Abstract
Pro-inflammatory status has been implicated in depression and suicidal behaviors. Polyunsaturated fatty acids (PUFAs) and cytokines, two types of inflammatory biomarkers, have been associated with suicide, independent of depression severity. How these biomarkers relate to each other is less clear. We measured plasma phospholipid levels of arachidonic acid (AA%), docosahexaenoic acid (DHA%), and eicosapentaenoic acid (EPA%) as a percentage of total phospholipids, as well as serum interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), in 80 patients with major depressive disorder (MDD) and 24 healthy controls (HC). Individual PUFA and cytokine species were compared using ANOVA across four suicide risk-stratified groups: 1) highest-risk, recent (within 5 years) suicide attempters (n=20); 2) high-risk, severe current suicidal ideators (having intent or plan) with no recent attempt history (n=22); 3) low-risk, current non-ideators who were also lifetime non-attempters (n=38); and 4) HC (n=24). None of the participants were enrolled following an acute suicide attempt. Of biomarkers studied, only DHA% (p=0.012) and IL-1β (p=0.002) differed between groups. In post-hoc testing, DHA% was lower in attempters than ideators (p=0.018) or MDD non-ideators (trend level, p=0.073). IL-1β was lowest in attempters, differentiating them from ideators (p=0.009) and HC (p=0.004). Recent suicide attempt, one of the most powerful predictors of suicide risk, was also most closely tied to inflammatory indices in this study. Low DHA% as an indicator of suicide risk is consistent with previous reports; however, lower IL-1β was unexpected and may relate to acuity/chronicity of inflammation. There is a need for prospective studies of immune status with respect to suicidal behaviors.
Keywords: Inflammation, cytokines, omega-3 polyunsaturated fatty acids (PUFA), suicide, depression
1. INTRODUCTION
According to the World Health Organization, 800,000 people die by suicide annually, with a rate of 10.7 per 100,000 individuals (WHO, 2020). Among psychiatric illnesses, depression is most commonly associated with suicide, accounting for 30% of cases (Bachmann, 2018). Despite efforts to develop new interventions, the suicide rate has consistently risen over the past two decades (Hedegaard et al., 2018). We also know little about the fundamental neurobiological causes of suicidal behaviors (van Heeringen and Mann, 2014). Many previously identified risk factors are not modifiable, such as prior attempt history, sex and age (Hawton et al., 2013) although depression severity is modifiable and effects a reduction in suicide risk (Gibbons et al., 2012). Thus, a paramount goal of suicide research is the identification of etiologic processes amenable to clinical intervention.
Inflammation is one promising candidate for modification that may affect suicide risk, because pro-inflammatory states are associated with major depression and with suicidal behavior (reviewed in (Gananca et al., 2016)). Higher levels of pro-inflammatory (Lindqvist et al., 2009) cytokines are associated with suicidal ideation (Karlović et al., 2012; Martinez et al., 2012; Monfrim et al., 2014; O’Donovan et al., 2013), with suicide attempt in both blood (Janelidze et al., 2011) and CSF (Lindqvist et al., 2011; Lindqvist et al., 2009; Martinez et al., 2012), and with completed suicide in postmortem brain tissue (Hoyo-Becerra et al., 2013; Pandey et al., 2012; Tonelli et al., 2008) and in CSF of patients with previous violent suicide attempts and those who subsequently died by suicide (Lindqvist et al., 2011). Therefore, cytokine activation may exert some influence over suicidal behaviors in predisposed individuals (reviewed in (Gananca et al., 2016)). Increased cytokine levels are associated not only with lower serotonin concentrations, depression, and increased risk of suicidal behavior, but also with activation of the kynurenine pathway (Achtyes et al., 2019; Raison et al., 2010). In major depressive disorder (MDD), kynurenine is elevated in suicide attempters compared with non-attempters (Sublette et al., 2011a).
Essential polyunsaturated fatty acids (PUFAs) play a key role in inflammation regulation, mainly by affecting the expression of pro-inflammatory mediators, and resolution through the actions of their active metabolites, generally known as specialized pro-resolving mediators (SPMs) (Calder, 2017; Layé et al., 2018); omega-3 PUFAs correlate negatively with pro-inflammatory cytokines (Ferrucci et al., 2006; McNamara et al., 2010; Micallef et al., 2009) and positively with anti-inflammatory cytokines (Ferrucci et al., 2006). Moreover, omega-3 PUFA supplementation reduces pro-inflammatory cytokine levels and enhances anti-inflammatory cytokine secretion (Oliver et al., 2012). Low omega-3 PUFA levels are also observed in major depression (Lin et al., 2010), in suicide attempters (Huan et al., 2004) and in suicide decedents (Lewis et al., 2011), and predicted future suicide attempts in one small study (Sublette et al., 2006).
