PURPOSE
Larotrectinib is a highly selective and CNS-active tropomyosin receptor kinase (TRK) inhibitor that has demonstrated efficacy across TRK fusion–positive cancers, regardless of the tumor type. The aim of this study was to assess the efficacy and safety of larotrectinib in patients with TRK fusion–positive lung cancers.
MATERIALS AND METHODS
Data from two global, multicenter, registrational clinical trials of patients treated with larotrectinib were analyzed: a phase II adult and young adult basket trial (NCT02576431) and a phase I adult trial (NCT02122913). The primary end point was objective response rate (ORR).
RESULTS
By July 20, 2020, 20 patients with TRK fusion–positive lung cancer had been treated. The ORR by investigator assessment among 15 evaluable patients was 73% (95% CI, 45 to 92); one (7%) patient had a complete response, 10 (67%) had a partial response, three (20%) had stable disease, and one (7%) had progressive disease as best response. The median duration of response, progression-free survival, and overall survival were 33.9 months (95% CI, 5.6 to 33.9), 35.4 months (95% CI, 5.3 to 35.4), and 40.7 months (95% CI, 17.2 to not estimable), respectively. Among patients with baseline CNS metastases, the ORR was 63% (95% CI, 25 to 91). Adverse events were mainly grade 1 or 2.
CONCLUSION
Larotrectinib is highly active with rapid and durable responses, extended survival benefit, and a favorable long-term safety profile in patients with advanced lung cancer harboring NTRK gene fusions, including those with CNS metastases. These findings support routine testing for NTRK fusions in patients with lung cancer.
INTRODUCTION
The neurotrophic tyrosine receptor kinase genes (NTRK) NTRK1, NTRK2, and NTRK3 encode the tropomyosin receptor kinase (TRK) proteins TRKA, TRKB, and TRKC, respectively. TRK proteins play an important role in neurodevelopment and postdevelopmental physiologic processes such as balance maintenance, appetite control, and pain perception.1,2 Fusions involving NTRK1, NTRK2, or NTRK3 are oncogenic drivers that are found in a variety of adult and pediatric tumor types, including < 1% of non–small-cell lung cancers (NSCLCs).3,4 Structurally, these fusions are characterized by a 3′ end containing one of the three NTRK genes, including the full kinase domain, and an upstream partner gene in the 5′ position.5 In-frame activating fusions result in constitutive activation of the TRK kinase and uninterrupted downstream signaling that drives tumor development.2 In NSCLC, similar to ALK, ROS1, and RET fusions, TRK fusions are associated with adenocarcinomas from younger patients with a minimal or no history of cigarette smoking, although these fusions have been observed across a range of tumor histologies, ages, and smoking histories.3
CONTEXT
Key Objective
To our knowledge, we report the first analysis of the efficacy and safety of larotrectinib exclusively in patients with tropomyosin receptor kinase (TRK) fusion–positive lung cancer from a registrational data set.
Knowledge Generated
Larotrectinib was highly active in patients with TRK fusion–positive lung cancer, producing rapid, marked, and durable responses, including in patients with brain metastases. Larotrectinib had a favorable safety profile and was well tolerated.
Relevance
These findings validate TRK fusions as key therapeutic targets and underscore the need to include NTRK fusion testing as part of comprehensive molecular profiling in patients with lung cancer.
Larotrectinib is a first-in-class, highly selective, and CNS-active TRK inhibitor.6-8 Larotrectinib received tumor-agnostic approval for the treatment of adult and pediatric patients with TRK fusion–positive cancers in 20189 on the basis of the robust and durable antitumor efficacy observed in a pooled analysis of three phase I and II trials.6 This efficacy was sustained after further follow-up with an expanded patient population.7 Larotrectinib showed a favorable safety profile; only 8% and 2% of patients required a dose reduction or permanent treatment discontinuation, respectively, because of an adverse event (AE).7
To date, however, the prospective efficacy and safety of larotrectinib solely in patients with TRK fusion–positive lung cancers have not been published. The aim of this analysis was to provide data on these outcomes in patients treated on this registrational program.
MATERIALS AND METHODS
Study Design
Patients were eligible for inclusion in this analysis if they had advanced lung cancer harboring an NTRK fusion and participated in one of two global, multicenter trials of larotrectinib: a phase II basket trial in adult and pediatric patients age ≥ 12 years (NAVIGATE, NCT02576431) and a phase I trial in adults age ≥ 18 years (NCT02122913). Complete methodologies for these studies have been described in a previous publication.6
Briefly, patients were eligible for inclusion in the NAVIGATE trial if they had a locally advanced or metastatic solid tumor, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-3, had adequate major organ function, and had received prior standard therapy appropriate for their tumor type and stage of disease or in the opinion of the investigator would be unlikely to tolerate or derive clinically meaningful benefit from appropriate standard-of-care therapy. Patients must have received a platinum-based doublet with or without maintenance therapy (continuation or switch maintenance), unless they declined chemotherapy.
Patients were eligible for inclusion in the phase I study if they had a locally advanced or metastatic solid tumor that had progressed or was nonresponsive to available therapies, were considered unfit for standard chemotherapy, or had a tumor for which no standard or available curative therapy exists, and had an ECOG performance status of 0-2. Patients with asymptomatic CNS metastases were eligible. Patients also had to have at least one measurable lesion as defined by RECIST version 1.1.6
In patients included from both studies, treatment consisted of single-agent larotrectinib administered at 100 mg twice daily in continuous 28-day cycles. Treatment beyond progression was permitted if the patient continued to benefit. TRK fusion status was determined by molecular testing in Clinical Laboratory Improvement Amendments–certified or similarly accredited laboratories. All study protocols were approved by the institutional review board or independent ethics committees and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. All patients, or guardians for patients younger than age 18 years, provided written informed consent. The data cutoff for the current analysis was July 20, 2020.
