ABSTRACT
Background
Breastfeeding is associated with a lower risk of subsequent overweight or obesity, but it is uncertain whether this is a causal relation because most studies have not adequately reduced risk of bias due to confounding.
Objectives
The aim of this review was to examine whether 1) ever compared with never consuming human milk and 2) different durations of human milk consumption among infants fed human milk are related to later risk of overweight or obesity, with emphasis on sibling-pair and intervention studies.
Methods
The 2020 Dietary Guidelines Advisory Committee, together with the Nutrition Evidence Systematic Review team, conducted a systematic review of articles relevant to healthy full-term infants in countries with a high or very high level of human development. We searched PubMed, Embase, Cochrane, and CINAHL; dual-screened the results using predetermined criteria; extracted data from and assessed the risk of bias for each included study; qualitatively synthesized the evidence; developed conclusion statements; and graded the strength of the evidence.
Results
The review included 42 articles, including 6 cohorts with sibling-pair analyses and 1 randomized controlled trial of a breastfeeding promotion intervention. Moderate evidence suggested that ever, compared with never, consuming human milk is associated with a lower risk of overweight and obesity at ages 2 y and older, particularly if the duration of human milk consumption is >6 mo. However, residual confounding cannot be ruled out. Evidence was insufficient to determine the relation between the duration of any human milk consumption, among infants fed human milk, and overweight and/or obesity at age 2 y and older.
Conclusions
Further research, using strong study designs, is needed to disentangle the complex relation between infant feeding practices and the risk of subsequent overweight or obesity, as well as the biological and behavioral mechanisms if the relation is causal.
Keywords: breastfeeding, human milk, overweight, obesity, infant, toddler, child, systematic review, sibling
See corresponding editorial on page 1577.
Introduction
Birth to 24 mo of postnatal life is a critical phase of life for future health, and how and what infants are fed contributes to developmental programming (1). Breastfeeding provides health benefits for both the mother and the infant (2). Dissimilarities in growth trajectories have been documented in breastfed compared with formula-fed infants in the first year of life (3–5). Ever being breastfed has been associated with a 12–14% reduction in the risk of childhood obesity (6, 7), although associations are substantially attenuated in studies that have been able to control for important confounding factors (such as parental overweight, maternal socioeconomic status and physical activity), and in studies comparing siblings within the same family (8).
Every 5 y, a Federal Advisory Committee (9) reviews scientific evidence to make recommendations to the USDA and the US Department of Health and Human Services (HHS) before the USDA and HHS update the Dietary Guidelines for Americans. For the first time, the 2020–2025 Dietary Guidelines for Americans (10) includes dietary guidance for infants and toddlers from birth to 24 mo of age in response to the mandate from the Agricultural Act of 2014 (11). Accordingly, the 2020 Dietary Guidelines Advisory Committee reviewed the evidence about and provided recommendations for feeding this age group (12). Supported by the USDA Nutrition Evidence Systematic Review (NESR) scientists and librarians (nesr.usda.gov), the committee conducted several systematic reviews, including one on the relation between human milk consumption and subsequent overweight and obesity (13).
The full systematic review included numerous prospective observational studies. Such studies, however, are prone to bias due to confounding because infant feeding is strongly socially patterned (14, 15). Therefore, the committee gave special attention to studies that reduced this risk of bias by using more rigorous study designs, such as sibling-pair studies, which reduce confounding because siblings often share parental (i.e., genetic), familial, and environmental characteristics (15). When sibling pairs differ in infant feeding or in the outcome of interest, they are considered “discordant,” and those differences yield insights into whether breastfeeding practices influence later overweight or obesity, assuming all other factors are equal. The committee also focused on the results of the sole randomized intervention trial that is relevant to this question, the Promotion of Breastfeeding Intervention Trial (PROBIT) in the Republic of Belarus (16–18). That trial evaluated the effects on multiple outcomes of a breastfeeding promotion program that led to a longer duration of breastfeeding in the intervention group compared with the control group.
The objective of this article is to describe the results of the sibling-pair and PROBIT studies within the context of the overall systematic review addressing the relation between breastfeeding and later overweight.
Methods
The committee used NESR's rigorous, protocol-driven method to conduct the full systematic review. The methods are described in detail in the scientific report of the committee (19) and in the systematic review documentation on the NESR website (13). An overview of the methods follows.
Development of the systematic review protocol
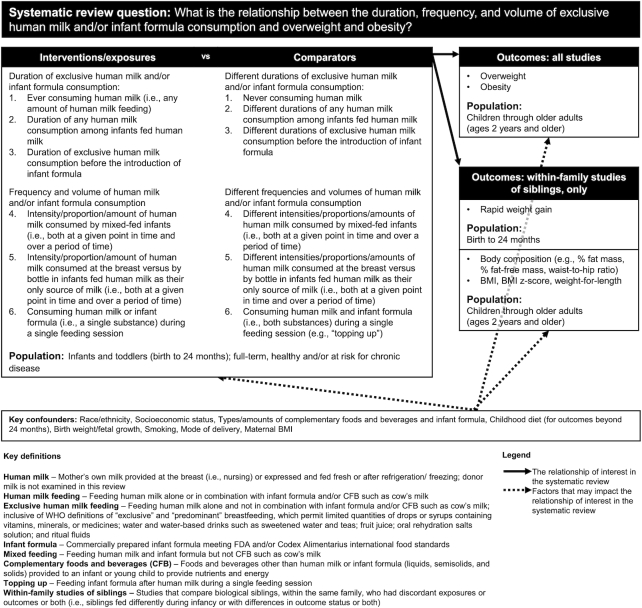
Committee members first developed a systematic review protocol that contained an analytic framework (Figure 1) and inclusion and exclusion criteria (13). The analytic framework described the population, interventions (for experimental studies) or exposures (for observational studies), comparators, and outcomes of interest for the systematic review. It also listed key confounders and how the committee defined key terms. The protocol and updates to the protocol were discussed by the committee in public meetings (20) and were posted at www.dietaryguidelines.gov for public comment and feedback and to ensure transparency to all stakeholders throughout the timeline of the committee's deliberations.
FIGURE 1.
Analytic framework for the full systematic review (13). The analytic framework illustrates the overall scope of the systematic review, including the population, the interventions and/or exposures, comparators, and outcomes of interest. It also includes definitions of key terms and identifies key confounders considered in the systematic review.
Population
The population of interest was healthy full-term infants in countries with a high or very high level of human development (21). We examined evidence about healthy, full-term infants because the purpose of the Dietary Guidelines for Americans is to promote health and prevent disease, rather than to treat specialized populations, such as infants born preterm. We examined evidence from infants in countries with a high or very high level of human development (21) to allow for generalizability to US infants.
Interventions/exposures and comparators
The full analytic framework included 6 comparisons of interventions/exposures compared with comparators, intended to align with the first feeding decisions caregivers make (Figure 1). In this article, we focus on the first 2 comparisons: 1) ever compared with never consuming human milk and 2) different durations of human milk consumption among infants fed human milk. Evidence for the other 4 comparisons [duration of exclusive human milk feeding prior to infant formula, extent of human milk feeding among infants fed both human milk and infant formula (mixed feeding), human milk fed by bottle compared with at the breast, and mixed feeding within a single feeding compared with not] was insufficient (13). We examined the consumption of mother's own milk fed at the breast or by bottle as well as infant formulas meeting US Food and Drug Administration (22) or Codex Alimentarius (23) food standards.
Outcomes
The original protocol listed a wide range of outcomes related to growth, size, and body composition. The committee subsequently updated the protocol to focus on outcomes reflecting overweight and obesity at ages 2 y and older, given their public health importance. Other growth and size outcomes were not examined because differences in growth and size between breastfed and formula-fed infants have already been well documented, including by an expert panel convened by the US government (5).
Inclusion and exclusion criteria
The systematic review included peer-reviewed articles published in English. The original protocol specified a publication date range from January 1980 to September 2019 when the literature search was conducted. We excluded articles published before 1980 because the Infant Formula Act of 1980 established nutrient requirements for commercial infant formulas in the United States, and thus health effects associated with formula consumption before 1980 might be different. For studies that did not assess sibling pairs, the updated protocol focused only on studies published after January 2011, because existing systematic reviews about infant feeding and later overweight and obesity include evidence from older studies (8). The committee included randomized and nonrandomized controlled trials, prospective and retrospective cohort studies, and nested case-control studies with at least 30 participants per study group, or a power analysis indicating sufficient statistical power to detect meaningful group differences. The updated protocol also specified that studies needed to account for at least one of the key confounders in the analytic framework to be eligible for inclusion.
