Abstract
Aims
The complementary studies FIDELIO-DKD and FIGARO-DKD in patients with type 2 diabetes and chronic kidney disease (CKD) examined cardiovascular and kidney outcomes in different, overlapping stages of CKD. The purpose of the FIDELITY analysis was to perform an individual patient-level prespecified pooled efficacy and safety analysis across a broad spectrum of CKD to provide more robust estimates of safety and efficacy of finerenone compared with placebo.
Methods and results
For this prespecified analysis, two phase III, multicentre, double-blind trials involving patients with CKD and type 2 diabetes, randomized 1:1 to finerenone or placebo, were combined. Main time-to-event efficacy outcomes were a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure, and a composite of kidney failure, a sustained ≥57% decrease in estimated glomerular filtration rate from baseline over ≥4 weeks, or renal death. Among 13 026 patients with a median follow-up of 3.0 years (interquartile range 2.3–3.8 years), the composite cardiovascular outcome occurred in 825 (12.7%) patients receiving finerenone and 939 (14.4%) receiving placebo [hazard ratio (HR), 0.86; 95% confidence interval (CI), 0.78–0.95; P = 0.0018]. The composite kidney outcome occurred in 360 (5.5%) patients receiving finerenone and 465 (7.1%) receiving placebo (HR, 0.77; 95% CI, 0.67–0.88; P = 0.0002). Overall safety outcomes were generally similar between treatment arms. Hyperkalaemia leading to permanent treatment discontinuation occurred more frequently in patients receiving finerenone (1.7%) than placebo (0.6%).
Conclusion
Finerenone reduced the risk of clinically important cardiovascular and kidney outcomes vs. placebo across the spectrum of CKD in patients with type 2 diabetes.
Key Question
Does finerenone, a novel selective, nonsteroidal mineralocorticoid receptor antagonist, added to maximum tolerated renin–angiotensin system inhibition reduce cardiovascular disease and kidney disease progression over a broad range of chronic kidney disease in patients with type 2 diabetes?
Key Finding
In a prespecified, pooled individual-level analysis from two randomized trials, we found reductions both in cardiovascular events and kidney failure outcomes with finerenone. Because 40% of the patients had an estimated glomerular filtration rate of >60 mL/min/1.73m2 they were identified solely on the basis of albuminuria.
Take Home Message
Finerenone reduces the risk of clinical cardiovascular outcomes and kidney disease progression in a broad range of patients with chronic kidney disease and type 2 diabetes. Screening for albuminuria to identify at-risk patients among patients with type 2 diabetes facilitates reduction of both cardiovascular and kidney disease burden.
Keywords: Cardiorenal outcomes, Chronic kidney disease, Finerenone, Hospitalization for heart failure, Hyperkalaemia, Type 2 diabetes
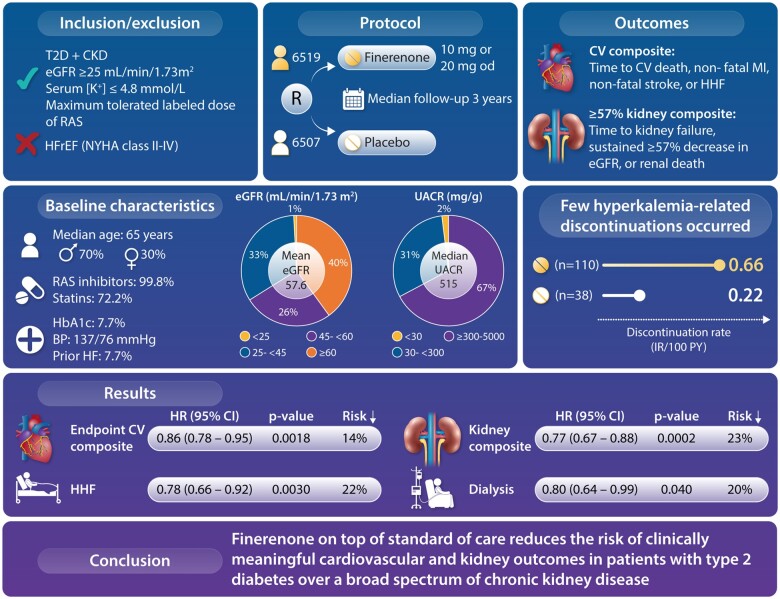
Graphical Abstract
Structured Graphical Abstract.
Finerenone reduced the risk of clinically important cardiovascular and kidney outcomes versus placebo across the spectrum of chronic kidney disease in patients with type 2 diabetes.
See the editorial comment for this article ‘Bringing FIDELITY to the estimate of treatment effects of finerenone in chronic kidney disease due to type 2 diabetes’, by Carly Adamson and Pardeep S. Jhund, https://doi.org/10.1093/eurheartj/ehab827.
Introduction
Patients with chronic kidney disease (CKD) and type 2 diabetes have high residual cardiorenal morbidity and mortality, despite current therapies,1–5 and the risks of progression towards kidney failure and cardiovascular events increase with severity and stage of CKD.6 Compared with patients with advanced kidney disease, who are more likely to progress to dialysis, patients with better preserved estimated glomerular filtration rate (eGFR) have a greater lifetime risk of cardiovascular morbidity such as heart failure, myocardial infarction (MI), stroke, or dying from cardiovascular causes.7
Evidence suggests that overactivation of the mineralocorticoid receptor (MR) leads to inflammation and fibrosis in the heart, kidneys, and vasculature where the MR is extensively expressed that can drive CKD and cardiovascular disease progression.8 Finerenone is a novel, selective, nonsteroidal MR antagonist (MRA) that blocks MR-mediated sodium reabsorption and MR overactivation and has demonstrated anti-inflammatory and anti-fibrotic effects in preclinical kidney and cardiovascular disease models.8 , 9 The FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease (FIDELIO-DKD) and FInerenone in reducinG cArdiovascular moRtality and mOrbidity in Diabetic Kidney Disease (FIGARO-DKD) phase III trials are complementary in nature due to features such as their similar designs and endpoints. Together, they form the largest cardiorenal outcomes programme in CKD in type 2 diabetes to date. They investigated the efficacy and safety of finerenone, on top of maximum tolerated renin–angiotensin system inhibition, on kidney and cardiovascular outcomes in patients with mild-to-severe CKD in type 2 diabetes (Supplementary material online, Figure S1).3 In FIDELIO-DKD, finerenone significantly reduced the risk of the primary kidney composite outcome and the key secondary cardiovascular composite outcome in patients with predominantly stage 3–4 CKD with severely increased albuminuria and type 2 diabetes.7 In FIGARO-DKD, finerenone significantly reduced the primary cardiovascular composite outcome risk in a broader patient population than studied in FIDELIO-DKD (patients with stage 2–4 CKD and moderately increased albuminuria, or stage 1–2 CKD with severely increased albuminuria).7 , 10
The FIDELIO-DKD trial was designed to detect a treatment effect of finerenone on kidney failure endpoints, whereas the FIGARO-DKD trial aimed to detect an effect on a cardiovascular composite primary endpoint.7 , 10 To improve the ability to detect a treatment effect on the kidney failure outcome, patients with a higher urine albumin-to-creatinine ratio (UACR) were preferentially selected in the FIDELIO-DKD trial. To provide a greater kidney failure-free interval to detect a treatment effect on cardiovascular events, in FIGARO-DKD, a population with moderate UACR and a wider eGFR range was selected. Thus, the two trials complemented each other with a slight overlap in the populations studied, and their similar designs and overlapping research sites allowed for the comparison and pooling of their results.
