Abstract
Background
Mitochondrial disorders are among the most common heritable diseases, with an overall lifetime risk of approximately one in 1500. Nonetheless, their diagnosis is often missed because of their extreme phenotypic and genotypic heterogeneity.
Methods
This review is based on publications retrieved by a selective literature search on the clinical features, genetics, pathogenesis, diagnosis, and treatment of mitochondrial diseases.
Results
Pathogenic defects of energy metabolism have been described to date in over 400 genes. Only a small number of these genes lie in the mitochondrial DNA; the corresponding diseases are either maternally inherited or of sporadic distribution. The remaining disease-associated genes are coded in nuclear DNA and cause diseases that are inherited according to Mendelian rules, mostly autosomal recessive. The most severely involved organs are generally those with the highest energy requirements, including the brain, the sensory epithelia, and the extraocular, cardiac, and skeletal musculature. Typical manifestations include epileptic seizures, stroke-like episodes, hearing loss, retinopathy, external ophthalmoparesis, exercise intolerance, and diabetes mellitus. More than two manifestations of these types should arouse suspicion of a disease of energy metabolism. The severity of mitochondrial disorders ranges from very severe disease, already evident in childhood, to relatively mild disease arising in late adulthood. The diagnosis is usually confirmed with molecular-genetic methods. Symptomatic treatment can improve patients’ quality of life. The only disease-modifying treatment that has been approved to date is idebenone for the treatment of Leber hereditary optic neuropathy. Intravitreal gene therapy has also been developed for the treatment of this disease; its approval by the European Medicines Agency is pending.
Conclusion
Patients with mitochondrial diseases have highly varied manifestations and can thus present to physicians in practically any branch of medicine. A correct diagnosis is the prerequisite for genetic counseling and for the initiation of personalized treatment.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submission is 4 November 2022.
Primary mitochondrial disorders (MIDs) form a group of extremely heterogeneous diseases caused by genetic mitochondrial dysfunction. Taken as a whole, they are among the most common heritable diseases.
Consistent with the central importance of mitochondrial energy metabolism, defects can affect any tissue and any organ. Disorders can manifest with any form of symptom and at any age (table 1). Tissues with high energy requirements, such as brain, sensory epithelia, and extraocular, cardiac, and skeletal musculature, are particularly vulnerable, explaining why patients with an MID present primarily to neurology, neuropediatrics, ophthalmology, and cardiology departments.
Table 1. Phenotypic spectrum of mitochondrial disorders in children and adults, classified according to categories in the Human Phenotype Ontology (HPO).
| Phenotypes | Children (%)*1 | Adults (%)*2 |
| Nervous system | 79.4 | 52.1 |
| Metabolism | 69.8 | 21.0 |
| Muscles | 53.6 | 43.8 |
| Heart and circulation | 24.2 | 13.2 |
| Eyes | 23.8 | 62.0 |
| Growth and weight | 21.3 | 4.9 |
| Gastrointestinal tract | 17.8 | 2.9 |
| Facial features | 10.8 | 4.6 |
| Lungs | 10.6 | 2.5 |
| Hearing | 10.5 | 18.6 |
| Blood | 9.1 | 0.3 |
| Urogenital tract | 7.0 | 2.1 |
| Endocrine system | 4.3 | 11.6 |
| Skin | 1.5 | 0.5 |
| Immune system | 1.5 | 0.5 |
| Skeleton | 0.9 | 0.2 |
*1 In 1234 patients with disease onset < 18 years of age; based on data from mitoNET and the Institute of Human Genetics at the TU Munich, Germany
*2 In 985 patients with disease onset ≥ 18 years of age; based on data from mitoNET
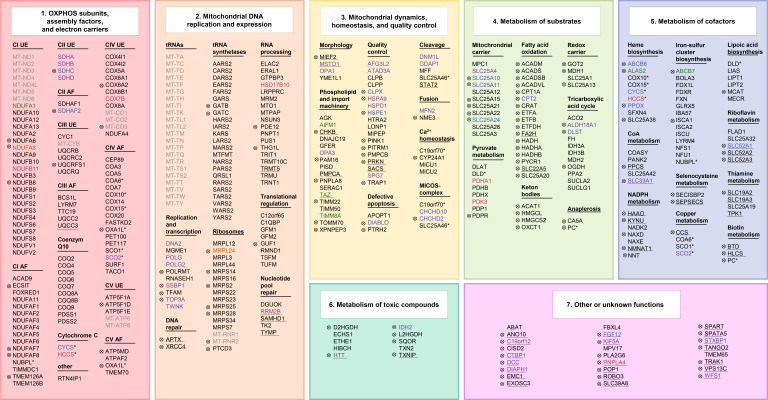
The structure and function of mitonchondria are determined by, among others, the interaction of approximately 1500 proteins. Pathogenic defects of energy metabolism have now been attributed to mutations in >400 of the associated 1500 genes. The majority of disorders involve impairment of the main mitochondrial function: aerobic energy production. eFigure 1 provides an overview of affected genes and their function.
eFigure 1.
Gene defects in mitochondrial disorders (legend see eBox1)
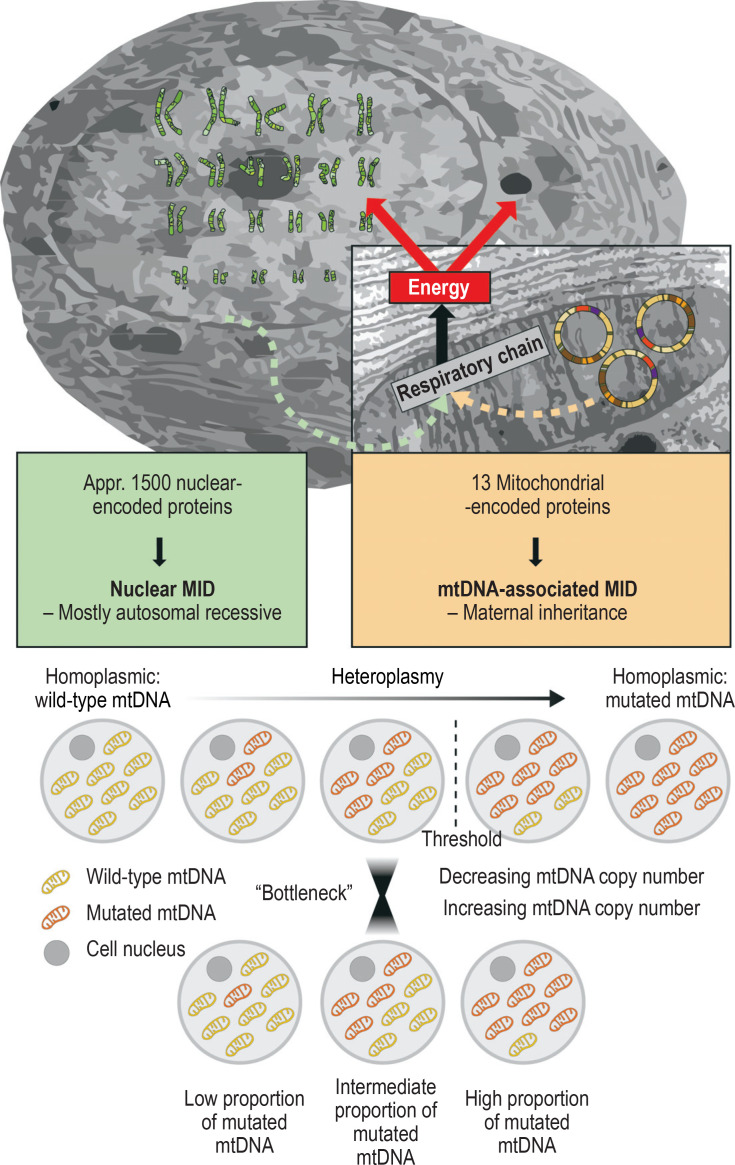
As a relic of their evolutionary past in the form of endosymbiotic bacteria, mitochondria have their own mitochondrial DNA (mtDNA) made up of 16,569 base pairs. This encodes for 13 structural proteins of the respiratory chain, as well as for two ribosomal RNAs (rRNA) and 22 transfer RNAs (tRNA), which are needed for the semi-autonomous transcription and translation of the structural proteins. Pathogenic variants of all these 37 genes have been described.
