Abstract
Objectives:
Sepsis is a major cause of death around the world. Complicated scoring systems require time to have data to predict short-term survival. Intensivists need a tool to predict survival in sepsis patients easily and quickly.
Materials and Methods:
This retrospective study reviewed the medical records of adult patients admitted to the surgical intensive care units between January 2009 and December 2011 in National Taiwan University Hospital. For this study, 739 patients were enrolled. We recorded the demographic and clinical variables of patients diagnosed with sepsis. A Cox proportional hazard model was used to analyze the survival data and determine significant risk factors to develop a prediction model. This model was used to create a nomogram for predicting the survival rate of sepsis patients up to 3 months.
Results:
The observed 28-day, 60-day, and 90-day survival rates were 71.43%, 52.53%, and 46.88%, respectively. The principal risk factors for survival prediction included age; history of dementia; Glasgow Coma Scale score; and lactate, creatinine, and platelet levels. Our model showed more favorable prediction than did Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment at sepsis onset (concordance index: 0.65 vs. 0.54 and 0.59). This model was used to create the nomogram for predicting the mortality at the onset of sepsis.
Conclusion:
We suggest that developing a nomogram with several principal risk factors can provide a quick and easy tool to early predict the survival rate at different intervals in sepsis patients.
KEYWORDS: Cox proportion hazard model, Nomogram, Sepsis, Survival
INTRODUCTION
Sepsis is a severe disease related to dysregulated host response to infections [1]. Despite the international Surviving Sepsis Campaign (SSC) guidelines, which were established to improve survival rates [2,3], sepsis is a major cause of multiple organ failure and death in critically ill patients [4,5]. The prognosis of sepsis has varied across different decades and countries [6] and is influenced by the site of infection, ethnicity differences, pathogens, and medical resources [7,8,9]. Several studies have reported the outcome of sepsis [10,11,12, and the survival rate in sepsis is related to comorbidities [7,11], severity score [7,11,13], organ dysfunction score [11,13,14], age, and lactate level [15,16]. There are several clinical scoring systems, such as Sequential Organ Failure Assessment (SOFA), which may predict survival [17,18,19]. However, it is complicated and time-consuming, and quick prediction tool is important for clinical physician. In this study, we analyzed risk factors using the database of a medical center in Taiwan and identified principal risk factors to develop our model for survival prediction at different intervals. Our primary aim was to create a quick nomogram for the early prediction of the 28-day survival rate up to 90-day at the onset of sepsis.
MATERIALS AND METHODS
Patients and definitions
The model was developed according to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis, TRIPOD, and Strengthening the Reporting of Observational studies in Epidemiology, STROBE) checklist for prediction model development and validation. In this retrospective study, we reviewed the medical records of patients with sepsis who were admitted to the multiple surgical intensive care units (SICUs) in National Taiwan University Hospital, Taiwan, which is a 1175 general-bed and 186 ICU-bed tertiary referral medical center, between January 2009 and December 2011. Sepsis patients who underwent either surgery or not were all screened according to sepsis definition. This study was approved by the Research Ethics Committee of National Taiwan University (IRB: 201305085RINC) and registered at ClinicalTrials.gov on August 7, 2013 (NCT01919138). Patient informed consent was waived by the IRB because our study was a retrospective study. Sepsis patients are defined as patients with sepsis and sepsis-induced organ dysfunction according to the SSC guidelines [20]. We screened all the adult patients older than 18 years who were admitted to ICUs. We screened all data and modified the inclusion of sepsis patients according to new definition [1]. All sepsis patients received treatments according to the SSC guidelines [21]. Patients who met the diagnostic criteria of sepsis were enrolled into this study. Patients with missing data or those transferred from other hospitals were excluded.
