Abstract
The number of stable discriminant biochemical characters is limited in the genera Alcaligenes and Agrobacterium, whose species are consequently difficult to distinguish from one another by conventional tests. Moreover, genomic studies have recently drastically modified the nomenclature of these genera; for example, Alcaligenes xylosoxidans was transferred to the genus Achromobacter in 1998. Twenty-five strains of Achromobacter xylosoxidans, three strains of an Agrobacterium sp., five strains of an Alcaligenes sp., and four unnamed strains belonging to the Centers for Disease Control and Prevention group IVc-2 were examined. These strains were characterized by conventional tests, including biochemical tests. The assimilation of 99 carbohydrates, organic acids, and amino acids was studied by using Biotype-100 strips, and rRNA gene restriction patterns were obtained with the automated Riboprinter microbial characterization system after cleavage of total DNA with EcoRI or PstI restriction endonuclease. This polyphasic approach allowed the two subspecies of A. xylosoxidans to be clearly separated. Relationships between five strains and the Ralstonia paucula type strain were demonstrated. Likewise, three strains were found to be related to the Ochrobactrum anthropi type strain. We showed that substrate assimilation tests and automated ribotyping provide a simple, rapid, and reliable means of identifying A. xylosoxidans subspecies and that these two methods can be used as alternative methods to characterize unidentified strains rapidly when discriminant biochemical characters are missing.
The taxonomic position of the genus Alcaligenes has been changing for a few years as a result of genomic studies. Alcaligenes species have been transferred to the genera Carbophilus (14), Halomonas (9), Ralstonia (18, 20), and Variovorax (19). Alcaligenes xylosoxidans, Alcaligenes ruhlandii, and Alcaligenes piechaudii were recently reassigned to the genus Achromobacter (21). The subspecies Achromobacter xylosoxidans subsp. denitrificans has been proposed and the subspecies Achromobacter xylosoxidans subsp. xylosoxidans was automatically created. Likewise, some Agrobacterium species now belong to the genera Ruegeria and Stappia (17).
A. xylosoxidans was found in aqueous environmental sources and isolated from a wide range of clinical samples. This organism is recognized as an opportunistic pathogen responsible for serious infections (8, 10). Medical equipment and solutions have been found to be contaminated with this organism (8).
Due to the limited number of stable discriminating characteristics, many Alcaligenes and Agrobacterium species remain difficult to distinguish from one another by conventional tests. Ribotyping was proposed as a taxonomic tool a few years ago and was shown to differentiate genera and species (4, 11, 13, 15, 16). More recently, a phenotypic approach based on an auxanogram using the Biotype-100 identification system revealed the taxonomic diversity of the pseudomonads (12) and also successfully distinguished Rhodococcus and Gordonia strains (2).
Twenty-five strains of A. xylosoxidans subsp. xylosoxidans or subsp. denitrificans, five Alcaligenes strains, three Agrobacterium strains, and four unnamed bacteria belonging to the Centers for Disease Control and Prevention (CDC) group IVc-2 from the Collection de l'Institut Pasteur (CIP) were identified to the species or subspecies level by a polyphasic approach based on conventional tests, auxanogram, and automated ribotyping.
MATERIALS AND METHODS
Bacterial strains.
Twenty-five strains of A. xylosoxidans, three strains of Agrobacterium, five strains of Alcaligenes, and four unnamed strains belonging to CDC group IVc-2 were studied. All the strains belonged to the CIP (Table 1).
TABLE 1.
