Abstract
Melanocytic nevi, dysplastic nevi, and melanoma are all derived from the pigment-producing cells, namely melanocytes. Concerning the clinical spectrum, cutaneous melanoma is the most aggressive skin cancer with a low survival rate, while nevi are the most common benign lesions in the general population, and dysplastic nevi place in between nevi and melanoma. Ultraviolet (UV) radiation is a well-recognized extrinsic risk factor for all three. BRAFV600E is a well-recognized driver mutation that activates the RAS-BRAF-mitogen-activated protein kinase (MAPK) signaling pathway among 40%–60% of melanoma cases. Interestingly, BRAFV600E mutation is detected even more in acquired nevi, approximately 80%. However, in nevi, several tumor suppressors such as p53 and phosphatase and tensin homolog (PTEN) are intact, and senescence factors, including p15INK4b, p16INK4a, p19, and senescence-associated acidic β-galactosidase, are expressed, leading to cell senescence and cell cycle arrest. Although loss of p53 function is rarely found in melanoma, decreased or loss of PTEN with an activated PI3k/Akt signaling pathway is common in nevi, which may abolish senescence status and allow further progression into dysplastic nevi or melanoma. At present, mouse models closely resembling human nevi are used for investigating these phenomena. Melanocortin 1 receptor deficiency, an intrinsic risk factor for melanomagenesis, is related to the production of procarcinogenic pheomelanin and the inhibition of PTEN function. Immune response escape via programmed cell death-1/programmed cell death ligand-1 interaction plays further roles in monitoring the spectrum. Here, we review the current literature on the molecular and immune mechanisms involving the transition from benign nevi to malignant melanoma.
KEYWORDS: Dysplastic nevi, Melanocortin 1 receptor, Melanoma, Nevi, Programmed cell death-1/programmed cell death ligand-1
INTRODUCTION
Melanocytes are neural crest-derived melanin-producing cells, which are located in several organs, including the skin, inner ears, eye, bone, and leptomeninges. Cutaneous melanoma (the most malignant skin cancer), melanocytic nevi (the most common benign skin tumor), and dysplastic nevi (in between nevi and melanoma) all result from melanocytes and share similar features including risk factors such as ultraviolet (UV) exposure and melanocortin 1 receptor (MC1R) deficiency, phenotypes on skin, and common gene mutations. Here, we introduce the clinical features of melanoma, nevi, and dysplastic nevi and review the molecular mechanisms and immune status which play critical roles in the transition from benign nevi to malignant melanoma.
CLINICAL CLASSIFICATION AND CHARACTERISTIC OF MELANOMAS
According to the WHO Classification of Skin Tumors (2018), melanomas are classified into cutaneous, mucosal, and uveal melanomas, based on the body part of origin [1]. Based on clinical morphology, cutaneous melanoma may be categorized as superficially spreading melanoma that grows slowly through outer skin layers into deeper layers; nodular melanoma which is extremely aggressive and grows quickly in the form of a blue or black lump; and lentigo maligna melanoma considered as in situ or stage 0 melanoma which appears as flat, brown patches on the skin and is the most treatable subtype. Based on the degree of cumulative solar damage (CSD) determined via histopathology, melanomas are also divided into low-CSD superficial spreading melanoma, high-CSD lentigo maligna melanoma, and desmoplastic melanoma. Melanomas not associated with CSD include Spitz, acral, mucosal, and uveal melanoma and those arising from congenital or blue nevi. Acral lentiginous melanoma is a specific type that appears on the palms, soles, and under nails, which is the most common type in Taiwan and Asian countries [2].
The percent 5-year survival rate for cutaneous malignant melanoma in Taiwan approximates 39%–46%, where low prognostic factors included distance metastasis, older age, male gender, and Breslow thickness exceeding 4 mm [2]. Melanomas exhibit symptoms such as Asymmetry, Border irregularity, Color variation, Diameter greater than 6 mm, and Evolving (a new or changing lesion), that are identified via “ABCDE” rules [3,4,5]. Melanomas are categorized into four prognostic stages based on tumor, node, and metastasis, including “Stage I” for low-risk primary melanoma, “Stage II” indicating high risk for recurrence, “Stage III” involving metastases into regional lymph nodes, and “Stage IV” involving distance metastasis [6]. Early diagnosis increases the survival rate of melanoma.
