Abstract
Background
Vancomycin-resistant enterococci (VRE) are major therapeutic challenges. Prospective contemporary data characterizing the clinical and molecular epidemiology of VRE bloodstream infections (BSIs) are lacking.
Methods
The Vancomycin-Resistant Enterococcal BSI Outcomes Study (VENOUS I) is a prospective observational cohort of adult patients with enterococcal BSI in 11 US hospitals. We included patients with Enterococcus faecalis or Enterococcus faecium BSI with ≥1 follow-up blood culture(s) within 7 days and availability of isolate(s) for further characterization. The primary study outcome was in-hospital mortality. Secondary outcomes were mortality at days 4, 7, 10, 12, and 15 after index blood culture. A desirability of outcome ranking was constructed to assess the association of vancomycin resistance with outcomes. All index isolates were subjected to whole genome sequencing.
Results
Forty-two of 232 (18%) patients died in hospital and 39 (17%) exhibited microbiological failure (lack of clearance in the first 4 days). Neutropenia (hazard ratio [HR], 3.13), microbiological failure (HR, 2.4), VRE BSI (HR, 2.13), use of urinary catheter (HR, 1.85), and Pitt BSI score ≥2 (HR, 1.83) were significant predictors of in-hospital mortality. Microbiological failure was the strongest predictor of in-hospital mortality in patients with E faecium bacteremia (HR, 5.03). The impact of vancomycin resistance on mortality in our cohort changed throughout the course of hospitalization. Enterococcus faecalis sequence type 6 was a predominant multidrug-resistant lineage, whereas a heterogeneous genomic population of E faecium was identified.
Conclusions
Failure of early eradication of VRE from the bloodstream is a major factor associated with poor outcomes.
Keywords: bacteremia, Enterococcus, VRE
Failure to eradicate enterococci from the bloodstream in the first 4 days after the index blood culture was the most consistent factor associated with increased risk of mortality. The association of vancomycin resistance with mortality changed throughout the course of the hospitalization.
Vancomycin-resistant enterococci (VRE) are leading causes of hospital-acquired infections affecting individuals who have multiple comorbidities or are immunocompromised [1–3]. The number of infections due to VRE reported in the Americas and Europe has increased during the last decade, becoming a significant burden to healthcare systems globally [4–7]. The Centers for Disease Control and Prevention (CDC) estimate that VRE are associated with 54 500 infections and 5400 deaths per year in the United States (US) [8]. Moreover, the CDC and World Health Organization have included VRE as high-priority bacteria against which new therapies are urgently needed [9]. Retrospective studies have shown that the presence of vancomycin resistance increases mortality in patients with enterococcal bloodstream infections (BSIs) compared to vancomycin-susceptible enterococci (VSE) [3, 10, 11]. However, the retrospective design of these studies makes it difficult to assess the role of vancomycin resistance in mortality, mostly because data related to patient illness severity and comorbidities are not widely available, making it difficult to make stringent adjustments. Additionally, the lack of isolate characterization and follow-up blood cultures preclude the evaluation of microbiological outcomes and prevent solid interpretations of the complex dynamics of these infections and response to therapy. Here, using a prospective cohort of patients with enterococcal BSI (both VRE and VSE), we sought to provide a comprehensive characterization of the contemporary clinical and genomic epidemiology of VRE BSIs in a multicenter study conducted in the US.
METHODS
Population
The Vancomycin-Resistant Enterococcal BSI Outcomes Study (VENOUS I) is a prospective cohort study of adult individuals (≥18 years old) with ≥1 blood culture positive for Enterococcus faecalis and Enterococcus faecium from 10 tertiary hospitals in Houston, Texas and 1 hospital in Detroit, Michigan (September 2016 to March 2018). The hospitals include the largest cancer center in the US and general hospitals with robust transplant and cardiovascular programs. Included patients must have ≥1 follow-up blood culture within 7 days after the initial bloodstream episode. Additionally, the initial enterococcal isolate must be available for further analyses. Only the first episodes of enterococcal bacteremia were included. Subsequent episodes of bacteremia were considered a recurrence (defined below) or new infection if patients were infected with a different species of Enterococcus.
Data
Clinical information was collected from the electronic medical records at each institution and managed using REDCap (Research Electronic Data Capture, Vanderbilt University). Data included demographics, past medical history, comorbid conditions, history of prior hospitalization (1 year), recent surgery (a surgical procedure within 2 weeks prior to the index BSI episode), chemotherapy, and receipt of immunosuppressive medications, including steroids (≥100 mg/day of hydrocortisone or equivalent given within 2 weeks prior to index BSI). Severity of illness and comorbidities were assessed using the Pitt BSI and Charlson scores, respectively, calculated within 48 hours of the index culture [12]. Source of the enterococcal BSI was based on treating physicians’ final diagnoses and available clinical/diagnostic data. Empiric therapy was defined as antibiotics given prior to final susceptibility results.
Definitive enterococcal antibiotic therapy was defined as antibiotics administered with in vitro activity against enterococci after final antibiotic susceptibility results or the following combinations: (1) daptomycin plus any of the following: ampicillin, ampicillin-sulbactam, ertapenem, amoxicillin-clavulanate, ceftriaxone, or piperacillin-tazobactam and (2) ceftriaxone plus ampicillin (only E faecalis). The antibiotics should have been administered for ≥48 hours to be considered for the analysis. Recurrent BSI was defined as a new positive enterococcal blood culture in a patient who had a previous negative enterococcal blood culture during the same hospitalization. Microbiological failure (MF) was defined as lack of clearance ≥4 days after the index blood culture [13]. All blood cultures were ordered by the treating physician and processed in the clinical microbiology laboratory of each hospital for identification and antibiotic susceptibility testing. All bacterial isolates were sent to the central study laboratory (Houston, Texas) for confirmatory testing and genomic characterization.