Low intake of omega-3 PUFAs may enhance suicide risk by affecting lipid raft composition, which in turn can impact: a) monoaminergic receptors and neurotransmission (Alfaidi et al., 2018; Liu et al., 2018), and b) toll-like receptors (TLR) (Wong et al., 2009), both of which are implicated in suicidal behaviors (Pandey et al., 2014). Possible mechanistic intermediates in relationships between PUFAs and suicide risk have been reviewed in detail in (Daray et al., 2018).
In this study we aimed to clarify relationships between suicide risk and specific pro-inflammatory cytokines or essential polyunsaturated fatty acids (PUFAs). From prior studies comparing these biomarkers in depressed patients with or without suicide risk (reviewed by us in (Gananca et al., 2016)), we selected three pro-inflammatory cytokines with strong evidence for an association between elevated levels and suicidal behaviors or ideation: IL-6 (Hoyo-Becerra et al., 2013; Janelidze et al., 2011; Karlović et al., 2012; Lindqvist et al., 2011; Lindqvist et al., 2009; Martinez et al., 2012; O’Donovan et al., 2013; Pandey et al., 2012), IL-1β (Martinez et al., 2012; Monfrim et al., 2014; Pandey et al., 2012; Pandey et al., 2018), and TNF-α (Janelidze et al., 2011; Martinez et al., 2012; Pandey et al., 2012).
Likewise, we studied three PUFA species commonly associated with suicide risk: docosahexaenoic acid (DHA, 22:6n-3), eicosapentaenoic acid (EPA, 20:5n-3), and arachidonic acid (AA, 20:4n-6) (Huan et al., 2004; Lewis et al., 2011; Sublette et al., 2006). As aggression also has been associated with suicide risk (Hartley et al., 2018), with PUFA status (Gajos and Beaver, 2016; Virkkunen et al., 1987; Zaalberg et al., 2016), and with inflammation (Suarez, 2003; Suarez et al., 2004; Suarez et al., 2002), we additionally explored whether aggression could affect the relationships between PUFAs or cytokines and risk of suicidal behavior.
2. MATERIAL AND METHODS
2.1. Sample
This research study was approved by the Institutional Review Board of the New York State Psychiatric Institute. We recruited adult participants with a diagnosis of DSM-IV Major Depressive Episode in the context of Major Depressive Disorder (MDD; n=80) who scored at least 16 on the 17-item Hamilton Depression Rating Scale (17-HDRS) (Hamilton, 1967), and HC (n=24). All participants gave informed consent to participate in the study. Depressed patients were recruited according to the following stratification criteria for risk of suicidal behavior: 1) low suicide risk: no history of lifetime suicide attempts and no suicidal ideation at the time of enrollment (n=38); 2) high suicide risk: severe suicidal ideation (with plan or intent) at the time of enrollment with no history of suicide attempts within the five years prior to enrollment (n=22); or 3) highest risk: history of suicide attempt within the five years prior to enrollment (n=20), regardless of suicidal ideation status at the time of study entry. Depressed patients with an ‘intermediate’ level of suicide risk at the time of enrollment were excluded: suicide attempts more distant than five years or no history of attempts with passive suicidal ideation (HDRS-17 suicide item with scores of 2). Participants also were excluded if they presented with a history of psychosis, serious active medical illness, chronic pain conditions, chronic allergies, anti-inflammatory or anti-allergy medication use on a regular basis or in the week previous to enrollment (if taken sporadically), substance use disorder in the 6 months prior to enrollment, or pregnancy. Taking psychotropic medication was not an exclusion criterion for the depressed group.
2.2. Clinical Assessments and Blood Sampling
All participants had a comprehensive psychiatric and medical assessment, physical examination and standard laboratory tests. Psychiatric diagnoses were based on the Structured Clinical Interview for DSM-IV and confirmed in consensus meetings. Depression severity was assessed using the HDRS-17 at study entry. Dietary intake of omega-3 PUFAs was assessed by a dietary questionnaire previously validated by us (Sublette et al., 2011b) for both healthy individuals and depressed patients, covering consumption of omega-3 fatty acids through self-reporting of the estimated weekly frequency and portion sizes of a list of specific fish, seafood, oils and nuts known to be major dietary omega-3 sources, for six months prior to study enrollment. Trait aggression was assessed using the Buss Perry Questionnaire (Buss and Perry, 1992). Clinical assessments and blood collection were all performed at the same time point, at study entry. Due to recruitment procedure constraints, blood collection was not performed in a fasted state or at the same time of the day for every participant, occurring between 12 pm and 4 pm, depending on the participantś scheduled appointment time. Blood samples were drawn into EDTA-containing tubes to obtain plasma for fatty acid measurement and tubes without anticoagulant to obtain serum for cyokine measurement. Samples were placed into an ice-water slurry until centrifugation at low speed for 10 min in refrigerated conditions, followed by transfer of supernatant to cryotubes and immediate storage at −80º. For each of the three batches of assays, the serum was defrosted on ice and immediately refrozen at −80º.