Strains studied
| Straina | Origin |
|---|---|
| Agrobacterium sp. | |
| CIP 102250 | Human (blood) |
| CIP 102460 | Contamination (HPLCb) |
| CIP 102731 | Human (conjunctivitis) |
| Alcaligenes sp. | |
| CIP 100007 | Unknown |
| CIP 100008 | Unknown |
| CIP 100998 | Human (blood) |
| CIP 101080 | Human (blood) |
| CIP 102485 | Human |
| A. xylosoxidans subsp. denitrificans | |
| CIP 60.83 | Unknown |
| CIP 77.15T | Soil |
| CIP 100012 | Unknown |
| CIP 100013 | Unknown |
| CIP 100015 | Unknown |
| CIP 100020 | Unknown |
| A. xylosoxidans subsp. xylosoxidans | |
| CIP 58.72 | Sputum |
| CIP 61.20 | Antiseptic solution (quaternary ammonium) |
| CIP 62.30 | Unknown |
| CIP 68.19 | Unknown |
| CIP 71.32T | Ear discharge |
| CIP 100005 | Human (blood) |
| CIP 100029 | Human (blood) |
| CIP 101719 | Human (blood) |
| CIP 101902 | Human (blood) |
| CIP 102041 | Human (blood) |
| CIP 102236 | Human (sputum) |
| CIP 102274 | Human (sputum) |
| CIP 102288 | Human (skin) |
| CIP 102498 | Human (bile) |
| CIP 102630 | Human (sputum) |
| CIP 102744 | Human (blood) |
| CIP 102768 | Human (trachea) |
| CIP 103938 | Blood |
| CIP 103961 | Sepsis |
| CIP 104044 | Skin |
| CIP 104045 | Human (lung) |
| Unnamed bacteria | |
| CIP 104521 | Blood |
| CIP 104522 | Blood |
| CIP 104523 | Blood |
| CIP 104524 | Bronchial source |
| R. paucula CIP 105943T | Human (respiratory tract) |
| O. anthropi CIP 82.115T | Unknown |
All the strains were from the CIP.
High-performance liquid chromatograph.
Conventional identification.
All strains were examined by the conventional tests described by Chester and Cooper (7).
Identification with Biotype-100 strips.
The assimilation of 99 carbohydrates, organic acids, and amino acids was studied by using Biotype-100 strips (BioMérieux, La Balme-les Grottes, France). Growth after 1 and 4 days of incubation was compared with that of the control without carbon source. The Recognizer, Adanson, and Dendrograf programs of the Taxotron package were used according to the user's manual for numerical analysis of Biotype-100 data. The distance coefficient selected was the complement of the Jaccard coefficient, and clustering was done by the unweighted pair group method of averages.
Identification with ribotyping method.
Ribotyping was carried out using the Riboprinter microbial characterization system (Qualicon, Inc., Wilmington, Del.). Colonies were picked from solid medium, suspended in sample buffer, and heat treated. The lysing agent was added, and the samples were transferred to the Riboprinter system. Restriction endonuclease digestion, gel separation, transfer, and hybridization with a chemiluminescence-labeled DNA probe containing the rRNA operon from Escherichia coli were carried out by the automated instrument in 8 h.
Gel images were exported in TIFF and analyzed with the RestrictoScan, Restrictotyper, Adanson, and Dendrograf programs of the Taxotron package. The cubic Spline algorithm was used to calculate fragment sizes. A fixed fragment size tolerance value of 4% was chosen.
Antibiotic susceptibility.
Antibiotic susceptibility of the strains was tested by the agar diffusion method (5). The susceptibilities were determined according to the guidelines of the Comité de l'Antibiogramme de la Société Française de Microbiologie (1).
RESULTS
Conventional identification.
All the strains studied were gram-negative rods, motile, oxidase and catalase positive, and strictly aerobic. All the strains were negative for production of gelatinase, caseinase, β-galactosidase, DNase, and arginine-dihydrolase. Lysine and ornithine were also not decarboxylated.
The A. xylosoxidans strains were characterized by their capacity to grow anaerobically with nitrate as the electron acceptor and by the absence of enzymatic activities as well as by the lack of production of H2S and hydrolysis of esculin and tributyrin. Characteristics differentiating the 25 strains of A. xylosoxidans are shown in Table 2. Xylose oxidative degradation was the only positive test for the 20 A. xylosoxidans subsp. xylosoxidans strains. This test was negative for the five A. xylosoxidans subsp. denitrificans strains.
TABLE 2.
Characteristics differentiating the A. xylosoxidans strains
| Characteristics | No. of positive strains
|
|
|---|---|---|
| A. xylosoxidans subsp. xylosoxidans (20 strains) | A. xylosoxidans subsp. denitrificans (5 strains) | |
| Growth: | ||
| At 41°C | 20 | 2 |
| At 45°C | 1 | 0 |
| In 8% NaCl | 3 | 0 |
| On Simmon's citrate | 17 | 5 |
| On malonate | 1 | 2 |
| Activity of: | ||
| Gamma-glutamyltransferase | 2 | 2 |
| Urease | 7 | 0 |
| Xylose oxidative degradation | 20 | 0 |
| Hydrolysis of hippurate | 14 | 5 |
For the 12 remaining strains, the results obtained for conventional tests are shown in Table 3. Alcaligenes strains showed great variability in their conventional tests, whereas Agrobacterium strains had a lot of common traits. The unnamed bacteria all gave identical responses to the tests carried out.
