Abstract
The window period in hepatitis C virus (HCV) infection is still a major problem in ensuring blood safety. HCV RNA detection by nucleic acid amplification technology-based tests has contributed to reduce the infectivity of blood products, but it is expensive, time-consuming and affected by a high prevalence of false-positive results. The aim of this study was to assess the performance of a newly developed enzyme immunoassay for the detection of HCV core antigen and its suitability for use in the screening of blood units in order to identify infecting samples that do not contain specific antibodies. For evaluation of laboratory performance, different samples were selected: to evaluate specificity, we tested 2,586 sera from blood donors, 500 general population samples, and 58 “difficult sera”. All samples were tested by two screening assays, and results were negative. To estimate clinical sensitivity, 103 HCV RNA-positive, anti-HCV-negative samples, 6 natural seroconversion panels, and 9 commercial seroconversion panels were tested. Intra- and interassay precision were determined on two HCV-RNA-positive, anti-HCV-negative sera. Seventeen (0.66%) blood donor samples, 2 (0.4%) general population samples, and 2 (3.44%) difficult sera were initially reactive; 3 sera were positive on repetition. These 21 samples tested by reverse transcription-PCR were negative. The clinical sensitivity calculated with seroconversion panels and seroconverted patient samples was very similar to PCR sensitivity: 95% of PCR-positive, antibody-negative samples contained detectable HCV antigen. Data on intra- and interassay precision showed dispersion indices with values of less than 10%. In conclusion, the HCV antigen assay showed high sensitivity and specificity and could become a useful means of improving the safety of blood and blood products.
In the 1970s and 1980s, posttransfusional non-A, non-B hepatitis was the most frequent infection transmitted by blood and blood products, representing 80 to 90% of all cases of posttransfusional hepatitis (23).
In the first half of the 1990s, diagnosis of hepatitis C virus (HCV) infection was mainly based on detecting antibodies against recombinant and/or synthetic viral antigens, and with the introduction of tests to detect these antibodies in the screening of blood units, there was a sharp drop in posttransfusional hepatitis (5, 9, 12, 24).
Antibody tests are unable to identify subjects in the early stage of infection, in what is known as the diagnostic window period, during which specific antibodies have not yet been produced, but the virus is present in the plasma, sometimes in large quantities. This stage prior to seroconversion may last up to 2 months in immunocompetent subjects and as long as 6 to 12 months in immunodeficient patients (22). The risk of a blood donation occurring during the window period for HCV has been estimated at 1/103,000 with a 95% confidence interval of 1/28,000 to 1/280,000 (21).
With the introduction of nucleic acid amplification technology (NAT)-based tests based on nucleic acid amplification (PCR and transcription-mediated amplification) or signal amplification (branched DNA [bDNA]), it is possible to identify viremic samples in which antibodies are not yet present and therefore reduce the window period to 15 to 20 days (3, 15). There are, however, problems concerning their possible use in the screening of blood units. Any viral RNA present in the sample is fairly unstable, which means that the sera must be tested immediately or frozen as quickly as possible and stored at −20 or −70°C. NAT-based tests require considerable skill on the part of the operators, although the recent availability of semiautomatic PCR methods could limit problems connected with this aspect (18). The high sensitivity of these methods and the high viral load of samples in the early stage of infection may enable these techniques to be applied to pools of sera (13, 14). The European Medicinal Evaluation Agency (EMEA) planned to introduce testing of plasma pools used in the manufacture of blood products for HCV RNA from July 1999 (10). These recommendations were followed by numerous European countries, which are starting to assess whether to introduce these techniques into the screening of noninactivated products. Pool analysis methods are complex with regard to organization and require high quality standards (19). Furthermore, the risk of false positives (for nucleic acid amplification methods) should be considered, and some studies reported a significant percentage of false-positive results connected with environmental contamination and carryover (2, 7).
On the other hand, bDNA-based methods, although of simple execution, were designed mainly as quantitative tests and are characterized by low sensitivity and long incubation times (20).