Study End Points
The primary end point for the combined analysis was objective response rate (ORR) on the basis of RECIST version 1.1. Secondary end points included the duration of response (DoR), progression-free survival (PFS), and overall survival (OS), on the basis of investigator assessment. The occurrence of AEs, including treatment discontinuation and dose modifications, was also assessed per National Cancer Institute Common Terminology Criteria for AEs, version 4.03.
Study Assessments
Tumor assessment was conducted using computed tomography, magnetic resonance imaging, and clinical measurement. Tumors were assessed at baseline, every 8 weeks for 12 months, and then every 12 weeks thereafter until disease progression. Additional imaging was allowed at the investigator's discretion.
Statistical Analysis
DoR, PFS, and OS were estimated using Kaplan-Meier analysis. 95% CIs were calculated using the Clopper-Pearson method. These events were measured as previously described.7
RESULTS
Patient Population
A total of 20 patients with lung cancers harboring an NTRK fusion were included in this analysis. Baseline patient characteristics are shown in Table 1. The median age was 48.5 years (range, 25.0-76.0 years), and 18 (90%) patients had an ECOG performance status of 0 or 1. Tumor histology was adenocarcinoma in 19 (95%) patients and neuroendocrine carcinoma in one (5%) patient. Half of the patients had baseline CNS metastases, two of whom were previously treated with brain radiotherapy (Data Supplement).
TABLE 1.
Clinicopathologic Features
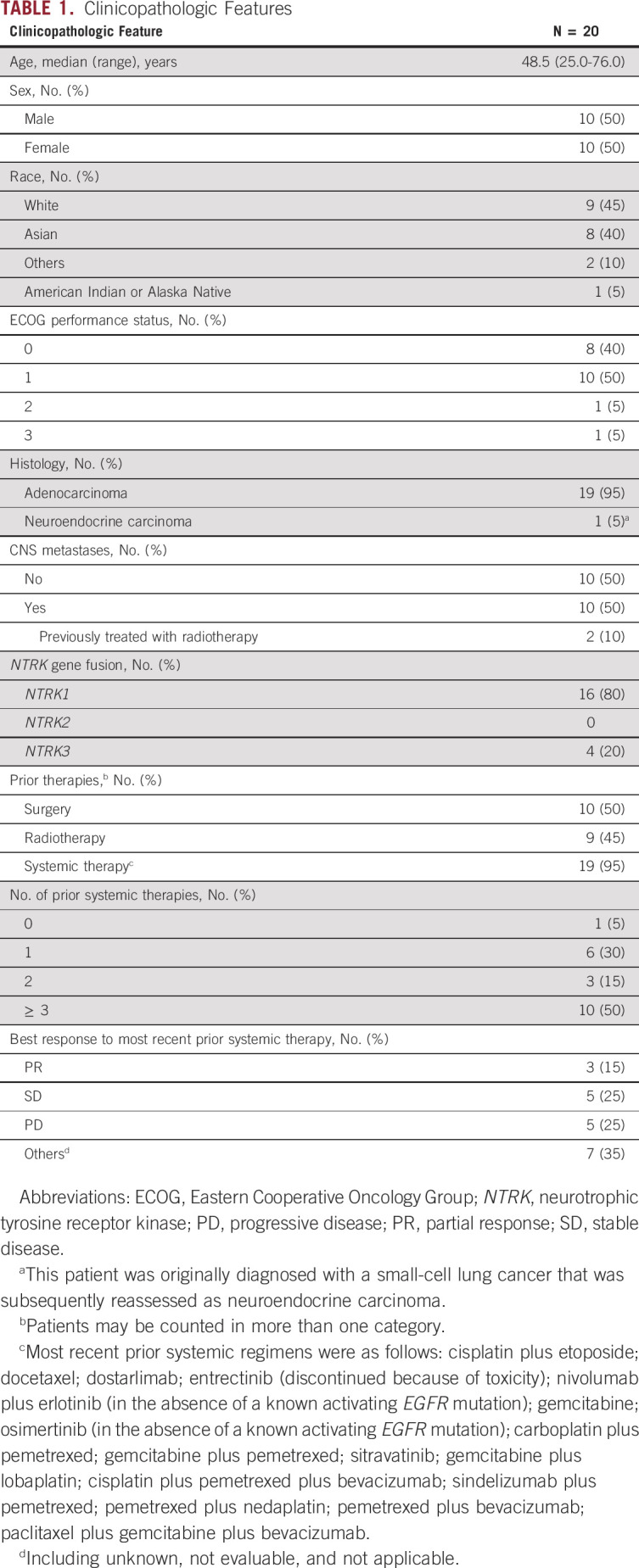
NTRK fusions were identified by RNA-based sequencing in seven (35%) patients (four by anchored multiplex polymerase chain reaction, two by targeted next-generation sequencing [NGS], and one by whole transcriptome sequencing) and by targeted DNA-based NGS in 13 (65%) patients. Sixteen (80%) patients had fusions involving NTRK1 (fusion partners: TPM3 [n = 6], EPS15 [n = 2], IRF2BP2 [n = 2], NOS1AP [n = 1], SQSTM1 [n = 1], TPR [n = 1], CD74 [n = 1], CLIP1 [n = 1], and PRDX1 [n = 1]), and four (20%) patients had fusions involving NTRK3 (fusion partners: SQSTM1 [n = 2] and ETV6 [n = 2]; Data Supplement).