2.3. Cytokine Analysis
Cytokine serum levels of human IL-1β, IL-6 and TNF-α were determined in duplicate by enzyme-linked immunosorbent assay (ELISA) with commercially available Quantakine® kits (R & D Systems, Inc., Minneapolis, MN). Briefly, 100 μL of serum/plasma or standard was added to each well and incubated for 2 hours at room temperature (RT) with gentle agitation using an orbital shaker. After 6 washes with wash buffer, 200 μL of conjugate was added to each well, incubated for 2 hours at room temperature, and again washed using wash buffer. Next, 50 μL of substrate solution was added to each well and incubated for 60 minutes, followed by addition of 50 μL of amplifier solution to each well, 30 minute incubation, and addition of 50 μL of stop solution to each well. For each well, the optical density (O.D.) was determined within 30 minutes using a microplate reader at 490 nm, with wavelength correction set to 650 nm. The standard curve was generated by plotting the mean absorbance for each standard, and data points were linearized. Cytokine concentrations were determined by comparing the measured O.D. against the standard curve. The minimum detectable dose ranges of ELISA for the different cytokines were 0.014–0.63 pg/ml for IL-1β, 0.016–0.11 pg/ml for IL-6, and 0.038–0.191 pg/ml for TNF-α.
2.4. Plasma PUFA Analysis
The plasma glycerophospholipid PUFAs EPA, DHA and AA were quantified using a modified high-throughput method (Glaser et al., 2010) in which plasma proteins were precipitated in cold methanol with the internal standard 1,2-pentadecanoyl-sn-glycero-3-phosphocholine. Sodium methoxide was added to the methanol extract containing mainly polar lipids to selectively esterify the glycerophospholipids. After brief mixing, the mixture was acidified and fatty acid methyl esters were extracted with hexane, concentrated, and analyzed by gas chromatography using a 30m capillary column (DB-FFAP- J&W Scientific, Folsom, CA, USA).
Individual PUFA levels were expressed as a percentage of total plasma phospholipids (DHA%, EPA % and AA%).
2.5. Statistical Analyses
Statistical analyses were performed using IBM SPSS (version 22 for Mac, Apple Inc, Cupertino, CA). Normality of distribution was tested for continuous variables; natural log-transformed values of serum cytokine and PUFA concentrations were used in order to attenuate distributional skew and reduce outliers. The remaining extreme outliers identified using boxplots on SPSS were winsorized.
Sociodemographics, clinical characteristics and inflammatory biomarkers were compared among the stratified suicide risk groups and HC using ANOVA for continuous and chi-square tests for categorical variables. When variances were unequal, unequal variance ANOVA was run. Where the ANOVA was significant, post-hoc comparisons were performed using Tukey’s HSD (Honest Significant Differences) testing, to compare each risk-stratified group individually with the other three groups with respect to inflammatory markers, and correlations were performed between biomarkers when significant.
To understand the effects of the candidate biomarkers on suicide risk, logistic regression was used with suicide attempt history as the response variable, and the candidate biomarkers as predictor variables. Due to the low number of attempters, age, sex, depression severity, aggression and current antidepressant medication use status were included as covariates in separate analyses.
To evaluate the effects of antidepressant medication use, a sensitivity analysis was performed by removing all participants using antidepressants from the entire sample and repeating the ANOVA with post-hoc comparisons to compare biomarker means by the four stratified risk groups. Additionally, biomarkers showing any group differences in the previous analysis were compared between antidepressant and non-antidepressant users. Finally, antidepressant use was controlled for in the logistic regression models.
For all analyses, p-values ≤ 0.05 were considered significant. For the ANOVA post-hoc analyses, multiple comparisons were adjusted for using the Tukey’s HSD.
3. RESULTS
3.1. Sample Characteristics
Eighty patients with DSM-IV MDD, ages 18–63 yrs, and twenty-four healthy controls (HC), ages 18–51 yrs, were recruited. Demographic and clinical characteristics are presented in Table 1. No significant differences were found between groups with regard to sex, age, smoker status, BMI, race or ethnicity. The depressed group presented with moderate depression severity and, as expected, patients with current suicidal ideation had higher HDRS severity scores than past attempters and low suicide risk patients (p=0.003). After removing the HDRS suicidal item, however, depression severity did not differ between groups (p=0.360). Among the past attempter group (n=20), the mean number of suicide attempts within 5 years before enrollment was 1.85 (SD 1.09, range 1–4), and the median interval between the last suicide attempt and time of study enrollment was 10.5 months (interquartile range, 46). Of these, three participants had their only or most recent suicide attempt within one month of study recruitment. Although allowed by our inclusion criteria, none of the past suicide attempter participants were enrolled during the acute aftermath of a suicide attempt. Around half of the patients with high or highest suicide risk (current suicidal ideation and past attempters) were currently taking antidepressant medication, compared to only 8% of depressed patients with low suicide risk (p<0.001). The depressed past attempter group showed higher levels of trait aggression (p=0.001) than participants from the other stratified suicide risk groups.