Data collection and clinical outcome follow-up
Patients were followed up from the onset of sepsis to death or up to 1 year. When patients were discharged from the hospital, we tracked their status through telephone interviews in 2013, but follow-up data for 52 patients (7%) were unavailable because the data were censored. We recorded demographics, history, infection sites, laboratory test results, Acute Physiology and Chronic Health Evaluation (APACHE) II scores [22], SOFA scores [15], Pitt scores [19], Charlson Comorbidity Index (CCI) [23], ICU stay, and survival status up to 1 year. Twenty-three patients had SOFA score <2 and were excluded according to new definition of sepsis.
The history of the following variables was recorded: smoking without quitting within 6 months prior to the study; diabetes mellitus; hypertension; cerebrovascular accident; dementia; gastrointestinal disease; renal insufficiency with chronic kidney disease stage IV or V; liver cirrhosis with Child–Pugh class A, B, or C; cardiovascular disease; chronic obstructive pulmonary disease (COPD); acquired immune deficiency syndrome; organ transplantation; immunocompromised status; and cancer. Sepsis was defined as systemic inflammatory response syndrome plus sepsis-induced organ systemic dysfunction. Organ systemic failure was defined as altered mental status (Glasgow Coma Scale [GCS] score of <10 or 9T); respiratory failure with PaO2/fraction of inspired oxygen (FiO2) ratio of <300; renal dysfunction with urine output of <0.5 mL/Kg/h or creatinine level of >2 mg/dL, metabolic acidosis with hyperlactatemia (lactate level >2 mmol/L), and pH <7.3; liver dysfunction with bilirubin level of >4 mg/dL and coagulatory dysfunction with platelet count of <100 k/μL or a platelet count that decreased to <50% of the baseline count; international normalized ratio of >1.5; or activated partial thromboplastin time of >60 s [24]. We excluded patients who had SOFA score <2 to meet the new definition of sepsis in 2016. Hospital (general ward) and ICU stay were recorded.
Statistical analyses, parsimonious prediction model, and nomogram
Statistical analyses were performed using SPSS 19 statistical software (IBM SPSS, Chicago, IL, USA) and R package 3.2.0. Original data were expressed as number and percentage (n [%]), mean ± standard deviation, and median (interquartile range) due to the different shapes of continuous data collected. We compared the demographic and clinical variables of sepsis patients using Student's t-test, Mann–Whitney U-test, Chi-square test, and Fisher's exact test. A Cox proportional hazard model was used to determine risk factors for mortality. On the basis of risk factors at onset, we attempted to establish a parsimonious prediction model. With the aim to develop an easy and quick application of the model, we tried to limit the number of risk factors to <7 for establishing the model. Compared with different models, our final model exhibited superior predictive ability, as indicated by the highest Harrell's concordance index (C-index) and smallest Akaike information criterion (AIC) [25]. Moreover, the survival area under the receiver operating characteristic (ROC) curve was used to determine the predictive ability of our model by comparing it with APACHE II and SOFA scores at sepsis onset, 24th h, and 48th h using the indices. Finally, we incorporated our prediction model into a nomogram, and we could easily evaluate the survival of sepsis patients using the nomogram. The two-tailed P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The number of critically ill patients admitted to surgical ICU was 16,439, among which 950 patients met the diagnostic criteria of sepsis. In 188 patients, the onset of sepsis was not available; hence, in this study, 739 patients were included, 23 patients with SOFA <2 were excluded, and their data were analyzed [Figure 1]. Patient characteristics are listed in Tables 1 and 2. The number of male patients (68.2%) was twice of that of female patients (31.8%), and the average age was 64.8 years. The common primary infection sites were the respiratory tract (46.7%), abdomen (39.1%), and wound infections (15.2%) [Table 1]. The mean CCI and Pitt scores were 4.1 and 2.5, respectively, at sepsis onset. The APACHE II score increased from sepsis onset (21.8 ± 6.9) to the 24th h (27.2 ± 15.0) and decreased at the 48th h (21.7 ± 9.8). The SOFA score decreased from sepsis onset (8.0 ± 3.2) to the 24th h (7.1 ± 3.6) and increased at the 48th h (7.2 ± 3.9). The observed survival rate on the 28th day was 71.43%, which decreased to 46.88% on the 90th day [Table 2].