Patients were heavily pretreated. The median number of prior lines of systemic therapy was three (range, 0-6), and 10 (50%) patients had received three or more prior systemic treatments (Data Supplement). Six (30%) patients had received prior immune checkpoint inhibitors.
Efficacy
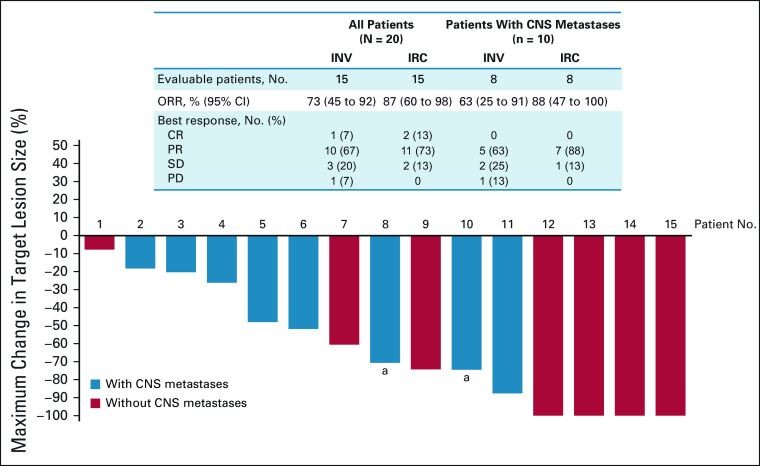
The investigator-assessed ORR among 15 evaluable patients was 73% (95% CI, 45 to 92); one (7%) patient had a complete response, 10 (67%) patients had a partial response, three (20%) patients had stable disease, and one (7%) had progressive disease (extracranial nontarget lesion) as the best response (Fig 1). The ORR by independent review committee assessment was consistent with the investigator-assessed results. The activity of larotrectinib in an exemplary responder is shown in Figure 2. Responses were achieved regardless of ECOG performance status, and a reduction in target tumor size was observed in all patients with measurable disease. Five of the 20 patients overall were not evaluable for response because they had not yet had a postbaseline tumor assessment.
FIG 1.
Response to larotrectinib. A waterfall plot of the maximum change in the target lesion size (investigator assessment) with larotrectinib treatment is shown for 15 evaluable patients whose lung cancers harbored a TRK fusion. aTwo patients had CNS metastases included as target lesions with a 100% and 59% reduction observed by cycle 4, respectively. Each patient number refers to the same patient across all tables and figures. CR, complete response; INV, investigator; IRC, independent review committee; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; TRK, tropomyosin receptor kinase.
FIG 2.
Response to larotrectinib. A 46-year-old man diagnosed with stage IV (T4N0M1) non–small-cell lung cancer previously progressed on first-line platinum-based chemotherapy (after 9 months) and on the PD-1 inhibitor dostarlimab (after 2 months). A TPM3-NTRK1 fusion was identified on molecular profiling, and larotrectinib was initiated on trial. A brisk partial response was achieved at 1.8 months, which was subsequently confirmed; the patient discontinued treatment because of progressive disease after a durable 39 months of disease control. NTRK, neurotrophic tyrosine receptor kinase; PD-1, programmed cell death-1.