For sibling-pair studies, the committee retained the original publication date range of January 1980 to September 2019 and also retained a broader list of outcomes that included rapid weight gain from birth to 24 months (as defined by study investigators), as well as BMI (in kg/m2) and measures of body composition at ages 2 y and older. In addition, sibling-pair studies with a cross-sectional design were eligible for inclusion.
Search process and screening of potentially relevant studies
A biomedical librarian from the NIH and systematic review librarians from NESR developed, peer-reviewed, and implemented the literature search to identify potentially relevant articles in PubMed, Embase, Cochrane, and CINAHL published from January 1980 to September 2019. Two NESR analysts used the inclusion and exclusion criteria to independently screen the titles, abstracts, and full texts of each search result in a stepwise manner with DistillerSR software (Evidence Partners). NESR analysts also manually reviewed the references of the included articles to identify articles to screen that were not retrieved by the literature search (Figure 2).
FIGURE 2.
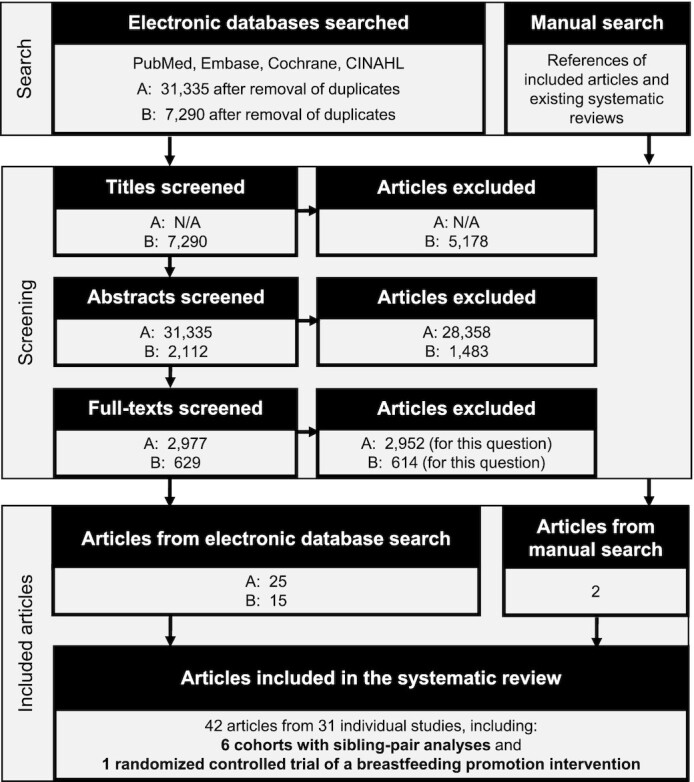
Literature search and screen flowchart. Two literature searches were used to identify articles. The first search (A) was conducted during the Pregnancy and Birth to 24 Months (P/B-24) Project to identify studies for a family of systematic reviews that examined human milk and infant formula consumption and several outcomes (https://nesr.usda.gov/infant-milk-feeding-practices-technical-expert-collaborative). Some of the intended reviews, including the review on overweight and obesity, were not completed before the end of the P/B-24 Project. The second search (B) was conducted during the work of the 2020 Dietary Guidelines Advisory Committee to identify studies published since the P/B-24 literature search.
Data extraction and assessment of risk of bias
For each included article, 1 NESR analyst extracted data into DistillerSR and a second analyst verified the accuracy and completeness of the extracted data. The 2 analysts completed independent risk-of-bias assessments using study design–specific tools for each article (13, 19).
Synthesis and grading of the evidence
The committee conducted a qualitative synthesis of the evidence to develop conclusion statements that pertain to the entire body of evidence, which included the sibling-pair studies and PROBIT trial (13). To grade the strength of the evidence, the committee used NESR's grading criteria for consistency, precision, generalizability, directness, and risk of bias (19) (see Supplemental Tables 1 and 2). The evidence underlying each conclusion statement was graded as strong, moderate, limited, or grade not assignable by the committee. Evidence from sibling-pair studies and the PROBIT trial was synthesized separately, but we did not develop distinct conclusion statements for these categories of studies.
Results
Results of the full systematic review are available online (13). In the sections below, we present results of studies that included sibling-pair analyses (Tables 1 and 2) for the 2 key comparisons, ever compared with never consuming human milk and different durations of human milk consumption (among infants fed human milk). In the PROBIT intervention trial, all infants initiated breastfeeding, so results relevant to ever compared with never consuming human milk are not available. However, results from the PROBIT trial are relevant to the second comparison regarding the duration of human milk consumption and are thus presented in that section (Table 3). Although the search criteria specified articles published in English, none of the articles excluded due to language were sibling-pair or intervention studies.
TABLE 1.
Evidence examining the relation between ever, compared with never, consuming human milk and outcomes reflecting overweight and obesity at ages 2 y and older from studies with sibling-pair analyses1
| Cohort | Article | Exposure2 | Outcome3 | Full-sample findings | Sibling-pair findings |
|---|---|---|---|---|---|
| Linked CENTURY Study (United States) | Hawkins et al. 2019 (24) | Initiated breastfeeding vs. did not initiate breastfeeding (reference)Birth years: 1987–2003 | BMI z score at 2 y | β (95% CI): −0.06 (−0.09, −0.04)(n = 55,058) | β (95% CI): −0.04 (−0.10, 0.03)(n = 2260 siblings with discordant outcomes) |
| BMI z score at 5 y | β (95% CI): −0.09 (–0.11, –0.07)(n = 43,893) | β (95% CI): −0.07 (−0.13, −0.01)(n = 3249 siblings with discordant outcomes) | |||
| Obesity at 2 y | OR (95% CI): 0.80 (0.73, 0.87)(n = 55,058) | OR (95% CI): 0.97 (0.72, 1.32)(n = 2260 siblings with discordant outcomes) | |||
| Obesity at 5 y | OR (95% CI): 0.77 (0.72, 0.83)(n = 43,893) | OR (95% CI): 0.94 (0.74, 1.20)(n = 3249 siblings with discordant outcomes) | |||
| Children of NLSY79 (United States) | Anderson et al. 2003 (25) | Ever breastfed vs. never breastfed (reference)Birth years:1986–1996 | Obesity vs. No obesity (reference) at 3–11 y | β ± SE: −0.018 ± 0.0084(n = 16,650 observations, probit model5)β ± SE: −0.016 ± 0.010(n = 15,050 observations, instrumental variable model5) | β ± SE: −0.021 ± 0.023(n = 4471 observations in siblings at the same age, mean 6.6 y)β ± SE: 0.012 ± 0.017(n = 7919 observations in siblings at the same point in time, mean 5.9 y for younger siblings and 9.2 y for older siblings) |
| Colen and Ramey 2014 (26) | Ever breastfed vs. never breastfed (reference)Birth years:1972–2006 | BMI at 4–14 y | β ± SE: −0.449 ± 0.094; P < 0.001(n = 8237) | β ± SE: −0.141 ± 0.188(n = 1773 siblings with discordant exposures) | |
| Obesity at 4–14 y | β ± SE: −0.342 ± 0.066; P < 0.001(n = 8237) | β ± SE: −0.173 ± 0.164(n = 1773 siblings with discordant exposures) | |||
| Add Health (United States) | Evenhouse and Reilly 2005 (27) | Ever breastfed vs. never breastfed (reference)Birth years:1976–1985 | BMI at 10–18 y | β ± SE: −0.41 ± 0.07; P < 0.10(n = 16,903) | β ± SE: 0.40 ± 0.33(n = 576 siblings with discordant exposures) |
| Overweight plus obesity at 10–18 y | OR ± SE from logit: 0.79 ± 0.03; P < 0.10(n = 16,903) | OR ± SE from logit: 1.32 ± 0.21; P < 0.10(n = 576 siblings with discordant exposures) | |||
| Obesity at 10–18 y | OR ± SE from logit: 0.77 ± 0.04; P < 0.10(n = 16,903) | OR ± SE from logit: 1.17 ± 0.25(n = 576 siblings with discordant exposures) | |||
| Nelson et al. 2005 (28) | Ever breastfed vs. never breastfed (reference)Birth years:1976–1985 | Overweight plus obesity at 12–21 y | OR (95% CI): 0.90 (0.76, 1.05)(n = 5929 males)OR (95% CI): 0.83 (0.72, 0.95)(n = 6069 females) | OR (95% CI) that the sibling without overweigh/obesity was ever breastfed and the sibling with overweight/obesity was never breastfed vs. both siblings had overweight/obesity and were never breastfed (reference): 0.52 (0.22, 1.24)OR (95% CI) that the sibling without overweight/obesity was ever breastfed and the sibling with overweight/obesity was never breastfed vs. both siblings had no overweight/obesity and were never breastfed (reference): 1.22 (0.64, 2.32)OR (95% CI) that the sibling with overweight/obesity was ever breastfed and the sibling without overweight/obesity was never breastfed vs. both siblings had overweight/obesity and were never breastfed (reference): 2.03 (0.64, 6.43)OR (95% CI) that the sibling with overweight/obesity was ever breastfed and the sibling without overweight/obesity was never breastfed vs. both siblings had no overweight/obesity and were never breastfed (reference): 1.27 (0.65, 2.50)(n = 224 siblings with discordant exposures and outcomes) | |
| BMI z score difference at 12–21 y | Not reported | β ± SE when the lighter sibling was breastfed: −0.004 ± 0.11β ± SE when the heavier sibling was breastfed: −0.09 ± 0.11(n = 224 siblings with discordant exposures and outcomes) | |||
| CDS (United States) | Metzger and McDade 2009 (29) | Ever breastfed vs. never breastfed (reference)Birth years: 1984–1993 | BMI z score at 9–19 y | Mean ± SE: −0.150 ± 0.065; P < 0.05(n = 2591) | Mean ± SE: −0.397 ± 0.176; P < 0.05(n = 976 siblings = 488 sibling pairs including 59 pairs with discordant exposures) |
| BMI >50th percentile vs. <50th percentile (reference) at 9–19 y | Not reported | OR: ∼1.006(ordinary least squares model7)OR: ∼1.30(fixed-effects model)(n = 30–44 siblings with discordant exposures and outcomes) | |||
| Overweight plus obesity vs. no overweight or obesity (reference) at 9–19 y | Not reported | OR: ∼0.60; P < 0.01(ordinary least squares model)OR: ∼0.40; P < 0.05(fixed-effects model)(n = 30–44 siblings with discordant exposures and outcomes) | |||
| Obesity vs. no obesity (reference) at 9–19 y | Not reported | OR: ∼0.70(ordinary least squares model)OR: ∼0.20; P < 0.01(fixed-effects model)(n = 30–44 siblings with discordant exposures and outcomes) |
Results are significant if a P-value < 0.05 is shown or the 95% CI does not include 1; otherwise they are not significant. Add Health, National Longitudinal Study of Adolescent Health; CDS, Child Development Supplement of the Panel Study of Income Dynamics; NLSY79, National Longitudinal Survey on Youth 1979 Cohort.