Outcomes
The main outcome of the study was all-cause in-hospital mortality, defined as death occurring from any cause during admission. This definition was preferred to 30-day mortality in order to minimize confounding from follow-up as not all centers consistently documented out-of-hospital mortality. Patients were followed until discharge or in-hospital death. Secondary outcomes were mortality at 4, 7, 10, 12, and 15 days after the index culture.
Data and Statistical Analyses
Median and interquartile range (IQR) were used as measures of central tendency and dispersion to describe baseline characteristics. Categorical variables were compared using χ2 or Fisher exact test when appropriate, and continuous variables were compared using the Wilcoxon-Mann-Whitney U test. Kaplan-Meier survival curves were used to estimate the median time to death and were compared using the log-rank test. Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence interval (CI) for in-hospital mortality and secondary outcomes (independent models were created for each secondary outcome). Patient follow-up started at the time of blood culture collection and ended when death occurred or the patient was discharged from the hospital, whichever came first. The Cox model was adjusted for comorbidity indexes (ie, Pitt BSI and Charlson scores). The proportional hazards assumptions were tested using the Schoenfeld residuals test. Variable selection was performed using a purposeful selection method using variables depicted in Table 1 and collinearity was checked during the variable selection process. The residual variation due to hospital site was accounted by inclusion of the hospital site variable as a random effect (frailty) term in the fixed-effects model. A similar analysis was performed for patients having a bloodstream infection secondary to E faecium.
Table 1.
Characteristics of Patients With Bloodstream Infections due to Enterococci
| Variables | VSE(n = 176) | VRE(n = 56) | Total Population (N = 232) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, median (IQR) | 66 (57.4–74.60) | 59 (51.4–66.60) | 64 (53–71) | .011 |
| Sex, male | 107 (60.80) | 29 (51.79) | 136 (58.62) | |
| Current admission | ||||
| Intensive care unit admission | 41 (23.30) | 23 (41.07) | 64 (27.59) | .009 |
| Reason of admission—medical | 166 (94.32) | 51 (91.07) | 217 (93.53) | |
| Length of hospitalization, d, median (IQR)a | 13 (3–23) | 25 (13.1–36.9) | 14 (8–31.5) | <.001 |
| Medical history | ||||
| Baseline comorbidities | ||||
| Heart/cardiovascular diseaseb | 78 (44.32) | 19 (33.93) | 97 (41.81) | |
| Diabetes mellitus | 60 (34.09) | 21 (37.50) | 81 (34.91) | |
| Chronic obstructive pulmonary disease | 19 (10.80) | 4 (7.14) | 23 (9.91) | |
| Chronic kidney disease | 34 (19.32) | 11 (19.64) | 45 (19.40) | |
| Liver disease | 12 (6.82) | 6 (10.71) | 18 (7.76) | |
| Solid malignancy | 52 (29.55) | 7 (12.50) | 59 (25.43) | |
| Hematological malignancy | 57 (32.39) | 30 (53.57) | 87 (37.50) | .004 |
| Charlson Comorbidity Index, median (IQR) | 4 (3–5.5) | 4 (3–5) | 4 (3–6) | |
| Solid organ transplant | 4 (2.27) | 4 (7.14) | 8 (3.45) | .098 |
| Bone marrow transplant | 17 (9.66) | 14 (25) | 31 (13.36) | .003 |
| Immunosuppressive therapy | 70 (39.77) | 23 (41.07) | 93 (40.09) | |
| Cardiac device and cardiac valve | 26 (14.77) | 5 (8.93) | 31 (13.36) | |
| Hemodialysis | 26 (14.77) | 11 (19.64) | 37 (15.95) | |
| Previous hospitalization within 1 y | 121 (68.75) | 50 (89.29) | 171 (73.71) | .002 |
| Nursing home/long-term facility | 12 (6.82) | 4 (7.14) | 16 (6.90) | |
| Microbiological failurec | 27 (15.34) | 12 (21.43) | 39 (16.81) | |
| Recurrent BSId | 7 (3.98) | 8 (14.29) | 15 (6.47) | .005 |
| At the time of blood culture collection | ||||
| Recent surgical procedure | 11 (6.25) | 5 (8.93) | 16 (6.90) | |
| Steroid use | 21 (11.93) | 14 (25) | 35 (15.09) | .017 |
| Neutropenia, defined as <500 cells/µL | 42 (23.86) | 27 (48.21) | 69 (29.74) | .001 |
| Central line placement | 83 (47.16) | 44 (78.57) | 127 (54.74) | |
| Urinary catheter | 35 (19.89) | 18 (32.14) | 53 (22.84) | .057 |
| Mechanical ventilation | 16 (9.09) | 12 (21.43) | 28 (12.07) | .014 |
| Pitt bacteremia score ≥2 | 73 (41.48) | 23 (41.07) | 96 (41.38) | |
| Index BSI episode | ||||
| Polymicrobial BSIe | 43 (24.43) | 13 (23.21) | 56 (24.14) | |
| Enterococcus faecium | 36 (20.45) | 50 (89.29) | 86 (37.07) | <.001 |
| Enterococcus faecalis | 140 (79.55) | 6 (10.71) | 146 (62.93) | <.001 |
| Infectious diseases consult | 146 (82.95) | 50 (89.29) | 196 (84.48) | |
| Endocarditis | 15 (8.52) | 4 (7.14) | 19 (8.19) | |
| Subjects with echocardiogram | 87 (49.43) | 28 (50.00) | 115 (49.57) | |
| Both (transthoracic and transesophageal) | 1 (1.15) | 5 (17.86) | 6 (5.22) | .015 |
