PURPOSE
In the era of personalized medicine, physicians rely on their understanding of clinical utility to assess the value of rapidly evolving genetic and genomic tests. Current definitions of the clinical utility of genetic testing sufficiently capture a range of benefits and risks that derive from positive and negative results of tests that assess one gene or a few genes. However, these definitions of clinical utility are inadequate to recognize the wider scope of benefits that accrue from more comprehensive genomic tests, which can develop data sets that inform clinical decision making as well as population health and scientific advancement in novel ways.
METHODS
An expert roundtable discussion with leaders from multiple sectors of the health care ecosystem was convened to develop a contemporary, fuller definition of the clinical utility of genomic testing in cancer care.
RESULTS
We present an updated definition and offer recommendations for successful implementation.
CONCLUSION
Applying this expanded definition will encourage evidence-based use of genomic testing in cancer care by helping physicians and other health care decision makers account for the broader range of benefits and risks of testing for individual patients, health systems, population health, and scientific advancement.
INTRODUCTION
The appropriate use of genomic tests (whole-genome, exome, or multigene tumor profiling acquired through a sequencing platform) in clinical oncology largely depends on an evidence-based assessment of all the benefits and risks that accrue from test results, commonly known as clinical utility.1 In the currently evolving context of personalized medicine, the capacity of genomic testing is expanding to deliver greater benefits for patients and health systems. Many stakeholders consider a broader set of elements comprising the value of new medical technologies encompassing additional streams of potential benefits and risks.2 Health care decision makers need clarity regarding the definition of the clinical utility of genomic tests in this new era. This study seeks to advance an improved definition of clinical utility for genomic testing in cancer care as follows:
The clinical utility of genomic testing in cancer care is the net benefit to patients with cancer and health systems that are derived from applying information generated by multigene testing to screening, prevention, and treatment strategies that can improve health care outcomes, including through enrollment in clinical trials, facilitate shared decision making, and reduce health care disparities. The utility of genomic profiling depends on its ability to provide information that is used to guide patients more efficiently to safer and more effective prevention and treatment strategies and its ability to improve the practical knowledge base for health system decision making.
Applying this definition of clinical utility of genomic testing in cancer care will help health care decision makers recognize how different aspects of clinical utility apply in different contexts.
BACKGROUND
The use of advanced diagnostic tests to detect predictive and prognostic biomarkers that may help guide screening, prevention, and treatment strategies is a cornerstone of personalized medicine in cancer care.3 The discovery over the past 20 years of a steadily increasing number of genetic biomarkers has added to the complexity of cancer care. Genetic biomarkers are used for early detection and prognosis, to inform treatment options, and to guide the development of targeted therapies that have improved outcomes for responder patients.
Despite an expanding body of evidence supporting the clinical value of genomic testing, it remains underutilized in clinical practice.4 The efficient use of genomic tests faces several implementation and policy barriers.5 Studies that have examined the clinical and economic value of genomic sequencing show that many patients with cancer never receive indicated genomic testing.6,7 Even for those who do, only 60%-75% of patients with actionable mutations receive targeted treatments indicated by their test results.8 A more complete understanding of the clinical utility of biomarker testing can help overcome these challenges by fostering a more complete appreciation of the value of ordering genomic testing and the importance of acting on relevant biomarkers.
A widely cited definition of the clinical utility of genetic testing was presented by Grosse and Khoury of the US Centers for Disease Control and Prevention (CDC) in a commentary published in 2006 in Genetics in Medicine.9 The authors wrote, “Clinical utility in its narrowest sense refers to the ability of a screening or diagnostic test to prevent or ameliorate adverse health outcomes such as mortality, morbidity, or disability through the adoption of efficacious treatments conditioned on test results. A screening or diagnostic test alone does not have inherent utility; because it is the adoption of therapeutic or preventative interventions that influence health outcomes. The clinical utility of a test depends on effective access to appropriate interventions.”
This definition pertains to all genetic testing and was developed in an era when multigene assessments were not available or feasible in practice. As such, it does not delineate the expanded scope of utility that is realized with multigene testing or consider elements of utility specifically associated with cancer care, which has evolved significantly since 2006. Since that time, important summaries of the clinical utility of genetic testing have been conducted by the Evaluation of Genomic Applications in Practice and Prevention initiative at the CDC10 and the National Academies of Sciences, Engineering, and Medicine,11 and clinical utility of genomics has been the subject of many public fora and expert commentaries.12-16 Still, a widely accepted modernized definition of clinical utility focused on genomic testing in cancer care has not yet emerged. The Grosse Khoury definition needs to be modified to align with the current and evolving applications of this testing to individual and population health and health system benefits in oncology.