Exposure that addresses ever compared with never consuming human milk or vice versa.
All of the studies in this table used the CDC growth reference for BMI z scores, BMI percentiles, overweight plus obesity (BMI ≥85th percentile), and obesity (BMI ≥95th percentile)
Anderson et al. (25) did not explicitly state that this finding was statistically significant, but the text of the article implied that it was.
Probit models are a standard type of regression model that estimate the probability of a dichotomous outcome. A limitation of this modeling approach is that one cannot account for unobserved confounding that may bias the relation of interest. Instrumental variables estimation is an econometric approach that attempts to account for unobserved confounding. In this approach, a third variable, or “instrument” that is related to the exposure but is not related to the probability of the outcome, is used in the regression models as a substitute for the actual exposure.
Approximated odds ratios taken from a bar graph.
The authors used a least squares model for the sibling sample and a fixed-effects model for the sibling difference model. The fixed-effects model uses differences between siblings as the dependent and independent variables in an ordinary least squares or logistic regression, so that characteristics shared by siblings are differenced out of the model.
TABLE 2.
Evidence examining the relation between the duration of any human milk consumption, among infants fed human milk, and outcomes reflecting overweight and obesity at ages 2 y and older from studies with sibling-pair analyses1
| Cohort | Article | Exposure2 | Outcome3 | Full-sample findings | Sibling-pair findings |
|---|---|---|---|---|---|
| Children of NLSY79 (United States) | Colen and Ramey 2014 (26) | Breastfeeding duration (wk)Birth years:1972–2006 | BMI at 4–14 y | β ± SE: −0.007 ± 0.002; P < 0.01(n = 8237) | β ± SE = 0.005 ± 0.003(n = 1773 siblings with discordant exposures) |
| Obesity at 4–14 y | β ± SE: −0.007 ± 0.002; P < 0.01(n = 8237) | β ± SE = 0.001 ± 0.004(n = 1773 siblings with discordant exposures) | |||
| Add Health (United States) | Evenhouse and Reilly 2005 (27) | Breastfeeding duration (mo; quasi-continuous using midpoints of the ranges 0–3, 3–6, 6–9, 9–12, 12–24, and >24 mo)Birth years:1976–1985 | BMI at 10–18 y | β ± SE: –0.03 ± 0.006; P < 0.10(n = 7417) | β ± SE = 0.01 ± 0.03(n = 470 siblings with discordant exposures) |
| Overweight plus obesity at 10–18 y | OR ± SE from logit: 0.98 ± 0.00; P < 0.10(n = 7417) | OR ± SE from logit: 1.01 ± 0.01(n = 470 siblings with discordant exposures) | |||
| Obesity at 10–18 y | OR ± SE from logit: 0.98 ± 0.01; P < 0.10(n = 7417) | OR ± SE from logit: 1.00 ± 0.02(n = 470 siblings with discordant exposures) | |||
| GUTS (United States) | Gillman et al. 2006 (30) | Breastfeeding duration, per 3.7 mo (the mean difference in breastfeeding duration for discordant siblings)Birth years:1982–1987 | Overweight plus obesity at 9–14 y | OR (95% CI): 0.88 (0.82, 0.94) when adjusted for the same confounders as the discordant sibling analysisOR (95% CI): 0.94 (0.88, 1.00) when also adjusted for maternal BMI and smoking, as well as household income(n = 5614 siblings) | (below) |
| Breastfeeding longer vs. shorter (reference) than the mean duration of the participant's sibship | Overweight plus obesity at 9–14 y | (above) | OR (95% CI): 0.92 (0.76, 1.11)(n = 2372 siblings with discordant exposures) | ||
| Helsinki Birth Cohort (Finland) | O'Tierney et al. 2009 (31) | Trend across the breastfeeding duration categories <2, 3–4, 5–7, and ≥8 moBirth years:1934–1944 | BMI based on self-reported height and weight at ∼62 y | Not reported | Mean ± SD: 26.6 ± 4.3 (<2 mo), 26.3 ± 4.3 (3–4 mo), 26.2 ± 4.2 (5–7 mo), 26.5 ± 4.0 (≥8 mo); P = 0.80 linear, 0.90 quadratic(n = 831 siblings with discordant exposures) |
| BMI based on clinical measurement at ∼62 y | Not reported | Mean ± SD: 29.5 ± 4.3 (<2 mo), 26.8 ± 5.2 (3–4 mo), 26.9 ± 4.2 (5–7 mo), 28.2 ± 4.9 (≥8 mo); P = 0.80 linear, 0.08 quadratic(n = 129 siblings with discordant exposures) | |||
| Overweight based on self-reported height and weight at ∼62 y | Not reported | Percent: 42.7 (<2 mo), 42.9 (3–4 mo), 41.2 (5–7 mo), 50.0 (≥8 mo); P = 0.08 linear, 0.50 quadratic(n = 831 siblings with discordant exposures) | |||
| Percent fat mass at ∼62 y | Not reported | Mean ± SD: 32.6 ± 9.7 (<2 mo), 27.6 ± 8.5 (3–4 mo), 28.4 ± 6.6 (5–7 mo), 30.8 ± 8.0 (≥8 mo); P = 0.50 linear, 0.03 quadratic(n = 121 siblings with discordant exposures) | |||
| Breastfed <2 mo vs. 5–7 mo (reference) | BMI based on self-reported height and weight at ∼62 y | Not reported | Mean difference (95% CI): 0.3 (–0.5, 1.1)(n = 439 siblings with discordant exposures) | ||
| BMI based on clinical measurement at ∼62 y | Not reported | Mean difference (95% CI): 2.3 (–0.1, 4.7)(n = 60 siblings with discordant exposures) | |||
| Overweight based on self-reported height and weight at ∼62 y | Not reported | Mean difference in percent (95% CI): 1.6 (–7.7, 11.0)(n = 439 siblings with discordant exposures) | |||
| Percent fat mass at ∼62 y | Not reported | Mean difference (95% CI): 3.9 (0.3, 7.4)(n = 57 siblings with discordant exposures) | |||
| Breastfed 3–4 mo vs. 5–7 mo (reference) | BMI based on self-reported height and weight at ∼62 y | Not reported | Mean difference (95% CI): 0.3 (–0.5, 1.1)(n = 401 siblings with discordant exposures) | ||
| BMI based on clinical measurement at ∼62 y | Not reported | Mean difference (95% CI): –0.4 (–2.7, 1.8)(n = 68 siblings with discordant exposures) | |||
| Overweight based on self-reported height and weight at ∼62 y | Not reported | Mean difference in percent (95% CI): 1.9 (–8.0, 11.8)(n = 401 siblings with discordant exposures) | |||
| Percent fat mass at ∼62 y | Not reported | Mean difference (95% CI): 0.3 (–3.1, 3.7)(n = 62 siblings with discordant exposures) | |||
| Breastfed ≥8 mo vs. 5–7 mo (reference) | BMI based on self-reported height and weight at ∼62 y | Not reported | Mean difference (95% CI): 0.2 (–0.5, 1.0)(n = 457 siblings with discordant exposures) | ||
| BMI based on clinical measurement at ∼62 y | Not reported | Mean difference (95% CI): 1.2 (–0.9, 3.2)(n = 77 siblings with discordant exposures) | |||
| Overweight based on self-reported height and weight at ∼62 y | Not reported | Mean difference in percent (95% CI): 8.8 (–0.3, 18.0)(n = 457 siblings with discordant exposures) | |||
| Percent fat mass at ∼62 y | Not reported | Mean difference (95% CI): 1.3 (–1.7, 4.3)(n = 74 siblings with discordant exposures) |
Results are significant if a P-value < 0.05 is shown or the 95% CI does not include 1; otherwise they are not significant. Add Health, National Longitudinal Study of Adolescent Health; GUTS, Growing Up Today Study; NLSY79, National Longitudinal Survey on Youth 1979 Cohort.