TABLE 3.
Characteristics differentiating unidentified strains
| Characteristic | Result for straina
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CIP 100007 | CIP 100008 | CIP 100998 | CIP 101080 | CIP 102485 | Unnamed bacteria | CIP 102250 | CIP 102460 | CIP 102731 | |
| Growth: | |||||||||
| At 5, 41, 45°C | −, −, − | −, +, − | −, −, − | −, +, − | −, +, − | −, +, − | −, −, − | −, −, − | −, −, − |
| On Simmon's citrate | + | + | + | + | + | + | − | − | + |
| On malonate | − | + | + | + | + | + | − | − | − |
| Denitrification | + | − | + | − | − | − | + | + | + |
| Hydrolysis of: | |||||||||
| Hippurate | − | + | + | + | + | + | + | + | + |
| Tributyrin | + | + | − | + | + | + | − | − | − |
| Esculin | − | − | − | − | − | − | − | + | + |
| Production of H2S | − | − | − | − | − | − | + | + | + |
| Activity of: | |||||||||
| Gamma-glutamyltransferase | + | + | + | − | + | + | + | + | + |
| Urease | + | − | − | − | + | + | + | + | + |
| Phenylalanine desaminase | − | − | − | − | + | − | + | + | + |
| Tween 80-esterase | + | + | − | + | + | + | ND | ND | ND |
| Lipase | − | + | − | − | − | + | − | − | − |
| Lecithinase | − | − | − | − | + | + | − | − | − |
| Protease | − | − | − | − | − | + | − | − | − |
CIP 100007, CIP 100008, CIP 100998, CIP 101080, and CIP 102485 were identified as Alcaligenes; CIP 102250, CIP 102460, and CIP 102731 were identified as Agrobacterium; and the unnamed bacteria (CIP 104521, CIP 104522, CIP 104523, and CIP 104524) were not identified at all and had the same responses in the conventional tests. All the strains were from the CIP. ND, not determined.
Biotype data.
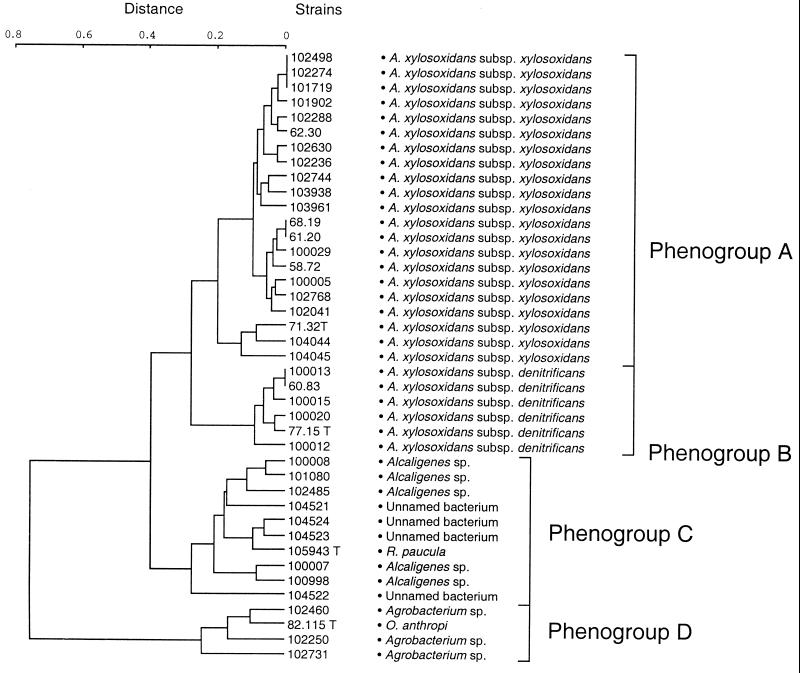
The 37 strains studied could all use a wide range of organic compounds as their sole energy and growth sources. The use of l-tartrate, trans-aconitate, d-gluconate, and caprate as carbon sources allowed us to differentiate the two subspecies of A. xylosoxidans. Whereas A. xylosoxidans subsp. xylosoxidans was able to grow on trans-aconitate, d-gluconate, and caprate, A. xylosoxidans subsp. denitrificans was not. In contrast, the A. xylosoxidans subsp. denitrificans used l-tartrate while A. xylosoxidans subsp. xylosoxidans did not. The phenogram in Fig. 1 shows the relationships, in terms of carbon source utilization, between these strains and the A. xylosoxidans subsp. xylosoxidans, A. xylosoxidans subsp. denitrificans, Ralstonia paucula, and Ochrobactrum anthropi type strains. Examination of the phenogram gave clear groupings and allowed us to define four phenogroups (A, B, C, and D) for the 41 strains tested. Phenogroup A was composed of A. xylosoxidans subsp. xylosoxidans strains. Two strains, CIP 104044 and CIP 104045, were more closely related to the type strain of A. xylosoxidans subsp. xylosoxidans than the others. Phenogroup B included the A. xylosoxidans subsp. denitrificans strains. The five strains of Alcaligenes and the four unnamed bacteria fell into phenogroup C, as did the R. paucula type strain. Phenogroup D contained the three Agrobacterium strains studied and the O. anthropi type strain.