Oocytes contain hundreds of mitochondria, whereas the few mitochondria that a sperm possesses are eliminated during fertilization. For this reason, mtDNA mutations and the related clinical pictures (Figure, Table 2) are inherited only down the maternal line. In most cases, a mixture of mutated and wild-type mtDNA is present (heteroplasmy), which can vary from generation to generation and tissue to tissue, and which determines to a great extent symptom severity (figure).
Figure.
Mitochondrial DNA (mtDNA) encodes for only 13 proteins in the mitochondrial respiratory chain. A total of around 1500 proteins, which are primarily encoded in the cell nucleus on the chromosomes, are needed for the structure and function of mitochondria. Accordingly, disorders that are attributed to mutations of nuclear-encoded genes are inherited according to Mendelian rules, mostly in an autosomal recessive pattern. In contrast, disorders caused by mtDNA mutations are maternally inherited. In most cases, not all mtDNA molecules carry the mutation, and a mixture of mutated and wild-type mtDNA is present (heteroplasmy). Cellular dysfunction mostly develops above a threshold of approximately 60% mutated mtDNA. The degree of heteroplasmy can vary from generation to generation due to the decline and subsequent increase in mtDNA copy number in embryogenesis (mitochondrial bottleneck), as well as from tissue to tissue, and plays a major role in determining symptom severity. MID, mitochondrial disorder
Table 2. Characteristics of selected mitochondrial disorders.
| Disorder | Commonest mutations | Characteristics |
| mtDNA point mutations: maternal inheritance | ||
| LHON | ND4-m.11778, ND1-m.3460, ND6-m.14484 |
Subacute, high-grade, central visual impairment P: approximately 1:30.000 (12) |
| MELAS/MIDD | tRNA-Leu- m.3243 > m.3271 |
Full-blown disease rare; oligosymptomatic disease common, for example, MIDD P: approximately 1:30.000 symptomatic m.3243-mutation carriers. of which approximately 10% have the full set of MELAS symptoms, around 30% MIDD (21) |
| MERRF | tRNA-Lys- m.8344 > m.8356 |
Eponymous symptoms not obligatory: myoclonus: 59%, epilepsy: 61%; RRF: 63%; in addition, frequently hearing loss: 72%, ataxia: 70%, psychiatric abnormalities: 54%, multiple lipomas P: approximately 1:500,000 symptomatic m.8344-mutation carriers (2) |
| NARP/MILS | ATP6- m.8993 > m.9176 |
55% Leigh syndrome, 8% NARP, other than that, numerous mono- or oligosymptomatic manifestations, primarily ataxia and/or neuropathy → Should be considered in multiple DD (32) |
| mtDNA deletions: occur mostly sporadically | ||
| CPEO (plus) | Single mtDNA deletion | Chronic progressive ptosis and external ophthalmoplegia, with or without additional non-muscular symptoms; double vision in around 50% of cases (33); usually occurs sporadically, maternally inherited only in exceptional cases (< 5%) (34); study results with elamipretide hitherto inconsistent (35) |
| KSS | Single mtDNA deletion | Historically defined as CPEO, retinitis pigmentosa, onset before 20 years of age, and additional symptoms |
| Nuclear gene mutations: autosomal dominant inheritance | ||
| ADOA | OPA1 > OPA3 | Slowly progressive loss of central vision (36); idebenone possibly beneficial, but hitherto based only on retrospective analysis of an uncontrolled cohort (37) |
| adPEO | POLG1 > ANT1, Twinkle → Secondary multiple DNA deletions |
Mutations in nuclear-encoded genes cause impaired mtDNA replication. thus multiple mtDNA deletions; clinically, adPEO is indistinguishable from the sporadic form; crucial to genetic counseling: risk of inheritance of 50% in adPEO, < 5% in cpeo |
| Nuclear gene mutations: X-chromosomal inheritance | ||
| PDH deficiency | PDHA1 | Ranging from severe neonatal lactic acidosis to Leigh syndrome and on to later-onset neurological disturbances; exacerbated in the case of intercurrent infections or fever; prognosis mostly poor |
| Nuclear gene mutations: autosomal recessive inheritance (selection from > 200 defects) | ||
| Leigh syndrome | > 80 Genes including SURF1 | Subacute necrotizing encephalomyelopathy ([38]; Figure 1d) I: approximately 1:40.000 live births (39) Depending on the underlying defect, trial use of the dietary supplements thiamine, coenzyme Q10, and riboflavin (40) |
| SANDO | POLG1 → Secondary multiple mtDNA deletions and depletion |
Biallelic POLG1 mutations cause varying combinations of epilepsy, ataxia, neuropathy, myopathy, and ophthalmoplegia |
| MNGIE | TYMP1 → Secondary multiple mtDNA deletions and depletion |
TYMP1 defect results in impaired mtDNA synthesis via mitochondrial nucleotide pool imbalances; clinical onset usually in late childhood; CPEO, leukoencephalopathy, peripheral neuropathy; gastrointestinal disorders result in pronounced cachexia, which significantly reduces life expectancy |
ADOA, autosomal dominant optic atrophy; adPEO, autosomal dominant progressive external ophthalmoplegia; CPEO, chronic progressive external ophthalmoplegia;
DD, differential diagnosis; I, incidence; KSS, Kearns–Sayre syndrome; LHON, Leber hereditary optic neuropathy;
MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; MERRF, myoclonic epilepsy with ragged-red fibers;
MIDD, maternally inherited diabetes and deafness; MILS, maternally inherited Leigh syndrome; MNGIE, mitochondrial neurogastrointestinal encephalomyopathy;
NARP, neuropathy, ataxia, retinitis pigmentosa; P, prevalence; PDH, pyruvate dehydrogenase; RRF, ragged red fibers;
SANDO, sensory ataxia, neuropathy, dysarthria, and ophthalmoplegia
The majority of the approximately 1500 mitochondrial proteins, however, are encoded by nuclear DNA (nDNA) in the cell nucleus; the associated messenger RNA is translated on cytoplasmic ribosomes into proteins, which enter the mitochondria via their own protein import mechanism. Accordingly, numerous MIDs follow Mendelian rules of inheritance, mostly autosomal recessive, rarely autosomal dominant or X-chromosomal (Table 2, Figure). Therefore, precise genetic classification of a disorder is of the utmost importance for the genetic counseling of affected families.