| Duration of anti-enterococcal therapy, d (days)f | 10 (7–15) | 12 (6–17.2) | 10 (6.75–15) | |
| Infection source | ||||
| Central line infection | 39 (22.16) | 17 (30.36) | 56 (24.14) | |
| Genitourinary | 25 (14.20) | 3 (5.36) | 28 (12.07) | .099 |
| Abdominal/gastrointestinal | 46 (26.14) | 11 (19.64) | 57 (24.57) | |
| Unknown/primary source | 63 (35.80) | 21 (37.50) | 84 (36.21) | |
| Wound/osteoarticular | 3 (1.70) | 4 (7.14) | 7 (3.02) | |
| Definitive antimicrobial therapyg | ||||
| Monotherapy | 110 (62.50) | 35 (62.50) | 145 (62.50) | |
| β-lactamsh | 47 (26.70) | 6 (10.71) | 53 (22.84) | .007 |
| Daptomycin | 30 (17.05) | 23 (41.07) | 53 (22.84) | <.001 |
| Daptomycin dose, mg/kg, median (IQR) | 8 (6–8) | 8 (8–10) | 8 (6–10) | .045 |
| Daptomycin ≥10 mg/kg | 6 (20) | 9 (39.13) | 15 (28.30) | |
| Vancomycin | 30 (17.05) | 2 (3.57) | 32 (13.79) | |
| Linezolid | 2 (1.14) | 4 (7.14) | 6 (2.59) | .031 |
| Tigecycline | 1 (0.57) | 0 | 1 (0.43) | |
| Combination therapy | 55 (31.25) | 14 (25) | 69 (29.74) | |
| Dual β-lactamsh | 15 (8.52) | 2 (3.57) | 17 (7.33) | |
| Gentamicin plus β-lactamsh | 9 (5.11) | 0 | 9 (3.88) | .084 |
| Vancomycin plus β-lactamsh | 9 (5.11) | 0 | 9 (3.88) | .05 |
| Daptomycin plus β-lactamsh | 8 (4.55) | 4 (7.14) | 12 (5.17) | |
| Daptomycin plus linezolid | 4 (2.27) | 4 (7.14) | 8 (3.45) | .098 |
| Otheri | 10 (5.68) | 4 (7.14) | 14 (6.03) | |
| Daptomycin dose, mg/kg, median (IQR) | 8 (8–10) | 8 (8–8) | 8 (8–10) | |
| Empirical therapyj | 138 (78.41) | 47 (83.93) | 185 (79.74) | |
| Vancomycin | 74 (42.05) | 18 (32.14) | 92 (39.66) | |
| β-lactamsh | 71 (40.34) | 19 (33.93) | 90 (38.79) | |
| Daptomycin | 27 (15.34) | 24 (42.86) | 51 (21.98) | <.001 |
| Linezolid | 23 (13.07) | 11 (19.64) | 34 (14.66) | |
| Tigecycline | 3 (1.70) | 4 (7.14) | 7 (3.02) | |
| Clinical outcomes | ||||
| In-hospital mortality | 22 (12.50) | 20 (35.71) | 42 (18.10) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BSI, bloodstream infection; IQR, interquartile range; VRE, vancomycin-resistant enterococci; VSE, vancomycin-susceptible enterococci.
Total days of hospitalization, including days before and after treatment of enterococcal bacteremia.
Categories of heart/cardiovascular diseases are not mutually exclusive. Conditions include ischemic heart disease, cerebrovascular disease, chronic heart failure, and peripheral vascular disease.
Microbiological failure was defined as lack of clearance of BSI after ≥4 days of the index blood culture, while receiving at least 48 hours of active antibiotic therapy.
Recurrent BSI was defined as the presence of a new positive enterococcal blood culture in a patient who had previous negative blood culture.
Polymicrobial BSI was defined as the presence of ≥1 bacterial species other than enterococci in the same blood culture.
Including empiric and definitive therapy.
Definitive enterococcal therapy was defined as a drug with in vitro activity against the enterococcal isolate recovered from the bloodstream of the individual (after release of antibiotic susceptibility results).
β-lactams include ampicillin, ampicillin-sulbactam, amoxicillin-clavulanic acid, ceftriaxone, or piperacillin-tazobactam.
Others include linezolid plus β-lactams (n = 6), daptomycin plus vancomycin (n = 2), tigecycline plus β-lactams (n = 2), gentamicin plus vancomycin (n = 1), daptomycin plus quinupristin-dalfopristin (n = 1), and daptomycin plus tigecycline (n = 2).
Defined as antibiotics given before antimicrobial susceptibility was available.
Statistical significance was set at 2-tailed 5% level (P < .05). Variables with >10% of missing data and/or a value ≤5 per category were excluded from the analysis. All analyses were performed using Stata 16.0 (StataCorp, College Station, Texas) and R version 3.6.0. We also performed an unadjusted desirability of outcome ranking (DOOR) [14, 15] analysis of the cumulative clinical events at 4, 7, 10, 12, and 15 days after the index culture. Analyses consisted of estimating the probability that a randomly selected patient with VRE vs VSE BSI had a more desirable DOOR, with a probability of 50% implying no difference between DOOR distributions of the groups (eg, VRE vs VSE). A probability of >50%—with a 95% bootstrap CI that excludes 50%—implies superiority of one group over the other. CIs were calculated using 5000 bootstrap resamples. Major clinical events included MF and/or recurrence of BSI. The best outcome was defined as being alive without MF and/or recurrence and the worst outcome was death. Thus, 3 levels were included: (1) alive, (2) MF and/or recurrence, and (3) death. We also performed a sensitivity analysis using an inverse probability weighing Cox analysis to evaluate the association of VRE with hospital mortality using the inverse of the propensity score as weights (Supplementary Table 1).