Figure 1.
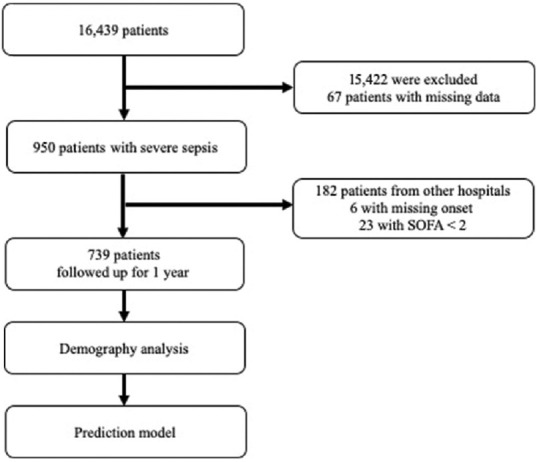
Flow diagram of the patient selection procedure
Table 1.
Demographic data
| Patient characteristics | Value, n (%) |
|---|---|
| Number of patients | 739 |
| Female | 235 (31.8) |
| Male | 504 (68.2) |
| Age (years) | 64.8±15.3 |
| Past history | |
| Smoking | 99 (13.4) |
| Diabetes mellitus | 205 (27.7) |
| Hypertension | 328 (44.4) |
| Liver cirrhosis | 54 (7.3) |
| Cardiovascular diseases | 115 (15.6) |
| COPD | 30 (4.1) |
| Renal insufficiency | 129 (17.5) |
| Immunocompromise | 62 (8.4) |
| CVA | 53 (7.2) |
| Dementia | 11 (1.5) |
| Cancers | 268 (36.3) |
| Infection sites | |
| Respiratory tract | 345 (46.7) |
| Abdomen | 289 (39.1) |
| Wound | 112 (15.2) |
| Bloodstream | 104 (14.1) |
| Urinary tract | 84 (11.4) |
| Others | 61 (8.3) |
COPD: Chronic obstructive pulmonary disease, CVA: Cerebral vascular accident
Table 2.
Severity, systemic failure, and mortality rate
| Patient characteristics | Value |
|---|---|
| Conditions at onset | |
| CCI | 4.1±3.4 |
| APACHE II score | 21.8±6.9 |
| SOFA score | 8.0±3.2 |
| qSOFA score | 1.4±0.8 |
| Pitt’s score | 2.5±2.0 |
| Number of OSF, n (%) | |
| 1-3 | 348 (47.1) |
| 4 or more | 391 (21.8) |
| Conditions at 24 h | |
| APACHE II score | 27.2±15.0 |
| SOFA score | 7.1±3.6 |
| Conditions at 48 h | |
| APACHE II score | 21.7±9.8 |
| SOFA score | 7.2±3.9 |
| Outcomes | |
| ICU stay (days [IQR]) | 33 (17-60) |
| Hospital mortality, n (%) | 393 (53.2) |
| Survival rate, days (%) | |
| 28 | 71.43 |
| 60 | 52.53 |
| 90 | 46.88 |
| 180 | 40.22 |
| 365 | 38.88 |
APACHE II: Acute Physiology and Chronic Health Evaluation II, CCI: Charlson comorbidity index, OSF: Organ systemic failure, SOFA: Sequential Organ Failure Assessment, qSOFA: Quick SOFA, ICU: Intensive care unit, IQR: Interquartile range
Mortality risk factors
Using the Cox proportional hazard analysis, we identified the risk factors according to patients’ characteristics and evaluated scores for all patients [Table 3]. According to the Cox proportional hazard analysis, the patients’ histories of dementia and liver cirrhosis were substantial risk factors. According to the Cox proportional hazard analysis, the patients’ histories of dementia and liver cirrhosis, clinical measurements, body temperature, GCS, albumin, total bilirubin, aspartate transaminase, blood urine nitrogen (BUN), creatinine, WBC, hemoglobin, hematocrit, platelet, partial thromboplastin time (PTT), the FiO2, and lactate were the substantial risk factors [Table 3]. Moreover, the high APACHE II and SOFA scores at the 3 time points, quick SOFA (qSOFA) scores higher than 2, high Pitt score, and high CCI were found to be associated with high mortality [Table 4].