With regard to epidemiology, a lifetime risk for developing an autosomal recessive MID of 48.4:100,000 individuals in Europe has been calculated using population genetic methods (1); for mtDNA-related MIDs, population-based data show a lifetime risk of 20.4:100,000 individuals (2). Together, this yields an estimated lifetime risk of 68.8:100,000 individuals. This means that one in 1470 newborns will develop an MID over the course of their lifetime.
General diagnosis
The most important diagnostic step is taken right at the outset: thinking of the possibility of an MID. Specific symptoms or symptom constellations, as well as an unusual combination of multiple organ manifestations, may provide pointers. Laboratory and imaging investigations (for example, elevated lactate in blood and cerebrospinal fluid, pathognomonic findings on cranial magnetic resonance imaging [cMRT]) may strengthen suspicion. Muscle biopsy, as a screening test for morphological (ragged red fibers [RRF]) and biochemical indications of an MID, has recently lost much of its importance. Instead, molecular genetic methods have become established as first-line diagnostics. This was initially in the form of targeted candidate gene sequencing (panel diagnostics), and is now increasingly performed in the form of whole exome sequencing (WES) or whole genome sequencing (WGS), both methods that best address the vast genetic heterogeneity of MIDs.
At up to 50%, WES and WGS achieve significantly higher diagnostic rates compared to panel diagnostics, and also enable the description of novel disease-associated genes (3). The cross-sectoral use of these methods is hampered by regulatory hurdles in the German healthcare system; as a result, they are currently used mainly following an application to the respective health insurance in individual cases or in the context of scientific projects. With the elimination of the application and authorization requirements for sequencing > 25 kb, the first steps have been taken in the direction of routine use of these methods. However, the indication should be made by appropriately qualified physicians, while the sequencing itself should be performed and interpreted by human geneticists. The aim of, among others, the German genome initiative, genomDE, is to establish these methods in routine practice in the sense of knowledge-generating patient care (www.genom.de). In order to further increase the diagnostic yield, multiomics data from transcriptome and proteome analyses are now being used in addition to functional investigations at the cellular level—to date, however, only in the context of scientific projects (4– 7). Here, unresolved cases of MID require a skin biopsy to be taken for fibroblast culture in order to generate the necessary data for a molecular diagnosis.
However, the aim of generating is not merely to elucidate the genetic cause and correctly classify the clinical picture. Due to the clinical heterogeneity of MID, comprehensive and regularly repeated phenotyping of all relevant organ systems is recommended, even if in the first instance there are no corresponding signs (deep phenotyping). This serves to detect hidden or subsequent organ involvement at an early stage and can have important therapeutic ramifications (8). For example, even in the absence of symptoms, we recommend annual cardiac investigations with long-term electrocardiogram (ECG) and echocardiography in order to promptly detect and treat conduction disorders and cardiomyopathy.
General treatment
Although MIDs are undoubtedly challenging to treat and the evidence for current treatment recommendations is mainly low-level, there are no grounds for therapeutic nihilism. Symptom-oriented treatments, such as the administration of antiepileptic drugs and the deployment of cochlear implants or pacemakers, can significantly improve quality of life and disease course. In order to counteract a vicious circle of exercise intolerance, reduced physical activity, and secondary deconditioning, individually tailored endurance and strength training is recommended (9). There is no evidence that taking vitamins and supplements confers any benefit, except in the case of a specific deficiency, for example, coenzyme Q10 deficiency disorders (10). Disease-modifying treatments are under development and include pharmaceuticals (for example, those with antioxidant activity or that promote mitochondrial biogenesis [11]), enzyme replacement therapies, and gene therapies. The only medication authorized to date is idebenone for Leber hereditary optic neuropathy (LHON). For information on the studies currently underway, the reader is referred to www.clinicaltrials.gov.
Registry and natural history studies
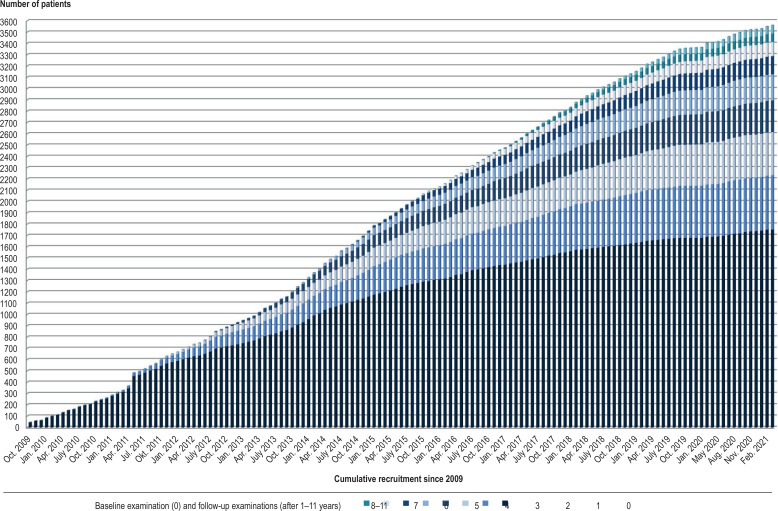
The German Network for Mitochondrial Diseases (mitoNET, www.mitoNET.org) has been funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) since 2009. It operates, among other things, a patient registry and a biobank, and also conducts studies on the natural history of MIDs. It currently includes the data of > 1700 patients (efigure 2). As part of the EU-funded project GENOMIT (www.GENOMIT.eu), an internationally harmonized concept for data collection has been developed, and a global MID registry will shortly open.
Registries of this kind make it possible to gain new insights into the phenotypic and genotypic spectrum of MIDs thanks to the large number of patients, and to promptly contact suitable patients regarding inclusion in clinical trials (trial readiness). Furthermore, monitoring the natural history of the disease through regular follow-ups is of tremendous importance for the planning of urgently needed randomized treatment studies.
Individual disorders
Leber hereditary optic neuropathy
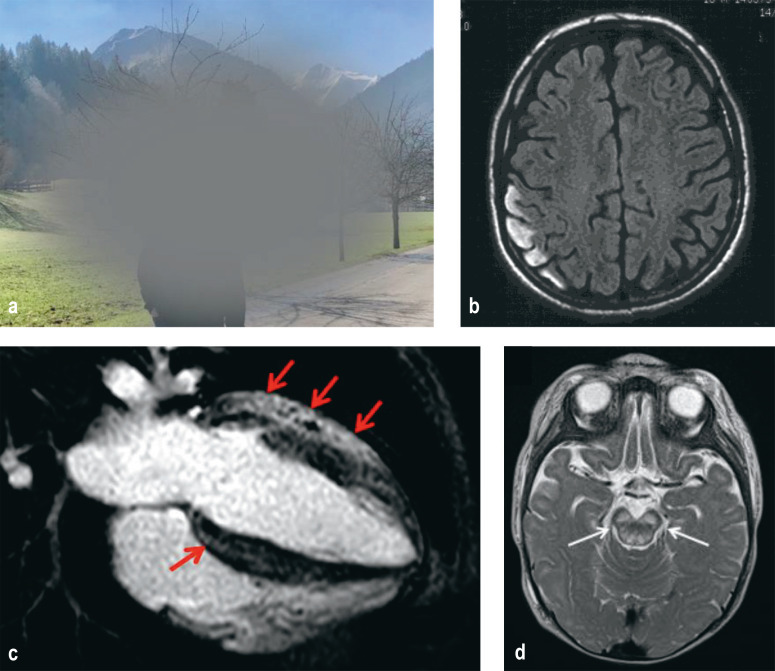
The retina and optic nerve are among the tissues with particularly high energy demands. For this reason, visual disorders are a frequent symptom of MID. Visual loss is particularly pronounced in LHON, which is considered the most common MID with a prevalence of approximately 1:30,000 (12). Although LHON can develop at any age, onset is predominantly in adolescence and young adulthood. Males are significantly more frequently affected than are women; a protective effect for estrogen is mooted. Following subacute onset, central vision becomes impaired to a high degree (Figure 1a). Either both eyes are affected simultaneously or, more frequently, initially only one eye, followed some weeks later by the second. In the majority of patients, visual acuity remains at < 0.1 in the long-term course.