Genome Sequencing and Analyses
Extraction of genomic DNA, library preparation, genome sequencing (Illumina Platform), and initial analyses were performed as described previously [16–19] (see Supplementary Materials for details). Paired-end sequencing data and genome assemblies are available under National Center for Biotechnology Information BioProject PRJNA665052. Species information was determined using BLASTn searches against specific DNA sequences [20, 21] in a customized in silico polymerase chain reaction bioinformatics pipeline [22]. Multilocus sequence typing was performed (https://github.com/tseemann/mlst) by scanning contig files against the PubMLST database to determine sequence type. Resistance genes were identified from genome assemblies using previously defined approaches for E faecium and adjusting the genomic characterization for E faecalis (Supplementary Table 2 and Supplementary Materials) [23]. Separate midpoint-rooted maximum-likelihood phylogenetic trees based on core genome alignment were created for E faecalis and E faecium using RAxML [18] version 8.2.12 with 100 bootstrap iterations. Clade A and clade B reference genomes (AUS0004 and Com15, respectively) were included in the E faecium tree to aid in determination of cladal division. Trees were visualized using iTOL [19]. Determination of single-nucleotide polymorphisms (SNPs) and SNP threshold for E faecium clade A isolates are described in the Supplementary Materials.
Patient Consent Statement
The protocol of this study was approved by the local institutional review board of participating institutions, which waived the requirement for written or verbal consent from the patients based on the observational nature of the study.
RESULTS
Between September 2016 and March 2018, 291 patients were identified and 232 patients were included in the VENOUS I study (Supplementary Figure 1). Among 232 patients, the median age was 64 years (IQR, 53–71 years), and 59% were male. A detailed characterization of the overall cohort and a comparison between patients with VRE vs VSE BSIs are presented in Table 1. Fifty-six (24%) individuals were infected with VRE, whereas 176 (76%) patients had a VSE BSI. Subjects with VRE BSI were younger (59 vs 66 years, P = .011) and more frequently admitted to the intensive care unit than those with VSE infections (41% vs 23%, P = .009). VRE BSI was found more often in subjects with hematological malignancy (53% vs 32%, P = .004) or bone marrow transplant (25% vs 10%, P = .003). In addition, patients with VRE BSI infection were more likely to be neutropenic at the time of diagnosis, have a central line, and on mechanical ventilation, as compared to those with VSE BSI (48% vs 26%, 78% vs 47%, and 21% vs 9%, respectively). Of note, the length of hospital stay was longer in individuals with VRE BSI compared to VSE (25 vs 13 days, P < .001). The frequency of polymicrobial BSI (isolation of bacterial species other than enterococci in the same blood culture) and the Pitt BSI score did not differ between VRE and VSE BSIs (Table 1). As expected, E faecium accounted for most VRE isolates (89%), whereas E faecalis predominated among the cases of VSE (79%) (Table 1).
Daptomycin was the most common antibiotic used as monotherapy in subjects with VRE infections (median dose of 8 mg/kg/day [IQR, 8–10 mg/kg]). Of note, 2 (3%) patients with VRE infection were treated with vancomycin. One patient had a VSE isolate but was later confirmed to be vancomycin-resistant E faecium by whole genome sequencing. The second isolate was deemed a contaminant by the infectious disease team since the blood culture was obtained directly from a line (patient was only treated with vancomycin for 48 hours after culture collection). Forty-two (18%) patients died during the study period and 39 (17%) had MF. Of note, 15 (6%) patients had a recurrent episode of enterococcal BSI. Recurrence was more frequent in VRE compared to VSE BSIs (14% vs 4%; P = .005). The median follow-up duration was 10 days (IQR, 2–71 days), and the median survival time was estimated to be 45 days (IQR, 38–51 days) after the first positive blood culture. The univariable analysis showed that mechanical ventilation (HR, 3.15 [95% CI, 1.60–6.10]), a Pitt BSI score ≥2 (HR, 2.72 [1.52–5.14]), MF (HR, 2.34 [95% CI, 1.22–4.47]), intensive care unit stay (HR, 2.22 [95% CI, 1.20–4.09]), VRE BSI (HR, 2.21 [95% CI, 1.20–4.10]), central line placement (HR, 2.25 [95% CI, 1.09–4.61]), urinary catheter (HR, 2.17 [95% CI, 1.17–4.02]), and absolute neutrophil count (ANC) <500 cells/μL (HR, 2.14 [95% CI, 1.50–5.14]) were associated with an increased rate of in-hospital mortality (Table 2; see Supplementary Table 3 for additional variables).
Table 2.
Estimated Hazard Ratios of In-Hospital Mortality When Fitting a Univariable and Multivariate Cox Regression Model
| Variable | Unadjusted | Adjusted Conventionala,b | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| Intensive care unit admission | 2.22 | (1.20–4.09) | .012 | … | … | |
| Pitt bacteremia score ≥2 | 2.72 | (1.52–5.14) | .001 | 1.83 | (1.47–2.28) | <.001 |
| Neutropenia, defined as <500 cells/µL | 2.78 | (1.50–5.14) | .001 | 3.13 | (2.89–3.39) | <.001 |
| Central line placement | 2.25 | (1.09–4.61) | .028 | … | … | |
| Urinary catheter | 2.17 | (1.17–4.02) | .014 | 1.85 | (1.17–2.93) | .009 |
| Mechanical ventilation | 3.15 | (1.60–6.10) | .001 | … | … | |
| VRE BSI | 2.21 | (1.20–4.10) | .011 | 2.13 | (1.54–2.93) | <.001 |
| Microbiological failure | 2.34 | (1.22–4.47) | .01 | 2.4 | (1.34–4.31) | .003 |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; HR, hazard ratio; VRE, vancomycin-resistant enterococci.
Inclusion of variables in the adjusted model were determined through purposeful variable selection.
A hospital-specific random effect intercept was included in the model and was stratified by hospital unit of admission.