Exposure that addresses the duration of any human milk consumption among infants fed human milk.
All of the US studies in this table used the CDC growth reference for overweight plus obesity (BMI ≥85th percentile) and obesity (BMI ≥95th percentile), and the study from Finland defined overweight as BMI 25–30 kg/m2.
TABLE 3.
Evidence examining the relation between the duration of any human milk consumption, among infants fed human milk, and overweight plus obesity and obesity at ages 2 y and older from the Promotion of Breastfeeding Intervention Trial1
| Article | Participant age, y | Odds of overweight plus obesity2 in the intervention vs. control group (reference), OR (95% CI) | Odds of obesity3 in the intervention vs. control group (reference), OR (95% CI) |
|---|---|---|---|
| Kramer et al. 2007 (16) | 6.5 (n = 13,889) | 1.1 (0.8, 1.4) | 1.2 (0.8, 1.6) |
| Martin et al. 2013 (18) | 11.5 (n = 13,879) | 1.18 (1.01, 1.39) | 1.17 (0.97, 1.41) |
| Martin et al. 2017 (17) | 16 (n = 13,557) | 1.14 (1.02, 1.28) | 1.09 (0.92, 1.29) |
In the Promotion of Breastfeeding Intervention Trial, the intervention group had a significantly longer duration of human milk consumption than the control group; all infants in the study were born in 1996–1997.
BMI ≥85th percentile using CDC growth reference
BMI ≥95th percentile using CDC growth reference.
Ever compared with never consuming human milk
Six articles, from 4 independent US cohorts, included sibling-pair analyses that examined outcomes associated with ever compared with never consuming human milk (24–29). All 6 studies used the CDC growth reference (32) to calculate BMI z scores or percentiles and defined overweight plus obesity as BMI ≥85th percentile and obesity as BMI ≥95th percentile.
Linked CENTURY study
Hawkins et al. (24) analyzed data from the Linked CENTURY Study. The full sample included 55,058 children at 2 y and 43,893 children at 5 y, and the subsample of sibling pairs with discordant outcomes included 2260 children at 2 y and 3249 at 5 y. In the full sample, initiating, compared with not initiating, human milk feeding was associated with a significantly lower BMI z score at 2 and 5 y [β (95% CI): –0.06 (–0.09, –0.04) and –0.09 (–0.11, –0.07), respectively] and significantly lower odds of obesity at both 2 and 5 y [OR (95% CI): 0.80 (0.73, 0.87) and 0.77 (0.72, 0.83), respectively]. In the subsample of siblings with discordant outcomes, the inverse association between initiating human milk feeding and BMI z score was not significant at 2 y [β (95% CI): –0.04 (–0.10, 0.03)] but was significant at 5 y [β (95% CI): –0.07 (–0.13, –0.01)]. In addition, there was no association of initiating human milk feeding with obesity at 2 or 5 y; odds ratios were closer to the null [OR (95% CI): 0.97 (0.72, 1.32) and 0.94 (0.74, 1.20), respectively] than was observed for the full sample.
Children of the National Longitudinal Survey on Youth 1979 cohort
Two articles (25, 26) examined children and adolescents whose mothers were part of the National Longitudinal Survey on Youth 1979 (NLSY79) cohort. Anderson et al. (25) conducted analyses on a full sample (n = 16,650 observations using a probit model and n = 15,050 observations using an instrumental variable model) and on subsamples of sibling pairs measured at the same age (mean age of 6.6 y; n = 4471 observations) or at the same point in time (mean age, 5.9 and 9.2 y for the younger and older siblings, respectively; n = 7919 observations). In the full sample, children who were ever fed human milk had a ∼1.8% decrease in the likelihood of being obese at 3–11 y of age compared with children never fed human milk (β ± SE: –0.018 ± 0.008 and –0.016 ± 0.010, from probit and instrumental variable models, respectively; P values were not reported, but the instrumental variable model was described as nonsignificant). In both sibling-pair subsamples, human milk feeding was not significantly associated with the likelihood of being obese at 3–11 y of age (β ± SE: –0.021 ± 0.023 and 0.012 ± 0.017, respectively).
Colen and Ramey (26) conducted analyses on a full sample of 8237 participants and on a subsample of sibling pairs with discordant infant feeding (n = 1773 participants). In the full sample, ever compared with never consuming human milk was associated with a significantly lower BMI at 4–14 y of age (β ± SE: –0.449 ± 0.094; P < 0.001) and a significantly lower log odds of obesity (β ± SE: –0.342 ± 0.066; P < 0.001). Within the discordant-feeding sibling-pairs subsample, the associations were in the same direction but were not statistically significant (for BMI, β ± SE: –0.141 ± 0.188; for obesity, β ± SE: –0.173 ± 0.164).
National Longitudinal Study of Adolescent Health
Two articles (27, 28) presented evidence from the National Longitudinal Study of Adolescent Health (Add Health), a nationally representative sample of 10- to 18-y-olds in 1994–1995 (baseline). At baseline, Evenhouse and Reilly (27) examined a full sample of 16,903 participants and a subsample of sibling pairs with discordant infant feeding (n = 576). In the full sample and the sibling-pairs subsample, ever compared with never consuming human milk was not significantly associated with BMI at 10–18 y of age (β ± SE: –0.41 ± 0.07, P < 0.10, and 0.40 ± 0.33, P ≥ 0.10, respectively) or with the odds of overweight plus obesity (OR ± SE from logit: 0.79 ± 0.03 and 1.32 ± 0.21, respectively; P < 0.10 for both) or obesity (OR ± SE from logit: 0.77 ± 0.04, P < 0.10, and 1.17 ± 0.25, P ≥ 0.10, respectively).
In a follow-up at 12–21 y of age, Nelson et al. (28) examined a full sample (n = 5929 males, 6069 females) and a subsample of siblings with discordant infant feeding and outcomes (i.e., in which the overweight sibling had been fed human milk and the nonoverweight sibling had not, and in which the overweight sibling had not been fed human milk and the nonoverweight sibling had; n = 224 discordant siblings). In the full sample, ever compared with never consuming human milk was associated with significantly lower odds of overweight plus obesity at 12–21 y of age among females [OR (95% CI): 0.83 (0.72, 0.95)] but not males [OR (95% CI): 0.90 (0.76, 1.05)]. Results for BMI z score were not presented for the full sample. The sibling-pair analyses did not show any significant associations between ever, compared with never, consuming human milk and overweight plus obesity or BMI z score at 12–21 y of age.
Child Development Supplement of the Panel Study of Income Dynamics
Metzger and McDade (29) examined children and adolescents in the Child Development Supplement of the Panel Study of Income Dynamics cohort at 9–19 y of age, with 2591 in the full sample, 976 in a subsample of siblings (488 sibling pairs, including 59 pairs with discordant exposures), and 30–44 in another subsample of siblings with differences in both feeding and BMI status. In the full sample, ever compared with never consuming human milk was associated with a lower BMI z score (mean ± SE: –0.150 ± 0.065; P < 0.05). In the sibling subsample, ever compared with never consuming human milk was associated with a lower BMI z score (mean ± SE: –0.397 ± 0.176; P < 0.05). In the subsample of siblings with differences in both feeding and BMI status, ever compared with never consuming human milk was not significantly associated with the odds of having a BMI >50th percentile (OR: ∼1.30 and ∼1.00 from fixed-effects and ordinary least squares regression models, respectively) but was associated with a lower odds of overweight plus obesity (OR: ∼0.40, P < 0.05 and ∼0.60, P < 0.01, respectively, from fixed-effects and ordinary least squares regression models) as well as a lower odds of obesity (OR: ∼0.20, P < 0.01 and OR ∼0.70, P ≥ 0.05, respectively, from fixed-effects and ordinary least squares regression models).