ENVIRONMENTAL FACTOR (ULTRAVIOLET EXPOSURE) AND GENETIC FACTOR (MELANOCORTIN 1 RECEPTOR DEFICIENCY) IN MELANOMAs
UV exposure is an environmental risk factor for melanoma. UV is divided into UVA (320–400 nm), UVB (280–320 nm), and UVC (<280 nm) depending on wavelength. Among these, UVC is almost completely absorbed by the ozonosphere. Protection of human skin from UV is based on epidermal melanocyte units, each of which is a functional unit composed of one melanocyte and 36 keratinocytes. Melanocytes contain dendrites that deliver melanosomes to keratinocytes within the unit. UVB is mostly absorbed by the epidermis, whereas UVA is mostly absorbed by the dermis. DNA is the main target of UVB- and UVA-induced skin carcinogenesis.
UVB exerts obvious effects on the epidermal layer of the skin, including burning and skin cancer. It also emits the UV wavelength that is most effective in inducing DNA photoproducts. The three major types of photoproducts are cyclobutane pyrimidine dimers, pyrimidine-pyrimidone 6–4 photoproducts (6–4 PPs), and Dewar valence isomers (DEWs), which act as premutagens that disrupt the nucleotide excision repair (NER) system. Genes significantly involved in melanomagenesis include phosphatase and tensin homologs (PTEN), CDKN2A and BRAF, as well as those associated with proliferation-related pathways such as NRAS, KIT, and NF1 [7]. BRAFV600E is the most recognized driver of mutation.
UVA reaches deep into skin causing photoaging and wrinkling. UVA is mainly absorbed by chromophores and triggers mutagenic oxidative reactions. While energy is directly transferred to DNA in type I reactions, in type II reactions, energy is transferred to molecular oxygen and then to reactive oxygen species, which damage DNA. Mutagenic 7,8-dihydro-8-oxoguanosine (8-oxoG) is an oxidative DNA lesion caused by type II reactions, which are repaired by the base excision repair (BER) mechanism. Mutated genes cause abnormal cell proliferation and differentiation as a part of the carcinogenesis process.
UV-induced tanning is dependent on p53, which stimulates transcriptional upregulation of proopiomelanocortin, which encodes α-melanocyte-stimulating hormone (α-MSH). Secreted α-MSH binds to the MC1R on melanocytes, thus producing eumelanin, which is transported back to keratinocytes as part of the protective tanning response to UV radiation [8]. MC1R, a member of G protein-coupled receptor family, is expressed in melanocytes and leukocytes, thereby activating UV protection and the anti-inflammation signaling pathway, respectively.
There are two aspects to the relationship between MC1R and melanomagenesis: pheomelanin/eumelanin ratio and PTEN/Akt pathway activation. MC1R shows polymorphism in the human population, and this mutation, which results in receptor inactivation, is caused by the substitution of cysteine residues with glycine or alanine [9]. MC1R loss of function results in synthesis of procarcinogenic pheomelanin rather than UV protective eumelanin. Populations with MC1R deficits present with fair skin, red hair, and increased risk of melanoma and non-melanoma skin cancers due to UV induced free radical production or PI3K/Akt signaling pathwat hyperactivation [10,11].
PTEN is protected from proteasomal degradation by activated MC1R in melanocytes, which inactivates Akt and suppresses melanomagenesis [10]. This explains individuals carrying the MC1R mutation exhibiting a higher risk for nevi-derived melanoma due to BRAF-activated mutation as well as PTEN degradation. MC1R variation was strongly associated with BRAF mutation, suggesting that MC1R mutation may play a critical role in the development of melanoma via UV exposure [12,13].
THE ROLE OF BRAFV600E MUTATION IN MELANOMAGENESIS: THE INITIATION STAGE
BRAF mutation is carried by 40%–60% of melanoma cases [14]. Mutated BRAF protein increases kinase activity and NIH-3T3 cell transformation [15]. BRAF, which is at chromosome 7q34 locus, encodes BRAF, a Ras-regulated serine/threonine protein kinase. Binding of extracellular growth factor to receptor tyrosine kinase (RTK) activates BRAF, which subsequently triggers the RAS-BRAF-MAPK/extracellular signal-regulated kinase (ERK) signaling pathway, leading to cell growth, proliferation, anti-apoptosis, and angiogenesis, among other processes [16,17].