Table 3.
Cox proportional hazard ratio of characteristics and measurements at onset
| HR | 95% CI | P | |
|---|---|---|---|
| Age | 1.009 | 1.00-1.02 | 0.010 |
| Past history | |||
| Liver cirrhosis | 1.484 | 1.06-2.07 | 0.021 |
| Cardiovascular diseases | 1.076 | 0.83-1.39 | 0.579 |
| COPD | 1.142 | 0.70-1.86 | 0.592 |
| Renal insufficiency | 1.020 | 0.79-1.31 | 0.878 |
| CVA | 0.985 | 0.68-1.42 | 0.937 |
| Dementia | 2.485 | 1.28-4.82 | 0.007 |
| Cancers | 1.217 | 1.00-1.48 | 0.052 |
| Vital signs and laboratory tests | |||
| Body temperature | 0.884 | 0.83-0.94 | <0.001 |
| Mean arterial pressure | 0.996 | 0.99-1.00 | 0.080 |
| GCS | 0.958 | 0.94-0.98 | <0.001 |
| Albumin | 0.837 | 0.69-1.02 | 0.077 |
| Total bilirubin | 1.036 | 1.02-1.05 | <0.001 |
| AST | 1.000 | 1.00-1.00 | 0.021 |
| Creatinine | 1.059 | 1.01-1.11 | 0.020 |
| BUN | 1.004 | 1.00-1.01 | 0.012 |
| White blood cell count | 1.002 | 1.00-1.01 | 0.076 |
| Hb | 0.929 | 0.89-0.97 | 0.001 |
| Platelet count | 0.998 | 1.00-1.00 | <0.001 |
| PTT | 1.013 | 1.01-1.02 | <0.001 |
| INR | 1.133 | 1.04-1.23 | 0.003 |
| FiO2 | 1.824 | 1.20-2.78 | 0.005 |
| Lactate level | 1.082 | 1.06-1.11 | <0.0001 |
HR: Hazard ratio, CI: Confidence interval, COPD: Chronic obstructive pulmonary disease, CVA: Cerebral vascular accident, AST: Aspartate transaminase, PTT: Partial thromboplastin time, INR: International normalized ratio, FiO2: Fraction of inspired oxygen, BUN: Blood urine nitrogen, Hb: Hemoglobin, GCS: Glasgow Coma Scale
Table 4.
Comparison of different predictive model
| HR | 95% CI | P | C-index | AIC | |
|---|---|---|---|---|---|
| APACHE II | |||||
| Onset | 1.020 | 1.01-1.03 | 0.005 | 0.5364 | 5033 |
| 24th h | 1.009 | 1.00-1.01 | 0.004 | 0.5686 | 5033 |
| 48th h | 1.025 | 1.02-1.04 | <0.001 | 0.5946 | 3662 |
| SOFA | |||||
| Onset | 1.102 | 1.07-1.14 | <0.001 | 0.5888 | 5003 |
| 24th h | 1.105 | 1.08-1.13 | <0.001 | 0.5942 | 4989 |
| 48th h | 1.135 | 1.10-1.17 | <0.001 | 0.6318 | 3613 |
| qSOFA score | |||||
| 0 | 1 (reference) | 0.5448 | 4868 | ||
| 1 | 1.199 | 0.85-1.69 | 0.300 | ||
| 2 | 1.535 | 1.09-2.16 | 0.014 | ||
| 3 | 1.688 | 1.08-2.63 | 0.021 | ||
| Pitt score | 1.085 | 1.03-1.14 | 0.001 | 0.5414 | 5030 |