FIG. 1.
Phenogram based on the carbon source utilization test.
Ribotyping data.
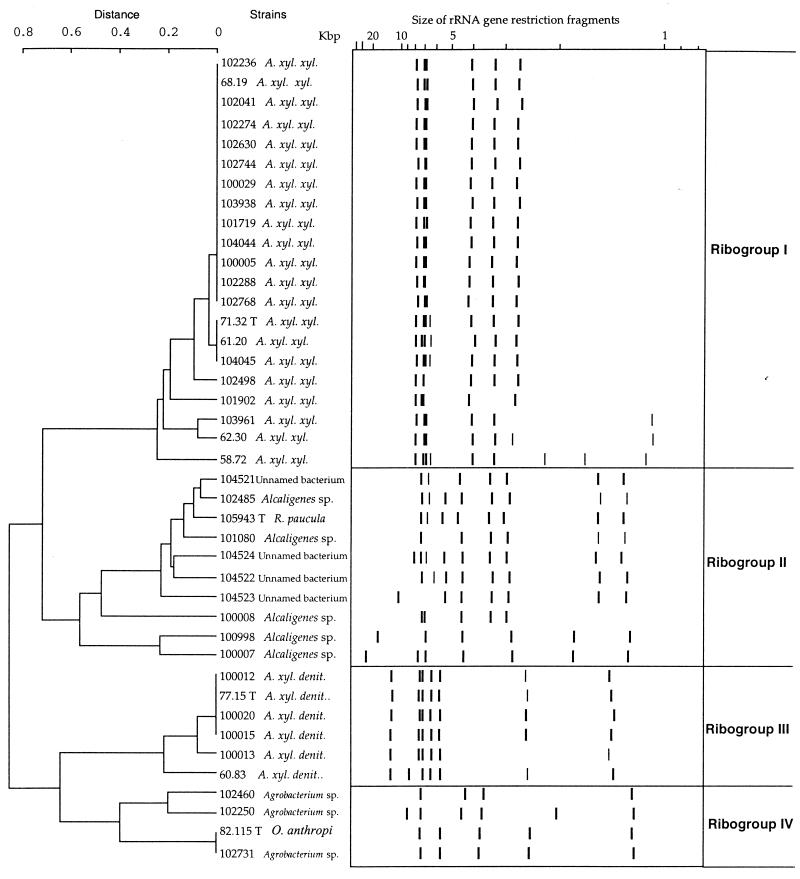
Ribotyping of the 41 strains listed in Table 1 was carried out by an automated system. The EcoRI and PstI enzymes always generated an appropriate number of restriction fragments to allow a comparative analysis to be made. A schematic representation of the banding patterns of all the strains and the deduced dendrograms are shown in Fig. 2 and 3.
FIG. 2.
Dendrogram based on the EcoRI ribotyping patterns of the 41 strains. A. xyl. xyl., A. xylosoxidans subsp. xylosidans; A. xyl. denit., A. xylosoxidans subsp. denitrificans.