Approximately 90% of BRAF mutations in non-CSD melanomas result from substitution of T by A at the 1799th nucleotide in exon 15, giving rise to valine (V) → glutamic acid (E) mutation at the 600th amino acid of BRAF. This causes an acidic change in the BRAF kinase domain, thereby increasing its kinase activity compared with wild-type BRAF, by mimicking phosphorylation in the activation segment [15,18]. Thus, the gain-of function mutation, BRAFV600E, plays a critical role in tumorigenesis and is detected in several types of cancer cells including those of colorectal cancers, gliomas, lung cancers, sarcomas, ovarian carcinomas, breast cancers, and liver cancers. The high frequency of BRAFV600E seen in melanoma cells also indicated that activation of downstream molecules such as ERK may play an important role in melanomagenesis. Apart from BRAFV600E, other BRAF mutations, such as BRAFG463V, BRAFG468A, and BRAFL696V, which also activate downstream ERK, have been reported in some melanoma cases [15].
Inhibition of the BRAF pathway provides a strategy for melanoma therapy. Two BRAF inhibitors, vemurafenib and dabrafenib, as well as an MEK inhibitor, trametinib, were approved as drugs for melanoma treatment by the Food and Drug Administration in 2012 and 2013, respectively [19,20]. However, manifestation of several side effects of vemurafenib, such as rashes, photosensitivity, photoaging, actinic keratosis, basal cell carcinoma, or cutaneous squamous cell carcinoma, due to loss of function of mismatch repair, BER or NER, especially under UVA exposure following vemurafenib treatment, have been reported [21]. Furthermore, UVB exposure following vemurafenib treatment causes DNA damage such as the formation of pyrimidine dimers [22].
THE ROLE OF P53 AND PHOSPHATASE AND TENSIN HOMOLOGS MUTATION IN MELANOMAGENESIS: THE ADVANCED STAGE
As opposed to that of BRAF, mutations of p53 and PTEN both arise later in primary melanoma [23,24]. Interestingly, p53 is not commonly associated with melanoma development. Evidently, 80%–95% of melanoma cases carry wild-type p53, which acts as a tumor suppressor leading to apoptosis, cell cycle arrest, and cell senescence. However, inactivation of other tumor-suppressor genes such as p16INK4A or p14ARF, leads to low ratio, unnecessary p53 mutations in melanoma [25]. The p53/retinoblastoma protein (pRB) apoptosis pathway is frequently downregulated in melanoma cases [26]. Although mechanisms underlying the inactivation of wild-type p53 is poorly understood, the expression levels of MDMx, TA-p63, and/or ΔNp63 isoform and iASPP, which inhibit p53 transactivation activity, leading to apoptosis, were enhanced in melanoma cases carrying wild type p53 [27]. Moreover, loss of expression of p53 apoptosis targets, APAF-1 and PUMA, was also detected in melanoma [28]. Restoration of p53, via the inhibition of MDM2 or iASPP phosphorylation, reduced melanoma progression as well as metastasis, and increased survival in mouse models. This may provide a further avenue for developing new melanoma therapies [29].
In contrast to low-frequency p53 mutations, PTEN mutations play an important role in melanoma as well as in most human cancers. It is a later-stage factor than the BRAF mutation [30]. In 1997, it was first reported that approximately 43% of melanoma cell lines harbored the PTEN mutation [31], indicating that PTEN alterations contributed to the tumorigenesis of melanoma. PTEN, which is located on chromosome 10q, is also known as a tumor-suppressor gene that causes a high-frequency loss of heterozygosity in dysplastic lesions and several cancer types including melanoma cases which also carried the BRAFV600E mutation. Of the lipid and protein phosphatase functions of PTEN, lipid phosphatase plays a more pivotal role in tumorigenesis by reducing PIP3 levels and downstream Akt activity, leading to cell cycle arrest at the G1/S phase by upregulating the cyclin-dependent kinase inhibitor, p27. In addition, stimulation by PTEN induces apoptosis via the upregulation of caspase and BID and the downregulation of anti-apoptosis factors, such as Bcl2. Overexpression of PTEN inhibited colony formation in melanoma, indicating the tumor-suppressor role played by PTEN [32].
THE CLINICAL AND MOLECULAR CHARACTERISTICS OF NEVI AND DYSPLASTIC NEVI
Similar to melanoma, melanocytic nevi also originate via melanocyte clonal proliferation. However, nevi are benign and form nest-like clusters within the lower epidermis, dermis, or both. These are known as junctional, intradermal, or compound nevi, respectively. Moreover, nevi may also be classified into congenital nevi, acquired nevi, blue nevi, and Spitz nevi, according to clinical and histologic characteristics. Congenital melanocytic nevi and blue nevi frequently harbor NRAS mutations and GNAQ mutations, respectively, while Spitz and atypical Spitz tumors often exhibit HRAS and kinase rearrangements [33]. Compared with congenital nevi that form at birth, the majority are acquired nevi, which usually form within the first 20 years of life. Acquired nevi are very common among people of European ancestry and less common among people of Asian or African heritage [34]. In regard to dermoscopic features, benign nevi exhibit regular shape, uniformity, aggregated morphology, and homogenous brown-to-black pigmentation [35]. Although both originate via melanocyte proliferation, nevus cells remain in senescence, as opposed to malignant melanoma [7]. Common and atypical nevi decrease significantly with age [36]. However, approximately 30% of nevi associated with BRAF or NRAS mutations are at risk for transforming into melanoma [37]. The exact mechanisms leading to the development of eruptive melanocytic nevi are unknown. UVB exposure or sunburn, light skin or hair color, inherited traits with a family history of skin cancer [33] or patients receiving bone marrow transplantation or immunosuppressive medications have been reported with increased risk of nevogenesis [34,35,36].