| CCI | 1.052 | 1.02-1.08 | <0.001 | 0.5315 | 5028 |
| Study modela | |||||
| Age | 1.010 | 1.00-1.02 | 0.015 | 0.6499 | 3559 |
| Lactate | 1.065 | 1.04-1.09 | <0.001 | ||
| Platelet | 0.998 | 1.00-1.00 | <0.001 | ||
| Dementia | 3.655 | 1.59-8.38 | 0.002 | ||
| Creatinine | 1.081 | 1.02-1.14 | 0.005 | ||
| GCS | 0.975 | 0.95-1.00 | 0.073 | ||
a Prediction model built in this study. HR: Hazard ratio, CI: Confidence interval, C-index: Harrell’s Concordance index, AIC: Akaike information criterion, APACHE II: Acute Physiology and Chronic Health Evaluation II, SOFA: Sequential Organ Failure Assessment, qSOFA: Quick SOFA, CCI: Charlson comorbidity index, GCS: Glasgow Coma Scale
Survival model analysis
A univariate analysis revealed that age, history of dementia, GCS, lactate, creatinine, and platelet at sepsis onset were the principal risk factors, and we established the prediction model using these factors. The indicators at different time points (APACHE II and SOFA at sepsis onset, 24th h, and 48th h), qSOFA score, Pitt score, and Charlson score were compared with our model [Table 3]. We employed the C-index and AIC to evaluate each model. At sepsis onset, our model showed more favorable prediction than did APACHE II and SOFA at sepsis onset (C-index: 0.65 vs. 0.54 and 0.59). APACHE II and SOFA scores showed more favorable prediction at the 48th h than at sepsis onset and 24th h [Table 4]. At sepsis onset, our model showed earlier and more favorable predictive ability of survival rate than did the individual indicators, such as APACHE II and SOFA scores (48 h), in the ROC, and more favorable predictive power and sensitivity were characterized [Figure 2]. To quantize the contribution of all factors, we visualized our model into a computable nomogram, as shown in Figure 3.
Figure 2.
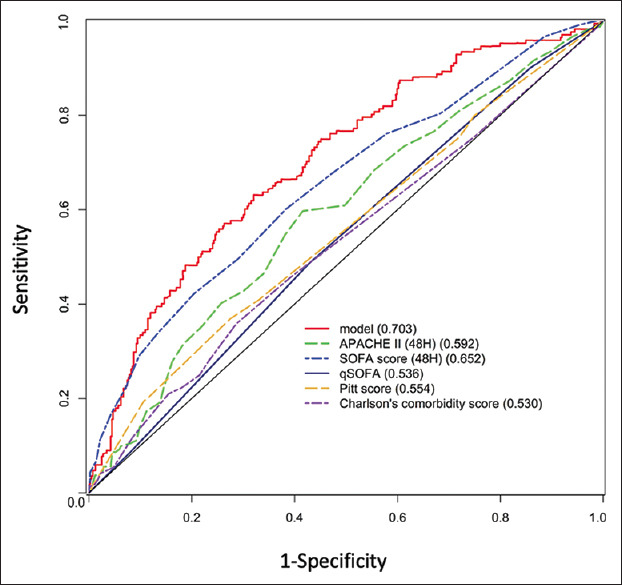
Receiver operating characteristic of different estimations. Our model at sepsis onset shows more favorable predictive ability than do qSOFA, Pitt score, Charlson comorbidity index, and SOFA/APACHE II scores at the 48 h. APACHE II: Acute Physiology and Chronic Health Evaluation II, SOFA: Sequential Organ Failure Assessment score, qSOFA: Quick SOFA
Figure 3.