Duration of human milk consumption among infants fed human milk
Four articles, based on 3 US cohorts and 1 cohort in Finland, included sibling-pair analyses examining associations between the duration of human milk consumption and subsequent BMI, body fat percentage, or overweight and/or obesity status (26, 27, 30, 31). In addition, the PROBIT trial in the Republic of Belarus resulted in a different duration of human milk feeding between intervention and control groups and thus is also described below (16–18). The study from Finland defined overweight in adulthood as BMI 25–30, and all other studies defined overweight plus obesity as BMI ≥85th percentile and obesity as BMI ≥95th percentile, based on the CDC growth reference (32).
Children of NLSY79
As reported in the previous section, Colen and Ramey (26) conducted analyses on a full sample of 8237 participants and on a subsample of sibling pairs with discordant infant feeding (n = 1773 participants). In the full sample, there were small but statistically significant inverse associations between weeks of human milk consumption and BMI (β ± SE: –0.007 ± 0.002; P < 0.01) as well as obesity (β ± SE: –0.007 ± 0.002; P < 0.01) at 4-14 y. In the sibling-pair subsample, these associations were not significant (β ± SE: 0.005 ± 0.003 for BMI; β ± SE: 0.001 ± 0.004 for obesity).
Add Health
Evenhouse and Reilly (27) examined a full sample of 16,903 participants in Add Health (of whom 7417 were fed human milk) and a subsample of sibling pairs with discordant infant feeding (i.e., siblings fed human milk for different durations; n = 470). In the full sample and sibling-pair subsample, there were no significant associations between the duration of any human milk consumption and BMI, overweight plus obesity, or obesity at 10–18 y of age.
Growing Up Today Study
Gillman et al. (30) assessed the association between the duration of human milk consumption and overweight plus obesity at 9–14 y of age in a sample of children and adolescents in the US-based Growing Up Today Study cohort. The full sample included 5614 siblings from 2709 families, and the subsample of siblings with discordant infant feeding (i.e., siblings fed human milk for different durations) included 2372 children and adolescents. In the full sample, each additional 3.7-mo increase in the duration of any human milk consumption (which was the mean difference in duration for discordant siblings) was associated with significantly lower odds of overweight plus obesity at 9–14 y of age when applying the same statistical adjustments for confounders as in the discordant sibling analysis [i.e., age, sex, Tanner stage, menarcheal status for girls, birthweight, birth order, inactivity, physical activity, and energy intake; OR (95% CI): 0.88 (0.82, 0.94)]. When the model was also adjusted for maternal BMI and smoking, as well as household income, the magnitude of the association was slightly attenuated and the confidence interval included the null [OR (95% CI): 0.94 (0.88, 1.00)]. In the discordant sibling analysis, no significant association was detected between consuming human milk for a duration longer than the mean duration within each family, compared with a duration shorter than the mean family duration, and odds of overweight plus obesity at 9–14 y of age. The odds ratio was of similar magnitude to the odds ratios in the full sample, but the confidence interval was wider and included the null [OR (95% CI): 0.92 (0.76, 1.11)].
Helsinki Birth Cohort
O'Tierney et al. (31) studied the Helsinki Birth Cohort from Finland. Offspring were born between 1934 and 1944. The study sample consisted of members of the cohort who were fed human milk, had a sibling in the cohort, and provided follow-up data in the year 2000, along with their sibling. The outcomes of interest were BMI and overweight from self-reported data (n = 831) and BMI and percent body fat from clinical measurement (n = 129) at about 62 y of age. The analyses compared siblings fed human milk for different durations (<2, 3–4, 5–7, and ≥8 mo). Duration of human milk feeding was not associated with offspring BMI or prevalence of overweight. However, when BMI was based on measurements conducted in the clinic (i.e., rather than self-report), the quadratic trend approached significance (P = 0.08), suggesting a U-shaped association. Compared with a 5- to 7-mo duration of human milk feeding, BMI tended to be higher with durations of <2 mo (+2.3; 95% CI: –0.1, 4.7) and ≥8 mo (+1.2; 95% CI: –0.9, 3.2) but not with a duration of 3–4 mo (–0.4; 95% CI: –2.7, 1.8). There was a significant quadratic trend (P = 0.03) in the percent body fat of participants fed human milk for <2, 3–4, 5–7, and ≥8 mo, suggesting a similar type of U-shaped association between duration of breastfeeding and percent body fat.
PROBIT
The PROBIT study conducted in the Republic of Belarus was a cluster randomized controlled trial of an intervention to promote prolonged duration and exclusivity of human milk feeding among mothers who chose to feed human milk. The study enrolled 17,046 infants at birth and followed 13,889 children to 6.5 y (16), 13,879 children to 11.5 y (18), and 13,557 adolescents to 16 y of age (17). The intervention group had higher rates of any human milk consumption measured at 3, 6, 9, and 12 mo of age compared with the control group (72.7% compared with 60.0% at 3 mo, 49.8% compared with 36.1% at 6 mo, 36.1% compared with 24.4% at 9 mo, and 19.7% compared with 11.4% at 12 mo). At 6.5 y of age, odds of overweight plus obesity or obesity did not differ by intervention group. At both 11.5 and 16 y of age, the intervention group had significantly higher odds of overweight plus obesity than the control group [OR (95% CI): 1.18 (1.01, 1.39) and 1.14 (1.02, 1.28), respectively]. For obesity, the confidence interval was wider and included the null [OR (95% CI): 1.17 (0.97, 1.41) and 1.09 (0.92, 1.29) at 11.5 and 16 y, respectively].
Discussion
This systematic review took a novel approach to the question of whether human milk feeding is related to subsequent risk of overweight or obesity by focusing on studies with lower risk of confounding (i.e., sibling-pair and intervention studies). It thus adds a new dimension to this important topic. The entire body of evidence, from all studies included in the review (12, 13), was considered when the committee developed conclusion statements. Because the conclusions differed for the 2 exposures examined, ever compared with never consuming human milk and different durations of human milk consumption among infants fed human milk, these 2 sets of evidence are discussed in separate sections below.
Ever compared with never consuming human milk
Based on evidence from 21 observational cohort studies published between 2011 and 2019, including the 4 sibling-pair studies described herein, the committee concluded that ever, compared with never, consuming human milk is associated with a lower risk of overweight and obesity at ages 2 y and older, particularly if the duration of human milk consumption is 6 mo or longer (13). This conclusion statement was graded as “moderate.” The observational cohort studies were strongly consistent, with 14 of 21 studies showing a significantly lower risk of overweight and/or obesity in those who were ever fed human milk and another showing a marginal association in the same direction; several of the remaining studies may have lacked statistical power to detect an association. Five of the 7 studies that compared infants who consumed human milk for different durations with infants who never consumed human milk suggested that a longer duration of human milk consumption (e.g., ≥6 mo) is most protective. However, these 21 studies were limited by potential confounding because none of them controlled for all of the key confounders specified in the analytic framework. In particular, few studies accounted for complementary feeding practices and childhood diet, both of which are likely to be highly correlated with whether the child was fed human milk and may also influence the risk of overweight and obesity.
Sibling-pair studies greatly reduce the risk of bias introduced by confounding in observational studies because siblings share genetic, familial, and environmental risk factors. The sibling-pair analyses generally showed an attenuation of the significant associations that were found in full-sample analyses in those 4 studies, suggesting that confounding may explain a substantial proportion of the association between ever compared with never consuming human milk and subsequent overweight and obesity. Nevertheless, 1 of the sibling-pair analyses (29) did show a significant association between ever compared with never consuming human milk and lower odds of overweight plus obesity and obesity at 9–19 y of age. In another sibling-pair analysis (24), initiating human milk feeding was associated with a significantly lower BMI z score at 5 y of age, although not with risk of overweight or obesity. Sibling-pair studies are often limited by the smaller sample size available for such analyses, given that discordance between siblings is likely less common than concordance (15). The lower statistical power of such analyses makes it less likely to detect significant associations. Although risk of confounding is reduced in sibling-pair analyses, it is not eliminated entirely. For example, if a relation is found between infant feeding and child overweight, it is possible that the reason for discordance in infant feeding (e.g., cesarean section delivery or a change in family structure) is the actual causal factor predisposing to child overweight. Among these 4 studies, several other limitations also were of concern, including maternal recall of infant feeding 4–18 y after birth in 2 of the cohorts (27–29), self-report of weight and height (25, 26), and incomplete description of methods used to collect outcome data (27, 28). In addition, none of these studies included sibling-pair analyses that compared infants who consumed human milk for different durations with infants who never consumed human milk. Therefore, it is not possible to evaluate whether the trend described in the conclusion statement (i.e., that longer durations of human milk consumption may be important) is observed in sibling-pair analyses.