Approximately 80% of nevi harbor BRAF mutations and the BRAF-driven RAS-RAF-MAPK/ERK signaling pathway plays a pivotal role in triggering both benign nevogenesis and malignant melanomagenesis [33]. Although BRAFV600E mutation is found in most nevi, several senescence markers or tumor suppressors, such as p53, p15INK4b, p16INK4a, and p19, as well as senescence-associated acidic β-galactosidase are also present, resulting in cell cycle arrest at the G0/G1 phase in nevi cells, regardless of the BRAFV600E mutation [28,38]. However, one-third of melanomas are derived from nevi, implying that nevi, considered as melanocytes during the senescence stage, may be transformed to melanoma due to subsequent abnormalities in other genes, such as UV-induced PTEN, MC1R, or p53 mutations, or immunosuppression [39,40]. A small portion of nevi possesses the ability to proliferate and retain mitosis occasionally [7]. UVB exposure may enhance the expression of several proliferation markers, such as HMB-45, PCNA, Ki67, and topoisomerase IIα [39], and UVB may activate nevi in senescence stage, resulting in transformation to melanoma [4].
In addition to common nevi, dysplastic nevi, also known as atypical moles, were defined as intermediates between acquired nevi and melanoma cells. Clinical characteristics of dysplastic nevi include irregular shape, color variation, and a diameter >5 mm. Dysplastic nevi exhibit a 2–12-fold risk of transforming into melanoma [41,42]. Dysplastic nevi were found in 34%–59% of patients with a history of melanoma [43,44]. Due to the overlap between features of common nevi and melanoma, dysplastic nevi lack predictive markers and are difficult to diagnose [45].
Mechanistically, approximately 58% of dysplastic nevi carry the BRAF mutation [33]. However, compared with only one BRAFV600E mutation in benign nevi, dysplastic nevi exhibit other mutations, such as the TERT promoter mutation, hemizygous substitution of CDKN2A, and other mutations leading to MAPK activation [7]. NRAS and BRAFnon-V600E mutations have also been detected in a few dysplastic nevi cases. Moreover, the PI3K/Akt pathway, when activated by either decreased PTEN or increased AKT3 expression, altered the senescence state of nevi, enabling further transformation into malignant melanoma. Suppression of the PI3K/Akt pathway inhibited tumor proliferation and also induced expression of the tumor suppressor, p15INK4b. In addition, increased PI3K/Akt activity was found in 17% of benign nevi, 43% of dysplastic nevi, 49% of primary melanoma, and 77% of metastatic melanoma [46,47]. This indicated that mutation of PTEN may play a crucial role in benign nevi escaping senescence and transforming into melanoma. However, in spite of being considered an intermediate between benign nevi and unequivocal melanoma, dysplastic nevi are also classified as mild, moderate, or severe, according to the grade as well as clinical and histological features, but show less association with melanoma risk, leading to controversy.
THE IMMUNE STATUS IN MELANOMA AND NEVI
During melanoma progression, the tumor microenvironment interacts with melanoma cells including fibroblasts and endothelial cells, as well as immune cells such as dendritic cells, T cells, and monocytes, and also with the extracellular matrix [48]. Melanoma cells are able to avoid immune response via the programmed cell death-1/programmed cell death ligand-1 (PD-1/PD-L1) pathway. PD-1 is highly expressed on activated T cell surfaces for purposes of immune response, leading to T cell proliferation and activation. Activated T cells and tumor-infiltrating lymphocytes are blocked by the binding of PD-1 with PD-L1 induced by tumor cells, which act as a negative factor for immunomodulation [49]. Hence, strategies targeting the PD-1/PD-L1 pathway (anti-PD-1 or anti-PD-L1) or other immune-checkpoints, such as CTLA4, have been developed for cancer therapy [50,51]. However, the effects of anti-PD-1 or PD-1 inhibitors on melanoma patients were weak. In 2018, Chen et al. reported that melanoma secretes exosomes carrying PD-L1 on their surface, which contribute to immunosuppression by inhibiting CD8+ T cell proliferation. The amount of circulating exosomal PD-L1 was positively correlated with T helper type 1 (IFN-γ) in metastatic melanoma cases. This indicated that exosomal PD-L1 may act as a predictor of melanoma, for purposes of immune therapy [52]. Accordingly, we hypothesized that immune status may play a role in the transformation of nevi to dysplastic nevi to melanoma. However, studies directed at this aspect are still lacking.