Furthermore, both methods involve expensive reagents and long execution times, aspects that have a significant effect on the final cost of each test, which is around $50 (U.S.)/test when the test is performed in diagnostic laboratories. The cost of NAT-based screening of blood donations decreases when tests are performed in pools. Therefore, a quick, inexpensive, sensitive, and specific test is clearly needed to identify potentially infective blood units that have not been identified by specific antibody tests.
A new test has recently been developed to detect the HCV core protein (HCV antigen [Ag]), which is coded for by one of the most conserved regions of the virus genome and which in anti-HCV-positive patients appears to be correlated with HCV-RNA levels (4, 11, 16). This protein may be an ideal target for the development of methods to detect an HCV Ag and more importantly to identify samples from individuals in the early stage of infection (17).
A preliminary study with a small study population was performed, and more definitive and complete data are needed (6).
The aim of this study was to assess the performance of this test for detecting HCV Ag in terms of its specificity and sensitivity and its suitability for use in the screening of blood units to identify infecting samples that do not contain specific antibodies.
MATERIALS AND METHODS
Serological assay.
The newly developed Ortho Antibody to HCV Core Antigen Elisa Test System (Ortho Diagnostics, Raritan, N.J.) is a sandwich immunoenzymatic test designed to detect the HCV core protein. Monoclonal antibodies reactive with HCV core Ag were used to coat each well in the assay microplate. Other monoclonal antibodies capable of recognizing the N-terminal region of the core protein were conjugated with horseradish peroxidase.
The first step of the procedure entailed incubation with shaking of the controls and diluted samples in the well for 90 min at 37°C, followed by washing with an automatic washer. Two-hundred microliters of conjugate was added, and the microwells were incubated at 37°C for 30 min. After washing, an enzymatic detection system comprising o-phenylenediamine (OPD) and hydrogen peroxide was dispensed. If there was a conjugate bonded to the antigen-antibody complexes, the OPD was oxidized and a final colored product was formed. Sulphuric acid was then added to stop the reaction. Absorbance intensity was measured with a photometer and read at 492 nm with a 620- to 630-nm reference. Color intensity was proportional to the quantity of bonded conjugate and therefore depended on the concentration of HCV Ag. The cutoff value was determined by adding 0.040 to the mean absorbance of the three negative standards supplied in the kit. The specimen was considered negative if the absorbance value was less than the cutoff absorbance and reactive if the optical density (OD) was greater than the cutoff value. A grey area between 80% of the cutoff absorbance and the cutoff absorbance and a borderline reactivity area between the cutoff absorbance and 120% of it were also considered.
Anti-HCV antibodies were detected with the Ortho HCV Ab enzyme-linked immunosorbent assay (ELISA) (Ortho Diagnostics) and Innotest HCV Ab III (Innogenetics, Innogenetics N.V., Zwijnaarde, Belgium) tests.
Molecular biological assay.
Qualitative and quantitative detection of HCV RNA was performed with the Cobas Amplicor Hepatitis C Virus Test, version 2.0, and the Cobas Amplicor HCV Monitor Test, version 2.0 (Roche Diagnostics, Branchburg, N.J.), respectively. The Cobas Amplicor Hepatitis C Virus Test, version 2.0, is able to detect HCV RNA at a concentration of 50 IU/ml, with a positive rate of 95% or greater, whereas the Cobas Amplicor HCV Monitor Test, version 2.0, detects viral genomes at a concentration of 600 IU/ml, with a positive rate of 95% or greater.
Design of the evaluation and procedure. (i) Specificity.
In order to evaluate the specificity of the assay, we tested 2,586 fresh or frozen sera taken from blood donors, 500 samples from the general population, and 58 difficult sera. The last group was taken from patients with dysproteinemia, systemic lupus erythematosus, or autoimmune hepatitis. It also included samples positive for rheumatoid factor, antinucleus antibodies (anti-ANA), anti-hepatitis A virus (HAV) immunoglobulin M (IgM), HbsAg, and anti-HBc IgM. All of the samples were screened for anti-HCV by two immunoenzymatic assays, and none was found to be reactive. They were all tested with the HCV Ag test. The initially reactive samples were retested with the HCV Ag test and screened for HCV RNA by qualitative PCR.