Because of the risk of confounding in observational studies and the limitations of the sibling-pair studies, it is difficult to determine whether a causal relation exists between ever compared with never consuming human milk and risk of later overweight or obesity. Other systematic reviews and meta-analyses on this topic have generally come to similar conclusions. For example, a systematic review of systematic reviews (8) concluded that breastfeeding is consistently associated with a reduction in the odds of overweight or obesity in childhood and adulthood, by about 13% in high-quality studies, but residual confounding could not be ruled out.
Duration of any human milk consumption among infants fed human milk
The committee concluded that the evidence was insufficient to determine the relation between the duration of any human milk consumption, among infants fed human milk, and overweight and/or obesity at age 2 y and older (13). This was based not on a lack of evidence (18 observational cohort studies, including 4 with sibling-pair analyses, and the PROBIT randomized controlled trial were included in the review) but rather on the inconsistency in the findings. Five studies showed significant inverse associations; 3 studies showed significant positive associations; 1 study reported significant associations, in opposite directions, at different ages; and 10 studies reported no significant associations between duration of human milk consumption and risk of overweight or obesity. Notably, all of the sibling-pair analyses showed no association, and the PROBIT trial found a higher risk of overweight or obesity in the intervention group compared with the control group. The relevance of the PROBIT trial to the US population has been questioned, given the much lower prevalence of child obesity in the Republic of Belarus at the time of the study relative to the US prevalence (17). Nonetheless, our conclusion is consistent with the systematic review of systematic reviews (8), which suggested that although breastfeeding of very short duration may be less protective than breastfeeding of longer duration with regard to subsequent overweight and obesity, residential confounding cannot be excluded.
Potential mechanisms and research needs
Despite the challenges of establishing a causal relation between human milk feeding exposures and risk of subsequent overweight or obesity, several lines of evidence suggest potential biological or behavioral mechanisms for such a relation (33). Rapid weight gain during infancy (particularly during the first 6 mo) is consistently related to subsequent risk of overweight or obesity (34–36), and rapid weight gain is more likely among formula-fed than among breastfed infants (35). Although the reasons for more rapid weight gain among formula-fed infants are not yet fully understood, infant self-regulation of energy intake may potentially differ between breastfed and formula-fed infants (37). In addition, higher protein intake among formula-fed infants drives hormonal differences that may stimulate greater weight gain and fat deposition (38), although the precise mechanisms are not yet clear, and this is an active area of investigation (39, 40). Randomized controlled trials of reduced protein formulas have demonstrated less rapid infant weight gain and reduced obesity at school age (41–45). The concentrations of free amino acids in human milk, when compared with infant formula, also may be important. For example, free glutamate, which is much higher in human milk than in conventional infant formulas, is a key signal for satiation. An experimental study comparing extensively hydrolyzed formula, with higher free glutamate content, with a standard infant formula reported a significant difference in early rapid weight gain between the groups (46).
Overfeeding of formula-fed infants also is a possibility, as feeding by bottle may make it more difficult for the infant to communicate satiety signals, and in some cases, the caregiver may urge the infant to finish the bottle so as to avoid wastage (47–49). The feeding dynamics of feeding at the breast may differ from those during bottle feeding. In a small pilot study using a within-subject approach (50), mothers were more sensitive to infant cues during breastfeeding, and the latency from feeding session midpoint to the first satiation cue was significantly longer when they were breastfeeding compared with when they were bottle feeding. Other investigators have reported that infants feeding directly from the breast exhibit more engagement and disengagement cues than do formula-fed infants (51). Differences in the dyadic approach of mothers and infants during feeding may have longer-term implications for programming of appetite regulation. At 3–6 y of age, children who were fed human milk in a bottle as infants were less likely to have high satiety responsiveness compared with directly breastfed children, after controlling for child age, child weight status, maternal race/ethnicity, and maternal education (48). All of the above studies were relatively small, however; thus, additional research on satiety signals and responsiveness is needed.
Future research studies on infant milk-feeding practices and health outcomes should be designed to reduce bias from confounding factors as much as possible. Sibling-pair studies are one example of this type of study design, but few such studies have been conducted, they tend to have much smaller sample sizes than do other types of observational studies, and causes of discordance in infant feeding between siblings complicate interpretation. Larger sibling-pair studies are needed that include consideration of reasons for discordance, and they need to examine siblings who differ in terms of the duration of human milk consumption (e.g., <6 mo, ≥6 mo), not just with respect to ever compared with never consuming human milk. Additional large randomized controlled trials of breastfeeding promotion, like the PROBIT trial (52), are also needed. If the trial achieves substantial differences in duration or exclusivity of breastfeeding between intervention groups, this provides an opportunity to examine effects on subsequent overweight or obesity (and many other outcomes).
Observational studies that make use of large data sets, especially those that follow participants longitudinally and, in particular, link children with siblings and parents, also would be useful for robustly assessing associations and providing more confidence in conclusions regarding potential causality. This could be achieved by linking surveillance systems that collect data about infant feeding and health outcomes (including overweight and obesity) and making use of emerging electronic medical record data. In general, observational studies need to take into account all of the key confounders in the analytical framework of this review, including aspects of the child's diet (complementary feeding and later dietary patterns). The use of instrumental variables, such as Mendelian randomization approaches that make use of genetic traits linked with breastfeeding, also could help minimize confounding (53). In both observational and intervention studies, researchers should consider effect modification in their study design whenever possible (e.g., child sex, parental obesity, socioeconomic status, race or ethnicity, child diets, child activity levels) to examine the impact of infant feeding on these outcomes within key subgroups.
Given the high prevalence of mixed feeding in the United States and elsewhere, additional research is also needed to investigate how the patterns and proportions of human milk feeding across the day and night and within each feeding, in the context of mixed feeding, are related to health outcomes. Similarly, very little evidence is available on the consequences of feeding human milk by bottle compared with from the breast. The composition of human milk varies during the day and within a feeding, which may affect the infant's physiology (54); bottle feeding human milk may modify these patterns, as well as the feeding dynamics of breastfeeding and bottle-feeding mothers and their infants.
We conclude that further carefully designed research is needed to disentangle the complex relation between infant feeding practices and the risk of subsequent overweight or obesity, as well as the biological and behavioral mechanisms if the relation is causal. This review was designed to be relevant to healthy infants in countries with a high or very high level of human development and may not be generalizable to other situations. Further research in countries undergoing the nutrition transition, greater use of stronger study designs, and comparing results across studies with different types of limitations is required to advance our understanding. Despite uncertainty about the relation of human milk feeding to the prevention of subsequent overweight, there are still many reasons to promote breastfeeding with regard to other outcomes for both the mother (55) [e.g., reduced risk of breast, ovarian, and endometrial cancers (56, 57); hypertension and cardiovascular disease (58); nonalcoholic fatty liver disease (59); and type 2 diabetes (60)] and the child [e.g., reduced risk of type 1 diabetes (61) and asthma (62), as well as greater cognitive development (15)].
Supplementary Material
Acknowledgments
We thank Lydia Bazzano for serving as a member of the Birth to 24 Months Subcommittee of the 2020 Dietary Guidelines Advisory Committee, which conducted this systematic review; Cria Perrine, Jennifer Lerman, and Kelly Scanlon for providing support to the Birth to 24 Months Subcommittee; the entire 2020 Dietary Guidelines Advisory Committee for reviewing and providing feedback about the systematic review protocol, evidence synthesis, and conclusion statements; Steve A. Abrams, Leila Beker, Tova Jacobovits, Kirsi Järvinen-Seppo, Laurie Nommsen-Rivers, Kimberly O'Brien, Emily Oken, Rafael Pérez-Escamilla, and Ekhard Ziegler for their foundational work during the Pregnancy and Birth to 24 Months Project that helped inform the development of our systematic review protocol; Yat Ping Wong, a former NESR team member, for helping to develop and conduct the literature search and retrieving articles; Perrine Nadaud and Carol Dreibelbis, former NESR team members, for screening search results; members of the public who provided comments about the systematic review; and the federal scientists who peer-reviewed the systematic review.