The immune microenvironment of dysplastic nevi displayed strong expression of IFN-γ, T helper type 2 (IL3) cytokines, oncostatin M, and CXCL1, compared with common nevi. Dual specificity protein phosphatase 3, which negatively regulates members of the MAPK subfamily, such as MEK or ERK, was also identified as a distinct factor associated with senescence between dysplastic nevi and common melanocytic nevi [53]. Dendritic cells act as antigen-presenting cells that participate in immune surveillance during tumorigenesis. Large numbers of CD1c+ dendritic cells were detected in dysplastic nevi specimens, compared with both invasive and primary melanomas, indicating that CD1c+ dendritic cells showed potential for participating in melanomagenesis [54].
CONCLUSION AND FUTURE PERSPECTIVES
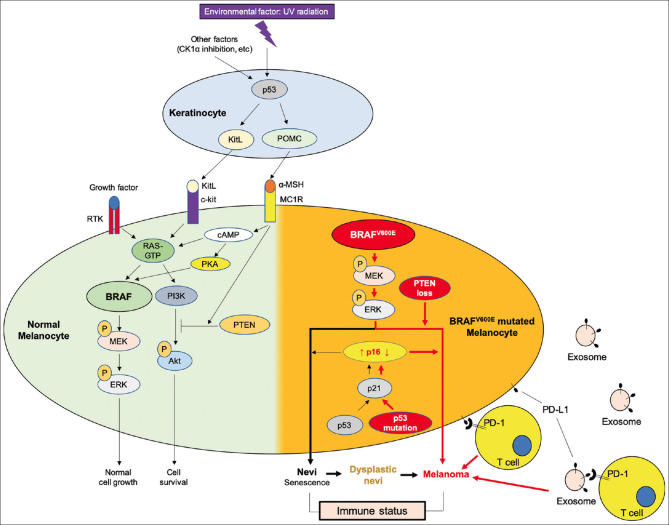
Melanoma, dysplastic nevi, and nevi commonly share the BRAFV600E mutation that triggers the MEK/MAPK pathway, leading to cell proliferation. The activation of RTK or kit ligand (KitL)/c-kit is insufficient for melanoma tumorigenesis due to the expression of several senescence makers, unless other additional mutations, such as PTEN loss, p53 mutation, or downregulation of p16, occur [Figure 1].
Figure 1.
Schematic summary of the molecular mechanisms involving the formation of nevi, dysplastic nevi and melanoma from a normal melanocyte. In skin, keratinocytes and melanocytes function together as epidermal melanin unit to respond to ultraviolet irradiation, the well-known environmental risk factor for skin cancers. In normal skin, ultraviolet induces p53/proopiomelanocortin signaling pathway activation in keratinocytes to stimulate α-melanocyte-stimulating hormone production, which binding to melanocortin 1 receptor on melanocytes to enhance eumelanin formation (the natural sunscreen) in one way, and to induce tumor suppressor, phosphatase and tensin homologs, which inhibits the PI3k/Akt pathway in the other way. Wild-type BRAF in melanocytes can be activated by binding of kit ligand from ultraviolet-irradiated keratinocytes to receptor c-kit or binding of other growth factors to receptor tyrosine kinase, to promote normal melanocyte growth and proliferation. Therefore, α-melanocyte-stimulating hormone/melanocortin 1 receptor/phosphatase and tensin homologs signaling pathway plays an important role to counteract BRAF/MEK/ERK signaling pathway activation induced melanocyte proliferation to prevent melanoma formation. Once the BRAFV600E mutation occurs (mostly with ultraviolet signature), if p53 is intact, ultraviolet will induce p16 expression which in turn induces melanocyte senescence, that is nevi. If phosphatase and tensin homologs loss or p53 mutated or p16 suppressed, the BRAFV600E mutation induced proliferation will become exaggerated, that is melanoma. If phosphatase and tensin homologs partial loss, BRAFV600E mutated nevi may become dysplastic nevi. Abnormal melanocytes may secret exosome carrying programmed cell death ligand-1, which binding to programmed cell death-1 receptor on T cells to escape immune surveillance. Therefore, BRAFV600E mutation is frequently detected in nevi, the senescence status and also with the potential to develop melanoma, if together with melanocortin 1 receptor deficiency, phosphatase and tensin homologs loss, p53 mutation, or p16 suppression. Immune suppression in endogenous environment may impact a lot on the behavior of nevi, dysplastic nevi or melanoma. CK1α inhibition is an ultraviolet-sparing approach to activate p53/kit ligand/Kit pathway to stimulate eumelanin formation, which may provide a rescue for α-melanocyte-stimulating hormone/melanocortin 1 receptor deficiency associated with pheomelanin formation
