates with high reliability. Following strain culture in plates, it was possible to obtain an identification after 18 h, most of this time being spent on the PCR (6 h) and electrophoretic resolution of the amplification products (6 h). Although a shorter time response is desirable, it should not compromise the quality of results. Particularly, we verified that the purity of the DNA samples, the annealing temperature, and the ramping steps in the PCR were essential to obtain reliable and consistent results. Variations in these experimental conditions may explain differences in results obtained by other authors (18), even using the same primers. Moreover, the amplification reaction and programs used in this study were designed to prevent the formation of unspecific products, such as single-stranded or heteroduplex structures (9, 10). All these factors were essential to obtain well-defined ITS patterns and to ensure the stability and reproducibility of the ITS-PCR patterns, which were verified by testing different DNA preparations of the same strains in several independent gel runs performed by different operators. The use of high-resolution agarose avoided the utilization of polyacrylamide gels used in previous works (10), with consequent simplification of the protocol.
In our experience, the restriction of the amplicons with restriction enzymes as proposed by other authors (10, 18) is not necessary for the identification of staphylococci of human origin, since the direct analysis of the amplification products provides enough resolution for the identification of the clinically more frequent species as well as those potentially relevant but not frequently associated with disease. In fact, the majority of these species were easily identified by direct comparison with the control strain profile, and therefore this additional resolution step may not be necessary.
Four staphylococcal species presented more than one ITS-PCR pattern. Of these, S. aureus was the species showing the highest number of different polymorphisms (Fig. 3). Nevertheless, the S. aureus isolates characterized in this work showed an ITS-PCR pattern which was identical or very similar to that of the control strain, which simplified their identification. When working with larger or more heterogeneous collections of isolates, S. aureus identification by ITS-PCR should be based on recognition of the characteristic nuclei of central bands (see Fig. 3 and also reference 10) and confirmed by additional testing, such as production of coagulase.
Because of the highly polymorphic nature of S. aureus ITS regions, several authors have already described the use of ITS-PCR for typing both methicillin-susceptible (MSSA) and -resistant strains (4, 7, 16, 20, 21). However, our own results using ITS-PCR to type strains representative of well-characterized MRSA clones showed that the discriminatory power of ITS-PCR for MRSA typing is limited (I. Couto et al., unpublished data).
In addition to the polymorphisms described in this work, intraspecific variations of the ITS profiles have also been described for S. saprophyticus and S. epidermidis (10, 18). In our work, and despite the number of S. epidermidis and S. saprophyticus isolates studied (302 and 14, respectively), we found no polymorphisms for the ITS-PCR patterns of these two species.
A significant error percentage was found between the identification results provided by the clinical laboratories and those obtained with ITS-PCR. The most often misidentified isolates, also the most significant from the clinical point of view, were S. epidermidis and S. haemolyticus isolates, which were misidentified as S. aureus. The implications of these errors are clearly illustrated by a collection of eight methicillin-resistant staphylococcal isolates identified by the API STAPH system as S. aureus in two independent assays performed in our laboratory. However, they did not produce coagulase and their ITS-PCR profiles were identical to that of the S. haemolyticus type strain. The misidentification of these isolates as S. aureus resulted from an intermediate result in the test for the utilization of d-mannose. This example documents the need for reliable methods to identify clinical staphylococci.
We also detected several S. aureus isolates that failed to react either in the coagulase test tube or in mannitol fermentation. These atypical isolates may represent another important issue in clinical staphylococcus identification. Coagulase-negative variants of S. aureus have been described by other authors (6). In a preliminary study, Mlynarczyk et al. (A. Mlynarczyk, G. Mlynarczyk, M. Luczak, and J. Jeljaszewicz. Abstr. 9th International Symposium on Staphylococci and Staphylococcal Infections, abstr. 21, 2000) described S. aureus strains with a negative reaction for coagulase in the conventional tube test, which accounted for about 20% of all MSSA and MRSA strains tested. The coagulase-negative S. aureus strains found in our study were readily identified by their ITS-PCR patterns, identical to those of typical S. aureus strains.
In conclusion, ITS-PCR proved to be a rapid and reliable identification method for staphylococci of human clinical origin, providing high reliability and reproducibility at low cost. Furthermore, the results obtained are easy to interpret, with no subjectivity in their analysis. The method is simple to implement and to perform, being also versatile, since the DNA prepared can be used for further characterization of the strains, such as searching for antimicrobial resistance genes.
ACKNOWLEDGMENTS
This work was partially supported by contracts FCT 34872/99 and FCT 34842/99, Fundação para a Ciência e a Tecnologia (FCT), Portugal, and a grant from Project FCG/ITQB/HFA from Fundação Calouste Gulbenkian, Portugal awarded to H. de Lencastre. I. Couto and S. Pereira were supported by grants BPD/20187/99 and 023/BIC/2000 from FCT (Portugal), respectively. M. Miragaia received a research grant from Fundação Calouste Gulbenkian.
The help of Mónica Vaz in the preliminary characterization of some of the isolates is acknowledged.