The efficacy and safety of finerenone, however, have not been fully evaluated across the spectrum of CKD in type 2 diabetes. The FInerenone in chronic kiDney diseasE and type 2 diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial programme analYsis (FIDELITY) pools these complementary studies with similar designs, assessments, and conduct. The aim of the FIDELITY prespecified pooled analysis was to provide more robust estimates of finerenone efficacy and safety across the spectrum of patients with CKD and type 2 diabetes, to provide reassurance regarding outcomes in a wide range of patients with a degree of precision that was not possible to obtain by considering the two trials separately.
Methods
Study design
This prespecified pooled efficacy and safety analysis, which was prespecified in a formal statistical analysis plan, combines data from FIDELIO-DKD (NCT02540993) and FIGARO-DKD (NCT02545049), two phase III, randomized, double-blind, placebo-controlled, multicentre clinical trials (Table 1 and Supplementary material online, Figure S2). Trial design and study protocol details have been published previously.7 , 11
Table 1.
Pooled analysis study details
| Study name | FIDELIO-DKD7 | FIGARO-DKD10 |
|---|---|---|
| Publication year | 2020 | 2021 |
| Study design | Phase III, randomized, double-blind, placebo-controlled, multicentre clinical trial | Phase III, randomized, double-blind, placebo-controlled, multicentre clinical trial |
| Sample sizea | 5734 | 7437 |
| Inclusion criteria |
|
|
| Exclusion criteria | ||
| Follow-up period, median | 2.6 years | 3.4 years |
| Primary outcome | Time to kidney failure, sustained ≥40% decrease in eGFR from baseline, or renal death | Time to CV death, non-fatal MI, non-fatal stroke, or HHF |
| Secondary outcome | Time to CV death, non-fatal MI, non-fatal stroke, or HHF | Time to kidney failure, sustained ≥40% decrease in eGFR from baseline, or renal death |
| Trial registry information | NCT02540993 | NCT02545049 |
The key features of the FIDELIO-DKD and FIGARO-DKD studies that comprised the FIDELITY prespecified pooled analysis are described above, including publication year, study design, sample size, eligibility criteria, median follow-up, primary and main secondary endpoints, and links to the trials’ ClinicalTrials.gov webpages.
CKD, chronic kidney disease; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; MI, myocardial infarction; RAS, renin–angiotensin system; SBP, systolic blood pressure; T2D, type 2 diabetes; UACR, urine albumin-to-creatinine ratio.
A total of 145 randomized patients (60 patients in FIDELIO-DKD and 85 patients in FIGARO-DKD) were prospectively excluded prior to database lock from all analyses because of critical Good Clinical Practice violations. This affected one site in the USA that was subsequently closed during the conduct of the trial, leading to the exclusion of 66 patients. In addition, during trial conduct, it was detected that several patients were randomized simultaneously at multiple trial sites in the same locality in Florida, USA. This led to the prospective exclusion of a total of 79 patient IDs.
Mean sitting SBP ≥170 mmHg or mean sitting DBP ≥110 mmHg at the run-in visit, or mean sitting SBP ≥160 mmHg or mean sitting DBP ≥100 mmHg at the screening visit.
New York Heart Association class II–IV at the run-in visit.
Patients
Eligible patients were adults (aged ≥18 years) with type 2 diabetes and CKD treated with a maximum tolerated labelled dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB).
Chronic kidney disease in FIDELIO-DKD
CKD in FIDELIO-DKD was defined as either: (i) persistent (demonstrated at both the run-in and screening visits, which took place between a minimum of 4 to a maximum of 16 weeks apart), moderately increased albuminuria (UACR ≥30–<300 mg/g) with an eGFR of ≥25–<60 mL/min/1.73 m2 and the presence of diabetic retinopathy, or (ii) persistent, severely increased albuminuria (UACR ≥300–≤5000 mg/g) and an eGFR of ≥25–<75 mL/min/1.73 m2.
Chronic kidney disease in FIGARO-DKD
CKD in FIGARO-DKD was defined as either: (i) persistent, moderately increased albuminuria (UACR ≥30–<300 mg/g) with an eGFR of ≥25–≤90 mL/min/1.73 m2, or (ii) persistent, severely increased albuminuria (UACR ≥300–≤5000 mg/g) and an eGFR ≥60 mL/min/1.73 m2. Patients in both trials had to have serum potassium ≤4.8 mmol/L at both the run-in and screening visits. Other key exclusion criteria included clinical diagnosis of symptomatic chronic heart failure with reduced ejection fraction (i.e., a Class IA recommendation for MRA treatment). Inclusion and exclusion criteria are listed in the Supplementary material online, Appendix.
Procedures
The procedures for the FIDELIO-DKD and FIGARO-DKD studies have been described previously. Briefly, eligible patients were randomized 1:1 to receive oral finerenone (10 or 20 mg) or placebo. Both studies consisted of run-in, screening, double-blind treatment, and safety follow-up periods (Supplementary material online, Figure S2).7 , 10 The run-in period required ACEi or ARB therapy to be adjusted to a maximum tolerated labelled dose that did not lead to unacceptable side effects. Study drug was withheld if potassium concentrations exceeded 5.5 mmol/L and restarted when potassium levels fell to ≤5.0 mmol/L. Further details are found in the Supplementary material online, Appendix.