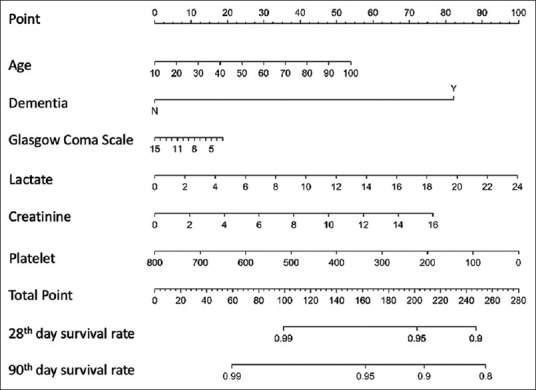
Nomogram for predicting survival on the 28th day and 90th day
DISCUSSION
Our results indicated that our model exhibited more favorable predictability than APACHE II and SOFA scores. The superior predictabilities of APACHE II and SOFA scores were observed at 48 h, but they were close to the predictability of our model at sepsis onset. Our model predicted survival 48 h earlier than did APACHE II and SOFA scores. Using our model, we developed a nomogram to help intensivists predict the survival rate at different intervals up to 3 months.
The primary aim of this study was to develop a nomogram to quickly predict survival rates at different intervals through graphical calculations. To apply the nomogram [Figure 3], each risk factor was plotted on a horizontal scale, and we could draw a vertical line to the point reference line to obtain the corresponding points. After summing all points, we could draw a vertical line from the corresponding point of total points line down to the 4 survival reference lines to get the survival rate at different intervals. To the best of our knowledge, few studies have reported the 3-month survival prediction of sepsis using a nomogram. Current tools require algebraic equations and complicated factors to calculate the final result, and clinical caregivers require a long time to collect numerous and complicated data. The nomogram has been applied for the prediction and diagnosis of many diseases, such as sepsis, COPD, and heart failure [26,27,28]. Furthermore, the nomogram is highly convenient in resource-limited areas and in resource-rich area, and it can be interpreted into an Excel formula or electronic medical system.
Multiple factors, such as APACHE II and SOFA score, contribute to different scale systems. In our model, age, GCS, and creatinine are included in APACHE II, and platelet, GCS, and creatinine are included in SOFA. Increasing age was documented as a risk in our study, which is in agreement with the finding of other studies [14,29]. Dementia was related to sepsis mortality, which is supported by previous research [30,31]. Our study and other studies have all observed that dementia increases the mortality up to 1 year, but only few studies have reported a relation between sepsis and dementia [30,32,33]. Dementia patients often exhibit obscure symptoms that might delay diagnosis and treatment, resulting in high mortality. GCS is documented as a risk factor for sepsis and is included in both SOFA and APACHE II scales [34]. GCS was reported as an early-detected tool in our and another study [35]. In our patients, the lactate level was determined as an obvious risk factor, and some studies have reported that the lactate level is valuable for determining the severity of sepsis [1,21,36]. Sepsis-related acute renal dysfunction is a well-known risk, and early hemodialysis improves the survival rate [11,37]. Creatinine, which indicates acute renal failure, was another risk factor observed in our and other studies [38,39]. Furthermore, sepsis-related thrombocytopenia might be the bone response to sepsis evolution, and improvement in the low platelet count might increase the survival rate [40,41].
In-hospital mortality is higher than some of previous studies, which ranges from 28% to 54.3% [6,8,10,42,43,44,45,46]. However, methodological differences with our study account for much of these differences. Vesteinsdottir et al. [44] reported a 28-day mortality of 24.6% and a 1-year mortality of 40.4%, but their patients had a much lower APACHE II score. Blanco et al. [6] reported similar patients’ characteristics with ours. The mortality of hospital (47.9%) and 28-day mortality (54.3%) in their study was within the range of our study between severe sepsis group and septic shock group. The study by Angus et al. [8] reported hospital mortality of 28.6%, but the proportion of organ failure might be the reason of lower mortality. Finfer et al. [43] patients were younger than others study, and their 28-day mortality was slight less than ours. Padkin et al. [42] reported that the patients who got the 24th-h of APACHE II ranging 23–55 had higher hospital mortality (68.7%), and this range of APACHE II score was similar to our study group. In Taiwan, a previous study by Shen et al. [45] reported a hospital mortality of 30.8%. However, they used ICD-9 code for patient group selection, and ICD-9 code might make different patient characteristics from ours, such as lower number of systemic organ failure.