METHODS
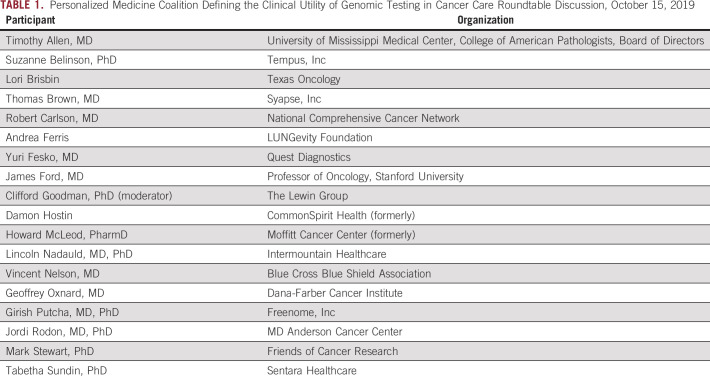
With this goal in mind, the Personalized Medicine Coalition convened a roundtable of leaders in personalized medicine (Table 1) representing various stakeholders, including providers, payers, patients, clinical guideline developers, and laboratory directors, to identify the components of an expanded definition of clinical utility of genomic testing in cancer care and develop recommendations for how to implement the expanded definition.
TABLE 1.
Personalized Medicine Coalition Defining the Clinical Utility of Genomic Testing in Cancer Care Roundtable Discussion, October 15, 2019
RESULTS
Although the collection of information through genomic testing is associated with benefits and some risks, the roundtable discussion revealed that a reframed definition of clinical utility should account for the ability of multiplex genomic tests to achieve a broader range of purposes than had been recognized in the 2006 definition. These include the following:
improved clinical outcomes;
health system and population benefits associated with data compilation and analysis and more efficient sample processing;
patient access to additional prevention and treatment strategies, including clinical trial eligibility assessment or enrollment; and
opportunities for shared decision making.
Improved Clinical Outcomes
The use of test results to inform clinical decision making that increases the likelihood of improved patient outcomes is generally acknowledged as a primary purpose of genomic testing. This purpose is consistent with standard practice in evidence-based medicine, which highlights objective information of health determinants to evaluate interventions.17 As such, genomic testing is used to help guide prevention or treatment plans that will improve health care outcomes and reduce adverse events. Similarly, such testing can improve treatment efficiency by ruling out interventions that are not safe or effective for a given patient. Information about individuals' probable responsiveness to treatment options, including their respective safety and efficacy, can prevent or ameliorate adverse health outcomes such as mortality, morbidity, disability, pain, or reduced function. Such information reduces clinical uncertainty, leading to optimal interventions earlier in care. Furthermore, this can increase patient confidence, improve adherence, and convey psychological benefits.
The primary risks that genomic testing can have on health outcomes include the possibility of attaining inaccurate or invalid test results. These risks can be minimized through strong biomarker validation and preanalytic processing standards.
Health System and Population Benefits Associated With Data Compilation and Analysis and Sample Processing
Appropriate genomic testing of patients and the compilation of patient data from clinical practice and clinical trials can yield high-quality evidence of health determinants that can advance understanding of how to select treatments for patients with specific characteristics and/or circumstances.18 In doing so, it can inform new and emerging prevention and treatment approaches.19,20 Analysis of large data sets pooled from multiple institutions can increase the power to discern optimal treatment and prevention strategies for particular patient subgroups.21
Genomic testing can also convey utility for health systems by enabling greater diagnostic processing efficiency, such as biospecimen conservation associated with analysis of multiple genetic markers in a single test when compared with processing multiple single-gene tests. In addition, genomic testing provides a means to assess the presence of additional biomarkers not originally contemplated but that are sought in later stages of care when additional biospecimens are not available.
The primary risk to health systems in incorporating genomic testing approaches involves additional costs associated with testing and interpretation, especially in those patients for whom it is unnecessary. However, analysis of practice-based data sets to inform clinical practice guidelines and standards can help manage such costs.
Patient Access to Additional Prevention and Treatment Strategies
Acquiring information about genetic determinants that can help predict response to prevention and treatment strategies can lead to health care options that may not have been considered otherwise. Germline genomic sequencing can provide information about susceptibilities and risks that can be applied to cancer screening and prevention strategies. Tumor genomic sequencing can include information that can help inform the use of approved interventions, provide incidental findings that uncover rare or infrequent actionable genetic alterations, inform potential new indications for therapies, and/or guide clinical trial recruitment. Furthermore, to the extent that genomic testing is demonstrated to improve clinical decision making by providing patient-specific results for guiding optimal care, it should become the standard of care in oncology, diminishing current disparities in cancer care and outcomes.