Figure 1.
Pathognomic findings in mitochondrial disorders
a) LHON: central scotoma from the patient’s perspective
b) MELAS: cortical T2 hyperintensity on cMRT in the case of fresh stroke-like episode
c) MELAS: cardiac involvement; the four-chamber view shows marked concentric left ventricular hypertrophy with contrast uptake (red arrows) consistent with myocardial fibrosis
d) Leigh syndrome: symmetrical signal changes in the brainstem
cMRT, cranial magnetic resonance imaging; LHON, Leber hereditary optic neuropathy; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes
The cause in > 90% of cases is one of the three primary LHON mutations, ND4-m.11778, ND6-m.14484, and ND1-m.3460 of the mtDNA, which result in defects of the complex I of the mitochondrial respiratory chain. Penetrance of these almost always homoplasmic (figure) mutations is incomplete. Only around 50% of male mutation carriers and around 10% of female mutation carriers develop disease, which explains why male patients predominate. Smoking is considered a trigger for the onset of symptoms (13). In the course of pathogenesis, retinal ganglion cell dysfunction develops, while in the chronic course, apoptotic loss of these cells and their axons (optic nerve) occurs.
Establishing the clinical diagnosis is challenging. Initially, there is no optic nerve atrophy, but instead discrete optic nerve swelling and peripapillary telangiectasia. In some cases, the optic disc is normal on fundoscopy and only optical coherence tomography shows discrete thickening of the nerve fiber layers. In the majority of patients, particularly in the case of unilateral onset, optic neuritis is initially suspected, and appropriate neurological diagnosis and treatment are carried out. It is crucial at this point to think of the possibility of LHON and arrange the simple and inexpensive genetic blood test.
Since mtDNA mutations are maternally inherited, all relatives in the maternal line are at risk of developing disease. Therefore, information regarding the harmful effect of smoking needs to be communicated to those at risk. However, as a result of the reduced penetrance, it is not unusual for no further cases to be known within a family.
In symptom-oriented treatment, magnifying visual aids and acoustic aids (for example, via smartphone) are important. Based on the results of a randomized controlled trial (14) and an expanded access program (15), conditional approval was granted in the European Union (EU) in 2015 for the drug idebenone (3 × 300 mg/day) (evidence level Ib, grade A recommendation [8]). Idebenone acts as an intramitochondrial antioxidant and is able to transfer electrons directly to complex III of the respiratory chain while bypassing the defective complex I, thereby producing overall less oxidative damage and more energy. Compared to the natural course, treatment improves the chances that visual acuity that is still good stabilizes (50% of patients) or that visual acuity that is already significantly reduced recovers in a clinically relevant manner (16). Recovery of this kind was observed in 46% of treated patients (versus 31% in a historical, untreated control group). Visual acuity in these patients improved by a mean of > 7 lines on the visual chart (15).
In addition, intravitreal gene therapy has been developed for patients with ND4-m.11778 mutation. Following unilateral injection 6–12 months after symptom onset, visual acuity improved in 37 patients at 96 weeks by a mean of 15 letters in the treated eye and 13 letters in the untreated contralateral eye on the eye chart. The contralateral effect could be explained in primate experiments by transfer of the gene therapy construct via the optic chiasm (17). Unilateral injection < 6 months following symptom onset produced similarly positive results in a further 38 patients (18). Gene therapy approval has been applied for.
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes
MELAS syndrome (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) is a mitochondrial multisystem disorder that usually begins in the first to second decade of life (table 2). In cases of severe disease, the disorder is apparent in childhood through delayed development, short stature, muscle weakness and exercise intolerance, migraines, epileptic seizures, and stroke-like episodes. In the further course, hearing loss, diabetes mellitus, as well as cardiac and gastrointestinal manifestations also develop. Stroke-like episodes were defined in a consensus paper as subacute-onset encephalopathic crises with neurological and psychiatric symptoms (including impaired consciousness, headache, epileptic seizures, visual deficits, visual hallucinations, agitation, and behavioral disturbances) that develop in the setting of epileptic activity (19). On cMRI, they appear as predominantly occipital cortical hyperintensities that are not confined to vascular territories (Figure 1b). They may either completely resolve, together with the clinical symptoms, or develop into cortical laminar necrosis. In the longer course, MELAS patients usually develop marked brain atrophy and dementia. Life expectancy is difficult to estimate in individual cases due to the highly variable course of the disease, but on average it is significantly reduced. Median survival in fully symptomatic patients was 16.9 years from the onset of neurological manifestations, while the average age of death was 34.5 ±19 years (20). The most frequent causes of death are epileptic state and cardiac events.
The cause of MELAS in >80% of cases is the m.3243A>G mutation in mtDNA, which leads to a change in conformation of the tRNA that transfers leucine, thereby resulting in less mitochondrial protein synthesis. In addition to this, numerous other mtDNA mutations are described. The diagnosis is confirmed by detection of the mutation in blood (leukocytes) or, in an even more sensitive manner, in urine sediment. Muscle biopsies are mostly no longer required for diagnostic purposes.
Disease severity correlates with the proportion of mutated mtDNA versus wild-type mtDNA. The full clinical picture of MELAS develops in only around 10% of m.3243-mutation carriers. More frequently, patients exhibit a combination of only diabetes and hearing loss (maternally inherited diabetes and deafness [MIDD], 30%), as well as other symptom constellations that do not meet the MELAS criteria (21). Heteroplasmy level and phenotype can also vary widely within a family.
In symptomatic treatment, consistent antiepileptic therapy is particularly important for the suppression and secondary prevention of seizures and stroke-like episodes. Primarily newer antiepileptic drugs such as levetiracetam, lamotrigine, and lacosamide are recommended, whereas older antiepileptic drugs may have mitochondrial toxicity, and valproate in particular is even contraindicated. The use of L-arginine is the subject of controversy and, in the absence of sufficient evidence, is not recommended in the consensus paper (19). To date, there are no specific treatments that positively affect disease course. A randomized controlled trial with dichloroacetate failed to demonstrate an effect and even had to be discontinued due to adverse effects (toxic neuropathy) (22).
Other important MIDs are presented in the eSupplement and in Table 2.