The authors' contributions were as follows—KGD, DG, SMD, EMM, TAD, REK, and EMT: developed the systematic review protocol and provided substantive input into the evidence synthesis; NT and GB: developed and conducted the literature search; DG, EMM, and SV: screened the search results, extracted data, and assessed risk of bias; JO, ES, and JdJ: provided oversight of the project; RLB and RN: peer-reviewed the systematic review content in the committee's scientific report; KGD: is responsible for the final content of the manuscript; and all authors: critically reviewed the manuscript and approved the final version.
Author disclosures: DG, EMM, SV, and GB worked under contract with the Food and Nutrition Service, USDA. Scientists who are employees of the funding source had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. All other authors report no conflicts of interest.
Notes
Supported by USDA, Food and Nutrition Service, Center for Nutrition Policy and Promotion, Alexandria, VA.
Data described in the manuscript are publicly and freely available without restriction at https://nesr.usda.gov/sites/default/files/2020-07/B24%20human-milk-infant-formula-%20overweight-obesity%20-%20full%20SR.pdf.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: Add Health, National Longitudinal Study of Adolescent Health; HHS, US Department of Health and Human Services; NESR, Nutrition Evidence Systematic Review team at the USDA Center for Nutrition Policy and Promotion; NLSY79, National Longitudinal Survey on Youth 1979; PROBIT, Promotion of Breastfeeding Intervention Trial.
Contributor Information
Kathryn G Dewey, Department of Nutrition, University of California, Davis, CA, USA.
Darcy Güngör, Panum Group, Bethesda, MD, USA; Nutrition Evidence Systematic Review team, Nutrition Guidance and Analysis Division (NGAD), Center for Nutrition Policy and Promotion (CNPP), Food and Nutrition Service (FNS), US Department of Agriculture (USDA), Alexandria, VA, USA.
Sharon M Donovan, Department of Food Science and Human Nutrition, University of Illinois, Urbana-Champaign, IL, USA.
Emily M Madan, Panum Group, Bethesda, MD, USA; Nutrition Evidence Systematic Review team, Nutrition Guidance and Analysis Division (NGAD), Center for Nutrition Policy and Promotion (CNPP), Food and Nutrition Service (FNS), US Department of Agriculture (USDA), Alexandria, VA, USA.
Sudha Venkatramanan, Panum Group, Bethesda, MD, USA; Nutrition Evidence Systematic Review team, Nutrition Guidance and Analysis Division (NGAD), Center for Nutrition Policy and Promotion (CNPP), Food and Nutrition Service (FNS), US Department of Agriculture (USDA), Alexandria, VA, USA.
Teresa A Davis, USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA.
Ronald E Kleinman, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Elsie M Taveras, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Regan L Bailey, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Rachel Novotny, Department of Human Nutrition Food and Animal Sciences, College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, Manoa, HI, USA.
Nancy Terry, National Institutes of Health Library, Office of Research Services, US Department of Health and Human Services (HHS), Bethesda, MD, USA.
Gisela Butera, Panum Group, Bethesda, MD, USA; Nutrition Evidence Systematic Review team, Nutrition Guidance and Analysis Division (NGAD), Center for Nutrition Policy and Promotion (CNPP), Food and Nutrition Service (FNS), US Department of Agriculture (USDA), Alexandria, VA, USA.
Julie Obbagy, Nutrition Evidence Systematic Review team, Nutrition Guidance and Analysis Division (NGAD), Center for Nutrition Policy and Promotion (CNPP), Food and Nutrition Service (FNS), US Department of Agriculture (USDA), Alexandria, VA, USA.
Janet de Jesus, Office of Disease Prevention and Health Promotion, HHS, Rockville, MD, USA.
Eve Stoody, NGAD, CNPP, FNS, USDA, Alexandria, VA, USA.
References
- 1. Koletzko B, Brands B, Poston L, Godfrey K, Demmelmair H. Early nutrition programming of long-term health. Proc Nutr Soc. 2012;71(3):371–8. [DOI] [PubMed] [Google Scholar]
- 2. Koletzko B, Godfrey K, Poston L, Szajewska H, van Goudoever J, de Waard M, Brands B, Grivell R, Deussen A, Dodd J. Nutrition during pregnancy, lactation and early childhood and its implications for maternal and long-term child health: the Early Nutrition Project recommendations. Ann Nutr Metab. 2019;74(2):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Neonatology. 1998;74(2):94–105. [DOI] [PubMed] [Google Scholar]
- 4. Gale C, Logan KM, Santhakumaran S, Parkinson JR, Hyde MJ, Modi N. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(3):656–69. [DOI] [PubMed] [Google Scholar]
- 5. Grummer-Strawn LM, Reinold CM, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States.Morb Mortal Wkly Rep. 2010;59(RR-9):1–15. [PubMed] [Google Scholar]
- 6. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:30–7. [DOI] [PubMed] [Google Scholar]
- 7. Arenz S, Rückerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity—a systematic review. Int J Obes. 2004;28(10):1247–56. [DOI] [PubMed] [Google Scholar]
- 8. Patro-Gołąb B, Zalewski BM, Kołodziej M, Kouwenhoven S, Poston L, Godfrey KM, Koletzko B, van Goudoever JB, Szajewska H. Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of overweight, obesity and body fat: a systematic review of systematic reviews. Obes Rev. 2016;17(12):1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US General Services Administration. FACA 101. [Internet] [cited 2021 Jan 27]. Available from: https://www.gsa.gov/policy-regulations/policy/federal-advisory-committee-management/finding-information-on-faca-committees/faca-101. [Google Scholar]
- 10. US Department of Agriculture and US Department of Health and Human Services . Dietary Guidelines for Americans, 2020–2025. 9th ed. 2020. [Internet] [cited 2021 Jan 27]. Available from: https://www.dietaryguidelines.gov/ [Google Scholar]
- 11. 113th Congress. Agricultural Act of 2014, Sec. 4204. Dietary Guidelines for Americans, Public Law 113–79, 128 Stat. 649. 2014. [Google Scholar]
- 12. 2020 Dietary Guidelines Advisory Committee . Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services, Part D, Chapters 4–7. Washington (DC): US Department of Agriculture, Agricultural Research Service; 2020. [Google Scholar]
- 13. 2020 Dietary Guidelines Advisory Committee and Nutrition Evidence Systematic Review Team . The duration, frequency, and volume of exclusive human milk and/or infant formula consumption and overweight and obesity: a systematic review. [Internet] [cited 2021 Jan 27]. Available from: https://nesr.usda.gov/sites/default/files/2020-07/B24%20human-milk-infant-formula-%20overweight-obesity%20-%20full%20SR.pdf [Google Scholar]
- 14. Beauregard JL, Hamner HC, Chen J, Avila-Rodriguez W, Elam-Evans LD, Perrine CG. Racial disparities in breastfeeding initiation and duration among US infants born in 2015. Morb Mortal Wkly Rep. 2019;68(34):745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smithers LG, Kramer MS, Lynch JW. Effects of breastfeeding on obesity and intelligence: causal insights from different study designs. JAMA Pediatr. 2015;169(8):707–8. [DOI] [PubMed] [Google Scholar]
- 16. Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet J-P, Martin RM. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86(6):1717–21. [DOI] [PubMed] [Google Scholar]
- 17. Martin RM, Kramer MS, Patel R, Rifas-Shiman SL, Thompson J, Yang S, Vilchuck K, Bogdanovich N, Hameza M, Tilling K. Effects of promoting long-term, exclusive breastfeeding on adolescent adiposity, blood pressure, and growth trajectories: a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2017;171(7):e170698–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin RM, Patel R, Kramer MS, Guthrie L, Vilchuck K, Bogdanovich N, Sergeichick N, Gusina N, Foo Y, Palmer T. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309(10):1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. 2020 Dietary Guidelines Advisory Committee . Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services, Part C. Washington (DC): US Department of Agriculture, Agricultural Research Service; 2020. [Google Scholar]
- 20. Dietary Guidelines for Americans. 2020 Dietary Guidelines Advisory Committee Meetings. [Internet] [cited 2021 Jan 27]. Available from: https://www.dietaryguidelines.gov/public-meetings. [Google Scholar]
- 21. United Nations Development Programme . Human development report 2014: sustaining human progress—reducing vulnerabilities and building resilience. [Internet] [cited 2021 Jan 21]. Available from: http://hdr.undp.org/en/content/human-development-report-2014 [Google Scholar]
- 22. US Food and Drug Administration. Federal requirements for the manufacture of infant formula for marketing in the United States, including registration requirements. [Internet] [cited 2021 Jan 21]. Available from: https://www.fda.gov/food/infant-formula-guidance-documents-regulatory-information/regulations-and-information-manufacture-and-distribution-infant-formula#manufacture. [Google Scholar]