Opportunities for Shared Decision Making
By placing actionable genomic information into the hands of patients, genomic testing empowers them to take a more active role in their own health care decision making. The potential value to individuals includes better understanding of their own susceptibility, risk, or prognosis related to cancer. This information can be used to ensure that the patient's course of care is best suited for them. In addition, this can improve awareness of implications for the patient's family and enable opportunities to inform family members of potential risks and encourage screening and prevention strategies accordingly. Health care policies that address benefits and risks to patients will affect the clinical utility of genomic testing in cancer care. Assuring data privacy and security and addressing ethical issues associated with individual risk information preferences and the delivery of information about variants with yet unknown significance can increase patient comfort with genomic testing and encourage shared decision making.
DISCUSSION
The clinical utility of genomic testing in cancer care can vary depending on the patient, condition, availability of effective interventions, and other factors. An expanded definition will likely allow more flexibility in determining and weighting the elements of clinical utility in an evaluation. However, a multifactorial definition could also increase the uncertainty of utility. It is, therefore, necessary to consider genomic testing subject to an understanding of the particular aspects of clinical utility that are most relevant in each context.
The outcomes to be considered in evaluating the utility of genomic testing may be prioritized depending on the health care decision maker and the purpose of the test. For example, for a community hospital system, the impact of testing on improving clinical outcomes and the health system benefits are likely the most important factors. Coverage decisions by health insurers may be based in large part on the impact of testing on population-level clinical outcomes, the test's impact on treatment efficiency (including to rule out interventions unlikely to be beneficial), and guiding access to available prevention and treatment options. For patients and caregivers, the utility of genomic testing for improving outcomes and treatment efficiency is critical, as are the impacts on shared decision making related to functional status, career, and risks to family. Among the practical matters of test implementation is ensuring that test results are returned to clinicians in a sufficiently timely manner to influence care decisions.
GENOMIC TESTING IMPLEMENTATION
Maximizing the clinical utility of genomic testing to all stakeholders will require improved policies and processes related to genomic testing use, including the following:
ensuring that physicians understand current developments in the rapidly evolving field of personalized medicine;
ensuring that genomic testing practices and processes are valid, consistent, and efficient;
deploying evidence-based clinical decision support via guidelines, pathways, and other tools;
ensuring access to targeted treatments; and
guiding patients to appropriate clinical trials when applicable.
The clinical utility of genomic testing is likely to grow over time. Increased implementation of genomic testing can contribute to clinical utility by building on comprehensive and curated genomic databases and deriving evidence to inform testing protocols, therapeutic options, and related clinical decision support.22
In some instances, the utility of genomic testing can bridge standard of care to clinical trials. This arises, for example, when one or more biomarkers indicate a sequence of therapies, starting with a first-line therapy and then a second-line therapy using approved drugs, followed by enrollment in clinical trials (eg, phase II, II-III, or III) if the patient's cancer progresses.23 In such instances, biomarkers have clinical utility not only for current standards of care but also for patients proceeding to subsequent-line therapies in approved clinical trial protocols. For some patients with few viable therapeutic options, investigational therapies in clinical trials may be considered for first-line therapeutic use when guided by comprehensive biomarker testing.
RECOMMENDATIONS
Consistent with the expanded definition of clinical utility and the potential for further advancing the clinical utility of genomic testing, we offer the recommendations in Table 2 to ensure its appropriate implementation.
TABLE 2.
Roundtable Recommendations for the Implementation of Genomic Testing in Cancer Care

ACKNOWLEDGMENT
Casey O'Neill, Elissa Quinn, and Lori Lanphere contributed to facilitating the expert roundtable discussion and provided helpful feedback for early versions of this report.
Daryl Pritchard
Honoraria: Xcenda, Genentech
Research Funding: Thermo Fisher, AstraZeneca (Inst)
Travel, Accommodations, Expenses: Genentech
Clifford Goodman
Employment: Lewin Group
Stock and Other Ownership Interests: United Health Group
Travel, Accommodations, Expenses: Bayer, Medtronic, BioMarin, Concert Genetics
Other Relationship: Several life sciences companies (see comments)
Lincoln D. Nadauld
This author is an Associate Editor for JCO Precision Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Ciitizen Corporation, Clariifi, Guidance Genomics
Consulting or Advisory Role: Roche, Roche/Genentech
Speakers' Bureau: Genentech
Travel, Accommodations, Expenses: Roche, Chugai Pharma
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
This article reflects a summary of an expert roundtable discussion held on October 15, 2019.