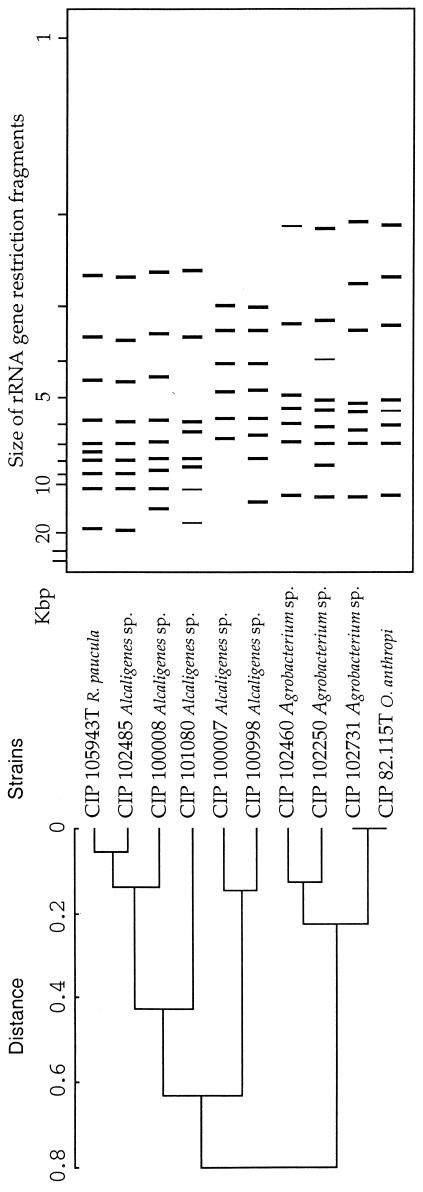
FIG. 3.
Dendrogram based on the PstI ribotyping patterns of the species unidentified strains.
The dendrogram obtained from EcoRI patterns revealed four ribogroups (Fig. 2). Ribogroup I included all the strains of A. xylosoxidans subsp. xylosoxidans. It was homogeneous and composed mostly of strains with a pattern identical to that of the species type strain. Most of the differences observed were within the smallest restriction fragments. Ribogroup III was composed of strains with a pattern similar or identical to that of the A. xylosoxidans subsp. denitrificans type strain. Ribogroup II, including the Alcaligenes strains and the four unnamed bacteria, could be subdivided into three clusters. In the first cluster the profiles observed for each strain were very similar to that of the R. paucula type strain and differed significantly from those of the two other clusters. Ribogroup IV was subdivided into two clusters, one containing two Agrobacterium strains and the other including the O. anthropi type strain and Agrobacterium sp. strain CIP 102731. These last two strains had identical ribotyping patterns. It is interesting that resistance to ticarcillin, piperacillin, cephalothin, cefotaxime, ceftazidime, kanamycin, and erythromycin and susceptibility to gentamicin and nalidixic acid were observed for Agrobacterium strains and for the O. anthropi type strain.
To verify whether EcoRI groupings were of taxonomic significance for the unidentified strains, they were ribotyped after digestion with PstI. The resulting dendrogram (Fig. 3) shows that Alcaligenes and Agrobacterium strains were separated into two groups. The R. paucula type strain was found in one of these groups, and the O. anthropi type strain was in the other. A significant distance between three of the Alcaligenes strains and R. paucula type strain was observed. This result was in agreement with that obtained with EcoRI for two of the strains. The third strain, CIP 101080, appeared to be more distant from R. paucula when cut with PstI than with EcoRI. Conversely, CIP 100008 appeared to be closer to R. paucula when cut with PstI than with EcoRI. The EcoRI and PstI results both gave the same groupings for the Agrobacterium strains.
DISCUSSION
We carried out a polyphasic taxonomic study to identify 37 strains. The strains were characterized by use of an auxanogram and ribotyping. The results were analyzed by computer and compared to the results given by conventional tests.
The oxidative degradation of xylose was the only biochemical characteristic found to differentiate the two A. xylosoxidans subspecies. Due to the lack of discriminating biochemical features, reliable tests were needed to distinguish the two subspecies. Our investigation demonstrated that although ribotyping cannot delineate the Staphylococcus subspecies (6), it can clearly discriminate the two A. xylosoxidans subspecies. The ribogroups obtained were homogeneous with respect to current nomenclature, and it is noteworthy that most A. xylosoxidans subspecies strains displayed identical ribotyping patterns. Moreover, ribogroups were consistent with phenogroups. However, the biotype data suggested that the two subspecies were more closely related than the ribotyping data.
Without consideration of the biochemical behavior, ribotyping and substrate assimilation tests grouped one strain of Alcaligenes, CIP 102485, and the four unnamed strains (CIP 104521, CIP 104522, CIP 104523, and CIP 104524) to R. paucula. Likewise, three strains of Agrobacterium, CIP 102460, CIP 102731, and CIP 102250, were related to the O. anthropi type strain.