(ii) Sensitivity.
In order to assess sensitivity, we applied the HCV Ag test to 103 qualitative PCR-positive and anti-HCV-negative sera, 6 natural seroconversion panels, and 9 commercial seroconversion panels (30994B by Serologicals; 6222, 6225, 6227, and 9041 by Bioclinical Partners, Inc.; and PHV 901, PHV 905, PHV 908, and PHV 917 by Boston Biomedica, Inc.).
To evaluate the correlation between HCV Ag and HCV-RNA, 45 anti-HCV-negative and HCV RNA-positive sera (25 from seroconversion panels and 20 of 103 single sera) were randomized and analyzed by quantitative PCR.
(iii) Intra-assay precision.
In order to assess intra-assay precision, we selected an HCV RNA-positive, anti-HCV-negative serum (serum A) with a cutoff index (expressed as the ratio of OD to cutoff value [OD/CO]) in a critical area, namely 1.5 to 2. The sample was tested 10 times in a single run. The results, expressed as the cutoff index, were assessed in terms of central trend index (mean) and absolute and relative dispersion (range and percentage of the coefficient of variation [CV]).
(iv) Interassay precision.
In order to assess interassay precision, we selected two HCV RNA-positive, anti-HCV-negative sera (sera A and B) with different concentrations of HCV Ag. The first one, just used for the intra-assay precision evaluation, was tested in duplicate for five runs. The second one, with more marked reactivity (approximate cutoff index of 3), was tested in duplicate for six runs. The results, expressed as cutoff index, were assessed in terms of central trend index (mean of individual runs) and relative and absolute dispersion (range and percentage of CV).
RESULTS
Specificity.
Tables 1 and 2 show that 17 (0.66%) of the 2,586 sera collected from blood donors presented initial reactivity, 4 with a borderline cutoff index. The distribution of cutoff indices showed that 2520 (97.44%) of the 2,569 nonreactive samples had a value of less than 0.5, and 42 (1.62%) fell within the range 0.5 to 0.8. Seven (0.27%) were within the grey zone. In the repeat test carried out with the 17 initially reactive samples, only 2 (0.08%) samples were reactive, and another had a cutoff index in the grey zone. Qualitative HCV RT-PCR in the 17 initially reactive sera revealed no viremic samples.
TABLE 1.
HCV Ag-reactive samples in specificity study population
| Samples (n) | No. (%) of samples
|
||
|---|---|---|---|
| HCV Ag initially reactive | HCV Ag repeated reactive | HCV RNA positive | |
| Blood donors (2,586) | 17 (0.66) | 2 (0.08) | 0 |
| Open population (500) | 2 (0.4) | 0 | 0 |
| Difficult sera (58) | 2 (3.44) | 1 (1.72) | 0 |
TABLE 2.
HCV Ag OD/CO distribution of blood donors, open population, and difficult sera
| Samples (n) | No. (%) of samples with OD/CO ratio of:
|
||||
|---|---|---|---|---|---|
| HCV Ag negative
|
Grey zone; 0.8–0.99 | Borderline; zone; 1–1.2 | HCV Ag positive; >1.2 | ||
| <0.5 | 0.5–0.79 | ||||
| Blood donors (2,586) | 2,520 (97.44) | 42 (1.62) | 7 (0.27) | 4 (0.15) | 13 (0.5) |
| Open population (500) | 498 (99.6%) | 0 | 0 | 0 | 2 (0.4) |
| Difficult sera (58) | 56 (96.55) | 0 | 0 | 1 (1.72) | 1 (1.72) |
Two (0.4%) of the 500 samples collected from the general population were initially reactive, both with plateau OD values. The remaining 498 sera had a cutoff index of less than 0.5. The repeat test on the two samples did not confirm any positivity, and both cutoff indices were less than 0.5. Qualitative HCV RT-PCR in the two initially reactive sera did not reveal any HCV RNA-positive samples.