Our study presented three limitations. First, it was a retrospective study; thus, extrapolating our results to the general population residing in different regions is difficult. Nevertheless, our results suggest that every hospital should create an optimal model using their database and develop a nomogram for predicting the survival rate of patients with sepsis. Second, we did not consider microbiological results as a risk factor, because many of our patients had multiple pathogens. Third, new clinical practice guidelines may affect the prediction of patient survival obtained using the developed nomogram [47]. Additional prospective studies should be conducted to investigate the predictive ability of this nomogram for the survival rate of patients with sepsis after it is adapted to new clinical practice guidelines.
CONCLUSIONS
We suggest that combining age, history of dementia, GCS, lactate, creatinine, and platelet in our model conferred it with earlier and more favorable predictive ability than a model incorporating only the APACHE II or SOFA score. The nomogram derived from the model offers a visualization tool for predicting the survival of patients with sepsis, and the properties of nomogram may offer an early and practical prediction method for low- and middle-income countries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank all intensivists and nurses who cared for the patients that enrolled for this study. We thank Miss Hsiao Jung Tseng, MS (Department of Clinical Trial Center, Chang Gung Memorial Hospital) for providing the consultation and statistical support. This manuscript was edited by Wallace Academic Editing.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salluh Jorge ÂI, Chen YC, Chang SC, Pu C, Tang GJ. The impact of nationwide education program on clinical practice in sepsis care and mortality of severe sepsis: A population-based study in Taiwan. PLoS One. 2013;8:e77414. doi: 10.1371/journal.pone.0077414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving spsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 4.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 5.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet. 2020;395:200–11. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: A Spanish multicentre study. Crit Care. 2008;12:R158. doi: 10.1186/cc7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35:2538–46. doi: 10.1097/01.CCM.0000284492.30800.00. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: The influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26:167–72. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 10.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–21. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 11.Brun-Buisson C, Meshaka P, Pinton P, Vallet B EPISEPSIS Study Group. EPISEPSIS: A reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30:580–8. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson S, Varpula M, Ruokonen E, Pettilä V, Parviainen I, Ala-Kokko TI, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: The Finnsepsis study. Intensive Care Med. 2007;33:435–43. doi: 10.1007/s00134-006-0504-z. [DOI] [PubMed] [Google Scholar]
- 13.Khwannimit B, Bhurayanontachai R. The epidemiology of, and risk factors for, mortality from severe sepsis and septic shock in a tertiary-care university hospital setting. Epidemiol Infect. 2009;137:1333–41. doi: 10.1017/S0950268809002027. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen HB, Kuan WS, Batech M, Shrikhande P, Mahadevan M, Li CH, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: A multi-national evaluation. Crit Care. 2011;15:R229. doi: 10.1186/cc10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arabi Y, Al Shirawi N, Memish Z, Venkatesh S, Al-Shimemeri A. Assessment of six mortality prediction models in patients admitted with severe sepsis and septic shock to the intensive care unit: A prospective cohort study. Crit Care. 2003;7:R116–22. doi: 10.1186/cc2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy Richards P, Levy H, Laterre PF, Charles Feldman P, Woodward B, Bates BM, et al. CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. Intensive Care Med. 2011;26:34–40. doi: 10.1177/0885066610383949. [DOI] [PubMed] [Google Scholar]