- 23. Food and Agriculture Organization of the United Nations and World Health Organization. Codex Alimentarius International Food Standards. [Internet] [cited 2021 Jan 27]. Available from: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B72-1981%252FCXS_072e.pdf. [Google Scholar]
- 24. Hawkins SS, Baum CF, Rifas-Shiman SL, Oken E, Taveras EM. Examining associations between perinatal and postnatal risk factors for childhood obesity using sibling comparisons. Child Obes. 2019;15(4):254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson PM, Butcher KF, Levine PB. Maternal employment and overweight children. J Health Econ. 2003;22(3):477–504. [DOI] [PubMed] [Google Scholar]
- 26. Colen CG, Ramey DM. Is breast truly best? Estimating the effects of breastfeeding on long-term child health and wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evenhouse E, Reilly S. Improved estimates of the benefits of breastfeeding using sibling comparisons to reduce selection bias. Health Serv Res. 2005;40(6, pt 1):1781–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nelson MC, Gordon-Larsen P, Adair LS. Are adolescents who were breast-fed less likely to be overweight? Analyses of sibling pairs to reduce confounding. Epidemiology. 2005;16:247–53. [DOI] [PubMed] [Google Scholar]
- 29. Metzger MW, McDade TW. Breastfeeding as obesity prevention in the United States: a sibling difference model. Am J Hum Biol. 2010;22(3):291–6. [DOI] [PubMed] [Google Scholar]
- 30. Gillman MW, Rifas-Shiman SL, Berkey CS, Frazier AL, Rockett HR, Camargo CA Jr, Field AE, Colditz GA. Breast-feeding and overweight in adolescence: within-family analysis. Epidemiology. 2006;17(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Tierney PF, Barker DJ, Osmond C, Kajantie E, Eriksson JG. Duration of breast-feeding and adiposity in adult life. J Nutr. 2009;139(2):422S–5S. [DOI] [PubMed] [Google Scholar]
- 32. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development.Vital Health Stat 11. 2002;May(246):1–190. [PubMed] [Google Scholar]
- 33. Bartok CJ, Ventura AK. Mechanisms underlying the association between breastfeeding and obesity. Int J Pediatr Obes. 2009;4(4):196–204. [DOI] [PubMed] [Google Scholar]
- 34. Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, Campbell K. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. 2018;19(3):321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young BE, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Adv Nutr. 2012;3(5):675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, Rich-Edwards JW, Gillman MW. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–8. [DOI] [PubMed] [Google Scholar]
- 37. Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. Maternal versus infant factors related to breast milk intake and residual milk volume: the DARLING study. Pediatrics. 1991;87(6):829–37. [PubMed] [Google Scholar]
- 38. Koletzko B, Demmelmair H, Grote V, Totzauer M. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin in Perinatol. 2019;43(7):151153. [DOI] [PubMed] [Google Scholar]
- 39. He X, Sotelo-Orozco J, Rudolph C, Lönnerdal B, Slupsky CM. The role of protein and free amino acids on intake, metabolism, and gut microbiome: a comparison between breast-fed and formula-fed rhesus monkey infants. Front Pediatr. 2020;7:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kouwenhoven SM, Antl N, Finken MJ, Twisk JW, van der Beek EM, Abrahamse-Berkeveld M, van de Heijning BJ, Schierbeek H, Holdt LM, van Goudoever JB. A modified low-protein infant formula supports adequate growth in healthy, term infants: a randomized, double-blind, equivalence trial. Am J Clin Nutr. 2020;111(5):962–74. [DOI] [PubMed] [Google Scholar]
- 41. European Childhood Obesity Trial Study Group . Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89(6):1836–45. [DOI] [PubMed] [Google Scholar]
- 42. Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries J-P, Dain E, Giovannini M, Verduci E, Gruszfeld D, Socha P. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99(5):1041–51. [DOI] [PubMed] [Google Scholar]
- 43. Putet G, Labaune J-M, Mace K, Steenhout P, Grathwohl D, Raverot V, Morel Y, Picaud J-C. Effect of dietary protein on plasma insulin-like growth factor-1, growth, and body composition in healthy term infants: a randomised, double-blind, controlled trial (Early Protein and Obesity in Childhood (EPOCH) study). Br J Nutr. 2016;115(2):271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ziegler EE, Fields DA, Chernausek SD, Steenhout P, Grathwohl D, Jeter JM, Nelson SE, Haschke F. Adequacy of infant formula with protein content of 1.6 g/100 kcal for infants between 3 and 12 months. J Pediatr Gastroenterol Nutr. 2015;61(5):596–603. [DOI] [PubMed] [Google Scholar]
- 45. Inostroza J, Haschke F, Steenhout P, Grathwohl D, Nelson SE, Ziegler EE. Low-protein formula slows weight gain in infants of overweight mothers. J Pediatr Gastroenterol Nutr. 2014;59(1):70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mennella JA, Inamdar L, Pressman N, Schall JI, Papas MA, Schoeller D, Stallings VA, Trabulsi JC. Type of infant formula increases early weight gain and impacts energy balance: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li R, Scanlon KS, May A, Rose C, Birch L. Bottle-feeding practices during early infancy and eating behaviors at 6 years of age. Pediatrics. 2014;134(Suppl):S70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DiSantis KI, Collins BN, Fisher JO, Davey A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle?. Int J Behav Nutr Phys Act. 2011;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li R, Magadia J, Fein SB, Grummer-Strawn LM. Risk of bottle-feeding for rapid weight gain during the first year of life. Arch Pediatr Adolesc Med. 2012;166(5):431–6. [DOI] [PubMed] [Google Scholar]
- 50. Whitfield KC, Ventura AK. Exploration of responsive feeding during breastfeeding versus bottle feeding of human milk: a within-subject pilot study. Breastfeed Med. 2019;14(7):482–6. [DOI] [PubMed] [Google Scholar]
- 51. Shloim N, Vereijken C, Blundell P, Hetherington M. Looking for cues—infant communication of hunger and satiation during milk feeding. Appetite. 2017;108:74–82. [DOI] [PubMed] [Google Scholar]
- 52. Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet J-P, Vanilovich I, Mezen I, Ducruet T. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–20. [DOI] [PubMed] [Google Scholar]
- 53. Brion M-JA, Lawlor DA, Matijasevich A, Horta B, Anselmi L, Araújo CL, Menezes AMB, Victora CG, Smith GD. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int J Epidemiol. 2011;40(3):670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hahn-Holbrook J, Saxbe D, Bixby C, Steele C, Glynn L. Human milk as “chrononutrition”: implications for child health and development. Pediatr Res. 2019;85(7):936–42. [DOI] [PubMed] [Google Scholar]
- 55. Feltner C, Weber RP, Stuebe A, Grodensky CA, Orr C, Viswanathan M. Breastfeeding programs and policies, breastfeeding uptake, and maternal health outcomes in developed countries. Comparative Effectiveness Review No. 210. Rockville (MD): Agency for Healthcare Research and Quality; 2018. [PubMed] [Google Scholar]
- 56. Collaborative Group on Hormonal Factors in Breast Cancer . Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. Lancet North Am Ed. 2002;360(9328):187–95. [DOI] [PubMed] [Google Scholar]
- 57. Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kirkegaard H, Bliddal M, Støvring H, Rasmussen K, Gunderson E, Køber L, Sørensen T, Nohr E. Breastfeeding and later maternal risk of hypertension and cardiovascular disease—the role of overall and abdominal obesity. Prev Med. 2018;114:140–8. [DOI] [PubMed] [Google Scholar]
- 59. Ajmera VH, Terrault NA, VanWagner LB, Sarkar M, Lewis CE, Carr JJ, Gunderson EP. Longer lactation duration is associated with decreased prevalence of non-alcoholic fatty liver disease in women. J Hepatol. 2019;70(1):126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gunderson EP, Lewis CE, Lin Y, Sorel M, Gross M, Sidney S, Jacobs DR, Shikany JM, Quesenberry CP. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Intern Med. 2018;178(3):328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Güngör D, Nadaud P, LaPergola CC, Dreibelbis C, Wong YP, Terry N, Abrams SA, Beker L, Jacobovits T, Järvinen KM. Infant milk-feeding practices and diabetes outcomes in offspring: a systematic review. Am J Clin Nutr. 2019;109(Suppl 1):817S–37S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Güngör D, Nadaud P, LaPergola CC, Dreibelbis C, Wong YP, Terry N, Abrams SA, Beker L, Jacobovits T, Järvinen KM. Infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span: a systematic review. Am J Clin Nutr. 2019;109(Suppl 1):772S–99S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.