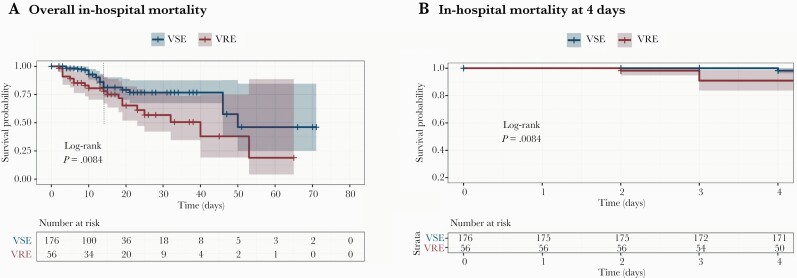
The difference in mortality between VRE vs VSE became apparent at day 2 of the BSI (Figure 1A and 1B). Of note, the survival curves of VRE vs VSE indicated that the effect in mortality was not uniform throughout the observation period (Figure 1, dashed line). While this effect did not violate the statistical test of the proportional hazards assumption, the Schoenfeld residuals were not linear (Supplementary Figure 2), so we chose to model it as a time-dependent variable to account for the nonuniform proportional hazard over time. In contrast, this situation was not observed with Pitt BSI score, neutrophil count, urinary catheter, or MF (Supplementary Figure 2A–E). The adjusted Cox analysis showed that an ANC <500 cells/µL, MF, VRE BSI, Pitt BSI score ≥2, and use of urinary catheter were associated with in-hospital mortality (Table 2). The interaction between VRE and MF on in-hospital mortality was not significant and was not included in the final model (Supplementary Table 4).
Figure 1.
Kaplan-Meier estimates. Survival curves of patients with enterococcal bloodstream infection. A, Overall in-hospital mortality; dotted line shows that the effect in mortality was not uniform throughout the observation period. B, Survival curve at day 2 of bacteremia. Curves are compared using the log-rank test and a value <.05 was considered significant. Shaded areas represent 95% confidence intervals. Abbreviations: VRE, vancomycin-resistant enterococci; VSE, vancomycin-susceptible enterococci.
Since our analyses suggested that the influence of VRE BSI on mortality changed during the course of the bloodstream episode, we evaluated the effect of VRE as a function of time at days 4, 7, 10, 12, and 15 after the first positive blood culture to test this hypothesis. The estimated HRs for these periods were 1.91, 1.68, 1.92, 2.48, and 7.02, respectively (Supplementary Table 5). Thus, these findings indicated that the highest impact on mortality of a VRE BSI was at day 15 (3.5-fold increase). We restricted the analysis by species, and under that scenario E faecium had a significantly higher impact on mortality compared to E faecalis (Supplementary Figure 3). Subsequently, we restricted the analysis to only E faecium BSIs because the small amount of vancomycin resistance in E faecalis (6 of 146; Table 1). Using this approach and the methods described above, MF was the strongest predictor of in-hospital mortality in the multivariate analysis for E faecium (HR, 5.03 [95% CI, 3.25–7.77]) (Supplementary Table 6). DOOR analyses confirmed that, compared to VSE, patients with a VRE infection were more likely to have a worse outcome at all tested time-points, including at day 15 (41% [95% CI, 34%–49%]) (Supplementary Table 7). We also performed a sensitivity analysis to examine the robustness of our results. Using inverse probability weighing with inverse propensity scores as the weights to account for covariates that predict the probability of having a VRE bloodstream infection, we confirmed that MF was the most consistent predictor of in-hospital mortality in our cohort and that the impact of VRE on mortality was not uniform throughout the observation period. However, the sensitivity analyses suggested that the impact of VRE on in-hospital mortality was statistically significant at day 7 of bacteremia and the effect continued to day 10 (Supplementary Table 8).