UV exposure is known as a risk factor for melanomagenesis. Hence, UV protection is a strategy that is used to prevent melanoma. Avoidance of mechanical injuries is important for preventing acral melanoma. Various sunscreens are used for UV protection, but the efficacy of commercial sunscreens for skin cancer prevention has been questioned. A recent publication of ours demonstrated that inhibition of CK1α, a component of the β-catenin degradation complex, stabilized both β-catenin and p53 and induced p53-dependent KitL expression in keratinocytes. KitL acts as a paracrine factor, which stimulates melanocytes into increasing eumelanin production and skin hyperpigmentation. Eumelanin, induced by CK1α inhibition, protects skin from acute sunburn with a resultant decrease in apoptosis of keratinocytes and reduced pro-inflammatory cytokine production in mouse models [55]. Therefore, CK1α inhibition may be considered a potential strategy for UV protection and prevention of UV injury, including melanoma, especially in MC1R-deficient individuals.
On the other hand, to investigate the molecular mechanism(s) underlying transformation from nevi to dysplastic nevi or melanoma, mouse model platforms have been established. A mouse model that mimics human nevi was generated. In this model, mutated BRAFV600E was expressed in melanocytes via the endogenous melanocyte-specific Tyrosinase promoter under tamoxifen or 4-hydroxytamoxifen (4-OHT) induction, resulting in nevi-like lesions being developed in the dermis and hair follicles [14]. In regard to the role of BRAF mutation in nevi or melanoma, mouse models may provide a platform for exploring the association between BRAF and other oncoproteins or tumor suppressors in nevogenesis or melanomagenesis.
Financial support and sponsorship
This work is supported by Research Collaboration Project for the Academia Sinica and Tzu Chi Foundation (TCAS107-01) granted by Buddhist Tzu Chi Medical Foundation and Ministry of Science and Technology (MOST-107-2314-B-303-010-MY3).
Conflicts of interest
Dr. Chung-Hsing Chang, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process of or decision to publish this article. The other author declared no conflict of interest in writing this paper.
REFERENCES
- 1.Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization Classification of cutaneous, mucosal, and uveal melanoma: Detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med. 2020;144:500–22. doi: 10.5858/arpa.2019-0561-RA. [DOI] [PubMed] [Google Scholar]
- 2.Tsai KC, Hung SJ, Wang JH, Huang SJ, Wang CH, Lee JT, et al. Cutaneous malignant melanoma in Eastern Taiwan: Clinicopathologic analysis of 56 cases. Dermatologica Sinica. 2019;37:187–93. [Google Scholar]
- 3.Daniel Jensen J, Elewski BE. The ABCDEF Rule: Combining the “ABCDE rule” and the “ugly duckling sign” in an effort to improve patient self-screening examinations. J Clin Aesthet Dermatol. 2015;8:15. [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi NR, Shaw HM, Rigel DS, Friedman RJ, McCarthy WH, Osman I, et al. Early diagnosis of cutaneous melanoma: Revisiting the ABCD criteria. JAMA. 2004;292:2771–6. doi: 10.1001/jama.292.22.2771. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann-Wellenhof R, Soyer HP, Wolf IH, Smolle J, Reischle S, Rieger E, et al. Ultraviolet radiation of melanocytic nevi: A dermoscopic study. Arch Dermatol. 1998;134:845–50. doi: 10.1001/archderm.134.7.845. [DOI] [PubMed] [Google Scholar]
- 6.Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–92. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16:345–58. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- 8.Wolf Horrell EM, Boulanger MC, D’Orazio JA. Melanocortin 1 receptor: Structure, function, and regulation. Front Genet. 2016;7:95. doi: 10.3389/fgene.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frändberg PA, Doufexis M, Kapas S, Chhajlani V. Cysteine residues are involved in structure and function of melanocortin 1 receptor: Substitution of a cysteine residue in transmembrane segment two converts an agonist to antagonist. Biochem Biophys Res Commun. 2001;281:851–7. doi: 10.1006/bbrc.2001.4429. [DOI] [PubMed] [Google Scholar]