The duration of treatment ranged from 0.03+ to 51.5+ months. By the data cutoff, treatment was ongoing in 11 (55%) patients (Data Supplement). Among patients with an objective response (n = 11), the median time to response was 1.8 months (range, 1.6-1.9 months; Fig 3), corresponding to the first follow-up imaging examination on trial.
FIG 3.
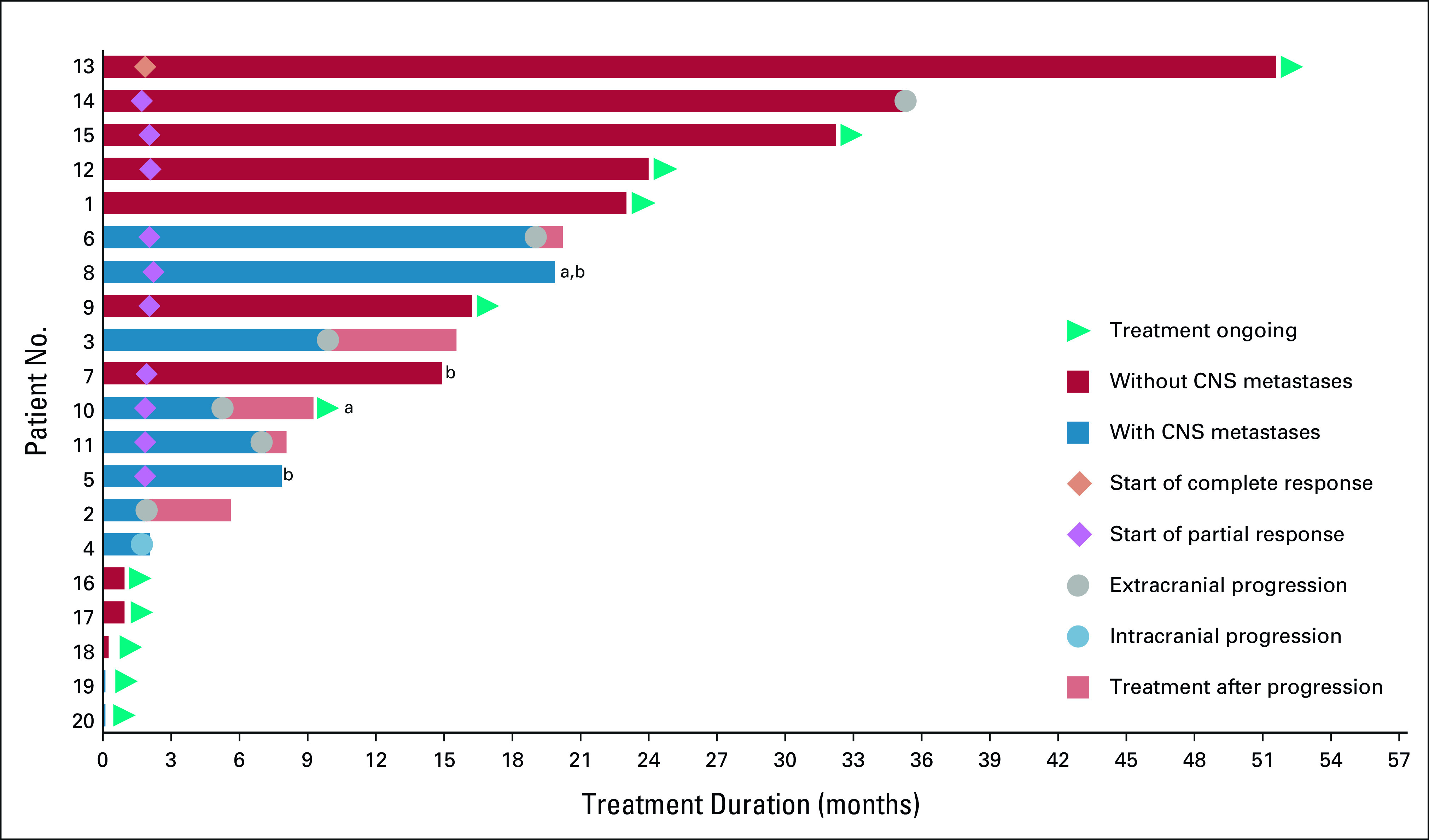
Larotrectinib treatment duration. A swimmer plot of the duration of larotrectinib treatment is shown for all 20 patients whose lung cancers harbored a TRK fusion. aTwo patients had CNS metastases included as target lesions with a 100% and 59% reduction observed by cycle 4, respectively. bPatient discontinued for reasons other than progression. Each patient number refers to the same patient across all tables and figures. TRK, tropomyosin receptor kinase.
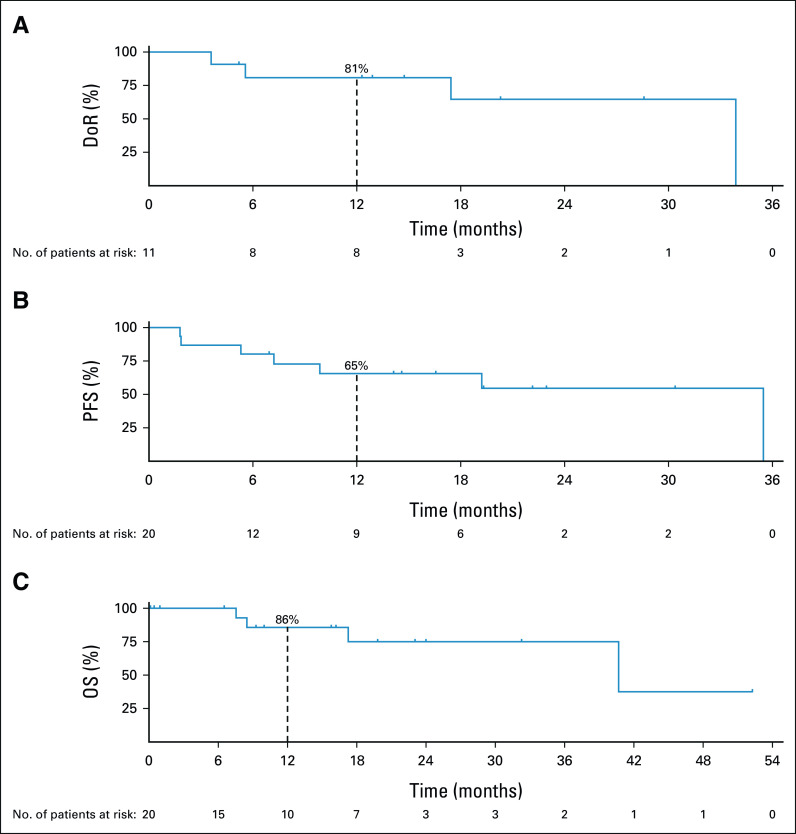
The median DoR was 33.9 months (95% CI, 5.6 to 33.9) at a median follow-up of 17.4 months; the DoR rates at 12 and 24 months were 81% and 65%, respectively. At a median follow-up of 16.6 months, the median PFS was 35.4 months (95% CI, 5.3 to 35.4). The 12- and 24-month PFS rates were 65% and 55%, respectively. Among 19 patients who had prior systemic therapy, 11 (58%) had > 2-fold longer PFS on larotrectinib compared with the time to progression or treatment failure on their most recent prior therapy (Data Supplement). Of the eight patients with < 2-fold longer PFS, seven were still on treatment and censored at the data cutoff. The median OS was 40.7 months (95% CI, 17.2 to not estimable) at a median follow-up of 16.2 months; the 12- and 24-month OS rates were 86% and 75%, respectively (Fig 4).
FIG 4.
DoR and survival. Kaplan-Meier curves of (A) DoR, (B) PFS, and (C) OS are shown for patients with TRK fusion–positive lung cancers treated with larotrectinib. At the data cutoff, the median DoR, PFS, and OS were 33.9 months (95% CI, 5.6 to 33.9), 35.4 months (95% CI, 5.3 to 35.4), and 40.7 months (95% CI, 17.2 to NE), respectively. DoR, PFS, and OS rates at 12 months are indicated with vertical dashed lines on each curve. DoR, duration of response; NE, not estimable; OS, overall survival; PFS, progression-free survival; TRK, tropomyosin receptor kinase.