Of the 58 difficult sera at the first determination, 2 (3.45%) were reactive, one having a borderline cutoff index. The repeat test confirmed reactivity for the sample with the higher cutoff index in the first test, whereas the borderline serum had a cutoff index approaching 0. In this case too, RT-PCR gave a negative result.
Sensitivity.
Of the 103 HCV-RNA-positive and anti-HCV-negative sera, 97 (94.2%) were positive with the HCV Ag test, all having a cutoff index greater than 1.2. Two of the six nonreactive samples in the HCV Ag test had a cutoff index in the grey zone (Table 3). The repeat HCV Ag test of the six initially nonreactive sera confirmed this result.
TABLE 3.
HCV Ag OD/CO ratio and viral load of the 6 HCV Ag-negative samples of 103 HCV RNA-positive, anti-HCV-negative samples
| Sample | HCV Ag OD/CO ratio | HCV RNA (IU/ml) |
|---|---|---|
| S.E.0001 | 0.23 | NDa |
| RA 1923 | 0.26 | 21,300 |
| RA 2041 | 0.31 | 4,430 |
| RA 2094 | 0.68 | 65,700 |
| RA 2095 | 0.93 | 75,700 |
| RA 2119 | 0.93 | 80,900 |
ND, not determined.
Table 4 sets out the results of the HCV Ag test, the HCV-RNA detection by means of PCR, and the anti-HCV determination performed with sera of the natural and commercial seroconversion panels. In 13 of the 15 panels used, the sampling date on which the first positivity in the HCV Ag test and the PCR occurred was the same. Discordant data were found with natural panel 65064, in which HCV-RNA positivity (day 34) preceded HCV positivity (day 36) by 2 days, and commercial panel BBI PHV 905, in which PCR was positive at the first sample available (day 0), whereas the HCV Ag test was reactive at the subsequent sampling (day 4). With regard to panel PHV 905, it should be pointed out that the day 0 sample analyzed by the HCV Ag test showed a cutoff index of 0.84, thus, falling within the grey zone. The reductions in diagnostic window period resulting from use of the HCV Ag test and PCR HCV in the 15 panels were equal on average to 33 days (range, 17 to 71 days) and 33.4 days (range 19 to 71 days), respectively.
TABLE 4.
Seroconversion sensitivity calculated with six natural and nine commercial serocoversion panels
| Panel | No. of days to detection from first available bleed
|
Windows reduction by HCV Ag test | ||
|---|---|---|---|---|
| HCV Ag | HCV RNA | Anti-HCVa | ||
| Natural | ||||
| 0001 | 30 | 30 | 66 | 36 |
| 0002 | 28 | 28 | 50 | 22 |
| 6045 | 0 | 0 | 37 | 37 |
| 6047 | 0 | 0 | 28 | 28 |
| 65064 | 36 | 34 | 67 | 31 |
| 65345 | 93 | 93 | 123 | 30 |
| Commercial | ||||
| 30994B | 0 | 0 | 48 | 48 |
| 6222 | 16 | 16 | 39 | 23 |
| 6225 | 17 | 17 | 49 | 32 |
| 6227 | 41 | 41 | 73 | 32 |
| 9041 | 24 | 24 | 61 | 37 |
| PHV 901 | 72 | 72 | 104 | 32 |
| PHV 905 | 4 | 0 | 21 | 17 |
| PHV 908 | 0 | 0 | 19 | 19 |
| PHV 917 | 12 | 12 | 83 | 71 |
Test is third generation, i.e., the third of its kind designed.
Overall, in the 15 seroconversion panels, there were 67 anti-HCV-negative and HCV RNA-positive samples. Of these, the HCV Ag test recognized 65 (97%) as positive, and—as already stated—1 had a cutoff index in the grey area.