Table 1.
Sociodemographic and clinical characteristics stratified by suicide risk.
| MDD High Suicide Risk | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Healthy Volunteers (n=24) | MDD Low Suicide Risk (n=38) | Current High Suicidal Ideation (n=22) | Past Attempters (n=20) | Statistics | ||
|
| |||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | df | F-test | p-value | |
|
| |||||||
| Age | 33.67 (9.84) | 35.08 (9.72) | 37.72 (12.02) | 32.70 (13.44) | 3 | 0.813 | 0.490 |
| BMI | 27.08 (6.30) | 24.65 (6.70) | 23.98 (7.70) | 24.92 (5.1) | 3 | 0.904 | 0.442 |
| 16-HDRS * | N/A | 19.81 (3.57) | 20.50 (3.24) | 18.95 (3.10) | 2 | 1.035 | 0.360 |
| BPQ | 52.36 (13.50) | 66.78 (21.99) | 70.28 (17.61) | 76.63 (23.76) | 2 | 5.605 | 0.001 |
|
| |||||||
| n (%) | n (%) | n (%) | n (%) | df | χ2 | p-value | |
|
| |||||||
| Male sex | 15 (62.5) | 6 (16) | 12 (55) | 10 (50) | 3 | 2.011 | 0.57 |
| White race | 10 (41.6) | 19 (50) | 17 (77.3) | 10 (50) | 3 | 6.636 | 0.08 |
| Non-Hispanic ethnicity | 10 (41.6) | 33 (86.8) | 20 (90.9) | 17 (85) | 3 | 1.360 | 0.715 |
| Smoker | 0 (0) | 7 (18) | 3 (14) | 3 (15) | 3 | 4.744 | 0.192 |
| Current antidepressant use | N/A | 3 (7.9) | 11 (50) | 9 (45) | 2 | 15.496 | <0.001 |
BMI - Body mass index;
16-HDRS - Hamilton depression rating scale, without suicide ideation item; BPQ -
Buss Perry aggression questionnaire; MDD – major depressive disorder
3.2. Biological markers and suicide risk
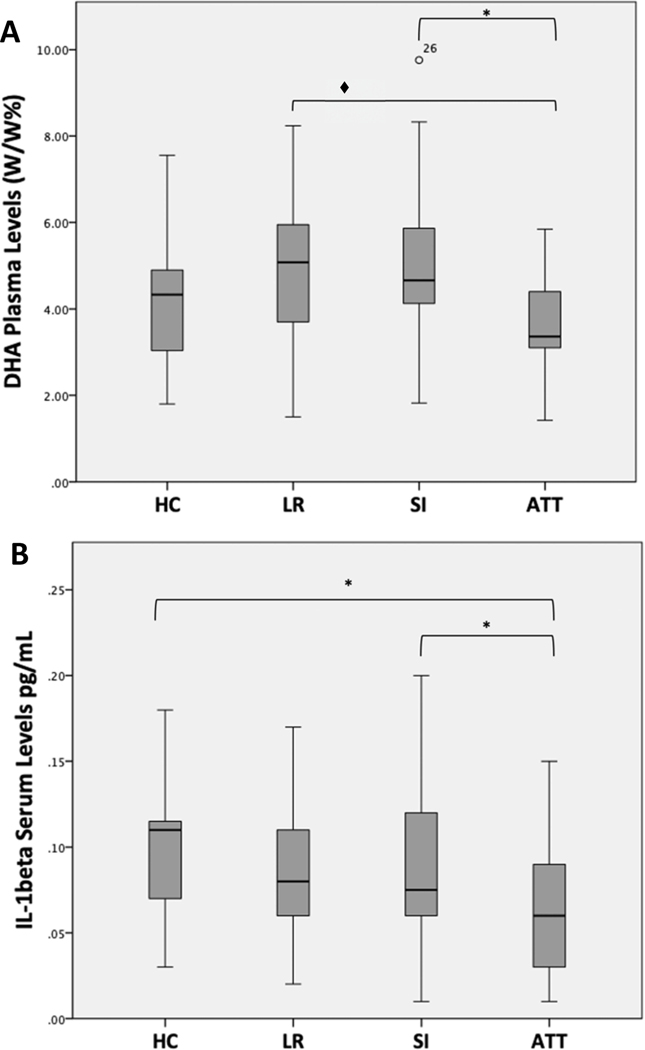
PUFA and cytokine characteristics are shown in Table 2. Among all biomarkers, only DHA% (p=0.012) and IL-1β (p=0.002) differed between groups. Post-hoc pairwise comparisons showed DHA% was lower in the highest-risk group (suicide attempters) than in the high-risk (severe ideators) (p=0.018) or low-risk (no ideation or attempts) groups (trend level, p=0.073) (Figure 1A). IL-1β also was lower in the highest-risk, suicide attempter group than in high-risk severe ideators (p=0.009) or HC (p=0.004) (Figure 1B). EPA% differed between groups at a trend level (p=0.058); no differences between groups were found for AA%, IL-6 or TNF-α (data not shown).