CNS Efficacy
All eight evaluable patients with CNS metastases at baseline had reductions in overall (systemic) target lesions, ranging from 18% to 88%. The ORR by investigator assessment was 63% (95% CI, 25 to 91); five (63%) patients had a partial response, two (25%) had stable disease, and one (13%) had progressive disease (Fig 1). Although intracranial response was not a study end point, CNS metastases were included as target lesions for two patients, with measured reductions in the CNS metastases of 59% and 100% by cycle 4, respectively. Neither of these patients previously received brain radiotherapy.
The duration of treatment among all 10 patients with CNS metastases ranged from 0.03+ to 20.2 months, with three patients still on treatment at the data cutoff. Five patients continued to receive treatment after extracranial-only progression with maintained disease control in the CNS (Fig 3).
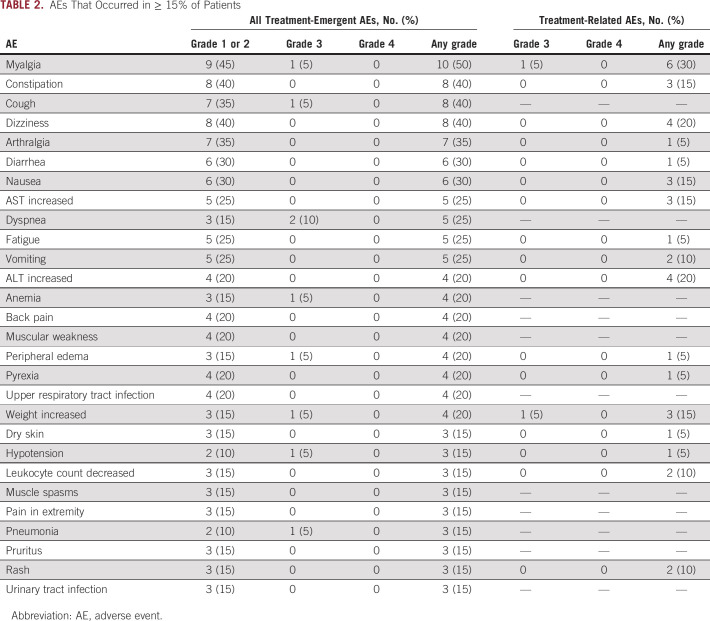
Safety
Treatment-related AEs were reported by 16 (80%) patients. AEs that occurred in ≥ 15% of patients are shown in Table 2; AEs were mostly grade 1 or 2, and there were no unexpected or new safety signals. Eight (40%) patients experienced a grade 3 treatment-emergent AE. Two (10%) patients experienced grade 3 AEs considered related to larotrectinib (hypersensitivity, increased weight, and myalgia). There was one grade 5 AE (cardiac arrest) that was not considered related to larotrectinib.
TABLE 2.
AEs That Occurred in ≥ 15% of Patients
Two (14%) patients required dose reductions because of AEs: grade 2 ALT and grade 2 AST increase in one patient (see the case report below), and grade 2 neutrophil count decrease in the other patient (all considered related to larotrectinib). Three (15%) patients had dose interruptions because of treatment-related AEs: grade 2 neutrophil count decrease in one patient, grade 2 AST and ALT increase in one patient, and grade 3 hypersensitivity in one patient. No patients experienced an AE that resulted in permanent discontinuation of larotrectinib.
DISCUSSION
In this global, multicenter, registrational data set, larotrectinib was found to be highly active in patients with TRK fusion–positive lung cancers. The drug resulted in rapid, marked, and durable responses. The ORR by investigator assessment was 73%, and the median DoR, PFS, and OS were 33.9, 35.4, and 40.7 months, respectively. Furthermore, activity in the CNS was achieved. These results exceed the same outcome measures reported historically for chemotherapy10 and immunotherapy in NSCLCs. For example, first-line chemoimmunotherapy regimens have demonstrated a median PFS of 5.1-10.3 months and a median OS of 17.1-30.0 months.11-17
These data support the recommendation that a TRK inhibitor is the preferred up-front systemic therapy for advanced lung cancers that harbor a TRK fusion regardless of programmed cell death-ligand 1 expression levels, in line with current clinical practice guidelines. International expert consensus recommendations from the Japan Society of Clinical Oncology, European Society for Medical Oncology, ASCO, and the Taiwan Oncology Society strongly recommend the use of a TRK inhibitor during the course of therapy for any TRK fusion–positive cancer. Should a TRK fusion be discovered while a patient is already on another systemic therapy, switching to a TRK inhibitor on progression is recommended and can be considered if the response to the current systemic therapy is suboptimal.18
Similar to lung cancers in general,19 oncogene-driven lung cancers have a propensity for CNS metastasis. A multicenter registry previously reported that 36% of patients with TRK fusion–positive lung cancer had brain metastases,3 and prospective clinical trial data sets including this one have reported an incidence of 50%-69%.20 Larotrectinib demonstrated rapid and durable responses in patients with baseline brain metastases, consistent with previous findings from all larotrectinib-treated TRK fusion–positive cancers with baseline brain metastases where the ORR was 75%.7 In the latter data set, larotrectinib demonstrated intracranial activity in three patients with evaluable intracranial disease: complete intracranial disease resolution in a patient with NSCLC and intracranial tumor reductions of 14% and 46% in two patients with thyroid cancer.7 These findings support the use of larotrectinib in TRK fusion–positive cancers with brain metastases.