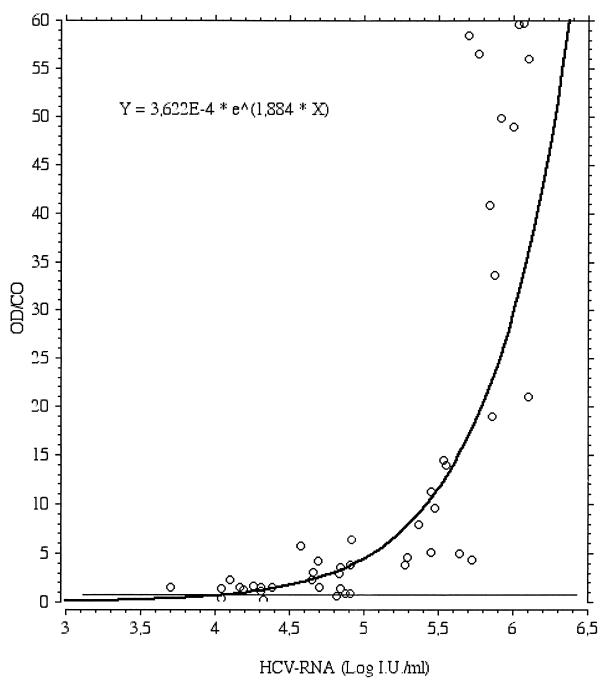
The viral load and cutoff index in the HCV Ag test of the 45 sera randomized for quantitative HCV-RNA dosage were correlated by means of a regression curve. The curve best fitting the distribution of the data is an exponential curve, shown in Fig. 1.
FIG. 1.
Regression plot between HCV Ag OD/CO and Log HCV-RNA level in 45 HCV RNA-positive anti-HCV-negative sera.
Intra-assay and interassay precision.
Data on intra-assay and interassay precision are shown in Table 5. The dispersion indices observed in both assays had values of less than 10%.
TABLE 5.
Intra- and interassay precision
| Serum | Precision
|
|||
|---|---|---|---|---|
| Intra-assay
|
Interassay
|
|||
| Mean OD/CO (range, min–max) | % CV | Mean OD/CO (range, min–max) | % CV | |
| A | 1.79 (1.56–2.07) | 9.4 | 2.06 (1.78–2.29) | 10 |
| B | 3.19 (2.86–3.58) | 9.7 | ||
DISCUSSION
The ELISA for detecting HCV core Ag assessed in our study was the first to be standardized and ready to be marketed. This assay meets the need for a method capable of detecting a viral replication marker before routinely used tests can detect specific HCV antibodies.
With regard to laboratory performance, there is no recognized “gold standard” for determining the presence or absence of infection in the window period. Therefore, it was not possible to determine the absolute specificity and sensitivity of the test under study. Considering the possible applications of the HCV Ag test, we took as a reference the most standardized, most commonly used NAT-based test—PCR.
With regard to specificity, the algorithm adopted entailed performance of the PCR assay with samples that were reactive in the HCV Ag test. Only 21 (0.67%) of the 3,144 sera tested overall were initially HCV Ag reactive, and being HCV RNA negative, they were considered as false positives. However, considering the sera were repeatedly reactive and 3,141 out of 3,144 sera (99.9%) were identified as HCV Ag negative, they could be false-negative PCRs (i.e., below the detection limits of the PCR test used in this study). Therefore, the HCV Ag test had a specificity comparable to that of ELISA kits routinely used in screening for blood-transmitted infections such as hepatitis B and human immunodeficiency virus infection (8, 25). Inter- and intra-assay reproducibility data also showed identical performances to ELISA tests currently on the market. Furthermore, the test for detecting HCV Ag has been shown to have excellent discriminatory ability. Indeed, virtually all (97.78%) of the 3,144 samples tested for specificity gave absorbance values of less than 50% of the cutoff value. Only 7 of the 3,144 samples (0.22%) had a cutoff index in the range 0.8 to 1, the grey zone.