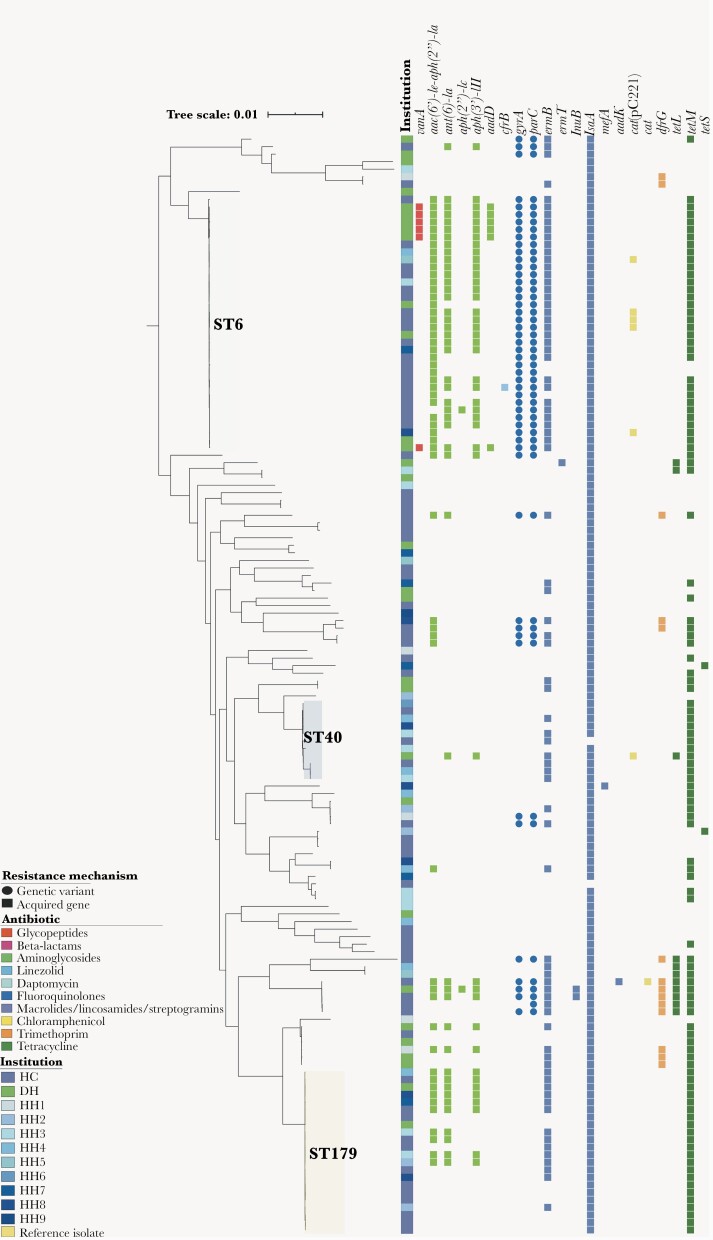
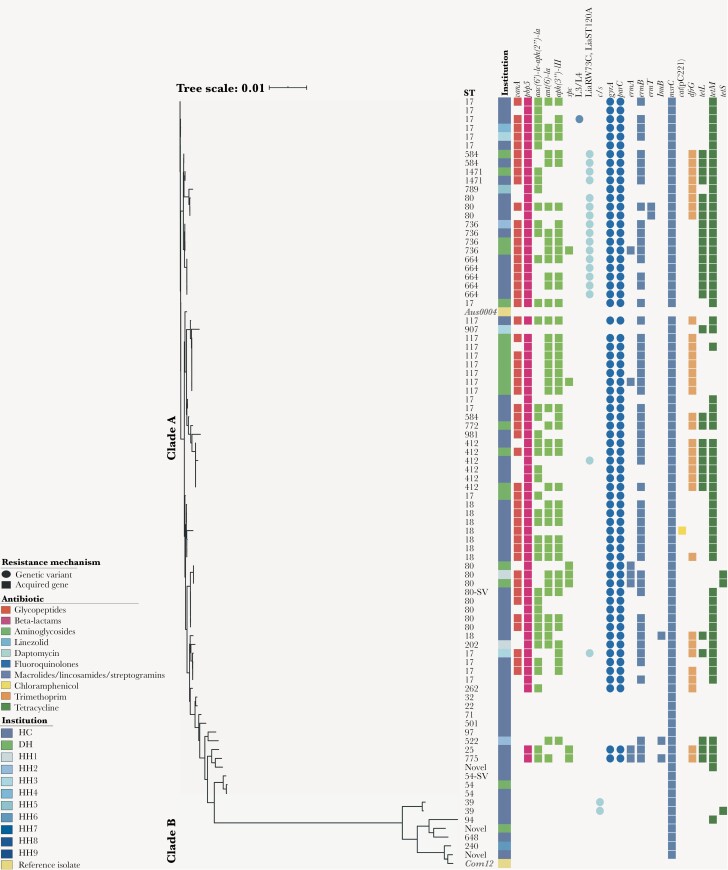
The most common sequence types among the 146 E faecalis isolates were ST6, ST179, and ST40 (Figure 2). Most of the vanA-containing E faecalis (6/146) isolates were ST6 and concentrated in a single institution, and 5 of 6 appeared to be highly related (Figure 2). Of note, all but one ST6 E faecalis harbored aac(6’)-Ie-aph(2”)-Ia conferring high-level resistance to gentamicin, and most were multidrug-resistant (MDR). No genetic evidence of resistance to ampicillin (genes coding for β-lactamase), linezolid (G2576 mutation on 23S ribosomal RNA), or daptomycin (mutations in liaFSR) was identified in E faecalis. The E faecium subset included 86 isolates representing 28 sequence types (Figure 3). Phylogenetic analysis showed the previously described split between clade A (hospital-adapted) and clade B (community-associated), with a variety of clade B isolates causing BSIs [24]. More than half of E faecium isolates (50 of 86 [58.1%]) harbored the vanA gene cluster and the majority of clade A isolates contained the “resistant” allele of pbp5, resulting in increased minimum inhibitory concentrations (MICs) of ampicillin. Interestingly, we identified 18 vancomycin-resistant E faecium strains that harbored the W73C and T120A substitutions in LiaR and LiaS, respectively, that had been previously associated with daptomycin resistance [25, 26]. These isolates belonged to ST584, ST1471, ST80, ST736, ST664, ST412, and ST17 (Figure 3). Two major institution-specific clade A E faecium clusters (≥5 isolates differing by <20 SNPs) were identified based on SNP distances (Supplementary Figure 4) at a Houston cancer center (n = 5) and Detroit hospital (n = 7), indicating potential sharing of clonally related isolates between patients at these locations.
Figure 2.
Phylogenetic and resistome analyses of Enterococcus faecalis from the Vancomycin-Resistant Enterococcal BSI Outcomes Study (VENOUS I). Enterococcus faecalis core gene-aligned, midpoint-rooted maximum likelihood phylogenetic tree with associated antibiotic resistance mechanisms (n = 146). The presence of a resistance mechanism is denoted by a colored circle (genetic variant) or square (acquired resistance gene), with colors representing the class or type of antibiotic resistance conferred. Gene abbreviations and descriptions can be found in Supplementary Table 2. Abbreviations: DH, Detroit hospital; HC, Houston cancer center; HH, Houston hospitals 1–9; ST, sequence type.
Figure 3.
Phylogenetic and resistome analyses of Enterococcus faecium from the Vancomycin-Resistant Enterococcal BSI Outcomes Study (VENOUS I). Enterococcus faecium core gene-aligned, midpoint-rooted, maximum likelihood phylogenetic tree with associated antibiotic resistance mechanisms (n = 86) and cladal reference isolates. The presence of a resistance mechanism is denoted by a colored circle (genetic variant) or square (acquired resistance gene), with colors representing the class or type of antibiotic resistance conferred. Gene abbreviations and descriptions can be found in Supplementary Table 2. Abbreviations: DH, Detroit hospital; HC, Houston cancer center; HH, Houston hospitals 1–9; ST, sequence type; ST-SV, single allelic variant of the respective sequence type.