Table 2.
Lipid and inflammatory characteristics stratified by suicide risk.
| MDD High Suicide Risk | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Healthy Volunteers | MDD Low Suicide Risk | Current High Suicidal Ideation | Past Attempters | Statistics | ||
|
| |||||||
| Cytokines (pg/mL) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F-test | df | p-value |
|
| |||||||
| IL-1B | 0.101 (0.41) | 0.08(0.45) | 0.095 0.49) | 0.061 (0.36) | 5.201 | 3 | 0.002 |
| IL-6 | 1.73 (0.89) | 1.39(0.91) | 1.69 (1.00) | 1.63 (0.83) | 0.440 | 3 | 0.725 |
| TNF-α | 0.97 (0.49) | 0.97(0.29) | 1.10 (0.79) | 0.89 (0.31) | 0.322 | 3 | 0.809 |
|
| |||||||
| PUFA% | |||||||
|
| |||||||
| AA % | 13.64 (2.64) | 13.28(2.70) | 14.10(2.42) | 12.88 (2.71) | 0.440 | 3 | 0.475 |
| EPA % | 0.85 (0.43) | 1.13 (1.06) | 1.38 (0.43) | 0.76 (0.29) | 2.574 | 3 | 0.058 |
| DHA % | 4.25 (1.61) | 4.97 (1.78) | 5.18 (1.72) | 3.67 (1.07) | 3.826 | 3 | 0.012 |
|
| |||||||
| Dietary Intake (g/d) | |||||||
|
| |||||||
| EPA | 0.02 (0.03) | 0.03(0.04) | 0.03(0.04) | 0.01(0.005) | 2.832 | 3 | 0.043 |
| DHA | 0.05(0.06) | 0.06(0.06) | 0.06(0.08) | 0.02(0.02) | 2.056 | 3 | 0.111 |
| ALA | 0.22(0.29) | 0.28(0.33) | 0.15(0.19) | 0.20(0.25) | 0.884 | 3 | 0.452 |
IL – interleukin; TNF – tumor necrosis factor; AA – arachidonic acid; ALA – alpha – linoleic acid; EPA - eicosapentaenoic acid; DHA – docosahexaenoic acid; MDD – major depressive disorder
Figure 1.
Docosahexaenoic Acid (A) and Interleukin-1β (B) Levels Grouped by Suicide Risk Stratification.
*[ = p<0.01; ◆[ = trend level p=0.073. P-values are after correction for multiple comparisons.
Abbreviations: HC- Healthy controls; LR - low risk (no current suicidal ideation, no lifetime history of suicide attempts); SI - current suicidal ideators with plan or intent; ATT - suicide attempters within 5 years
In a logistic regression model including DHA%, IL-1β and aggression as predictors, each variable independently predicted suicide attempt status (DHA%, p=0.019; IL-1 β, p=0.036; aggression, p=0.025). However, no correlations were found between DHA% and IL-1β, or between either of these biomarkers and aggression, number of suicide attempts (within the 5 years previous to enrollment) or depression severity, after removing the suicide item, within each of the four individual subgroups (data not shown).
Sensitivity analyses performed to understand the role of antidepressant use found similar results in the relationships between the inflammatory biomarkers and suicide risk status when current antidepressant users were excluded. DHA% (p=0.025) and IL-1 β (p<0.001) still differed between groups. On post-hoc pairwise comparisons, DHA% still was lower in highest-risk suicide attempters (n= 11) than in high-risk severe ideators (n=11; p=0.045), and IL-1β still was lower in attempters compared to severe ideators (p<0.001) and HC (n=24; p=0.005). Likewise, within the attempter group, DHA% and IL-1β means did not differ when antidepressant users (n=9) were compared to non-users (n=11) (data not shown). Similarly, controlling for antidepressant use in our logistic regression model described above, DHA%, IL-1β and aggression still predicted suicide attempt status, (DHA%, b=0.543, p=0.02; IL-1β, b=0.884, p=0.04; aggression, b=−0.031, p=0.039), while antidepressant use did not (b=0,691, p=0.289). Finally, similar significant results were obtained when controlling for BMI in the same model (DHA%, b=0.577, p=0.019; IL-1β, b=0.899, p=0.039; aggression, b=−0.033, p=0.028; and BMI, b=0.020, p=0.734).