Another approved therapy for patients with TRK fusion–positive cancers is the multikinase inhibitor entrectinib, which showed an ORR of 69% in 13 patients with lung cancer and a median PFS and OS of 14.9 months at a median follow-up of 14.2 months.20
Larotrectinib was well tolerated in this series, and no new or unexpected safety signals were observed compared with the larger data set of all larotrectinib-treated TRK fusion–positive cancers,7 making the drug amenable to long-term use. As with any potent TRK inhibitor, occasional unique on-target AEs can occur; these include dizziness, weight gain, paresthesias, and TRK inhibitor withdrawal pain that providers should monitor in the clinic.21 Also consistent with the larger larotrectinib data set, local therapy extended the duration of disease control in TRK fusion–positive lung cancers with oligoprogression or solitary site progression. This is in line with guidelines for other oncogene-driven NSCLCs, which recommend targeted therapy continuation after local therapy for disease progression.22
The identification of numerous actionable drivers in lung cancer supports the use of appropriately designed, comprehensive NGS panels for molecular profiling. Clinical practice guidelines recommend routine testing for EGFR mutations, ALK fusions, and ROS1 alterations23-25 and the inclusion of NTRK1–3, ERBB2, MET, BRAF, KRAS, and RET in NGS panels.25 Our analysis demonstrates that patients with TRK fusion–positive lung cancers had better outcomes on larotrectinib compared with prior systemic therapy, supporting early testing. Orthogonal confirmatory assays including pan-TRK immunohistochemistry are available,26 particularly in practice environments without payer coverage for NGS.27 Furthermore, the fact that DNA-based NGS may not detect all fusions28 supports the use of complementary RNA-based NGS to maximize the likelihood of identifying NTRK (in addition to ALK, ROS1, and RET) fusions.
Acquired resistance to TRK inhibitors may occur through on-target or off-target mechanisms. On-target resistance involves the development of TRK kinase domain mutations, which sterically restrict the binding of first-generation TRK inhibitors. Next-generation TRK inhibitors with activity against TRK kinase domain resistance mutations are in clinical development.8,29 Off-target resistance mechanisms involve bypass signaling activation because of genomic alterations in other oncogenic drivers, and these alterations may often be targetable with existing agents.30
In summary, the highly selective TRK inhibitor larotrectinib is active and well tolerated in patients with TRK fusion–positive lung cancers, including those with CNS metastases. These findings validate TRK fusions as key therapeutic targets and underscore the need for TRK fusion–inclusive early molecular testing strategies in patients with lung cancer.
ACKNOWLEDGMENT
The authors would like to thank the patients, their families, and all investigators involved in this study. Medical writing support was provided by Francesca Murphy, and editorial support was provided by George Chappell, MSc, both of Scion, London, UK, supported by Bayer according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288). The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicians' Education Resource, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, PeerView
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Millennium, BerGenBio, MORE Health, Lilly, AbbVie, 14ner Oncology/Elevation Oncology, Remedica, Archer, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Melendi, Repare Therapeutics
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
Daniel S. W. Tan
Honoraria: Bristol Myers Squibb, Takeda, Novartis, Roche, Pfizer
Consulting or Advisory Role: Novartis, Merck, Loxo, AstraZeneca, Roche, Pfizer
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Roche
Ulrik N. Lassen
Honoraria: Bayer, Pfizer, Novartis
Consulting or Advisory Role: Bayer, Pfizer
Research Funding: BMS (Inst), Roche (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Lilly (Inst)
Serge Leyvraz
Consulting or Advisory Role: Bayer, Immunocore
Travel, Accommodations, Expenses: Bayer, Immunocore
Jyoti D. Patel
Consulting or Advisory Role: AbbVie, AstraZeneca, Takeda Science Foundation
Research Funding: Bristol Myers Squibb (Inst)
Lee Rosen
Research Funding: Pfizer (Inst), Bayer (Inst), PsiOxus Therapeutics (Inst), Rgenix (Inst)
Benjamin Solomon
Consulting or Advisory Role: Bayer
Research Funding: AstraZeneca/Merck (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/358846
Ricarda Norenberg
Employment: Bayer Health
Consulting or Advisory Role: Bayer Health (Inst)
Laura Dima
Employment: Bayer
Nicoletta Brega
Employment: Bayer
Leadership: Bayer
Stock and Other Ownership Interests: Bayer
Travel, Accommodations, Expenses: Bayer
Other Relationship: Bayer
Lin Shen
Consulting or Advisory Role: MSD, Bristol Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji biopharmaceutical, Harbour BioMed, Merck
Speakers' Bureau: Hutchison Whampoa, MSD
Research Funding: Yaojie Ankang (Nanjing) Technology Co, Ltd (Inst), Baiji Shenzhou (Beijing) Biotechnology Co, Ltd (Inst), Beijing Xiantong Biomedical Technology Co, Ltd (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst)
Victor Moreno
Employment: START
Consulting or Advisory Role: Merck, Bristol Myers Squibb, Bayer, Janssen Oncology, Roche, Basilea
Speakers' Bureau: Bayer
Research Funding: AbbVie (Inst), ACEA Biosciences (Inst), Adaptimmune (Inst), Amgen (Inst), AstraZeneca (Inst), Bayer (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Eisai (Inst), E-therapeutics (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Menarini (Inst), Merck (Inst), Nanobiotix (Inst), Novartis (Inst), Pfizer (Inst), PharmaMar (Inst), PsiOxus Therapeutics (Inst), Puma Biotechnology (Inst), Regeneron (Inst), RigonTEC (Inst), Roche (Inst), Sanofi (Inst), Sierra Oncology (Inst), Synthon (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Tesaro (Inst), Transgene (Inst)