Outcomes
The outcome definitions for FIDELIO-DKD and FIGARO-DKD have been described previously.7 , 10 The efficacy outcomes selected for this analysis were either a primary or a secondary outcome or those prespecified in the hierarchical outcomes in the complementary studies. The efficacy outcomes of interest for this pooled analysis were a composite cardiovascular outcome of time to cardiovascular death, non-fatal MI, non-fatal stroke, or hospitalization for heart failure (HHF), and a composite kidney outcome of time to first onset of kidney failure, sustained ≥57% decrease in eGFR from baseline over ≥4 weeks, or renal death. In the composite kidney outcome, kidney failure was defined as end-stage kidney disease (ESKD) or a sustained decrease in eGFR to <15 mL/min/1.73 m2, and ESKD was defined as initiation of chronic dialysis (for ≥90 days) or kidney transplantation. Other prespecified outcomes included: a second composite kidney outcome of time to first occurrence of kidney failure, sustained ≥40% decrease in eGFR from baseline over ≥4 weeks, or renal death; time to all-cause mortality; time to all-cause hospitalization; and change in UACR from baseline to Month 4.
The eGFR ≥40% composite kidney outcome was the primary or secondary outcome in the complementary trials.7 , 10 However, a sustained ≥57% decrease in eGFR (equivalent to doubling of serum creatinine) was selected in FIDELITY because it is a classic outcome in diabetic nephropathy studies2 , 12–14 and is a more robust kidney failure surrogate outcome than a ≥40% decrease in eGFR, particularly when initial changes in eGFR occur.14 , 15 This outcome was selected before data pooling and analysis. Furthermore, the eGFR ≥57% outcome was a predefined outcome in the complementary trials.
In the safety analyses, adverse events were considered treatment emergent if they started or worsened during study drug intake or up to 3 days after any temporary or permanent interruption. The hyperkalaemia management procedure has been described previously.7 , 10
Statistical analyses
Statistical analyses were prespecified exploratory evaluations rather than hypothesis confirming. Statistical tests where P-values are provided were exploratory in nature; therefore, no adjustment for multiplicity was performed.
The full analysis set comprised all randomized patients [except those with critical Good Clinical Practice (GCP) violations, who were prospectively excluded from all analyses]. Safety analyses were performed in the safety analysis set, defined as all randomized patients without critical GCP violations who took ≥1 dose of study drug. Study outcomes were analysed using stratified Cox proportional hazards models fitted using the stratification factors: study, region (North America, Latin America, Europe, Asia, and others), eGFR category at screening (25–<45, 45–<60, and ≥60 mL/min/1.73 m2), albuminuria category (moderately increased and severely increased) at screening, and a history of cardiovascular disease (present or absent; see Supplementary material online, Appendix, for further details). P-values for the comparison of treatment groups are presented based on a stratified log-rank test. Treatment effects are expressed as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) from the stratified Cox proportional hazards models. Events were counted from randomization up to the end-of-study visit and patients without an event were censored at the date of their last contact with complete information on all components of the respective outcome. The time-to-event analyses reporting included first events only. Events based on a sustained decrease in eGFR were considered in the analysis from randomization up until 5 months after the last eGFR was recorded at a clinic visit. For subgroup analyses, HRs were derived from stratified Cox proportional hazards models, including treatment subgroup and a subgroup by treatment interaction term as fixed effects.
Cumulative incidences based on Aalen–Johansen accounting for mortality as competing risk and corresponding numbers needed to treat were calculated in 6-month intervals for the composite cardiovascular outcome and the key composite kidney outcome. Cumulative incidences based on Kaplan–Meier were calculated for all-cause mortality. An on-treatment sensitivity analysis was performed for outcomes considering only events occurring up until 30 days after study drug cessation in the full analysis set.
The sponsor, Bayer, conducted the statistical analyses, and all authors had access to the data and participated in its interpretation. All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). Additional statistical methods are found in the Supplementary material online, Appendix.
Results
From September 2015 through October 2018, 33 292 patients from 48 countries were screened and 13 171 patients were randomized (Supplementary material online, Figure S3). Critical GCP violations related to site or patient misconduct resulted in the prospective exclusion of 145 patients (see Supplementary material online, Appendix), leaving a population of 13 026 patients on whom statistical analyses were performed.
Patient characteristics, medications, and demographics at baseline were balanced between patients randomized to finerenone and placebo (Table 2 and Supplementary material online, Table S1; published previously3). The mean eGFR was 57.6 mL/min/1.73 m2 and median UACR 515 mg/g. Overall, 10.2%, 41.0%, and 48.3% of patients had moderate, high, and very high Kidney Disease Improving Global Outcomes risk scores, respectively (Supplementary material online, Figure S4). History of cardiovascular disease was reported in 45.6% of patients. Blood glucose (mean glycated haemoglobin 7.7%) and blood pressure (mean systolic blood pressure 136.7 ± 14.2) were moderately well controlled. Baseline cardiovascular medications included statins, anti-platelets, and diuretics in 72.2%, 56.0%, and 51.5% of patients, respectively, and renin–angiotensin system inhibition in 99.8% of patients. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose co-transporter-2 (SGLT-2) inhibitors were used by 7.2% and 6.7% of patients, respectively. Medications started after study drug initiation are summarized in Supplementary material online, Table S2. The median follow-up period was 3.0 years [interquartile range (IQR) 2.3–3.8 years].
Table 2.