- 10.Cao J, Wan L, Hacker E, Dai X, Lenna S, Jimenez-Cervantes C, et al. MC1R is a potent regulator of PTEN after UV exposure in melanocytes. Mol Cell. 2013;51:409–22. doi: 10.1016/j.molcel.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–53. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherer D, Rachakonda PS, Angelini S, Mehnert F, Sucker A, Egberts F, et al. Association between the germline MC1R variants and somatic BRAF/NRAS mutations in melanoma tumors. J Invest Dermatol. 2010;130:2844–8. doi: 10.1038/jid.2010.242. [DOI] [PubMed] [Google Scholar]
- 13.Dong F, R Cui, Liu F. Additional roles of A-MSH/MC1R signaling in melanomagenesis beyond melanogenesis. J Dermatolog Clin Res. 2013;1:1001–3. [Google Scholar]
- 14.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 16.Niault TS, Baccarini M. Targets of Raf in tumorigenesis. Carcinogenesis. 2010;31:1165–74. doi: 10.1093/carcin/bgp337. [DOI] [PubMed] [Google Scholar]
- 17.Wang DG, Xu XH, Ma HJ, Li CR, Yue XZ, Gao J, et al. Stem cell factor combined with matrix proteins regulates the attachment and migration of melanocyte precursors of human hair follicles in vitro. Biol Pharm Bull. 2013;36:1317–25. doi: 10.1248/bpb.b13-00172. [DOI] [PubMed] [Google Scholar]
- 18.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dossett LA, Kudchadkar RR, Zager JS. BRAF and MEK inhibition in melanoma. Expert Opin Drug Saf. 2015;14:559–70. doi: 10.1517/14740338.2015.1011618. [DOI] [PubMed] [Google Scholar]
- 20.Haferkamp S, Borst A, Adam C, Becker TM, Motschenbacher S, Windhövel S, et al. Vemurafenib induces senescence features in melanoma cells. J Invest Dermatol. 2013;133:1601–9. doi: 10.1038/jid.2013.6. [DOI] [PubMed] [Google Scholar]
- 21.Kamenisch Y, Berneburg M. Progeroid syndromes and UV-induced oxidative DNA damage. J Investig Dermatol Symp Proc. 2009;14:8–14. doi: 10.1038/jidsymp.2009.6. [DOI] [PubMed] [Google Scholar]
- 22.Kiss F, Anstey AV. A review of UVB-mediated photosensitivity disorders. Photochem Photobiol Sci. 2013;12:37–46. doi: 10.1039/c2pp25275a. [DOI] [PubMed] [Google Scholar]
- 23.Castresana JS, Rubio MP, Vázquez JJ, Idoate M, Sober AJ, Seizinger BR, et al. Lack of allelic deletion and point mutation as mechanisms of p53 activation in human malignant melanoma. Int J Cancer. 1993;55:562–5. doi: 10.1002/ijc.2910550407. [DOI] [PubMed] [Google Scholar]
- 24.Birck A, Ahrenkiel V, Zeuthen J, Hou-Jensen K, Guldberg P. Mutation and allelic loss of the PTEN/MMAC1 gene in primary and metastatic melanoma biopsies. J Invest Dermatol. 2000;114:277–80. doi: 10.1046/j.1523-1747.2000.00877.x. [DOI] [PubMed] [Google Scholar]
- 25.Box NF, Vukmer TO, Terzian T. Targeting p53 in melanoma. Pigment Cell Melanoma Res. 2014;27:8–10. doi: 10.1111/pcmr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gembarska A, Luciani F, Fedele C, Russell EA, Dewaele M, Villar S, et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nat Med. 2012;18:1239–47. doi: 10.1038/nm.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terzian T, Torchia EC, Dai D, Robinson SE, Murao K, Stiegmann RA, et al. p53 prevents progression of nevi to melanoma predominantly through cell cycle regulation. Pigment Cell Melanoma Res. 2010;23:781–94. doi: 10.1111/j.1755-148X.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11:503–11. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–22. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 31.Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997;57:3660–3. [PubMed] [Google Scholar]
- 32.Tsao H, Zhang X, Fowlkes K, Haluska FG. Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res. 2000;60:1800–4. [PubMed] [Google Scholar]
- 33.Roh MR, Eliades P, Gupta S, Tsao H. Genetics of melanocytic nevi. Pigment Cell Melanoma Res. 2015;28:661–72. doi: 10.1111/pcmr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreani V, Richard MA, Blaise D, Gouvernet J, Grob JJ. Naevi in allogeneic bone marrow transplantation recipients: the effect of graft-versus-host disease on naevi. Br J DermatolW. 2002;147:433–41. doi: 10.1046/j.1365-2133.2002.04748.x. [DOI] [PubMed] [Google Scholar]