REFERENCES
- 1.Aires de Sousa M, Santos Sanches I, van Belkum A, van Leeuwen W, Verbrugh H, de Lencastre H. Characterization of methicillin-resistant Staphylococcus aureus isolates from Portuguese hospitals by multiple genotyping methods. Microb Drug Res. 1996;2:331–341. doi: 10.1089/mdr.1996.2.331. [DOI] [PubMed] [Google Scholar]
- 2.Barry T, Colleran G, Glennon M, Dunican L K, Gannon F. The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991;1:51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Bes M, Guerin-Faublee V, Meugnier H, Etienne J, Freney J. Improvement of the identification of staphylococci isolated from bovine mammary infections using molecular methods. Vet Microbiol. 2000;71:287–294. doi: 10.1016/s0378-1135(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 4.Dolzani L, Tonin E, Lagatolla C, Monti-Bragadin C. Typing of Staphylococcus aureus by amplification of the 16S–23S rRNA intergenic spacer sequences. FEMS Microbiol Lett. 1994;119:167–173. doi: 10.1111/j.1574-6968.1994.tb06884.x. [DOI] [PubMed] [Google Scholar]
- 5.Forsman P, Tilsala-Timisjärvi A, Alatossava T. Identification of staphylococcal and streptpcoccal causes of bovine mastitis using 16S–23S rRNA spacer regions. Microbiology. 1997;143:3491–3500. doi: 10.1099/00221287-143-11-3491. [DOI] [PubMed] [Google Scholar]
- 6.Fox L K, Besser T E, Jackson S M. Evaluation of a coagulase-negative variant of Staphylococcus aureus as a cause of intramammary infections in a herd of dairy cattle. J Am Vet Med Assoc. 1996;15:1143–1146. [PubMed] [Google Scholar]
- 7.Gürtler V, Barrie H D. Typing of Staphylococcus aureus strains by PCR-amplification of variable-length 16S–23S rDNA spacer regions: characterization of spacer sequences. Microbiology. 1995;141:1255–1265. doi: 10.1099/13500872-141-5-1255. [DOI] [PubMed] [Google Scholar]
- 8.Gürtler V, Stanisich V A. New approaches to typing and identification of bacteria using 16S–23S rDNA spacer region. Microbiology. 1996;142:3–16. doi: 10.1099/13500872-142-1-3. [DOI] [PubMed] [Google Scholar]
- 9.Jensen M A, Straus N. Effect of PCR conditions on the formation of heteroduplex and single-stranded DNA products in the amplification of bacterial ribosomal DNA spacer regions. PCR Methods Appl. 1993;3:186–194. doi: 10.1101/gr.3.3.186. [DOI] [PubMed] [Google Scholar]
- 10.Jensen M A, Webster J A, Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 1993;59:945–952. doi: 10.1128/aem.59.4.945-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleeman K T, Bannerman T L, Kloos W E. Species distribution of coagulase-negative staphylococcal isolates at a community hospital and implications for the selection of staphylococcal identification procedures. J Clin Microbiol. 1993;31:1318–1321. doi: 10.1128/jcm.31.5.1318-1321.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloos W E. Taxonomy and systematics of staphylococci indigenous to humans. In: Crossley B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 113–117. [Google Scholar]
- 13.Kloos W E, Schleifer K H. Genus IV, Staphylococcus Rosenbach 1884. In: Holt J G, Sneath P H A, Mair N S, Sharpe M S, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams and Wilkins; 1986. pp. 1013–1035. [Google Scholar]
- 14.Kloos W E, Bannerman T L. Staphylococcus and Micrococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. p. 282. [Google Scholar]
- 15.Kloos W E, Bannerman T L. Staphylococcus and Micrococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 264–282. [Google Scholar]
- 16.Kumari D N P, Keer V, Hawkey P M, Parnell P, Joseph N, Richardson J F, Cookson B. Comparison and application of ribosome spacer DNA amplicon polymorphisms and pulsed-field gel electrophoresis for differentiation of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1997;35:881–885. doi: 10.1128/jcm.35.4.881-885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lina G, Etienne J, Vandenesch F. Biology and pathogenicity of staphylococci other than Staphylococcus aureus and S. epidermidis. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogenes. Washington, D.C.: American Society for Microbiology; 2000. pp. 450–462. [Google Scholar]
- 18.Mendoza M, Meugnier H, Bes M, Etienne J, Freney J. Identification of Staphylococcus species by 16S–23S rDNA intergenic spacer PCR analysis. Int J Syst Bacteriol. 1998;48:1049–1055. doi: 10.1099/00207713-48-3-1049. [DOI] [PubMed] [Google Scholar]
- 19.Pattee P A, Lee H C, Bannantine J P. Genetical and physical mapping of the chromosome of Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 41–58. [Google Scholar]
- 20.Saruta K, Matsunaga T, Kono M, Hoshina S, Ikawa S, Sakai O, Machida K. Rapid identification and typing of Staphylococcus aureus by nested PCR amplified ribosomal DNA spacer. FEMS Microbiol Lett. 1997;146:271–278. doi: 10.1111/j.1574-6968.1997.tb10204.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz F J, Steiert M, Tichy H V, Hofmann B, Verhoef J, Heinz H P, Kohrer K, Jones M E. Typing of methicillin-resistant Staphylococcus aureus isolates from Dusseldorf by six genotypic methods. J Med Microbiol. 1998;47:341–351. doi: 10.1099/00222615-47-4-341. [DOI] [PubMed] [Google Scholar]