Baseline patient demographics, clinical characteristics, and medicationsa
| Finerenone (10 mg od or 20 mg od) (n = 6519) | Placebo (n = 6507) | All patients (n = 13 026) | |
|---|---|---|---|
| Age, years | 64.7 ± 9.4 | 64.8 ± 9.7 | 64.8 ± 9.5 |
| Sex, n (%) | |||
| Male | 4481 (68.7) | 4607 (70.8) | 9088 (69.8) |
| Female | 2038 (31.3) | 1900 (29.2) | 3938 (30.2) |
| Race or ethnic group, n (%) | |||
| White | 4449 (68.2) | 4420 (67.9) | 8869 (68.1) |
| Black/African American | 253 (3.9) | 269 (4.1) | 522 (4.0) |
| Asian | 1432 (22.0) | 1462 (22.5) | 2894 (22.2) |
| Others | 385 (5.9) | 356 (5.4) | 741 (5.8) |
| Duration of diabetes, years | 15.4 ± 8.7 | 15.4 ± 8.7 | 15.4 ± 8.7 |
| HbA1c, % | 7.7 ± 1.4 | 7.7 ± 1.4 | 7.7 ± 1.4 |
| Systolic blood pressure, mmHg | 136.8 ± 14.2 | 136.7 ± 14.3 | 136.7 ± 14.2 |
| History of cardiovascular disease, n (%) | 2979 (45.7) | 2956 (45.4) | 5935 (45.6) |
| Heart failure, n (%) | 485 (7.4) | 522 (8.0) | 1007 (7.7) |
| eGFR, mL/min/1.73 m2 | 57.5 ± 21.6 | 57.7 ± 21.8 | 57.6 ± 21.7 |
| eGFR, mL/min/1.73 m2, n (%) | |||
| ≥60 | 2603 (39.9) | 2592 (39.8) | 5195 (39.9) |
| 45–<60 | 1717 (26.3) | 1717 (26.4) | 3434 (26.4) |
| 25–<45 | 2117 (32.5) | 2115 (32.5) | 4232 (32.5) |
| <25 | 81 (1.2) | 81 (1.2) | 162 (1.2) |
| UACR, mg/g, median (IQR) | 514 (198–1129) | 515 (198–1163) | 515 (198–1147) |
| UACR, mg/g, n (%) | |||
| <30 | 120 (1.8) | 110 (1.7) | 230 (1.8) |
| 30–<300 | 2076 (31.8) | 2023 (31.1) | 4099 (31.5) |
| ≥300 | 4321 (66.3) | 4371 (67.2) | 8692 (66.7) |
| Serum potassium, mmol/L | 4.35 ± 0.44 | 4.35 ± 0.44 | 4.35 ± 0.44 |
| Baseline medications, n (%) | |||
| Renin–angiotensin system inhibitors | 6408 (99.8) | 6495 (99.8) | 13 003 (99.8) |
| Angiotensin-converting enzyme inhibitors | 2526 (38.7) | 2553 (39.2) | 5079 (39.0) |
| Angiotensin receptor blockers | 3987 (61.2) | 3950 (60.7) | 7937 (60.9) |
| Diuretics | 3325 (51.0) | 3385 (52.0) | 6710 (51.5) |
| Statins | 4657 (71.4) | 4742 (72.9) | 9399 (72.2) |
| Potassium bindersb | 94 (1.4) | 88 (1.4) | 182 (1.4) |
| Glucose-lowering therapies | 6354 (97.5) | 6366 (97.8) | 12 720 (97.7) |
| Insulin | 3866 (59.3) | 3764 (57.8) | 7630 (58.6) |
| GLP-1RAs | 497 (7.6) | 447 (6.9) | 944 (7.2) |
| SGLT-2 inhibitors | 438 (6.7) | 439 (6.7) | 877 (6.7) |
Details of key patient baseline demographic and clinical characteristics, and medication use for both treatment groups and the overall FIDELITY population.
eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated haemoglobin; IQR, interquartile range; od, once daily; SGLT-2, sodium-glucose co-transporter-2; UACR, urine albumin-to-creatinine ratio.
Plus or minus values indicate mean ± standard deviation.
Sodium polystyrene sulphonate, calcium polystyrene sulphonate, and potassium-binding agents.
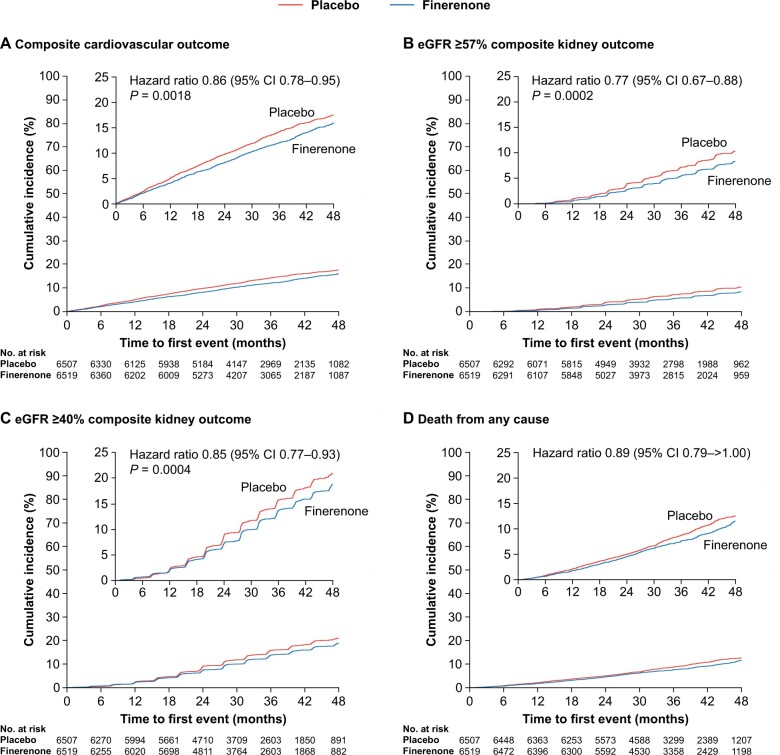
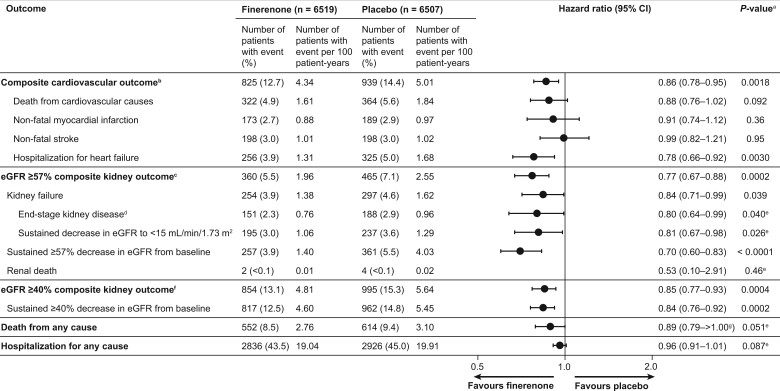
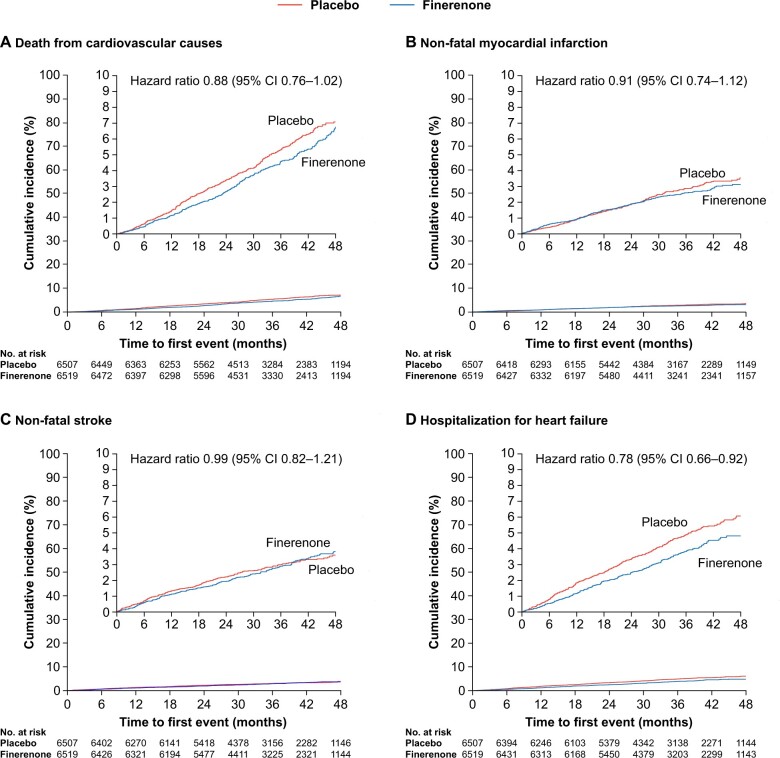
The composite cardiovascular outcome (time to cardiovascular death, non-fatal MI, non-fatal stroke, or HHF) occurred in 825 (12.7%) patients receiving finerenone and 939 (14.4%) patients receiving placebo (HR, 0.86; 95% CI, 0.78–0.95; P = 0.0018; Figures 1 and 2). Significantly lower incidences of HHF occurred with finerenone vs. placebo (HR, 0.78; 95% CI, 0.66–0.92 [P = 0.0030], Figures 2 and 3). Cardiovascular death and non-fatal MI incidences were directionally consistent with the cardiovascular composite outcome; non-fatal stroke was similar with finerenone and placebo (Figures 2 and 3). Based on a between-group risk difference of 2.2% after 3 years, 46 (95% CI, 29–109) patients would need to be treated with finerenone to prevent one composite cardiovascular outcome event. Consistent results were observed in prespecified subgroups (including those with SGLT-2 inhibitor or GLP-1RA baseline use; Supplementary material online, Figure S5), and a prespecified ‘on-treatment’ sensitivity analysis (including all events from randomization up to 30 days after last intake of study drug) confirmed the results of the main analysis (Supplementary material online, Table S3). Results for each of the cardiovascular and renal endpoints by individual trial in FIDELITY, including P interaction values, are presented in Supplementary material online, Figure S6.
Figure 1.