Special aspects
Cardiac involvement
Due to their high energy consumption, cardiomyocytes are particularly susceptible to impaired mitochondrial energy metabolism. In our cohort, cardiovascular symptoms were observed in 24% of patients with disease onset <18 years of age and in 13% of patients with onset ≥ 18 years of age (table 1). In the heart, both the myocardium (cardiomyopathy) and the cardiac conduction system can be affected (23). For example, hypertrophic cardiomyopathy in MELAS is characteristic (Figure 1c) with a typical pattern of scarring. From a clinical perspective, this cardiac disease causes exercise dyspnea and heart failure; the myocardial scarring can be the starting point for ventricular arrythmias (in some cases even sudden cardiac death) (24).
On the other hand, patients with chronic progressive external ophthalmoplegia (CPEO), and in particular Kearns-Sayre syndrome (KSS), are more likely to exhibit conduction disturbances (in the form of bundle branch or AV block) and circumscribed textural disturbances in the left ventricular myocardium (with systolic heart function often still preserved). As a result of primary bradycardic arrhythmias, these patients are also predisposed to sudden cardiac death (25), which has been reported as the cause of death in up to 20% of patients with KSS (26). As early on as at initial diagnosis of MID, a cardiac work-up with ECG and echocardiography is recommended, additionally including cardiac MRI if necessary. Regular long-term ECG follow-up is of central importance for treatment decisions (for example, whether or not a pacemaker or defibrillator is required).
Anesthesia
The organ systems particularly affected by MID (central nervous system, heart, muscles) are also target organs for anesthesia drugs. This often results in increased sensitivity to these drugs. Therefore, careful selection and dosage are required. Due to the heterogeneity of MID as well as limited evidence, anesthesia needs to be individually tailored to each patient. To this end, comprehensive preoperative assessment and patient information are needed. In principle, any anesthetic is capable of suppressing mitochondrial function. Before, during, and after anesthesia, it is essential to ensure that disturbances in glucose metabolism, body temperature, electrolytes, and fluid balance are kept to a minimum in patients with MID. Postoperatively, monitoring on an intensive care unit is indicated (27, 28).
Vaccinations
Infectious diseases pose a particular risk for MID patients. An inflammatory reaction, especially fever, can trigger a clinical exacerbation, in some cases with fatal consequences. Since there is no scientific evidence of a negative effect for vaccinations in MID, both the US Center for Disease Control and Prevention and the World Health Organization recommend the same vaccinations for MID patients as for the healthy population (29, 30).
COVID-19
As an infectious disease, COVID-19 may pose a particular risk to MID patients, for example, when diabetes mellitus, cardiomyopathy, or respiratory failure are present in the setting of MID. There is also experimental evidence showing that mitochondrial dysfunction may promote a severe COVID-19 course (31). Therefore, MID patients should take particular precautions to avoid infection and also get vaccinated against COVID-19.
Supplementary Material
eSupplement
Additional individual disorders
Autosomal dominant optic atrophy
Autosomal dominant optic atrophy (ADOA) begins almost unnoticed at pre-school age with symmetrical, slowly progressive visual loss. As a result, the diagnosis is often not made until a routine examination is carried out upon starting school or in adulthood as part of family check-ups. Approximately 40% of patients retain vision of ≥ 0.32 and 15% ≥ 0.5. In around 20% of cases, extraocular symptoms manifest (ADOA plus), in particular hearing loss (63%), ocular motility disturbances (46%), myopathy (36%), ataxia (30%), and neuropathy (30 %).
The cause of ADOA in approximately 70% of cases is a mutation in the nuclear gene OPA1, which encodes for a mitochondrial dynamin-like GTPase. Other genes are affected in the remaining cases, for example OPA3 (36). Here again, as in Leber hereditary optic neuropathy (LHON), the retinal ganglion cells and their axons (optic nerve) are particularly vulnerable. Since it is primarily the papillomacular bundles that are affected, central or paracentral scotomas develop, while the peripheral visual field mostly remains well preserved.
Symptomatic therapy is the same as in LHON. There are also early indications that idebenone may confer a benefit, but these are based to date only on retrospective analysis of an uncontrolled cohort (37).
Chronic progressive external ophthalmoplegia and Kearns–Sayre syndrome
Chronic progressive external ophthalmoplegia (CPEO) is one of the most common MIDs. Slowly progressive bilateral ptosis and increasingly limited eye movements in all directions of view are typical and associated with double vision in approximately 50% of patients (33). Proximal muscle weakness as well as muscular dysphagia and dysarthria may also develop. Since the transition from pure CPEO to mitochondrial multisystem disorders (CPEO plus) is fluid, a comprehensive neurological and internal medicine work-up should always be carried out. The most severe manifestation in this continuum is Kearns–Sayre syndrome (KSS), which, historically, is defined as a combination of CPEO, pigmentary retinopathy, onset before the age of 20, as well as one of the following additional symptoms: ataxia, elevated cerebrospinal fluid protein, or cardiac conduction disturbances.
These disorders can be caused by different gene defects, the precise classification of which is important for genetic counseling. Most commonly, they are due to single mtDNA deletions, which usually occur sporadically and are inherited through the maternal line only in exceptional cases (<5%) (34). In other cases, a nuclear gene mutation (for example, in the POLG1 gene, which codes for the mitochondrial DNA polymerase) causes multiple mtDNA deletions that are inherited in an autosomal dominant manner. More rarely, one finds mtDNA point mutations that are maternally inherited.
To confirm this diagnosis, it is still usually necessary to perform a muscle biopsy, since the mtDNA deletions can only be reliably detected there. Morphological demonstration of ragged red fibers (RRF) and cytochrome c oxidase (COX)-negative fibers also underpins the diagnosis.
As part of symptomatic treatment, surgical lid elevation is medically indicated if the eyelid covers the pupil. Disturbing double vision can be corrected with prism glasses, if necessary also with strabismus surgery. Early implantation of a cardiac pacemaker or defibrillator in the case of relevant arrhythmias can be life-saving. To date, there are no specific treatments that positively affect disease course. Positive effects seen in a phase-2 trial with elamipretide (35) were not confirmed in a phase-3 trial.
Leigh syndrome
Leigh syndrome (table 2), a typical and relatively common (approximately 1:40,000 live births) form of childhood MID, is defined as a combination of clinical and laboratory signs of mitochondrial dysfunction associated with symmetrical lesions in the basal ganglia and/or brainstem (38; Figure 1d). As such, rather than a unified clinical picture, Leigh syndrome is an umbrella term for >80 syndromes attributable to various genetic defects, with autosomal recessive mutations in the SURF1 gene or mutations at position m.8993 of the mtDNA being the most frequent (39). The same mtDNA mutation can also cause much milder disease with juvenile or adult onset and symptom constellations consisting primarily of ataxia, cognitive impairment, and neuropathy (table 2), as well as in rare cases full-blown NARP (neuropathy, ataxia, retinitis pigmentosa) syndrome (32). Contrary to previous assumptions, the degree of heteroplasmy of the m.8993 mutation does not correlate well with the severity of disease. Thus, there must be other unknown modifying factors. Due to the wide clinical and genetic heterogeneity of Leigh syndrome, prognosis is challenging and affected families can only be adequately counseled if they are aware of the underlying genetic abnormalities. However, it must be stressed that early-childhood Leigh syndrome very often follows an unfavorable course and that therapeutic intervention can be undertaken in only very few cases. Treatable causes of Leigh syndrome primarily include impaired thiamine metabolism and defects in coenzyme Q10 biosynthesis. In rare cases, riboflavin-responsive disorders (for example, ACAD9 deficiency) may also be present. Therefore, the dietary supplements thiamine, coenzyme Q10, and riboflavin, which have low side-effect profiles, should be given on a low-threshold trial basis in equivocal cases of Leigh syndrome (31). It is also important to avoid catabolic states (for example, in the case of refusal to eat, gastroenteritis, and bacterial infections). The parents of affected children need to be advised that such situations should be regarded as emergencies and that low-threshold hospital admission (for example, for rehydration, glucose administration, and, where necessary, antibiotic treatment) is indicated.