DISCUSSION
In this prospective, multicenter, observational cohort study that spanned 2 years in multiple US hospitals, we show that the most consistent factor impacting mortality in patients with enterococcal BSI is failure to eradicate the organism from the bloodstream. Indeed, MF remained the only factor affecting mortality when analyzing patients infected with vancomycin-resistant vs vancomycin-susceptible E faecium, the most recalcitrant and difficult-to-treat species. Our findings are unique when compared to previous studies, which associated only vancomycin resistance with increased risk of mortality regardless of species [3, 10, 11, 27, 28]. This discrepancy is likely due to the fact that previous studies are retrospective and lack microbiological assessments, since follow-up blood cultures were not considered or available. Moreover, the definition of mortality applied by several of these studies encompasses a broader period of observation (ie, 30-day mortality) rather than considering a clinically relevant time window throughout the duration of the BSI.
To dissect the temporal impact of vancomycin resistance on mortality, we used a time-covariate analysis in our cohort and showed that the influence of vancomycin resistance on mortality varied over the course of the disease. Furthermore, a DOOR ranking outcome that included MF and/or recurrence permitted comparison of the longitudinal effects of vancomycin resistance on the overall clinical outcome. These novel findings suggest that interventions targeted to early eradication of the organisms in the bloodstream should be a priority in patients with VRE BSI and that such benchmark should be included as a major outcome when designing interventional trials. Since there is an important degree of uncertainty on what is the best therapy for MDR enterococci (particularly ampicillin- and vancomycin-resistant E faecium), efforts to optimize therapies for these organisms are urgently needed.
Our comprehensive genomic analyses yielded several conclusions. First, the proportion of E faecium carrying vanA was lower than previously described (58%), and there was a marked heterogeneity of genetic lineages of E faecium causing invasive disease (rather than outbreaks). Nonetheless, some clustering of isolates was found [5, 29, 30]. Second, we found genetically related E faecium isolates harboring mutations associated with daptomycin resistance. Since daptomycin has become the front-line antibiotic against VRE infection, the possible dissemination of these linages is a cause of major concern, particularly with the current uncertainties in MIC determination [31]. Third, resistance to linezolid, the only US Food and Drug Administration–approved antibiotic for VRE, was uncommon both in E faecalis and E faecium in this cohort. Notably, the genotypic prediction of daptomycin and linezolid resistance remains to be confirmed by phenotypic tests since it was not performed as a routine test. Finally, MDR E faecalis ST6 is the predominant genetic lineage causing invasive disease in the participating centers, most of them carrying resistance determinants to aminoglycosides and compromising the use of gentamicin for deep-seated infections due to E faecalis.
Several limitations need to be discussed. First, the participant hospitals are located in the US (2 cities), and our findings may not be generalizable. Nonetheless, VRE are serious public health threats in the US and the hospitals had a broad representation of patients who are at risk of acquiring VRE infections, namely, critically ill, cancer, and immunocompromised patients [8]. Indeed, VRE tend to affect the same types of patients regardless of geographical location, and we believe our cohort is representative of the typical patients who develop enterococcal BSI. Second, since the study was exempt from informed consent, the only data available were those in the electronic medical record, and it is plausible that some data might have been missed. For example, subsequent blood cultures beyond 7 days after index culture were at the discretion of the treating physician, which may affect the determination of recurrence. Thus, we could not include this variable in the final mortality analyses due to this inconsistency in the cohort. Third, due to the heterogeneity of treatment approaches, lack of information related with infection source control, and low numbers, analyses to evaluate efficacy of antibiotics on MF and mortality were not possible. The VENOUS cohort is being expanded and we expect that, as the sample size increases, we will be able to make more meaningful therapeutic comparisons. Fourth, our study population was heterogeneous, and the observed impact of VRE on mortality might be due to the presence of special immunocompromised populations. Therefore, the weight of VRE on mortality might also differ among patient populations. Finally, the levels of mortality and DOOR analyses were arbitrarily chosen, but we believe they reflect “real-life” events of clinical relevance.
In conclusion, in this unique prospective study of patients with VRE and non-VRE BSI, MF was the most consistent factor affecting poor outcomes. We found a temporal association of VRE in outcomes, suggesting that early effective interventions are critical to improve the outcomes of these vulnerable patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. G. A. C., J. M. M., S. S., A. Q. D., T. T. T., B. M. H., C. A. A.: Substantial contribution to the financial support, conception, design, acquisition, analysis and interpretation of data, drafting the work, and critical revision for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. J. M. M., S. S., C. P.: Substantial contribution to the conception, statistical analysis, and interpretation of data; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. C. L., D. v. D.: Substantial contribution to the analysis and interpretation of data; final approval of the version to be published. K. R., G. S.-P., P. V. S.: Substantial contribution to the design and acquisition data. R. R., L. D.: Substantial contribution to the conception and analysis and interpretation of data; final approval of the version to be published. K. R., M. Z., C. L., Y. D., L. M. A., L. S., H. S., C. G., F. B., S. L. A., S. A. S.: Drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award numbers K24AI121296, R01AI134637, R01AI48342, and P01AI152999 to C. A. A.) and the University of Texas (Science and Technology Acquisition and Retention [STAR] Award to C. A. A.). J. M. M. was partially funded with an Early Stage Investigator Award from the Antimicrobial Resistance Leadership Group (NIAID grant number UM1AI104681 to Vance Fowler Jr) and by the Millennium Science Initiative, MICROB-R, NCN17_081 and FONDECYT regular 1211947 from the government of Chile. S. R. S. was partially funded under an NIH predoctoral T32 training grant (number 5T32AI055449-15 to Theresa M. Koehler). B. M. H. was partially funded by the NIAID, NIH (award number K01AI148593). C. L. was funded under an NIH predoctoral T32 training grant (number T32GM086330). C. P. was partially funded by the NIH (grant number R01AI134637). C.G. was partially funded by CIBERINFEC (CB21/13/00009), Instituto de Salut Carlos III, Madrid Spain.
