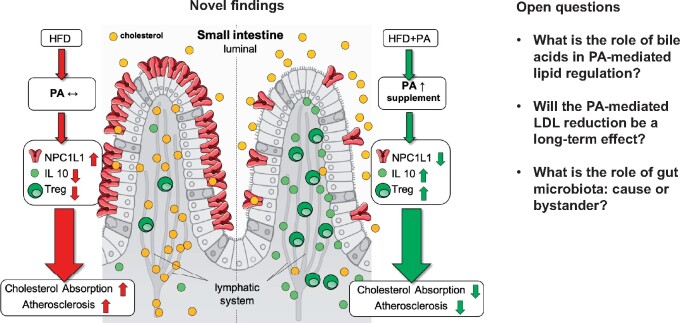
Graphical Abstract
This editorial refers to ‘Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism’, by A. Haghikia et al., https://doi.org/10.1093/eurheartj/ehab644.
Despite the tremendous progress in cardiovascular (CV) treatments in the past decades, the incidence of atherosclerotic CV diseases (ACVDs) is still increasing worldwide and will remain the leading cause of death and morbidity in the years ahead.1
Therefore, a paradigm shift in our understanding of the pathophysiology of ACVD is needed to identify novel players, which, in addition to traditional CV risk factors, contribute to the initiation, progression, and complications of CV disease. It has become evident that the gut microbiota is a pivotal ecosystem impacting CV health and disease,2 but our knowledge of its exact role is still fragmentary. Unveiling the cross-talk between the gut microbiota, its metabolites, and the CV system will move us towards novel and individualized treatments able to reduce the onset and progression of ACVD.
Across the millennia, the intestinal microbiota has evolved into an ecosystem of microorganisms living in a symbiotic relationship with the host. The human microbiota lives in the small and, mainly, in the large intestine, and protects the host from pathogen colonization, modulates the immune system, synthesizes vitamins, or extracts energy from undigested fibres through bacterial fermentation and production of short-chain fatty acids (SCFAs) or other metabolites absorbed into the systemic circulation and able to modulate the function of different organs.3
The short-chain fatty acid propionate
The main SCFAs are acetate, propionate (PA), and butyrate. SCFAs derive from intestinal anaerobic fermentation of dietary fibres and complex polysaccharides, and are considered to have beneficial properties in different disease conditions such as bowel, respiratory, and cardio-metabolic diseases, although their mode of action has yet to be fully elucidated. Under physiological conditions, SCFA levels in the peripheral blood are in the micromolar range, with acetate being the most abundant.4 Consumption of a high-fibre diet raises total SCFA concentrations in the blood, and the CV beneficial effects of the Mediterranean diet are partly attributed to its content of fruits, vegetables, and legumes, which are associated with increased SCFA levels and increased bacterial diversity.5 Accordingly, low fibre intake leads not only to a reduction in gut microbiota composition and diversity, but also to a reduction in the production and circulating levels of SCFAs.5
Acetate and butyrate improved endothelial dysfunction induced by the pro-inflammatory and vasoconstricting angiotensin II (AngII) by increasing the bioavailability of the vasoprotective gas nitric oxide.6 Interestingly, chronic AngII infusion in rats induced hypertension accompanied by a decrease in acetate- and butyrate-producing bacteria, and plasma butyrate was relatively depleted in hypertensive patients.7
In another experimental study, propionate treatment protected from cardiac hypertrophy, fibrosis, vascular dysfunction, and decreased blood pressure in experimental AngII-induced hypertension.8
SCFAs have potent anti-inflammatory and immunomodulatory effects. PA has been shown to induce the differentiation and enhance the suppressive capacity of the T regulatory (Treg) cells. Systemic depletion of Treg cells accelerates atherosclerosis in hypercholesterolaemic mice and is reported in patients with ACVD and immuno-metabolic inflammatory conditions.9 , 10 Supplementation of PA in immune-mediated inflammatory disease such as multiple sclerosis significantly increased functionally competent Treg cells, leading to reduced annual relapse rates together with reduced brain disease progression.9
Indeed, the anti-hypertensive and anti-atherogenic effects of PA have been recently linked to its beneficial immunomodulatory actions enhancing Treg cells.8
Novel findings
The study by Arash Haghikia et al. published in this issue of the European Heart Journal identifies a novel regulatory circuit where PA exerts immune-regulatory effects selectively in the gut to control intestinal cholesterol homeostasis and reduces the aortic atherosclerotic lesion area in the hypercholesterolaemic and atherosclerosis-prone apolipoprotein E-deleted (ApoE– / –) mice fed a high-fat diet (HFD).11
Mechanistically, PA increased Treg cell numbers, which in turn released high concentrations of interleukin (IL)-10, the major Treg cytokine, in the intestinal wall. IL-10 suppressed the expression of Niemann–Pick C1-like 1 (NPC1L1), a major transmembrane transporter responsible for intestinal cholesterol absorption. As a consequence, treatment with PA prevented the increase in total and LDL cholesterol induced by HFD. Furthermore, the Authors performed a randomized, placebo-controlled, double-blind trial enrolling a total of 62 patients with LDL cholesterol levels >115 mg/dL, who were randomized to receive either oral placebo or PA (500 mg) twice daily for 8 weeks.