4. DISCUSSION
To our knowledge, this is the first published study to simultaneously assess the relationships of PUFA and pro-inflammatory cytokine levels to suicide risk stratification. Our finding of low DHA% in depressed patients with a history of suicide attempt is in accordance with previous studies linking low omega-3 status and suicide risk (Huan et al., 2004; Lewis et al., 2011; Sublette et al., 2006). Contrary to expectations, however, we did not find elevated pro-inflammatory cytokine levels in high-risk or highest-risk patients, even IL-6, which is elevated in association with suicide risk in most studies (reviewed in (Gananca et al., 2016)).
Lower IL-1β levels in attempters likewise was unexpected. However, three previous studies did find lower IL-1β related to suicidal behavior (Coryell et al., 2018; Lu et al., 2019) or depression (Ovaskainen et al., 2009). In contrast, some other studies found a positive association (Martinez et al., 2012; Monfrim et al., 2014; Pandey et al., 2012; Pandey et al., 2018) or no association (Gabbay et al., 2009; Huang and Lee, 2007; Isung et al., 2012; Lindqvist et al., 2009; Tonelli et al., 2008; Torres-Platas et al., 2014) between IL-1β and suicidal behaviors.
As expected from previous studies (Keilp et al., 2006; Oquendo et al., 2004), we also found higher trait aggression in suicide attempters; however, we found no relationship between aggression and the biological markers that we studied.
DHA has well-established anti-inflammatory properties, and low DHA levels have been consistently associated with pro-inflammatory states in animal (McNamara et al., 2010) and human studies (Ferrucci et al., 2006; Maes et al., 2000; Micallef et al., 2009). Furthermore, DHA metabolites (SPMs), namely resolvins, protectins and maresins, actively stimulate the inflammation resolution process (reviewed in (Joffre et al., 2019; Serhan, 2014)). In contrast, IL-1β is one of the main pro-inflammatory cytokines, having an important role in activating IL-6 and inducing the acute phase reactant C-reactive protein (CRP). Regarding the relationship between these two biological species, in prior studies DHA consistently opposes IL-1. For example, DHA: 1) causes decreased IL-1β production in vitro in activated macrophages, in a concentration-dependent manner (Solanki et al., 2013) and more potently than does EPA (Weldon et al., 2007); 2) inhibits IL-1β expression and apoptotic activity in chondrosarcoma cells (Xu et al., 2019); 3) decreases IL-1-mediated ERK1/2 signaling in hepatic cells (Ventro et al., 2017); 4) decreases IL-1 levels, in combination with ketamine, in hippocampal LPS-induced cells (Chang et al., 2019); and 5) in combination with antidepressants, counteracts the deleterious effects of IL-1 on hippocampal neurogenesis (Borsini et al., 2017). In animal models, DHA supplementation reduces plaque endothelial IL-1β (Alfaidi et al., 2018). In a clinical study, dietary supplementation with EPA plus DHA in aging adults with chronic venous leg ulcers decreased IL-1 β after 4 weeks of treatment (Tan et al., 2018). Moreover, DHA product resolvin D1 (RvD1) has a blunting effect on neuroinflammation, e.g. it decreases in vitro gene expression of LPS-stimulated microglial IL-1β, IL-6 and TNF-α (Rey et al., 2016) and decreases microglial activation and INF-γ production, in a rat Parkinsońs disease model (Krashia et al., 2019).
One explanation for our discrepant findings could be that the above studies demonstrate acute effects of DHA supplementation on IL-1β, which may not reflect effects of the chronic stress of low DHA and the subsequent responses within the inflammatory framework which are likely complex, non-linear, and modulated by multiple factors. Reinforcing this aspect is the fact that our suicide attempter sample was not recruited in the acute stage following a suicide attempt, which might yield results more similar to the abovementioned studies. In the context of PUFA-cytokine interactions, it is noteworthy that a few studies in cell cultures also found pro-inflammatory activity in response to DHA (Horowitz et al., 2014; Maes et al., 2007), that might be dose-dependent (Paschoal et al., 2013). That DHA may exhibit both anti- and pro-inflammatory properties in different contexts suggests a possible immune-regulatory role that may depend on the cell type, dosage/ concentration, and other components of the inflammatory milieu.
There is evidence suggesting that the inflammatory activation seen in subsets of depressed patients elicits an immune-suppressive response known as the compensatory immune-regulatory reflex system (CIRS) (Maes and Carvalho, 2018), adaptively attenuating excessive inflammatory responses. For instance, besides their actions initiating the acute phase of the immune response, pro-inflammatory cytokines also homeostatically stimulate the increased production of their cellular receptors, which when soluble after shedding from cell membranes, may in turn bind to the corresponding pro-inflammatory cytokines and dampen their actions. A meta-analysis (Köhler et al., 2017) in depression found increased levels of 1) the soluble IL-1 receptor antagonist (sIL-1RA), which binds competitively to the IL-1R and blocks further IL-1 activity, 2) soluble receptors for tumor necrosis factor (sTNF-R) and interleukin-2 (sIL-2R), and 3) the anti-inflammatory cytokine IL-10. It has been suggested that activation of CIRS might contribute to the recovery from the acute phases of depression, either spontaneously or in response to treatment (Maes and Carvalho, 2018). Also contributing to a return to homeostasis, pro-resolving metabolites (SPMs) might help attenuate pro-inflammatory cytokine levels. Although higher levels of SPMs might not be expected to be found in our sample of attempters with low DHA levels since SPM concentrations are largely dependent on PUFA dietary consumption, inflammatory states per se also induce enzymatic biosynthesis of these mediators. Recently, increased RvD1 levels have been described in mania and depression states compared to euthymia in bipolar patients and healthy controls (Kok Kendirlioglu et al., 2020), possibly consistent with subclinical inflammation. Further studies are needed to better understand these relationships.