With regard to sensitivity, this study showed that the HCV Ag test's ability to detect the virus is comparable to that of PCR. The most significant finding is undoubtedly the reduction in window period as calculated with the seroconversion panels used. The first positive sample in the HCV Ag test and PCR was on average 33 days (33 versus 33.4 days) prior to antibody detectability. Furthermore, when we considered the 170 anti-HCV-negative HCV RNA-positive samples analyzed (103 individual samplings and 67 samples from seroconversion panels), 162 (95.29%) were HCV Ag positive and 3 (1.76%) were in the grey zone.
The finding of some HCV RNA-positive and HCV Ag-negative samples was not entirely unexpected considering the high sensitivity of NAT-based tests, which have taken about 10 years to reach the present degree of standardization. Also, an analysis of the regression curve for the HCV Ag test cutoff index and viral load expressed in international units per milliliter shows that the curve meets the cutoff value at an HCV RNA concentration of about 4 log IU/ml. This shows that the analytical sensitivity of the test, which was not the subject of the analysis, was less marked than that of NAT-based tests. However, viral kinetics in the early stage of HCV infection, characterized by rapid growth in viral replication indices in only a few days, as shown in the natural and commercial panels, caused PCR and HCV Ag positivity to be virtually simultaneous.
In conclusion, the Ortho Antibody to HCV Core Antigen ELISA Test System has been shown to have high sensitivity and specificity, allowing it to be used in the screening of blood donations.
Our experience has also shown that the test is easy to use, which, together with the low cost of the kit, means it can be used to analyze individual blood donations and solve organizational problems connected with pool analysis (1, 13).
It is therefore a valid alternative to NAT-based tests for identification of HCV infection in the diagnostic window period, specially in situations in which organizational and technological problems connected with pool analysis are difficult to solve (e.g., in countries not able to set up nucleic acid tests). The HCV Ag test could become a useful means for improving the safety of blood and blood products and for detecting an early HCV infection in high risk individuals and late seroconverters.
REFERENCES
- 1.Allain J P. Genomic screening for blood-borne viruses in transfusion settings. Clin Lab Haematol. 2000;22:1–10. doi: 10.1046/j.1365-2257.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 2.Aslanzadeh J, Padilla B B, Shanley J D. Evaluation of PCR and nested PCR for laboratory diagnosis of hepatitis C virus infection. Mol Cell Probes. 1996;10:173–178. doi: 10.1006/mcpr.1996.0024. [DOI] [PubMed] [Google Scholar]
- 3.Barbara J A J, Garon J A. Polymerase chain reaction and transfusion microbiology. Vox Sang. 1993;64:73–80. doi: 10.1111/j.1423-0410.1993.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 4.Bukh J, Purcell R H, Miller R H. Sequence analysis of the core gene of 14 hepatitis C virus genotypes. Proc Natl Acad Sci USA. 1994;91:8239–8243. doi: 10.1073/pnas.91.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 6.Courouce A M, Le Marrec N, Bouchardeau F, Razer A, Maniez M, Laperche S, Simon N. Efficacy of HCV core antigen detection during preseroconversion period. Transfusion. 2000;40:1198–1202. doi: 10.1046/j.1537-2995.2000.40101198.x. [DOI] [PubMed] [Google Scholar]
- 7.Damen M, Cuypers H T, Zaijer H L, Resink H W, Schaasberg W P, Gerlich W H, Niesters H G, Lelie P N. International collaborative study on the second EUROHEP HCV-RNA reference panel. J Virol Methods. 1996;58:175–185. doi: 10.1016/0166-0934(96)02011-3. [DOI] [PubMed] [Google Scholar]
- 8.Decker R H. Diagnosis. In: Zuckermann A J, Thomas H C, editors. Viral hepatitis. Scientific basis and clinical management. New York, N.Y: Churchill Livingstone; 1993. pp. 165–184. [Google Scholar]