Time to efficacy outcomes. (A) The composite cardiovascular outcome defined as cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure (Aalen–Johansen curve). (B) The composite kidney outcome defined as kidney failure, sustained ≥57% decrease in estimated glomerular filtration rate from baseline over ≥4 weeks, or renal death (Aalen–Johansen curve). (C) The composite kidney outcome defined as kidney failure, sustained ≥40% decrease in estimated glomerular filtration rate from baseline over ≥4 weeks, or renal death (Aalen–Johansen curve). (D) All-cause mortality (Kaplan–Meier curve). Outcomes were assessed in time-to-event analyses.
Figure 2.
Efficacy outcomes. aStatistical tests where P-values are provided were exploratory in nature; therefore, no adjustment for multiplicity was performed. bThe composite of time to first onset of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for heart failure. cThe composite of time to first onset of kidney failure, sustained ≥57% decrease in estimated glomerular filtration rate from baseline over ≥4 weeks, or renal death. dInitiation of chronic dialysis for ≥90 days or kidney transplantation. eAnalyses for P-values not prespecified. fThe composite of time to first onset of kidney failure, sustained ≥40% decrease in estimated glomerular filtration rate from baseline over ≥4 weeks, or renal death. g P = 1.001 to 3 decimal places.
Figure 3.
Components of the composite cardiovascular outcome. Outcomes were assessed in time-to-event analyses (Aalen–Johansen curves). (A) Cardiovascular death. (B) Non-fatal myocardial infarction. (C) Non-fatal stroke. (D) Hospitalization for heart failure.
The incidence of the composite kidney outcome (time to first onset of kidney failure, sustained ≥57% decrease in eGFR from baseline over ≥4 weeks, or renal death) was significantly lower with finerenone vs. placebo (Figures 1 and 2), occurring in 360 (5.5%) patients receiving finerenone and 465 (7.1%) patients receiving placebo (HR, 0.77; 95% CI, 0.67–0.88; P = 0.0002); the number needed to treat based on a between-group risk difference of 1.7% at 3 years was 60 (95% CI, 38–142). Analysis of the sustained ≥57% decrease in eGFR component showed a 30% risk reduction (HR, 0.70; 95% CI, 0.60–0.83; P < 0.0001) and the ESKD component a 20% risk reduction with finerenone vs. placebo (HR, 0.80; 95% CI, 0.64–0.99; P = 0.040). The prespecified ‘on-treatment’ sensitivity analysis confirmed the results of the primary analysis (Supplementary material online, Table S3).
The composite kidney outcome of kidney failure, sustained ≥40% eGFR decrease, or renal death occurred in 854 (13.1%) and 995 (15.3%) patients receiving finerenone and placebo, respectively (HR, 0.85; 95% CI, 0.77–0.93; P = 0.0004; Figures 1 and 2). Incidences of all-cause mortality (Figures 1 and 2) and hospitalization for any cause (Figure 2) with finerenone were not significantly different from placebo (P = 0.051 and P = 0.087, respectively). The mean change in UACR from baseline to 4 months was 32% lower with finerenone vs. placebo (ratio of least-squares mean change from baseline, 0.68; 95% CI, 0.66–0.70), an effect maintained throughout the trial (Supplementary material online, Figure S7A).
Similar incidences of investigator-reported treatment-emergent adverse events were observed between treatment groups (Table 3 and Supplementary material online, Table S4). Serious adverse events occurred in 31.6% of patients in the finerenone group and 33.7% of patients in the placebo group. Renal-related adverse events, including acute kidney injury, occurred in similar proportions of patients between groups. Hyperkalaemia-related adverse events occurred more frequently with finerenone (14.0%) vs. placebo (6.9%), but no hyperkalaemia-related adverse events were fatal and only a small proportion led to permanent treatment discontinuation [1.7% (incidence rate 0.66 per 100 patient-years) and 0.6% (incidence rate 0.22 per 100 patient-years), respectively] or hospitalization (0.9% and 0.2%, respectively). After the fourth month of treatment, the change in serum potassium from baseline was +0.21 mmol/L [standard deviation (SD) 0.47 mmol/L] with finerenone and +0.02 mmol/L (SD 0.43 mmol/L) with placebo; the mean serum potassium was stable over time thereafter (Supplementary material online, Figure S7B). Hypokalaemia occurred less frequently with finerenone (1.1%) vs. placebo (2.3%), while gynaecomastia was similar in the finerenone group (0.1%) and the placebo group (0.2%). Patients receiving finerenone had a modest reduction in mean systolic blood pressure compared with patients receiving placebo [change in mean systolic blood pressure at 4 months was –3.2 mmHg (SD 15.0 mmHg) with finerenone and +0.5 mmHg (SD 14.6 mmHg) with placebo; Supplementary material online, Figure S8].