Only a few studies have investigated CIRS biomarkers with regard to suicidal behavior. Elevated sIL-2R was observed in suicide attempters (Nassberger and Traskman-Bendz, 1993). High IL-4 also has been reported in the prefrontal cortex of suicide adolescents (Tonelli et al., 2008), whereas on meta-analysis, plasma IL-4 levels were lower in depressed patients with suicide risk than in controls (Ducasse et al., 2015). No studies evaluating SPMs with regard to suicidal behavior have been reported.
Being cross-sectional, our data can provide no evidence for causal mechanisms. However, since we are considering suicidal behavior that occurred prior to the evaluation over a fairly large timeframe, it is possible that the chronic stress of low dietary DHA plus pro-inflammatory cytokine activation in the past might have contributed to suicidal behaviors, followed by attenuation of pro-inflammatory mediators through the CIRS and pro-resolving pathways.
To better understand the complex interactions between PUFAs and immune biomarkers, future studies should include assessment of both pro and anti-inflammatory molecules, including cytokine soluble cytokine receptors (sIL-1RA, sTNF-R1, sTNF-R2, sIL-2R) and their ratios with the respective circulating cytokines, anti-inflammatory cytokines such as IL-4 and IL-10 and PUFA pro-resolving metabolites involved in inflammation resolution. Although procedurally less feasible and conferring more burden on participants, concomitant CSF measurement of these biomarkers would be the ideal approach. Prospective studies are of paramount importance to characterize the evolution of these biomarkers according to the disease staging, whether in acute episodes, remission or chronicity. For instance, it would be beneficial to include patients from emergency room departments or from inpatient units with admissions due to acute suicide attempts. Lastly, instruments like the Food Frequency Questionnaire are helpful in cross-sectional studies like ours but are subject to recall bias. Including dietary tracking logs for participants in prospective studies could provide more accuracy in assessing omega-3 dietary consumption.
The clinical importance of our findings is that both DHA deficiency and inflammation are modifiable risk factors amenable to intervention and their evaluation could be part of the standard suicide risk assessment. Increasing omega-3 PUFA intake through diet or supplementation is an affordable and safe measure that could have an impact in preventing suicidal behaviors. Our results also suggest that within the spectrum of suicidal behavior, suicide attempt status is a more useful marker than suicidal ideation for suicide risk related to inflammation. This is consistent with clinical findings that suicide attempts also predict suicide more reliably than suicidal ideation (Hawton et al., 2013).
4.1. Limitations
Limitations to our study include the small sample size, particularly given the large variance seen in the PUFA and cytokine measurements. A procedural limitation was that serum for cytokine analysis underwent three thawing and refreezing cycles, which might have influenced the cytokine measurements, although most cytokines have been shown to be unaffected by up to three freeze-thaw cycles (Thavasu et al., 1992). Another limitation of the study design was not drawing blood in a fasted state and at the same time of the day for every participant. Since IL-6 secretion follows a circadian rhythm with two daily peaks, morning and evening (Vgontzas et al., 2005), a possible confound regarding our IL-6 results could be that the blood draws were not performed at the same time of day. Another possible confound related to our blood drawing procedure is that fasting state and content of meals can also impact cytokine circulating levels (Zhou et al., 2010). Additionally, in contrast to brain DHA levels that largely reflect dietary intake, cytokines do not easily cross the blood brain barrier, and peripheral levels may not mirror central levels (Lindqvist et al., 2009; Miller et al., 2019; Wang and Miller, 2018), complicating the interpretation of circulating cytokine measurement results. A final limitation is that although suicide attempt history is a known marker for future suicide risk, suicide attempts occurred at a median interval of 10.5 months previous to study entry, so connections between suicide attempt status and inflammatory responses fall into a chronic, rather than acute category. Strengths of the study design include comparisons between different, well-defined phenotypes of suicidal behavior, having controlled or excluded participants in regard to many relevant confounders described for cytokine research, namely presence of allergies, inflammatory illnesses, chronic pain, anti-inflammatory medications, aging, body weight, smoking, and excessive alcohol consumption (Himmerich et al., 2019). Although medication use was a potential confound, our analyses refute that.