- 9.Donahue J G, Munoz A, Ness P M. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327:369–373. doi: 10.1056/NEJM199208063270601. [DOI] [PubMed] [Google Scholar]
- 10.European Medicinal Evaluation Agency. Introduction of gene amplification (GAT) for detection of hepatitis C virus in plasma pools: addendum to notes for guidelines on plasma derived products 1997. CPMP/BWP/390/97. London, United Kingdom: European Medicinal Evaluation Agency; 1997. [Google Scholar]
- 11.Komatsu F, Takasaki K. Determination of serum hepatitis C virus (HCV) core protein using a novel approach for quantitative evaluation of HCV viremia in anti-HCV positive patients. Liver. 1999;19:375–380. doi: 10.1111/j.1478-3231.1999.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuo G, Choo Q L, Alter H J, Gitnick G L, Redeker A G, Purcell R H, Dienstag J L, Alter M J, Stevens C E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 13.Lefrere J J, Coste J, Defer C. Screening blood donation for viral genomes: multicenter study of real time simulation using pooled samples model of hepatitis C virus RNA detection. Transfusion. 1998;38:915–922. doi: 10.1046/j.1537-2995.1998.381098440855.x. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer J. Intersecting pools and their potential application in testing donated blood for viral genomes. Vox Sang. 1997;73:93–97. doi: 10.1046/j.1423-0410.1997.7320093.x. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Breitkreutz K, Baylis S A, Allain J P. Nucleic acid amplification tests for the detection of blood-borne viruses. Vox Sang. 1999;76:194–199. doi: 10.1159/000031050. [DOI] [PubMed] [Google Scholar]
- 16.Orito E, Mizokami M, Tanaka T, Lau J Y N, Suzuki K, Yamaguchi M, Ohra Y, Tanaka S, Kohara M. Quantification of serum hepatitis C virus core protein in patients chronically infected with different hepatitis C virus genotypes. Gut. 1996;39:876–880. doi: 10.1136/gut.39.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson J, Green G, Iida K, Caldwell B, Kerrison P, Bernich S, Aoyagi K, Lee S R. Detection of hepatitis C core antigen in the antibody negative “window” phase of hepatitis C infection. Vox Sang. 2000;78:80–85. doi: 10.1159/000031155. [DOI] [PubMed] [Google Scholar]
- 18.Poljak M, Seme K, Koren S. Evaluation of the automated COBAS AMPLICOR hepatitis C virus PCR system. J Clin Microbiol. 1997;35:2983–2984. doi: 10.1128/jcm.35.11.2983-2984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth W K, Weber M, Seifried E. Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus and HIV-1 in a blood-bank setting. Lancet. 1999;353:359–363. doi: 10.1016/S0140-6736(98)06318-1. [DOI] [PubMed] [Google Scholar]
- 20.Schiff E R, De Medina M, Kahan R S. New perspectives in the diagnosis of hepatitis C. Semin Liver Dis. 1999;19(Suppl. 1):3–15. [PubMed] [Google Scholar]
- 21.Schreiber G B, Busch M P, Kleinman S H, Korelitz J J. The risk of trasfusion trasmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 22.Van der Poel C L, Cuypers H T, Reesink H W. Hepatitis C virus six years on. Lancet. 1994;344:1475–1479. doi: 10.1016/s0140-6736(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 23.Van der Poel C L. Hepatitis C virus and blood transfusion: past and present risk. J Hepatol. 1999;31(Suppl. 1):101–106. doi: 10.1016/s0168-8278(99)80384-5. [DOI] [PubMed] [Google Scholar]
- 24.Wong Y L, Lee S D, Hwang S J. Incidence of post-transfusion hepatitis before and after screening for hepatitis C virus antibody. Vox Sang. 1994;67:187–190. doi: 10.1111/j.1423-0410.1994.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Operational characteristics of commercially available assays to determine antibodies to HIV-1 and/or HIV-2 in human sera. Report 3. Global Programme on AIDS/RES/DIA/91.1. Geneva, Switzerland: World Health Organization; 1991. [Google Scholar]