Table 3.
Safety outcomes
| Treatment-emergent AEsa | Number of patients with event (%) |
|
|---|---|---|
| Finerenone (n = 6510) | Placebo (n = 6489) | |
| Any AE | 5602 (86.1) | 5607 (86.4) |
| AE related to study drug | 1206 (18.5) | 862 (13.3) |
| AE leading to treatment discontinuation | 414 (6.4) | 351 (5.4) |
| Any serious AEb | 2060 (31.6) | 2186 (33.7) |
| Serious AEb related to study drug | 83 (1.3) | 61 (0.9) |
| Serious AEb leading to treatment discontinuation | 145 (2.2) | 154 (2.4) |
| Investigator-reported hyperkalaemiac | 912 (14.0) | 448 (6.9) |
| Hyperkalaemia related to study drug | 573 (8.8) | 249 (3.8) |
| Permanent discontinuation due to hyperkalaemia | 110 (1.7) | 38 (0.6) |
| Serious hyperkalaemiab | 69 (1.1) | 16 (0.2) |
| Hospitalization due to serious hyperkalaemia | 61 (0.9) | 10 (0.2) |
| Fatal hyperkalaemia | 0 (0.0) | 0 (0.0) |
| Investigator-reported hypokalaemia | 70 (1.1) | 149 (2.3) |
| Investigator-reported renal-related AEs | ||
| Acute kidney injuryd | 220 (3.4) | 234 (3.6) |
| Hospitalization due to acute kidney injuryd | 85 (1.3) | 86 (1.3) |
| Treatment discontinuation due to acute kidney injuryd | 14 (0.2) | 10 (0.2) |
| Adverse events affecting ≥5% of patients in either groupd | ||
| Hyperkalaemia | 781 (12.0) | 382 (5.9) |
| Nasopharyngitis | 559 (8.6) | 577 (8.9) |
| Arthralgia | 496 (7.6) | 459 (7.1) |
| Back pain | 436 (6.7) | 428 (6.6) |
| Urinary tract infection | 431 (6.6) | 432 (6.7) |
| Diarrhoea | 423 (6.5) | 411 (6.3) |
| Anaemia | 425 (6.5) | 397 (6.1) |
| Hypertension | 419 (6.4) | 581 (9.0) |
| Upper respiratory tract infection | 407 (6.3) | 394 (6.1) |
| Oedema peripheral | 384 (5.9) | 584 (9.0) |
| Glomerular filtration rate decreased | 348 (5.3) | 274 (4.2) |
| Hypoglycaemia | 340 (5.2) | 375 (5.8) |
| Dizziness | 341 (5.2) | 322 (5.0) |
| Bronchitis | 328 (5.0) | 332 (5.1) |
| Constipation | 317 (4.9) | 334 (5.1) |
| Pneumonia | 271 (4.2) | 387 (6.0) |
Summary of safety outcomes by treatment group (including events leading to treatment discontinuation or hospitalization) which included AEs, serious AEs, hyperkalaemia-related events, renal AEs, hypokalaemia events, and a list of the most commonly occurring AEs that had an incidence ≥5% in either treatment group.
AEs, adverse events; MedDRA, Medical Dictionary for Regulatory Activities.
Reported as treatment-emergent AEs relating to seriousness criteria.
A treatment-emergent event was considered to be a serious AE if it: (i) resulted in death; (ii) was life-threatening; (iii) required inpatient hospitalization (or prolongation of existing hospitalization); (iv) caused persistent or significant disability/incapacity; (v) was a congenital abnormality or birth defect; or (vi) was judged by the investigator to be a serious or important medical event.
Investigator-reported AEs using the MedDRA preferred terms ‘hyperkalaemia’ and ‘blood potassium increased’.
MedDRA preferred term.
Discussion
The pooled analysis of two complementary trials comprising 13 026 patients with a broad spectrum of CKD and type 2 diabetes, all treated with an optimized dose of an ACEi or ARB,7 , 10 provides robust evidence of both cardiovascular and kidney protection with finerenone vs. placebo. Based on the FIDELIO-DKD trial results, finerenone is currently indicated to reduce the risk of sustained eGFR decline, ESKD, cardiovascular death, non-fatal MI, and HHF in adult patients with CKD associated with type 2 diabetes.7 , 16 Across the FIDELITY population, the relative risk reduction was 14% for the composite cardiovascular outcome and 23% for the composite kidney outcome; cardiovascular outcomes across subgroups by baseline demographics and clinical characteristics were generally consistent. The results of this analysis provide reassurance regarding the safety and efficacy of finerenone across a wide spectrum of patients with CKD and type 2 diabetes with a degree of precision that was previously not possible to obtain by considering the two trials separately.
MRAs are indicated for the treatment of patients with chronic symptomatic heart failure with reduced ejection fraction17 , 18—such patients were excluded from the FIDELIO-DKD and FIGARO-DKD studies7 , 10—but data supporting use of MRAs to reduce the risk of heart failure developing in patients with CKD and type 2 diabetes are limited. The FIDELITY analysis provides evidence that finerenone use in patients with CKD and type 2 diabetes, a population at high risk of developing heart failure, prevents HHF. In the FIDELITY analysis, HHF was the main driver of the cardiovascular benefit with finerenone, with a relative risk reduction of 22% vs. placebo (P = 0.0030), in a population that excluded patients with chronic symptomatic heart failure with reduced ejection fraction at the run-in visit.7 , 10 This finding is important because heart failure is a major source of morbidity and healthcare costs among patients with CKD and type 2 diabetes19–21; therefore, prevention of such events can improve patient outcomes and potentially reduce healthcare costs. Furthermore, the fact that finerenone lowers HHF risk across the CKD spectrum emphasizes the need to screen all patients with type 2 diabetes for this risk factor.
The FIDELITY analysis shows not only a 30% reduction in the risk of a sustained ≥57% decrease in eGFR on top of optimized ACEi or ARB therapy but also a relative risk reduction of 20% in ESKD with finerenone vs. placebo (P = 0.0403). The relative risks of all components of the kidney composite outcome were reduced with finerenone vs. placebo, except time to renal death (this finding was likely due to low event numbers, although the HR of 0.53 still favoured finerenone over placebo). The relative risk of a sustained ≥57% decrease in eGFR was reduced by 30%, ESKD by 20%, sustained decrease in eGFR to <15 mL/min/1.73 m2 by 19%, and kidney failure by 16%. The requirement for dialysis is one of the most dreaded complications of CKD progression22; it is associated with substantial morbidity and costs.23 Reduction of this outcome, which is of great relevance to both patients and payers, is therefore notable.