Expert Testimony: Medscape/Bayer, Nanobiotix
Travel, Accommodations, Expenses: Sanofi/Regeneron
Other Relationship: Bristol Myers Squibb
Shivaani Kummar
Stock and Other Ownership Interests: PathomIQ, Arxeon Therapeutics
Consulting or Advisory Role: Seattle Genetics, Bayer, Boehringer Ingelheim, Mundipharma EDO GmbH, Harbor BioMed, SpringWorks Therapeutics, Gilead Sciences, Mirati Therapeutics, EcoR1 Capital, Cadila Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Dynavax Technologies (Inst), Pfizer (Inst), Loxo (Inst), Corvus Pharmaceuticals (Inst), Plexxikon (Inst), Jounce Therapeutics (Inst), ADC Therapeutics (Inst), Advenchen Laboratories (Inst), Incyte (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), Astex Pharmaceuticals (Inst), Seattle Genetics (Inst), Amgen (Inst), Genome & Company (Inst), Moderna Therapeutics (Inst), ADC Therapeutics (Inst), ORIC Pharmaceuticals (Inst), Elevation Oncology (Inst), Vincerx Pharma (Inst), Day One Therapeutics (Inst)
Travel, Accommodations, Expenses: Bayer
Jessica J. Lin
Honoraria: Pfizer, OncLive
Consulting or Advisory Role: C4 Therapeutics, Genentech, Nuvalent, Inc, Blueprint Medicines, Turning Point Therapeutics, Turning Point Therapeutics
Research Funding: Hengrui Therapeutics (Inst), Turning Point Therapeutics (Inst), Novartis (Inst), Neon Therapeutics (Inst), Relay Therapeutics (Inst), Elevation Oncology (Inst), Bayer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Pfizer
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Data from this analysis were presented at the 2021 ASCO congress. Abstract #9109
SUPPORT
Supported by Bayer Healthcare and Loxo Oncology, Inc, a wholly owned subsidiary of Eli Lilly and Company. AD was supported in part by Cancer Center and RO1 grants from the National Institutes of Health (P30 CA008748, RO1 CA226864) and Nonna's Garden. DT was supported in part by grants from the National Medical Research Council (NMRC; Singapore; NMRC/OFLCG/002-2018; NMRC/CSA/010/2019).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Alexander Drilon, Daniel S. W. Tan, Jyoti D. Patel, Laura Dima, Nicoletta Brega, Lin Shen, Shivaani Kummar
Financial support: Laura Dima
Provision of study materials or patients: Alexander Drilon, Daniel S. W. Tan, Ulrik N. Lassen, Yongmei Liu, Jyoti D. Patel, Lee Rosen, Shivaani Kummar, Jessica J. Lin
Collection and assembly of data: Alexander Drilon, Ulrik N. Lassen, Serge Leyvraz, Yongmei Liu, Jyoti D. Patel, Lee Rosen, Benjamin Solomon, Nicoletta Brega, Lin Shen, Victor Moreno, Shivaani Kummar, Jessica J. Lin
Data analysis and interpretation: Alexander Drilon, Daniel S. W. Tan, Ulrik N. Lassen, Jyoti D. Patel, Lee Rosen, Benjamin Solomon, Ricarda Norenberg, Nicoletta Brega, Victor Moreno, Shivaani Kummar, Jessica J. Lin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicians' Education Resource, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, PeerView
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Millennium, BerGenBio, MORE Health, Lilly, AbbVie, 14ner Oncology/Elevation Oncology, Remedica, Archer, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Melendi, Repare Therapeutics
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
Daniel S. W. Tan
Honoraria: Bristol Myers Squibb, Takeda, Novartis, Roche, Pfizer
Consulting or Advisory Role: Novartis, Merck, Loxo, AstraZeneca, Roche, Pfizer
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Roche
Ulrik N. Lassen
Honoraria: Bayer, Pfizer, Novartis
Consulting or Advisory Role: Bayer, Pfizer
Research Funding: BMS (Inst), Roche (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Lilly (Inst)
Serge Leyvraz
Consulting or Advisory Role: Bayer, Immunocore
Travel, Accommodations, Expenses: Bayer, Immunocore
Jyoti D. Patel
Consulting or Advisory Role: AbbVie, AstraZeneca, Takeda Science Foundation
Research Funding: Bristol Myers Squibb (Inst)
Lee Rosen
Research Funding: Pfizer (Inst), Bayer (Inst), PsiOxus Therapeutics (Inst), Rgenix (Inst)
Benjamin Solomon
Consulting or Advisory Role: Bayer
Research Funding: AstraZeneca/Merck (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/358846
Ricarda Norenberg
Employment: Bayer Health
Consulting or Advisory Role: Bayer Health (Inst)
Laura Dima
Employment: Bayer
Nicoletta Brega
Employment: Bayer
Leadership: Bayer
Stock and Other Ownership Interests: Bayer
Travel, Accommodations, Expenses: Bayer
Other Relationship: Bayer
Lin Shen
Consulting or Advisory Role: MSD, Bristol Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji biopharmaceutical, Harbour BioMed, Merck
Speakers' Bureau: Hutchison Whampoa, MSD
Research Funding: Yaojie Ankang (Nanjing) Technology Co, Ltd (Inst), Baiji Shenzhou (Beijing) Biotechnology Co, Ltd (Inst), Beijing Xiantong Biomedical Technology Co, Ltd (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst)
Victor Moreno
Employment: START
Consulting or Advisory Role: Merck, Bristol Myers Squibb, Bayer, Janssen Oncology, Roche, Basilea
Speakers' Bureau: Bayer
Research Funding: AbbVie (Inst), ACEA Biosciences (Inst), Adaptimmune (Inst), Amgen (Inst), AstraZeneca (Inst), Bayer (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Eisai (Inst), E-therapeutics (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Menarini (Inst), Merck (Inst), Nanobiotix (Inst), Novartis (Inst), Pfizer (Inst), PharmaMar (Inst), PsiOxus Therapeutics (Inst), Puma Biotechnology (Inst), Regeneron (Inst), RigonTEC (Inst), Roche (Inst), Sanofi (Inst), Sierra Oncology (Inst), Synthon (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Tesaro (Inst), Transgene (Inst)
Expert Testimony: Medscape/Bayer, Nanobiotix
Travel, Accommodations, Expenses: Sanofi/Regeneron
Other Relationship: Bristol Myers Squibb
Shivaani Kummar
Stock and Other Ownership Interests: PathomIQ, Arxeon Therapeutics