SUPPORT
Supported in part by a project grant from Genentech to the Personalized Medicine Coalition.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Daryl Pritchard
Provision of study materials or patients: Daryl Pritchard
Collection and assembly of data: Clifford Goodman, Lincoln D. Nadauld
Data analysis and interpretation: Clifford Goodman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daryl Pritchard
Honoraria: Xcenda, Genentech
Research Funding: Thermo Fisher, AstraZeneca (Inst)
Travel, Accommodations, Expenses: Genentech
Clifford Goodman
Employment: Lewin Group
Stock and Other Ownership Interests: United Health Group
Travel, Accommodations, Expenses: Bayer, Medtronic, BioMarin, Concert Genetics
Other Relationship: Several life sciences companies (see comments)
Lincoln D. Nadauld
This author is an Associate Editor for JCO Precision Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Ciitizen Corporation, Clariifi, Guidance Genomics
Consulting or Advisory Role: Roche, Roche/Genentech
Speakers' Bureau: Genentech
Travel, Accommodations, Expenses: Roche, Chugai Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Holtzman NA, Watson MS (eds): Promoting safe and effective genetic testing in the United States. Final report of the Task Force on Genetic Testing, 1997. http://www.genome.gov/10001733 [PubMed] [Google Scholar]
- 2.Lakdawalla DN, Doshi JA, Garrison LP Jr, et al. : Defining elements of value in health care—A health economics approach: An ISPOR Special Task Force Report [3]. Value Health 21:131-139, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Goldman DP, Gupta C, Vasudeva E, et al. : The value of diagnostic testing in personalized medicine. Forum Health Econ Policy 16:S87-S99, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Nelson RS, McLeod HL: How Precision Medicine Combats Underused Genomic Testing, Special Section: Laboratory Stewardship Focus April 2021. Clinical Laboratory News, 2021. https://www.aacc.org/cln/articles/2021/april/how-precision-medicine-combats-underused-genomic-testing [Google Scholar]
- 5.Pritchard DE, Moeckel F, Villa MS, et al. : Strategies for integrating personalized medicine into healthcare practice. Per Med 14:141-152, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Gray SW, Hicks-Courant K, Cronin A, et al. : Physicians' attitudes about multiplex tumor genomic testing. J Clin Oncol 32:1317-1323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabhan C: Barriers to genomic testing get in the way of precision medicine. Stat News, June 20, 2018. https://www.statnews.com/2018/06/20/genomic-testing-barriers-precision-medicine/ [Google Scholar]
- 8.Steuten L, Goulart B, Meropol NJ, et al. : Cost effectiveness of multigene panel sequencing for patients with advanced non-small-cell lung cancer. JCO Clin Cancer Inform 3:1-10, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Grosse S, Khoury M: What is the clinical utility of genetic testing? Genet Med 8:448-450, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Teutsch SM, Bradley LA, Palomaki GE, et al. : The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative: Methods of the EGAPP Working Group. Genet Med 11:3-14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Academies of Sciences, Engineering, and Medicine : An Evidence Framework for Genetic Testing. Washington, DC, The National Academies Press, 2017 [PubMed] [Google Scholar]
- 12.Joseph L, Cankovic M, Caughron S, et al. : The spectrum of clinical utilities in molecular pathology testing procedures for inherited conditions and cancer: A report of the Association for Molecular Pathology. J Mol Diagn 18:605-619, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Burke W: Clinical validity and clinical utility of genetic tests. Curr Protoc Hum Genet 9:Unit 9.15, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Farkas DH: Clinical validity and utility: Putting the patient front and center. J Mol Diagn 18:635-637, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Bunnik EM, Janssens AC, Schermer MH: Personal utility in genomic testing: Is there such a thing? J Med Ethics 41:322-326, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Medical Device Innovation Consortium (MDIC) : Developing Clinical Evidence for Regulatory and Coverage Assessments in In Vitro Diagnostics (IVDs): A Framework for Developing Credible Evidence of Analytical Validity, Clinical Validity, and Clinical Utility for IVDs, 2019. https://mdic.org/wp-content/uploads/2019/08/Clinical-Evidence-IVD-Framework-FINAL.pdf [Google Scholar]
- 17.Khoury MJ: Genetics and genomics in practice: The continuum from genetic disease to genetic information in health and disease. Genet Med 5:261-268, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Mandl KD, Glauser T, Krantz ID, et al. : The genomics research and innovation network: Creating an interoperable, federated, genomics learning system. Genet Med 22:371-380, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson MR, Tipney H, Painter JL, et al. : The support of human genetic evidence for approved drug indications. Nat Genet 47:856-860, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Spreafico R, Soriaga LB, Grosse J, et al. : Advances in genomics for drug development. Genes 11:942, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenson PD, Mort M, Ball EV, et al. : The human gene mutation database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 133:1-9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarty D, Gao J, Phillips S, et al. : OncoKB: A precision oncology knowledge base. JCO Precis Oncol 1:1-16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modest DP, Pant S, Sartore-Bianchi A: Treatment sequencing in metastatic colorectal cancer. Eur J Cancer 109:70-83, 2019 [DOI] [PubMed] [Google Scholar]