Prior trials of dual renin–angiotensin system blockade have failed to show cardiovascular or kidney protection among patients with CKD and type 2 diabetes, suggesting that the way in which the renin–angiotensin–aldosterone system is blocked is important.24–26 The FIDELITY analysis suggests a strong effect of MR overactivation in the pathogenesis of both cardiovascular disease and CKD progression in patients with CKD and type 2 diabetes.
Previous studies assessing SGLT-2 inhibitors in patients with CKD and type 2 diabetes, such as CREDENCE or DAPA-CKD, included patient populations representing a substantially smaller part of the CKD spectrum than the FIDELITY analysis.2 , 27 Because the trials differed in population, design, and other aspects described previously, their results cannot be directly compared with the FIDELITY analysis.7 , 10 In FIDELITY, 877 patients (6.7%) received SGLT-2 inhibitors and 944 patients (7.2%) received GLP-1RAs at baseline, and the cardiovascular and kidney benefits of finerenone are at least as large in patients on SGLT-2 inhibitors or GLP-1RAs as in those without. Preclinical data on the initial combination of finerenone and empagliflozin in a rat model of hypertension-induced end-organ damage also showed that the combination approach conferred a protective effect across various cardiorenal outcomes.28 It is possible that there is an additive effect of the combination of finerenone on top of SGLT-2 inhibitors or GLP-1RAs, which may relate to their distinct mechanisms of action. However, this was not a randomized trial of finerenone and SGLT-2 inhibitor or GLP-1RA combination therapy, and the efficacy and safety of these agents in combination will be determined in future clinical trials and analyses.
Owing to its mechanism of action, finerenone treatment is expected to increase serum potassium concentration through MR antagonism.8 In FIDELITY, hyperkalaemia was more frequent with finerenone vs. placebo. However, the incidence of hyperkalaemia-related adverse events with clinical impact was low, with hyperkalaemia-related permanent treatment discontinuation in only 1.7% of patients receiving finerenone vs. 0.6% with placebo over a median follow-up of 3.0 years (IQR 2.3–3.8 years). Hypokalaemia occurred less frequently in finerenone-treated patients than in placebo-treated patients. Potassium intake was not restricted during the trials, but finerenone or placebo was withheld in cases where potassium concentrations >5.5 mmol/L were detected, until potassium concentrations fell to ≤5.0 mmol/L. Therefore, finerenone improves cardiorenal outcomes in patients with CKD and type 2 diabetes with a manageable hyperkalaemia risk and a reduction in hypokalaemia. The hypokalaemia reduction is notable because it is causatively associated with adverse outcomes in heart failure.29
Although this prespecified pooled analysis of two studies with similar protocols included a large patient population with CKD and type 2 diabetes, limitations are that it did not include patients with non-albuminuric CKD and only a small proportion of Black patients were included. Since the design of the FIGARO-DKD and FIDELIO-DKD studies, SGLT-2 inhibitors have emerged as therapeutic options for patients with CKD and type 2 diabetes.30 Some patients in the finerenone phase III trials also received these novel agents at study commencement, and consistent cardiovascular benefits were observed in these groups.
In conclusion, finerenone reduced the risk of clinically important cardiovascular and kidney outcomes vs. placebo across the spectrum of CKD in patients with type 2 diabetes. The results highlight the importance of early treatment before CKD has progressed to improve outcomes in this patient population.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors and study sponsor are indebted to the patients and their families, as well as the investigators and sites participating in the studies. Medical writing assistance was provided by Lizahn Zwart, PhD, of Chameleon Communications International and was funded by Bayer AG.
Funding
This work was supported by Bayer AG, who funded by the FIDELIO-DKD and FIGARO-DKD studies and pooled analysis.
Role of the funder/sponsor
The Executive Committee, in collaboration with the study sponsor, designed the trials and protocols and supervised the trial conduct. In both trials, patient safety was overseen by an independent data monitoring committee. Analyses were conducted by the sponsor; all authors had access to and participated in the interpretation of the data. The study sponsor collected and analysed the data, and sponsor authors contributed to interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Conflict of interest: R.A. reported personal fees and non-financial support from Bayer Healthcare Pharmaceuticals Inc. during the conduct of the study; he also reported personal fees and non-financial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius, Janssen, Relypsa, Sanofi, and Vifor Pharma; he has received personal fees from Ironwood Pharmaceuticals, Lexicon, Merck & Co, and Reata, and non-financial support from E. R. Squibb & Sons, Opko Pharmaceuticals, and Otsuka America Pharmaceutical; he is a member of data safety monitoring committees for Amgen, AstraZeneca, and Celgene; a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen, and Relypsa; and a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim, and Janssen; he has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate; and he has received research grants from the U.S. Veterans Administration and the National Institutes of Health. G.F. reported that he is a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor Pharma; he is a senior consulting editor for JACC Heart Failure and has received research support from the European Union. B.P. reported consultant fees for AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Proton Intel, Sanofi/Lexicon, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; he has stock options for KBP Biosciences, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, Proton Intel, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent # 9931412) and a provisional patent for histone-acetylation-modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045784). S.D.A. has received research support from Abbott Vascular and Vifor International and personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier, and Vifor Pharma. P.R. reported personal fees from Bayer during the conduct of the study; he has received research support and personal fees from AstraZeneca and Novo Nordisk and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi, and Vifor; all fees are given to Steno Diabetes Center Copenhagen. A.J., C.N., and M.G. are full-time employees of Bayer AG, Division Pharmaceuticals, Germany. P.K. is a full-time employee of Bayer AG, Division Pharmaceuticals, Germany. He is a co-inventor of finerenone and holds US and European patents relating to finerenone (US8436180B2 and EP2132206B1). L.M.R. reported receipt of consultancy fees from Bayer. G.L.B. reported research funding, paid to the University of Chicago Medicine, from Bayer during the conduct of the study; he also reported research funding, paid to the University of Chicago Medicine, from Novo Nordisk and Vascular Dynamics; he acted as a consultant and received personal fees from for Alnylam, Merck, and Relypsa; he is an editor of the American Journal of Nephrology, Nephrology, and Hypertension and a section editor of UpToDate and is an associate editor of Diabetes Care and Hypertension Research.
Data availability
Data are not currently available.
Will data be available: Yes
Where: Electronic repository
When will data availability begin: Date to be confirmed by Bayer.