- 19.Rhee JY, Kwon KT, Ki HK, Shin SY, Jung DS, Chung DR, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: A comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009;31:146–50. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105–12. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 25.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto Y, Yamauchi Y, Yasunaga H, Takeshima H, Hasegawa W, Jo T, et al. Development of a nomogram for predicting in-hospital mortality of patients with exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1605–11. doi: 10.2147/COPD.S129714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu M, Zhong X, Cui X, Xu X, Zhang Z, Guan L, et al. Development and validation of a risk-prediction nomogram for patients with ureteral calculi associated with urosepsis: A retrospective analysis. PLoS One. 2018;13:e0201515. doi: 10.1371/journal.pone.0201515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlera S, Tavazzi L, Franzosi MG, Marchioli R, Raimondi E, Masson S, et al. Predictors of mortality in 6975 patients with chronic heart failure in the Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico-Heart Failure trial: Proposal for a nomogram. Circ Heart Fail. 2013;6:31–9. doi: 10.1161/CIRCHEARTFAILURE.112.967828. [DOI] [PubMed] [Google Scholar]
- 29.Tran DD, Groeneveld AB, van der Meulen J, Nauta JJ, Strack van Schijndel RJ, Thijs LG. Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Crit Care Med. 1990;18:474–9. doi: 10.1097/00003246-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Shen HN, Lu CL, Li CY. Dementia increases the risks of acute organ dysfunction, severe sepsis and mortality in hospitalized older patients: A national population-based study. PLoS One. 2012;7:e42751. doi: 10.1371/journal.pone.0042751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao LT, Sheu JJ, Lin HC, Tsai MC, Chung SD. Association between sepsis and dementia. J Clin Neurosci. 2015;22:1430–3. doi: 10.1016/j.jocn.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Lemay AC, Anzueto A, Restrepo MI, Mortensen EM. Predictors of long-term mortality after severe sepsis in the elderly. Am J Med Sci. 2014;347:282–8. doi: 10.1097/MAJ.0b013e318295a147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouza C, Martínez-Alés G, López-Cuadrado T. The impact of dementia on hospital outcomes for elderly patients with sepsis: A population-based study. PLoS One. 2019;14:e0212196. doi: 10.1371/journal.pone.0212196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastos PG, Sun X, Wagner DP, Wu AW, Knaus WA. Glasgow coma scale score in the evaluation of outcome in the intensive care unit: Findings from the acute physiology and chronic health evaluation III study. Crit Care Med. 1993;21:1459–65. doi: 10.1097/00003246-199310000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Jaimes F, Farbiarz J, Alvarez D, Martínez C. Comparison between logistic regression and neural networks to predict death in patients with suspected sepsis in the emergency room. Crit Care. 2005;9:R150–6. doi: 10.1186/cc3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels R, Nutbeam T, McNamara G, Galvin C. The sepsis six and the severe sepsis resuscitation bundle: A prospective observational cohort study. Emerg Med J. 2011;28:507–12. doi: 10.1136/emj.2010.095067. [DOI] [PubMed] [Google Scholar]
- 37.Neveu H, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French study group on acute renal failure. Nephrol Dial Transplant. 1996;11:293–9. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 38.Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: Predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–30. doi: 10.1097/01.asn.0000059863.48590.e9. [DOI] [PubMed] [Google Scholar]
- 39.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–28. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 40.Naime AC, Ganaes JO, Lopes-Pires ME. Sepsis: The involvement of platelets and the current treatments. Curr Mol Pharmacol. 2018;11:261–9. doi: 10.2174/1874467211666180619124531. [DOI] [PubMed] [Google Scholar]
- 41.Becchi C, Al Malyan M, Fabbri LP, Marsili M, Boddi V, Boncinelli S. Mean platelet volume trend in sepsis: Is it a useful parameter? Minerva Anestesiol. 2006;72:749–56. [PubMed] [Google Scholar]
- 42.Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31:2332–8. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 43.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 2004;30:589–96. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 44.Vesteinsdottir E, Karason S, Sigurdsson SE, Gottfredsson M, Sigurdsson GH. Severe sepsis and septic shock: A prospective population-based study in Icelandic intensive care units. Acta Anaesthesiol Scand. 2011;55:722–31. doi: 10.1111/j.1399-6576.2011.02437.x. [DOI] [PubMed] [Google Scholar]
- 45.Shen HN, Lu CL, Yang HH. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest. 2010;138:298–304. doi: 10.1378/chest.09-2205. [DOI] [PubMed] [Google Scholar]
- 46.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–23. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 47.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]