It was not surprising that the unnamed bacteria were found to be related to R. paucula, because they belong to the same CDC group as CIP 105943T (18). The observation that the ribotypes of unidentified strains fall into the same cluster as the R. paucula or O. anthropi type strains in both ribotyping assays strongly suggests that they belong to the corresponding species. Using two endonucleases instead of one reduces the probability of two unrelated strains having similar patterns. CIP 102460, CIP 102731, and CIP 102250 also had drug resistance profiles similar to that of O. anthropi, which also suggested that these strains belong to this species (3).
Although the ribotype and biotype groupings corresponded, the respective distribution of the strains within each group was slightly different. This explains why it was difficult to classify CIP 101080 and CIP 100008. Furthermore, the comparison with other type strains of neighboring species, like Ralstonia pickettii, Ralstonia gilardii, Ralstonia eutropha, and Ralstonia solanacearum, did not provide the best grouping for CIP 100998 and CIP 10007 (data not shown). Further analysis is required to resolve the taxonomic relationship of CIP 101080, CIP 100008, CIP 100998, and CIP 10007. Methods that may be used include 16S rRNA sequencing, followed by the comparison of the sequences obtained to those found in the databases.
This study emphasized the need for performant bacterial strain identification systems and the creation of databases which allow the strains identified by each system to be compared. The methods used in this study constitute a powerful tool for the rapid, simple, and reliable identification of strains which are difficult to separate using conventional tests. These methods cannot replace quantitative DNA-DNA hybridization studies for the estimation of relationships between the strains; however, they allow the rapid screening of unidentified strains for comparison with the type strains of species within the same genus.
REFERENCES
- 1.Acar J, et al. Comité de l'Antibiogramme de la Société Française de Microbiologie. Bull Soc Fr Microbiol. 1998;138:153–156. [Google Scholar]
- 2.Bizet C, Barreau C, Harmant C, Nowakowski M, Pietfroid A. Identification of Rhodococcus, Gordona and Dietzia species using carbon source utilization tests (Biotype-100 strips) Res Microbiol. 1997;148:799–809. doi: 10.1016/s0923-2508(97)82456-4. [DOI] [PubMed] [Google Scholar]
- 3.Bizet C, Bizet J. Sensibilité comparée de Ochrobactrum anthropi, Agrobacterium tumefaciens, Alcaligenes faecalis, Alcaligenes denitrificans subsp. denitrificans, Alcaligenes denitrificans subsp. xylosoxidans et Bordetella bronchiseptica vis à vis de 35 antibiotiques dont 17 β-lactamines. Pathol Biol. 1995;43:258–263. [PubMed] [Google Scholar]
- 4.Brosch R, Lefèvre M, Grimont F, Grimont P A. Taxonomic diversity of pseudomonads revealed by computer interpretation of ribotyping data. Syst Appl Microbiol. 1996;19:541–555. [Google Scholar]
- 5.Chabbert Y, Derlot E, Courvalin P. Etude du Centre National de Référence des antibiotiques sur l'inoculum de l'antibiogramme. Path Biol. 1986;34:317–319. [PubMed] [Google Scholar]
- 6.Chesneau O, Morvan A, Aubert S, El Sohl N. The value of rRNA gene restriction site polymorphism analysis for delineating taxa in the genus Staphylococcus. Int J Syst Evol Microbiol. 2000;50:689–697. doi: 10.1099/00207713-50-2-689. [DOI] [PubMed] [Google Scholar]
- 7.Chester B, Cooper L H. Achromobacter species (CDC group Vd): morphological and biochemical characterization. J Clin Microbiol. 1979;9:425–436. doi: 10.1128/jcm.9.3.425-436.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decré D, Arlet G, Bergogne-Bérézin E, Philippon P. Identification of a carbenicillin-hydrolyzing β-lactamase in Alcaligenes denitrificans subsp. xylosoxidans. Antimicrob Agents Chemother. 1995;39:771–774. doi: 10.1128/AAC.39.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobson S J, Franzmann P D. Unification of the genera Deleya (Baumann et al. 1983), Halomonas (Vreeland et al. 1980), and Halovibrio (Fendrich 1988) and the species Paracoccus halodenitrificans (Robinson and Gibbons 1952) into a single genus, Halomonas, and placement of the genus Zymobacter in the family Halomonadaceae. Int J Syst Bacteriol. 1996;46:550–558. [Google Scholar]