Contributor Information
Rajiv Agarwal, Indiana University School of Medicine and Richard L. Roudebush VA Medical Center, 1481 W. 10th St, Indianapolis, IN 46202, USA.
Gerasimos Filippatos, Department of Cardiology, Attikon University Hospital, Rimini 1, Chaidari 124 62, Athens, Greece.
Bertram Pitt, Department of Medicine, University of Michigan School of Medicine, 1500 E. Medical Centre Dr #6303, Ann Arbor, MI 48109, USA.
Stefan D Anker, Department of Cardiology (CVK) and Berlin Institute of Health Center for Regenerative Therapies, German Centre for Cardiovascular Research Partner Site Berlin, CharitéUniversitätsmedizin, Charitépl. 1, 10117 Berlin, Germany.
Peter Rossing, Steno Diabetes Center Copenhagen, Niels SteensensVej 2-4, 2820 Gentofte, Denmark; Department of Clinical Medicine, University of Copenhagen, Blegdamsvej 3b 33.5, DK-2200 Copenhagen, Denmark.
Amer Joseph, Cardiology and Nephrology Clinical Development, Bayer AG, Müllerstraße 178, 13353 Berlin, Germany.
Peter Kolkhof, Research and Development, Preclinical Research Cardiovascular, Bayer AG, Friedrich-Ebert-Straße 217/333, 42117, Wuppertal, Germany.
Christina Nowack, Research and Development, Clinical Development Operations, Bayer AG, Friedrich-Ebert-Straße 217/333, 42117, Wuppertal, Germany.
Martin Gebel, Research and Development, Integrated Analysis Statistics, Bayer AG, Friedrich-Ebert-Straße 217/333, 42117, Wuppertal, Germany.
Luis M Ruilope, Cardiorenal Translational Laboratory and Hypertension Unit, Institute of Research imas12, Instituto de Investigación Hospital 12 de OctubreCentro de ActividadesAmbulatorias, 6ª Planta Bloque DAvda. de Córdoba, s/n28041 Madrid, Spain; CIBER-CV, Hospital Universitario 12 de Octubre, Av. de Córdoba, s/n, 28041, Madrid, Spain; Faculty of Sport Sciences, European University of Madrid, C. Tajo, s/n, 28670 Villaviciosa de Odón, Madrid, Spain.
George L Bakris, Department of Medicine, University of Chicago Medicine, 5841 South Maryland Avenue, MC 6092, 60637 Chicago, IL, USA.
References
- 1. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017;12:2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perkovic V, Jardine MJ, Neal B et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 3. Agarwal R, Anker SD, Bakris G et al. ; FIDELIO-DKD and FIGARO-DKD Investigators. Investigating new treatment opportunities for patients with chronic kidney disease in type 2 diabetes: the role of finerenone. Nephrol Dial Transplant 2020. doi:10.1093/ndt/gfaa294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neuen BL, Young T, Heerspink HJL et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845–854. [DOI] [PubMed] [Google Scholar]
- 5. Zelniker TA, Wiviott SD, Raz I et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–2031. [DOI] [PubMed] [Google Scholar]
- 6. Fox CS, Matsushita K, Woodward M et al. ; Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bakris GL, Agarwal R, Anker SD et al. ; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal R, Kolkhof P, Bakris G et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2021;42:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolkhof P, Jaisser F, Kim SY, Filippatos G, Nowack C, Pitt B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol 2017;243:271–305. [DOI] [PubMed] [Google Scholar]
- 10. Pitt B, Filippatos G, Agarwal R et al. ; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021. doi:10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 11. Ruilope LM, Agarwal R, Anker SD et al. ; FIGARO-DKD Study Investigators. Design and baseline characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial. Am J Nephrol 2019;50:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis EJ, Hunsicker LG, Clarke WR et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860. [DOI] [PubMed] [Google Scholar]
- 13. Brenner BM, Cooper ME, de Zeeuw D et al. ; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 14. Coresh J, Turin TC, Matsushita K et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AS, Inker LA, Matsushita K et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014;64:821–835. [DOI] [PubMed] [Google Scholar]
- 16. Bayer Healthcare Pharmaceuticals Inc. KERENDIA (Finerenone) Prescribing Information; 2021. https://labeling.bayerhealthcare.com/html/products/pi/Kerendia_PI.pdf (17 August 2021).
- 17. Yancy CW, Jessup M, Bozkurt B et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240–327–e327. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, Bueno H et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 19. Zareini B, Blanche P, D'Souza M et al. Type 2 diabetes mellitus and impact of heart failure on prognosis compared to other cardiovascular diseases: a nationwide study. Circ Cardiovasc Qual Outcomes 2020;13:e006260. [DOI] [PubMed] [Google Scholar]
- 20. Lawson CA, Seidu S, Zaccardi F et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine 2021;32:100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. United States Renal Data System. Cardiovascular disease in patients with CKD. In: USRDS Annual Data Report. Volume 1: Chronic Kidney Disease. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020, Chapter 4. https://adr.usrds.org/2020/chronic-kidney-disease/4-cardiovascular-disease-in-patients-with-ckd (1 June 2021).
- 22. Frontini R, Sousa H, Ribeiro O, Figueiredo D. “What do we fear the most?”: Exploring fears and concerns of patients, family members and dyads in end-stage renal disease. Scand J Caring Sci 2020. doi:10.1111/scs.12940. [DOI] [PubMed] [Google Scholar]
- 23. United States Renal Data System. Healthcare expenditures for persons with CKD. In: USRDS Annual Data Report. Volume 1: Chronic Kidney Disease. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. (Chapter 6). https://adr.usrds.org/2020/chronic-kidney-disease/6-healthcare-expenditures-for-persons-with-ckd. (1 July 2021).
- 24. Fried LF, Emanuele N, Zhang JH et al. ; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903. [DOI] [PubMed] [Google Scholar]
- 25. Parving HH, Brenner BM, McMurray JJ et al. ; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–2213. [DOI] [PubMed] [Google Scholar]
- 26. Imai E, Chan JC, Ito S et al. ; ORIENT Study Investigators. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 2011;54:2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heerspink HJL, Stefansson BV, Correa-Rotter R et al. ; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 28. Kolkhof P, Hartmann E, Freyberger A et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol 2021;52:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sfairopoulos D, Arseniou A, Korantzopoulos P. Serum potassium and heart failure: association, causation, and clinical implications. Heart Fail Rev 2021;26:479–486. [DOI] [PubMed] [Google Scholar]
- 30. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care 2021;44:S151–S167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not currently available.
Will data be available: Yes
Where: Electronic repository
When will data availability begin: Date to be confirmed by Bayer.