Potential conflicts of interest. C. A. A. has received grant support from Merck, MeMed Diagnostics, and Entasis Therapeutics. D. v. D. has served on advisory boards for Allergan, Achaogen, Qpex, Shionogi, Tetraphase, Sanofi-Pasteur, T2 Biosystems, NeuMedicine, Roche, MedImmune, Astellas, Pfizer, and Merck. Y. D. has received grant support from Janssen, Pfizer, MSD, Shionogi, served on advisory boards for Janssen, Gilead, bioMérieux, and received speaking fee from AstraZeneca. J. M. M. has received unrestricted research grants from Pfizer, MSD and bioMérieux. H. S. reports research grants from the German Research Foundation, the German Centre for Infection Research, Accelerate, and Entasis, and personal fees from Basilea, Entasis, Gilead, MSD, and Shionogi. C. G. has received grant support from Pfizer and Merck. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Weber S, Hogardt M, Reinheimer C, et al. . Bloodstream infections with vancomycin-resistant enterococci are associated with a decreased survival in patients with hematological diseases. Ann Hematol 2019; 98:763–73. [DOI] [PubMed] [Google Scholar]
- 2. Kramer TS, Remschmidt C, Werner S, et al. . The importance of adjusting for Enterococcus species when assessing the burden of vancomycin resistance: a cohort study including over 1000 cases of enterococcal bloodstream infections. Antimicrob Resist Infect Control 2018; 7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papanicolaou GA, Ustun C, Young JH, et al. . Bloodstream infection due to vancomycin-resistant Enterococcus is associated with increased mortality after hematopoietic cell transplantation for acute leukemia and myelodysplastic syndrome: a multicenter, retrospective cohort study. Clin Infect Dis 2019; 69:1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiner LM, Webb AK, Limbago B, et al. . Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panesso D, Reyes J, Rincon S, et al. . Molecular epidemiology of vancomycin-resistant Enterococcus faecium: a prospective, multicenter study in South American hospitals. J Clin Microbiol 2010; 48:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vehreschild M, Haverkamp M, Biehl LM, Lemmen S, Fatkenheuer G.. Vancomycin-resistant enterococci (VRE): a reason to isolate? Infection 2019; 47:7–11. [DOI] [PubMed] [Google Scholar]
- 7. European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. Solna, Sweden: ECDC; 2018. [Google Scholar]
- 8. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: CDC; 2019. [Google Scholar]
- 9. Boucher HW, Talbot GH, Bradley JS, et al. . Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1–12. [DOI] [PubMed] [Google Scholar]
- 10. Prematunge C, MacDougall C, Johnstone J, et al. . VRE and VSE bacteremia outcomes in the era of effective VRE therapy: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2016; 37:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hefazi M, Damlaj M, Alkhateeb HB, et al. . Vancomycin-resistant Enterococcus colonization and bloodstream infection: prevalence, risk factors, and the impact on early outcomes after allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. Transpl Infect Dis 2016; 18:913–20. [DOI] [PubMed] [Google Scholar]
- 12. Henderson H, Luterbach CL, Cober E, et al. . The Pitt bacteremia score predicts mortality in nonbacteremic infections. Clin Infect Dis 2020; 70:1826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shukla BS, Shelburne S, Reyes K, et al. . Influence of minimum inhibitory concentration in clinical outcomes of Enterococcus faecium bacteremia treated with daptomycin: is it time to change the breakpoint? Clin Infect Dis 2016; 62:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evans SR, Rubin D, Follmann D, et al. . Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 2015; 61:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Duin D, Lok JJ, Earley M, et al. . Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolger AM, Lohse M, Usadel B.. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014; 30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bankevich A, Nurk S, Antipov D, et al. . SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Letunic I, Bork P.. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 2019; 47:W256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dutka-Malen S, Evers S, Courvalin P.. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol 1995; 33:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dutka-Malen S, Evers S, Courvalin P.. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol 1995; 33:24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Github. EnterococcusSppPCR. 2021. https://github.com/rriosn/EnterococcusSppPCR/. Accessed 26 October 2021.
- 23. Rios R, Reyes J, Carvajal LP, et al. . Genomic epidemiology of vancomycin-resistant Enterococcus faecium (VREfm) in Latin America: revisiting the global VRE population structure. Sci Rep 2020; 10:5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lebreton F, van Schaik W, McGuire AM, et al. . Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. mBio 2013; 4:e00534-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller WR, Tran TT, Diaz L, et al. . LiaR-independent pathways to daptomycin resistance in Enterococcus faecalis reveal a multilayer defense against cell envelope antibiotics. Mol Microbiol 2019; 111:811–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davlieva M, Wu C, Zhou Y, Arias CA, Shamoo Y.. Two mutations commonly associated with daptomycin resistance in Enterococcus faecium LiaS(T120A) and LiaR(W73C) appear to function epistatically in LiaFSR signaling. Biochemistry 2018; 57:6797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacAllister TJ, Stohs E, Liu C, et al. . 10-year trends in vancomycin-resistant enterococci among allogeneic hematopoietic cell transplant recipients. J Infect 2018; 77:38–46. [DOI] [PubMed] [Google Scholar]
- 28. Ye JJ, Shie SS, Cheng CW, et al. . Clinical characteristics and treatment outcomes of vancomycin-resistant Enterococcus faecium bacteremia. J Microbiol Immunol Infect 2018; 51:705–16. [DOI] [PubMed] [Google Scholar]
- 29. Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN.. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 2007; 58:163–70. [DOI] [PubMed] [Google Scholar]
- 30. Corso AC, Gagetti PS, Rodriguez MM, et al. . Molecular epidemiology of vancomycin-resistant Enterococcus faecium in Argentina. Int J Infect Dis 2007; 11:69–75. [DOI] [PubMed] [Google Scholar]
- 31. Campeau SA, Schuetz AN, Kohner P, et al. . Variability of daptomycin MIC values for Enterococcus faecium when measured by reference broth microdilution and gradient diffusion tests. Antimicrob Agents Chemother 2018; 62:e00745-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.