- 10.Duggan J M, Goldstein S J, Chenoweth C E, Kauffman C A, Bradley S F. Achromobacter xylosoxidans bacteremia: report of four cases and review of the literature. Clin Infect Dis. 1996;23:569–576. doi: 10.1093/clinids/23.3.569. [DOI] [PubMed] [Google Scholar]
- 11.Grimont F, Grimont P A D. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tool. Ann Inst Pasteur/Microbiol. 1986;137B:165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- 12.Grimont P A D, Vancanneyt M, Lefèvre M, Vandeemeulebroecke K, Vauterin L, Brosch R, Kesters K, Grimont F. Ability of Biolog and Biotype-100 systems to reveal the taxonomic diversity of pseudomonads. Syst Appl Microbiol. 1996;19:510–527. [Google Scholar]
- 13.Janvier M, Grimont P A D, Grimont F. Characterization of Methylophaga species by rRNA gene restriction patterns (ribotyping) Syst Appl Microbiol. 1999;22:372–377. [Google Scholar]
- 14.Meyer O, Stackebrandt E, Auling G. Reclassification of ubiquinone Q-10 containing carbooxidotrophic bacteria: transfer of “[Pseudomonas] carboxydovorans” OM5 to Oligotropha, gen. nov., as Oligotropha carboxidovorans, comb. nov., transfer of “[Alcaligenes] carboxydus ” DSM 1086T to Carbophilus, gen. nov., as Carbophilus carboxidus, comb. nov., transfer of “[Pseudomonas] compransoris ” DSM 1231T to Zavarzinia, gen. nov., as Zavarzinia compransoris, comb. nov., amended descriptions of the new genera. Syst Appl Microbiol. 1993;16:390–395. [Google Scholar]
- 15.Pignato S, Giammanco G M, Grimont F, Grimont P A D, Giammanco G. Molecular characterization of the genera Proteus, Morganella and Providencia by ribotyping. J Clin Microbiol. 1999;37:2840–2847. doi: 10.1128/jcm.37.9.2840-2847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quilici M L, Bizet C. Ribotyping of Chryseobacterium meningosepticum: its use as an epidemiological tool and its correlation with serovars. Res Microbiol. 1996;147:415–425. doi: 10.1016/0923-2508(96)84716-4. [DOI] [PubMed] [Google Scholar]
- 17.Uchino Y, Hirata A, Yokota A, Sugiyama J. Reclassification of marine Agrobacterium species: proposals of Stappia stellulata gen. nov., comb. nov., Stappia aggregata sp. nov., nom. rev., Ruegeria atlantica gen. nov., comb. nov., Ruegeria gelatinovora comb. nov., Ruegeria algicola comb. nov., and Ahrensia kieliense gen. nov. sp. nov., nom. rev. J Gen Appl Microbiol. 1998;44:201–210. doi: 10.2323/jgam.44.201. [DOI] [PubMed] [Google Scholar]
- 18.Vandamme P, Goris J, Coenye T, Hoste B, Janssen D, Kesters K, De Vos P, Falsen E. Assignment of Centers for Disease Control group IVc-2 to the genus Ralstonia as Ralstonia paucula sp. nov. Int J Syst Evol Microbiol. 1999;49:663–669. doi: 10.1099/00207713-49-2-663. [DOI] [PubMed] [Google Scholar]
- 19.Willems A, De Ley J, Gillis M, Kersters K. Comamonadaceae, a new family encompassing the Acidovorans rRNA complex, including Variovorax paradoxus gen. nov., comb. nov., for Alcaligenes paradoxus (Davis 1969) Int J Syst Bacteriol. 1991;41:445–450. [Google Scholar]
- 20.Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol Immunol. 1995;39:897–904. doi: 10.1111/j.1348-0421.1995.tb03275.x. [DOI] [PubMed] [Google Scholar]
- 21.Yabuuchi E, Kawamura Y, Kosako Y, Esaki T. Emendation of genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and proposal of Achromobacter ruhlandii (Packer and Vishniac) comb. nov., Achromobacter piechaudii (Kiredjian et al.) comb. nov. and Achromobacter xylosoxidans subsp. denitrificans (Rüger and Tan) comb. nov. Microbiol Immunol. 1998;42:429–438. doi: 10.1111/j.1348-0421.1998.tb02306.x. [DOI] [PubMed] [Google Scholar]