Questions on the article in issue 44/2021:
Mitochondrial Disorders
The submission deadline is 4 November 2022. Only one answer is possible per question.
Please select the answer that is most appropriate.
Question 1
Which pattern of inheritance do genetic mutations in mitochondrial DNA (mtDNA) follow?
Autosomal dominant
Autosomal recessive
Down the father’s line (paternal)
Down the mother’s line (maternal)
Intermediate autosomal
Question 2
Approximately how high is the lifetime risk for mitochondrial disorders?
10:100 000 Persons
20:100 000 Persons
50:100 000 Persons
70:100 000 Persons
90:100 000 Persons
Question 3
Which organ systems are most frequently affected in adults with mitochondrial disorders (and disease onset ≥ 18 years of age) according to data from mitoNET?
Eyes, nervous system, and muscles
Hearing, eyes, and immune system
Endocrine system, muscles, and cardiovascular system
Metabolism, hearing, and immune system
Urogenital tract, muscles, and hearing
Question 4
How many mitochondrial respiratory chain proteins are encoded in mitochondrial DNA (mtDNA)?
Three proteins
13 Proteins
30 Proteins
1000 Proteins
1500 Proteins
Question 5
To date, there is an approved disease-modifying pharmacological therapy for only one mitochondrial disorder. Which disorder is this?
Chronic progressive external ophthalmoplegia (CPEO)
Leigh syndrome
Leber hereditary optic neuropathy (LHON)
Kearns–Sayre syndrome (KSS)
Myoclonic epilepsy with ragged-red fibers (MERRF)
Question 6
What does the abbreviation MELAS stand for?
Mitochondrial epilepsy, lactic acidosis, and sleepless episodes
Myocarditis, encephalomyopathy, lactic acidosis, and sight loss
Myocarditis, epilepsy, lung edema, acidosis, and stroke
Muscle weakness, encephalomyopathy, lactic acidosis, and stroke-like episodes
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes
Question 7
Which antiepileptic drug is contraindicated for the treatment of epileptic seizures in the setting of a mitochondrial disorder?
Levetiracetam
Gabapentin
Valproate
Lamotrigine
Lacosamide
Question 8
Which of the following disorders/syndromes are caused exclusively by nuclear gene mutations?
PDH (pyruvate dehydrogenase) deficiency
LHON (Leber hereditary optic neuropathy)
NARP (neuropathy, ataxia, retinitis pigmentosa)
KSS (Kearns–Sayre syndrome)
MIDD (maternally inherited diabetes and deafness)
Question 9
Which of the following statements applies to vaccinations for patients with mitochondrial disorders?
Due to stronger vaccination side effects, only the most essential vaccinations should be performed.
The same vaccinations as in the general population are recommended.
Patients with mitochondrial disorders are not allowed to receive any vaccinations.
Vaccinations should only be administered in an inpatient setting with patient monitoring.
The intervals between vaccinations in the basic immunization schedule should be halved in each case, since the immune response is weaker.
Question 10
Which term describes the presence of a mixture of mutated and wild-type mtDNA (mtDNA, mitochondrial DNA) in a tissue/cell?
Homoplasmy
Haploidy
Heteroploidy
Heteroplasmy
Heterozygosity
eBOX. Legend to eFigure 1:
Gene defects in mitochondrial disorders
An association with mitochondrial disorders has been reported for 413 genes. The genes have been grouped into subcategories according to their function:
(1) OXPHOS subunits (SU), assembly factors (AF), and electron carriers (112 of 413 genes)
(2) Mitochondrial DNA replication and expression (104 of 413 genes)
(3) Mitochondrial dynamics, homeostasis, and quality control (55 of 413 genes)
(4) Metabolism of substrates (57 of 413 genes)
(5) Metabolism of cofactors (49 of 413 genes)
(6) Metabolism of toxic compounds (10 of 413 genes)
(7) Other or unknown functions (26 of 413 genes).
The mode of inheritance is indicated by color. Some genes (n = 13) have different functions within these categories (indicated by *).
Of the 413 genes, 141 are not in the core set of all four publications (indicated by ![]() ). Genes encoding proteins that are not localized in the mitochondria according to MitoCarta2.0 are highlighted with an underscore (54 of 413 genes).
). Genes encoding proteins that are not localized in the mitochondria according to MitoCarta2.0 are highlighted with an underscore (54 of 413 genes).
eFigure 2.
History of recruitment in the mitoNET registry. Between October 2009 and February 2021, 1753 patients with mitochondrial disorders (confirmed or highly suspected) were included in the mitoNET registry (0 = baseline examination). In addition, follow-up examinations (at 1 year, 2 years, etc. up to 11-year follow-up) are shown, from which it is possible to reconstruct the natural history of disorders.
Acknowledgments
Translated from the original German by Christine Rye.
Acknowledgments
We would like to thank Dr. Sarah Stenton, Dr. Boriana Büchner, and Stefan Weißinger for their support in producing the figures, as well as Prof. Dr. Thomas Meitinger for engaging in critical discussions. We would also like to thank the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) for research funding of the German Network for Mitochondrial Diseases (Deutsche Netzwerk für mitochondriale Erkrankungen, mitoNET, 01GM1906A for TK, CK, FD, and HP) and the E-Rare project GENOMIT (01GM1920B for TK and HP).
Footnotes
Conflict of interest statement
Prof. Klopstock received third-party funding to conduct clinical trials from Santhera Pharmaceuticals, GenSight Biologics, Khondrion, and Stealth BioTherapeutics. He is chairman of the non-profit association Deutsches mitoNET e. V., which received donations from Santhera Pharmaceuticals and GenSight Biologics. He received consulting fees from Santhera Pharmaceuticals, GenSight Biologics, Chiesi GmbH, and Pretzel Therapeutics. For lectures he received speaker’s fees from Santhera Pharmaceuticals, GenSight Biologics, and Chiesi GmbH. In addition, the abovementioned companies partially reimbursed congress fees as well as travel and accommodation costs.
PD Dr. Priglinger received study support (third-party funding) from Gensight Biologics and Iveric Bio. She received speaker’s fees from Novartis and Chiesi GmbH, and had travel and accommodation costs reimbursed by Recordati Pharma.
Prof. Yilmaz received speaker’s and consulting fees from Alnylam Therapeutics GmbH, Pfizer Pharma GmbH, and Akcea Therapeutics GmbH; he also has scientific collaborations with Philips and Circle Cardiovascular Imaging Inc.