- 35.Song JS, London WB, Hawryluk EB, Sridharan M, Fisher DE, et al. Risk of melanocytic nevi and non-melanoma skin cancer in children after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2017;52:989–97. doi: 10.1038/bmt.2017.57. [DOI] [PubMed] [Google Scholar]
- 36.Vena GA, Fargnoli MC, Cassano N, Argenziano G. Drug-induced eruptive melanocytic nevi. Expert Opin Drug Metab Toxicol. 2017;13:293–300. doi: 10.1080/17425255.2017.1247155. [DOI] [PubMed] [Google Scholar]
- 37.Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16:267–73. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 38.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–6. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 39.Rudolph P, Tronnier M, Menzel R, Möller M, Parwaresch R. Enhanced expression of Ki-67, topoisomerase IIalpha, PCNA, p53 and p21WAF1/Cip1 reflecting proliferation and repair activity in UV-irradiated melanocytic nevi. Hum Pathol. 1998;29:1480–7. doi: 10.1016/s0046-8177(98)90019-3. [DOI] [PubMed] [Google Scholar]
- 40.McNeal AS, Liu K, Nakhate V, Natale CA, Duperret EK, Capell BC, et al. CDKN2B Loss Promotes Progression from Benign Melanocytic Nevus to Melanoma. Cancer Discov. 2015;5:1072–85. doi: 10.1158/2159-8290.CD-15-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grob JJ, Gouvernet J, Aymar D, Mostaque A, Romano MH, Collet AM, et al. Count of benign melanocytic nevi as a major indicator of risk for nonfamilial nodular and superficial spreading melanoma. Cancer. 1990;66:387–95. doi: 10.1002/1097-0142(19900715)66:2<387::aid-cncr2820660232>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 42.Halpern AC, Guerry D, 4th, Elder DE, Clark WH, Jr, Synnestvedt M, Norman S, et al. Dysplastic nevi as risk markers of sporadic (nonfamilial) melanoma. A case-control study. Arch Dermatol. 1991;127:995–9. [PubMed] [Google Scholar]
- 43.Tucker MA, Halpern A, Holly EA, Hartge P, Elder DE, Sagebiel RW, et al. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. JAMA. 1997;277:1439–44. [PubMed] [Google Scholar]
- 44.Holly EA, Kelly JW, Shpall SN, Chiu SH. Number of melanocytic nevi as a major risk factor for malignant melanoma. J Am Acad Dermatol. 1987;17:459–68. doi: 10.1016/s0190-9622(87)70230-8. [DOI] [PubMed] [Google Scholar]
- 45.Duffy K, Grossman D. The dysplastic nevus: From historical perspective to management in the modern era: Part II. Molecular aspects and clinical management. J Am Acad Dermatol. 2012;67:19.e1–12. doi: 10.1016/j.jaad.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–69. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: A clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–82. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 48.Brandner JM, Haass NK. Melanoma's connections to the tumour microenvironment. Pathology. 2013;45:443–52. doi: 10.1097/PAT.0b013e328363b3bd. [DOI] [PubMed] [Google Scholar]
- 49.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–0. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 52.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitsui H, Kiecker F, Shemer A, Cannizzaro MV, Wang CQ, Gulati N, et al. Discrimination of dysplastic nevi from common melanocytic nevi by cellular and molecular criteria. J Invest Dermatol. 2016;136:2030–40. doi: 10.1016/j.jid.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 54.Dyduch G, Tyrak KE, Glajcar A, Szpor J, Ulatowska-Białas M, Okoń K. Melanomas and dysplastic nevi differ in epidermal CD1c+ dendritic cell count. Biomed Res Int 2017. 2017 doi: 10.1155/2017/6803756. 6803756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang CH, Kuo CJ, Ito T, Su YY, Jiang ST, Chiu MH, et al. CK1α ablation in keratinocytes induces p53-dependent, sunburn-protective skin hyperpigmentation. Proc Natl Acad Sci U S A. 2017;114:E8035–E8044. doi: 10.1073/pnas.1702763114. [DOI] [PMC free article] [PubMed] [Google Scholar]