The PA-treated group showed a reduction in LDL [–15.9 mg/dL (–8.1%)] and non-HDL cholesterol levels. In line with the findings from the experimental studies, phenotyping of peripheral T cells displayed significant increase of Tregs in the PA group without significant alteration of Th17 or Th1 cell numbers.
These novel results suggests that PA may serve as a specific prebiotic to selectively modulate the intestinal immune system, namely the Treg cell–IL-10 axis, leading to reduced intestinal cholesterol absorption with lowering effects of plasmatic LDL.
A major strength of the work by Haghikia et al. is the convergent findings of a PA-dependent immunomodulatory and lipid-lowering effect in hypercholestorelaemic rodents and patients.
Open questions
Given its clinical relevance, this translational work also raises a number of important questions (Graphical Abstract).
Graphical Abstract.
Novel insights and open questions about the anti-atherosclerotic actions of propionate through immune-dependent regulation of intestinal cholesterol metabolism; adapted from Haghikia et al.11
What is the role of bile acids in PA-mediated lipid regulation?
Cholesterol 7 alpha-hydroxylase is a critical regulatory enzyme of bile acid biosynthesis that was up-regulated in hepatocytes of ApoE–/– mice upon PA treatment. Bile acids are products of cholesterol catabolism and natural ligands for the farnesoid X receptor (FXR), a member of the nuclear receptor hormone superfamily.12
FXR activation changes the circulating and intestinal bile acid pool composition, leading to inhibition of intestinal cholesterol absorption12 and higher intestinal cholesterol excretion, which was indeed increased in ApoE–/– mice after PA treatment in the present study. Interestingly, HFD expression of apical sodium–bile acid transporter, which mediates active uptake of conjugated bile acids to maintain their enterohepatic recirculation12 remained up-regulated upon PA treatment, possibly leading to increased circulatory bile acids. Although mRNA expression of FXR was not significantly altered by a HFD and PA treatment, a potential activation of its direct downstream effectors in the hepatic and intestinal compartments may still be present and needs further investigation. Thus, whether the early lipid-lowering effects of PA may be mediated in part by a bile acid-dependent mechanism remains untested.
Will the PA-mediated lipid reduction be a long-term effect?
In-depth investigation in future studies is needed to confirm whether lipid-lowering actions of PA supplementation persist and potentially increase during long-term treatment. If efficacy will be equal in both sexes, in people with different geographic origins and diets, concomitant diseases (especially disarrangements of glucose control) and medications, PA oral supplements may become an appealing, add-on option to existing cholesterol-lowering treatments, for instance to synergize treatment with ezetimibe which acts by blocking the sterol-induced internalization of NPC1L1 or as an alternative in cases of intolerance to it.
Adherence to currently available LDL-lowering treatments is still unsatisfactory and thus a prebiotic approach with PA may hold the promise of an inexpensive treatment with an excellent tolerability and safety profile, with a potential better patient compliance. Unfortunately, at present, poor treatment adherence is a key predictor of unsatisfactory LDL cholesterol targets and cardiovascular mortality in statin-treated patients.13
The work of Haghikia et al. describes reduction of the atherosclerotic plaque burden in hypercholesterolaemic and atherosclerosis-prone mice under a HFD. Clinical trials with PA supplementation in patients with established ACVD would then be the next step to assess whether the promise of atherosclerotic plaque burden reduction holds true and whether PA contributes to the transformation of vulnerable atherosclerotic lesions into more stable plaques. Further animal and human studies may also provide us with a comprehensive understanding of the underlying (i.e. anti-inflammatory?) mechanisms.
Gut microbiota: causal or bystander?
The last and most challenging question raised by this proof-of-concept study is about which gut bacterial species may be responsible for the PA production and may therefore be therapeutically targeted by personalized interventions able to modulate PA production.
Despite accumulating literature reporting different subsets of gut bacteria such as Lactobacillus strains capable of metabolizing propionate with cholesterol-lowering properties,14 Haghikia et al. found that gut microbiota composition and diversity were unchanged in faecal samples obtained from patients at baseline and after PA supplementation over 8 weeks.
Thus, is it conceivable that some gut microbiota species may alter their metabolism in response to oral application of PA, leading to the production of other metabolites (e.g. bile acids), at least partly contributing to the lipid-regulatory effects of PA. According to recent reports, PA or other SCFAs may, for instance, stimulate the colonic L-cells with intestinal production of glucagon like peptide-1 (GLP-1) and peptide-YY (PYY),15 which, in turn, are known to mediate beneficial CV effects.16
Haghikia et al. show that in mice with depleted gut microbiota after treatment with antibiotics, PA was not able to significantly attenuate atherosclerotic lesion size as it did in mice with intact gut microbiota. This finding supports the existence of other gut microbial-dependent anti-atherogenic metabolites or mechanisms beyond the described down-regulation of NPC1L1-dependent intestinal cholesterol absorption.