Prof. Kornblum received consulting fees and travel cost reimbursement from Stealth Biotherapeutics. She received speaker’s fees from Novartis and Santhera Pharmaceuticals. Study support (third-party funding) was provided to her by Stealth Biotherapeutics. She coordinates the German DGN/AWMF guidelines on mitochondrial disorders.
Prof. Distelmaier received travel cost reimbursement and speaker’s fees from Santhera Pharmaceuticals.
Dr. Prokisch declares that no conflict of interest exists.
References
- 1.Tan J, Wagner M, Stenton SL, et al. Lifetime risk of autosomal recessive mitochondrial disorders calculated from genetic databases. EBioMedicine. 2020;54 doi: 10.1016/j.ebiom.2020.102730. 102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorman GS, Schaefer AM, Ng Y, et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol. 2015;77:753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenton SL, Prokisch H. Genetics of mitochondrial diseases: Identifying mutations to help diagnosis. EBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102784. 102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings BB, Marshall JL, Tukiainen T, et al. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal5209. eaal5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer LS, Bader DM, Mertes C, et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat Commun. 2017;8 doi: 10.1038/ncomms15824. 15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer LS, Wortmann SB, Prokisch H. „Transcriptomics“: molecular diagnosis of inborn errors of metabolism via RNA-sequencingJ. Inherit Metab Dis. 2018;41:525–532. doi: 10.1007/s10545-017-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenton SL, Prokisch H. Advancing genomic approaches to the molecular diagnosis of mitochondrial disease. Essays Biochem. 2018;62:399–408. doi: 10.1042/EBC20170110. [DOI] [PubMed] [Google Scholar]
- 8.Kornblum C., et al. Deutsche Gesellschaft für Neurologie, editor. Mitochondriale Erkrankungen, S1-Leitlinie, Leitlinien für Diagnostik und Therapie in der Neurologie. https://dgn.org/leitlinien/ll-030-049-mitochondriale-erkrankungen-2021/ last accessed on 17 September 2021. 2021 [Google Scholar]
- 9.Stefanetti RJ, Blain A, Jimenez-Moreno C, et al. Measuring the effects of exercise in neuromuscular disorders: a systematic review and meta-analyses. Wellcome Open Res. 2020;5 doi: 10.12688/wellcomeopenres.15825.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinzii CM, Emmanuele V, Hirano M. Clinical presentations of coenzyme q10 deficiency syndrome. Mol Syndromol. 2014;5:141–146. doi: 10.1159/000360490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirinen E, Auranen M, Khan NA, et al. Niacin cures systemic NAD(+) deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab. 2020;31:1078–1090. doi: 10.1016/j.cmet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Carelli V, La Morgia C, Klopstock T. Mancuso M, Klopstock T, editors. Mitochondrial optic neuropathies. Diagnosis and management of mitochondrial disorders. Cham, Switzerland. Springer Nature Switzerland AG. 2019:125–139. [Google Scholar]
- 13.Rabenstein R, Catarino C, Rampeltshammer V, et al. Smoking and alcohol, health-related quality of life and psychiatric comorbidities in Leber’s Hereditary Optic Neuropathy mutation carriers: a prospective cohort study. Orphanet J Rare Dis. 2021;16 doi: 10.1186/s13023-021-01724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber‘s hereditary optic neuropathy. Brain. 2011;134:2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catarino CB, von Livonius B, Priglinger C, et al. Real-world clinical experience with Idebenone in the treatment of Leber Hereditary Optic Neuropathy. J Neuroophthalmol. 2020;40:558–565. doi: 10.1097/WNO.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carelli V, Carbonelli M, de Coo IF, et al. International consensus statement on the clinical and therapeutic management of Leber Hereditary Optic Neuropathy. J Neuroophthalmol. 2017;37:371–381. doi: 10.1097/WNO.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 17.Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aaz7423. eaaz7423. [DOI] [PubMed] [Google Scholar]
- 18.Newman NJ, Yu-Wai-Man P, Carelli V, et al. Efficacy and safety of intravitreal gene therapy for Leber Hereditary Optic Neuropathy treated within 6 months of disease onset. Ophthalmology. 2021;128:649–660. doi: 10.1016/j.ophtha.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Ng YS, Bindoff LA, Gorman GS, et al. Consensus-based statements for the management of mitochondrial stroke-like episodes. Wellcome Open Res. 2019;4 doi: 10.12688/wellcomeopenres.15599.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann P, Engelstad K, Wei Y, et al. Natural history of MELAS associated with mitochondrial DNA m3. 243A>G genotype. Neurology. 2011;77:1965–1971. doi: 10.1212/WNL.0b013e31823a0c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesbitt V, Pitceathly RDS, Turnbull DM, et al. The UK MRC Mitochondrial Disease Patient Cohort Study: clinical phenotypes associated with the m3. 243A > G mutation-implications for diagnosis and management. J Neurol Neurosurg Psychiatry. 2013;84:1107–1112. doi: 10.1136/jnnp-2012-303528. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann P, Engelstad K, Wei Y, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324–330. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- 23.Bates MGD, Bourke JP, Giordano C, d‘Amati G, Turnbull DM, Taylor RW. Cardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. Eur Heart J. 2012;33:3023–3033. doi: 10.1093/eurheartj/ehs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malfatti E, Laforêt P, Jardel C, et al. High risk of severe cardiac adverse events in patients with mitochondrial m3. 243A>G mutation. Neurology. 2013;80:100–105. doi: 10.1212/WNL.0b013e31827b1a2f. [DOI] [PubMed] [Google Scholar]
- 25.Barends M, Verschuren L, Morava E, Nesbitt V, Turnbull D, McFarland R. Causes of Death in Adults with Mitochondrial Disease. JIMD Rep. 2016;26:103–113. doi: 10.1007/8904_2015_449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabunga P, Lau AK, Phan K, et al. Systematic review of cardiac-electrical disease in Kearns-Sayre syndrome and mitochondrial cytopathy. Int J Cardiol. 2015;181:303–310. doi: 10.1016/j.ijcard.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Yeoh C, Teng H, Jackson J, et al. Metabolic disorders and anesthesia. Curr Anesthesiol Rep. 2019;9:340–359. doi: 10.1007/s40140-019-00345-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A, Dunne E, Mannion M, et al. A review of anaesthetic outcomes in patients with genetically confirmed mitochondrial disorders. Eur J Pediatr. 2017;176:83–88. doi: 10.1007/s00431-016-2813-8. [DOI] [PubMed] [Google Scholar]
- 29.Doja A. Genetics and the myth of vaccine encephalopathy. Paediatr Child Health. 2008;13:597–599. doi: 10.1093/pch/13.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruk SK, Pacheco SE, Koenig MK, Bergerson JRE, Gordon-Lipkin E, McGuire PJ. Vulnerability of pediatric patients with mitochondrial disease to vaccine-preventable diseases. J Allergy Clin Immunol Pract. 2019;7:2415–2418. doi: 10.1016/j.jaip.2019.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenoy S. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm Res. 2020;69:1077–1085. doi: 10.1007/s00011-020-01389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stendel C, Neuhofer C, Floride E, et al. Delineating MT-ATP6-associated disease: from isolated neuropathy to early onset neurodegeneration. Neurol Genet. 2020;6 doi: 10.1212/NXG.0000000000000393. e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Wai Man CY, Smith T, Chinnery PF, Turnbull DM, Griffiths PG. Assessment of visual function in chronic progressive external ophthalmoplegia. Eye (Lond) 2006;20:564–568. doi: 10.1038/sj.eye.6701924. [DOI] [PubMed] [Google Scholar]