Consulting or Advisory Role: Seattle Genetics, Bayer, Boehringer Ingelheim, Mundipharma EDO GmbH, Harbor BioMed, SpringWorks Therapeutics, Gilead Sciences, Mirati Therapeutics, EcoR1 Capital, Cadila Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Dynavax Technologies (Inst), Pfizer (Inst), Loxo (Inst), Corvus Pharmaceuticals (Inst), Plexxikon (Inst), Jounce Therapeutics (Inst), ADC Therapeutics (Inst), Advenchen Laboratories (Inst), Incyte (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), Astex Pharmaceuticals (Inst), Seattle Genetics (Inst), Amgen (Inst), Genome & Company (Inst), Moderna Therapeutics (Inst), ADC Therapeutics (Inst), ORIC Pharmaceuticals (Inst), Elevation Oncology (Inst), Vincerx Pharma (Inst), Day One Therapeutics (Inst)
Travel, Accommodations, Expenses: Bayer
Jessica J. Lin
Honoraria: Pfizer, OncLive
Consulting or Advisory Role: C4 Therapeutics, Genentech, Nuvalent, Inc, Blueprint Medicines, Turning Point Therapeutics, Turning Point Therapeutics
Research Funding: Hengrui Therapeutics (Inst), Turning Point Therapeutics (Inst), Novartis (Inst), Neon Therapeutics (Inst), Relay Therapeutics (Inst), Elevation Oncology (Inst), Bayer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Amatu A, Sartore-Bianchi A, Bencardino K, et al. : Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol 30:viii5-viii15, 2019. (suppl 8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocco E, Scaltriti M, Drilon A: NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 15:731-747, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farago AF, Taylor MS, Doebele RC, et al. : Clinicopathologic features of non-small-cell lung cancer harboring an NTRK gene fusion. JCO Precis Oncol 2:1-12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsythe A, Zhang W, Phillip Strauss U, et al. : A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther Adv Med Oncol 12:1758835920975613, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaishnavi A, Le AT, Doebele RC: TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 5:25-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drilon A, Laetsch TW, Kummar S, et al. : Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 378:731-739, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong DS, DuBois SG, Kummar S, et al. : Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21:531-540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drilon A: TRK inhibitors in TRK fusion-positive cancers. Ann Oncol 30:viii23-viii30, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer HealthCare Pharmaceuticals Inc . VITRAKVI prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210861s006lbl.pdf
- 10.Goffin J, Lacchetti C, Ellis PM, et al. : First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: A systematic review. J Thorac Oncol 5:260-274, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. : Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 378:2078-2092, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Jotte RM, Cappuzzo F, et al. : Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288-2301, 2018 [DOI] [PubMed] [Google Scholar]
- 13.West H, McCleod M, Hussein M, et al. : Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924-937, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. : Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med 381:2020-2031, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Reck M, Ciuleanu T-E, Dols MC, et al. : Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 38:9501, 2020. (suppl 15; abstr 9501) [Google Scholar]
- 16.Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. : Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 38:1505-1517, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Reck M, Rodríguez-Abreu D, Robinson AG, et al. : Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37:537-546, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Yoshino T, Pentheroudakis G, Mishima S, et al. : JSCO—ESMO—ASCO—JSMO—TOS: International expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol 31:861-872, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Villano JL, Durbin EB, Normandeau C, et al. : Incidence of brain metastasis at initial presentation of lung cancer. Neurooncol 17:122-128, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drilon A, Paz-Ares L, Doebele RC, et al. : 543P Entrectinib in NTRK fusion-positive NSCLC: Updated integrated analysis of STARTRK-2, STARTRK-1 and ALKA-372-001. Ann Oncol 31:S474-S475, 2020 [Google Scholar]
- 21.Liu D, Flory J, Lin A, et al. : Characterization of on-target adverse events caused by TRK inhibitor therapy. Ann Oncol 31:1207-1215, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planchard D, Popat S, Kerr K, et al. : Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv192-iv237, 2018. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 23.Lindeman NI, Cagle PT, Beasley MB, et al. : Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 8:823-859, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leighl NB, Rekhtman N, Biermann WA, et al. : Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol 32:3673-3679, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindeman NI, Cagle PT, Aisner DL, et al. : Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 142:321-346, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Hechtman JF, Benayed R, Hyman DM, et al. : Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am J Surg Pathol 41:1547-1551, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchio C, Scaltriti M, Ladanyi M, et al. : ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol 30:1417-1427, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Benayed R, Offin M, Mullaney K, et al. : High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res 25:4712-4722, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laetsch TW, Hong DS: Tropomyosin receptor kinase inhibitors for the treatment of TRK fusion cancer. Clin Cancer Res 27:4974-4982, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Cocco E, Schram AM, Kulick A, et al. : Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med 25:1422-1427, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]