In line with this notion, Bartolomaeus et al. recently showed that PA treatment, thanks to its beneficial systemic immunomodulatory and anti-inflammatory effects, reduced aortic atherosclerotic lesion burden in AngII-infused ApoE–/– mice without affecting serum levels of total cholesterol, LDL, HDL, or triglycerides.8
Further studies are therefore needed to shed light on the exact role of the gut microbiota on the promise held by PA to be a novel antiatherosclerotic effector exerting beneficial effects on (i) immune cells, locally or systemically, (ii) a systemic reduction of plasma lipid levels, or (iii) both.
Take-home messages
Mounting evidence has linked changes in the composition or metabolic profiles of the microbiota with human disease, including frequent CV disorders such as heart failure, hypertension, and atherosclerosis.
To prove beyond doubt whether and how changes in the gut microbiota are causally associated with CVD, are influenced by CVD, or are simple bystanders represents the most difficult challenge to be faced within the next decade to make sure that the microbiome might become an integral part of clinical cardiovascular medicine.
Conflict of interest: E.O. is the recipient of a Swiss National Science Foundation PRIMA-Assistant Professorship (PR00P3_179861/1) at the Faculty of Medicine, University of Zurich, and has no conflicts to declare.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P; European Society of Cardiology. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J 2020;41:12–85. [DOI] [PubMed] [Google Scholar]
- 2. Schupack DA, Mars RAT, Voelker DH, Abeykoon JP, Kashyap PC. The promise of the gut microbiome as part of individualized treatment strategies. Nat Rev Gastroenterol Hepatol 2021; doi: 10.1038/s41575-021-00499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 4. Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021;13:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang DD, Nguyen LH, Li Y, Yan Y, Ma W, Rinott E, Ivey KL, Shai I, Willett WC, Hu FB, Rimm EB, Stampfer MJ, Chan AT, Huttenhower C. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med 2021;27:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robles-Vera I, Toral M, de la Visitacion N, Aguilera-Sanchez N, Redondo JM, Duarte J. Protective effects of short-chain fatty acids on endothelial dysfunction induced by angiotensin II. Front Physiol 2020;11:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 2015;65:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartolomaeus H, Balogh A, Yakoub M, Homann S, Marko L, Hoges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt KU, Dechend R, Rump LC, Forslund SK, Müller DN, Stegbauer J, Wilck N. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 2019;139:1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, Bader V, Haase S, Kaisler J, David C, Schneider R, Troisi R, Zent D, Hegelmaier T, Dokalis N, Gerstein S, Del Mare-Roumani S, Amidror S, Staszewski O, Poschmann G, Stühler K, Hirche F, Balogh A, Kempa S, Träger P, Zaiss MM, Holm JB, Massa MG, Nielsen HB, Faissner A, Lukas C, Gatermann SG, Scholz M, Przuntek H, Prinz M, Forslund SK, Winklhofer KF, Müller DN, Linker RA, Gold R, Haghikia A. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020;180:1067–1080. [DOI] [PubMed] [Google Scholar]
- 10. Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol 2016;13:167–179. [DOI] [PubMed] [Google Scholar]
- 11. Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, Heinze P, Kaisler J, Nageswaran V, Aigner A, Ceglarek U, Cineus R, Hegazy AN, van der Vorst EPC, Döring Y, Strauch CM, Nemet I, Tremaroli V, Dwibedi C, Kränkel N, Leistner DM, Heimesaat MM, Bereswill S, Rauch G, Seeland U, Soehnlein O, Müller DN, Gold R, Bäckhed F, Hazen SL, Haghikia A, Landmesser U. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J 2022;43:518–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perino A, Demagny H, Velazquez-Villegas LA, Schoonjans K. Molecular physiology of bile acid signaling in health, disease and aging. Physiol Rev 2021;101:683–731. [DOI] [PubMed] [Google Scholar]
- 13. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, Murphy J, Banach M, De Servi S, Gaita D, Gouni-Berthold I, Hovingh GK, Jozwiak JJ, Jukema JW, Kiss RG, Kownator S, Iversen HK, Maher V, Masana L, Parkhomenko A, Peeters A, Clifford P, Raslova K, Siostrzonek P, Romeo S, Tousoulis D, Vlachopoulos C, Vrablik M, Catapano AL, Poulter NR; DA VINCI study. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2021;28:1279–1289. [DOI] [PubMed] [Google Scholar]
- 14. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 2017;19:29–41. [DOI] [PubMed] [Google Scholar]
- 15. Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol 2018;315:G53–G65. [DOI] [PubMed] [Google Scholar]
- 16. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab 2018;20 Suppl 1:5–21. [DOI] [PubMed] [Google Scholar]