- 34.Chinnery PF, DiMauro S, Shanske S, et al. Risk of developing a mitochondrial DNA deletion disorder. Lancet. 2004;364:592–596. doi: 10.1016/S0140-6736(04)16851-7. [DOI] [PubMed] [Google Scholar]
- 35.Karaa A, Haas R, Goldstein A, Vockley J, Weaver WD, Cohen BH. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology. 2018;90:e1212–e1221. doi: 10.1212/WNL.0000000000005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finsterer J, Mancuso M, Pareyson D, Burgunder JM, Klopstock T. Mitochondrial disorders of the retinal ganglion cells and the optic nerve. Mitochondrion. 2018;42:1–10. doi: 10.1016/j.mito.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Romagnoli M, La Morgia C, Carbonelli M, et al. Idebenone increases chance of stabilization/recovery of visual acuity in OPA1-dominant optic atrophy. Ann Clin Transl Neurol. 2020;7:590–594. doi: 10.1002/acn3.51026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baertling F, Rodenburg RJ, Schaper J, et al. A guide to diagnosis and treatment of Leigh syndrome. J Neurol Neurosurg Psychiatry. 2014;85:257–265. doi: 10.1136/jnnp-2012-304426. [DOI] [PubMed] [Google Scholar]
- 39.Lake NJ, Compton AG, Rahman S, Thorburn DR. Leigh syndrome: one disorder, more than 75 monogenic causes. Ann Neurol. 2016;79:190–203. doi: 10.1002/ana.24551. [DOI] [PubMed] [Google Scholar]
- 40.Distelmaier F, Haack TB, Wortmann SB, Mayr JA, Prokisch H. Treatable mitochondrial diseases: cofactor metabolism and beyond. Brain. 2017;140 doi: 10.1093/brain/aww303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eSupplement
Additional individual disorders
Autosomal dominant optic atrophy
Autosomal dominant optic atrophy (ADOA) begins almost unnoticed at pre-school age with symmetrical, slowly progressive visual loss. As a result, the diagnosis is often not made until a routine examination is carried out upon starting school or in adulthood as part of family check-ups. Approximately 40% of patients retain vision of ≥ 0.32 and 15% ≥ 0.5. In around 20% of cases, extraocular symptoms manifest (ADOA plus), in particular hearing loss (63%), ocular motility disturbances (46%), myopathy (36%), ataxia (30%), and neuropathy (30 %).
The cause of ADOA in approximately 70% of cases is a mutation in the nuclear gene OPA1, which encodes for a mitochondrial dynamin-like GTPase. Other genes are affected in the remaining cases, for example OPA3 (36). Here again, as in Leber hereditary optic neuropathy (LHON), the retinal ganglion cells and their axons (optic nerve) are particularly vulnerable. Since it is primarily the papillomacular bundles that are affected, central or paracentral scotomas develop, while the peripheral visual field mostly remains well preserved.
Symptomatic therapy is the same as in LHON. There are also early indications that idebenone may confer a benefit, but these are based to date only on retrospective analysis of an uncontrolled cohort (37).
Chronic progressive external ophthalmoplegia and Kearns–Sayre syndrome
Chronic progressive external ophthalmoplegia (CPEO) is one of the most common MIDs. Slowly progressive bilateral ptosis and increasingly limited eye movements in all directions of view are typical and associated with double vision in approximately 50% of patients (33). Proximal muscle weakness as well as muscular dysphagia and dysarthria may also develop. Since the transition from pure CPEO to mitochondrial multisystem disorders (CPEO plus) is fluid, a comprehensive neurological and internal medicine work-up should always be carried out. The most severe manifestation in this continuum is Kearns–Sayre syndrome (KSS), which, historically, is defined as a combination of CPEO, pigmentary retinopathy, onset before the age of 20, as well as one of the following additional symptoms: ataxia, elevated cerebrospinal fluid protein, or cardiac conduction disturbances.
These disorders can be caused by different gene defects, the precise classification of which is important for genetic counseling. Most commonly, they are due to single mtDNA deletions, which usually occur sporadically and are inherited through the maternal line only in exceptional cases (<5%) (34). In other cases, a nuclear gene mutation (for example, in the POLG1 gene, which codes for the mitochondrial DNA polymerase) causes multiple mtDNA deletions that are inherited in an autosomal dominant manner. More rarely, one finds mtDNA point mutations that are maternally inherited.
To confirm this diagnosis, it is still usually necessary to perform a muscle biopsy, since the mtDNA deletions can only be reliably detected there. Morphological demonstration of ragged red fibers (RRF) and cytochrome c oxidase (COX)-negative fibers also underpins the diagnosis.
As part of symptomatic treatment, surgical lid elevation is medically indicated if the eyelid covers the pupil. Disturbing double vision can be corrected with prism glasses, if necessary also with strabismus surgery. Early implantation of a cardiac pacemaker or defibrillator in the case of relevant arrhythmias can be life-saving. To date, there are no specific treatments that positively affect disease course. Positive effects seen in a phase-2 trial with elamipretide (35) were not confirmed in a phase-3 trial.
Leigh syndrome
Leigh syndrome (table 2), a typical and relatively common (approximately 1:40,000 live births) form of childhood MID, is defined as a combination of clinical and laboratory signs of mitochondrial dysfunction associated with symmetrical lesions in the basal ganglia and/or brainstem (38; Figure 1d). As such, rather than a unified clinical picture, Leigh syndrome is an umbrella term for >80 syndromes attributable to various genetic defects, with autosomal recessive mutations in the SURF1 gene or mutations at position m.8993 of the mtDNA being the most frequent (39). The same mtDNA mutation can also cause much milder disease with juvenile or adult onset and symptom constellations consisting primarily of ataxia, cognitive impairment, and neuropathy (table 2), as well as in rare cases full-blown NARP (neuropathy, ataxia, retinitis pigmentosa) syndrome (32). Contrary to previous assumptions, the degree of heteroplasmy of the m.8993 mutation does not correlate well with the severity of disease. Thus, there must be other unknown modifying factors. Due to the wide clinical and genetic heterogeneity of Leigh syndrome, prognosis is challenging and affected families can only be adequately counseled if they are aware of the underlying genetic abnormalities. However, it must be stressed that early-childhood Leigh syndrome very often follows an unfavorable course and that therapeutic intervention can be undertaken in only very few cases. Treatable causes of Leigh syndrome primarily include impaired thiamine metabolism and defects in coenzyme Q10 biosynthesis. In rare cases, riboflavin-responsive disorders (for example, ACAD9 deficiency) may also be present. Therefore, the dietary supplements thiamine, coenzyme Q10, and riboflavin, which have low side-effect profiles, should be given on a low-threshold trial basis in equivocal cases of Leigh syndrome (31). It is also important to avoid catabolic states (for example, in the case of refusal to eat, gastroenteritis, and bacterial infections). The parents of affected children need to be advised that such situations should be regarded as emergencies and that low-threshold hospital admission (for example, for rehydration, glucose administration, and, where necessary, antibiotic treatment) is indicated.