4.2. Conclusions
Our results are consistent with prior observations of low DHA associated with suicidal behavior but do not support a role for a persisting pro-inflammatory state within five years of suicide attempt. For a nuanced understanding of inflammation and suicide risk, future studies focusing prospectively on the timing of cytokine responses related to suicidal behaviors may be of fundamental importance. Understanding the complex network of immune mediators and timing of the immune response related to suicidal behaviors can inform the development of individualized treatments targeting the biomarkers of interest.
Supplementary Material
Acknowledgments
Dr. Oquendo owns equity in Mantra, Inc. Her family owns stock in Bristol Myers Squibb. Drs. Oquendo and Mann receive royalties from the Research Foundation for Mental Hygiene for the commercial use of the C-SSRS
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2020.12.029.
Conflict of Interest. All other authors have no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achtyes E, Keaton SA, Smart L, Burmeister AR, Heilman PL, Krzyzanowski S, Nagalla M, Guillemin GJ, Escobar Galvis ML, Lim CK, Muzik M, Postolache T, Leach R, Brundin L, 2019. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaidi MA, Chamberlain J, Rothman A, Crossman D, Villa-Uriol MC, Hadoke P, Wu J, Schenkel T, Evans PC, Francis SE, 2018. Dietary Docosahexaenoic Acid Reduces Oscillatory Wall Shear Stress, Atherosclerosis, and Hypertension, Most Likely Mediated via an IL-1-Mediated Mechanism. J Am Heart Assoc 7(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann S, 2018. Epidemiology of Suicide and the Psychiatric Perspective. Int J Environ Res Public Health 15(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini A, Alboni S, Horowitz MA, Tojo LM, Cannazza G, Su KP, Pariante CM, Zunszain PA, 2017. Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav Immun 65, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Perry M, 1992. The aggression questionnaire. J. Pers. Soc. Psychol. 63(3), 452–459. [DOI] [PubMed] [Google Scholar]
- Calder PC, 2017. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 45(5), 1105–1115. [DOI] [PubMed] [Google Scholar]
- Chang D, Zhao J, Zhang X, Lian H, Du X, Yuan R, Wen Y, Gao L, 2019. Effect of ketamine combined with DHA on lipopolysaccharide-induced depression-like behavior in rats. Int Immunopharmacol 75, 105788. [DOI] [PubMed] [Google Scholar]
- Coryell W, Wilcox H, Evans SJ, Pandey GN, Jones-Brando L, Dickerson F, Yolken R, 2018. Aggression, impulsivity and inflammatory markers as risk factors for suicidal behavior. J Psychiatr Res 106, 38–42. [DOI] [PubMed] [Google Scholar]
- Daray FM, Mann JJ, Sublette ME, 2018. How lipids may affect risk for suicidal behavior. J. Psychiatr. Res. 104, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducasse D, Olie E, Guillaume S, Artero S, Courtet P, 2015. A meta-analysis of cytokines in suicidal behavior. Brain. Behav. Immun. 46, 203–211. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM, 2006. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 91(2), 439–446. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ, 2009. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J. Child Adolesc. Psychopharmacol. 19(4), 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajos JM, Beaver KM, 2016. The effect of omega-3 fatty acids on aggression: A meta-analysis. Neurosci Biobehav Rev 69, 147–158. [DOI] [PubMed] [Google Scholar]
- Gananca L, Oquendo MA, Tyrka AR, Cisneros-Trujillo S, Mann JJ, Sublette ME, 2016. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 63, 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Brown CH, Hur K, Davis J, Mann JJ, 2012. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch. Gen. Psychiatry 69(6), 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser C, Demmelmair H, Koletzko B, 2010. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J. Lipid Res. 51(1), 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CM, Pettit JW, Castellanos D, 2018. Reactive Aggression and Suicide-Related Behaviors in Children and Adolescents: A Review and Preliminary Meta-Analysis. Suicide Life. Threat. Behav. 48(1), 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawton K, Casanas ICC, Haw C, Saunders K, 2013. Risk factors for suicide in individuals with depression: a systematic review. J. Affect. Disord. 147(1–3), 17–28. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Curtin SC, Warner M, 2018. Suicide Rates in the United States Continue to Increase. NCHS Data Brief(309), 1–8. [PubMed] [Google Scholar]
- Himmerich H, Patsalos O, Lichtblau N, Ibrahim MAA, Dalton B, 2019. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front Psychiatry 10, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MA, Wertz J, Zhu D, Cattaneo A, Musaelyan K, Nikkheslat N, Thuret S, Pariante CM, Zunszain PA, 2014. Antidepressant compounds can be both pro- and anti-inflammatory in human hippocampal cells. Int J Neuropsychopharmacol 18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyo-Becerra C, Huebener A, Trippler M, Lutterbeck M, Liu ZJ, Truebner K, Bajanowski T, Gerken G, Hermann DM, Schlaak JF, 2013. Concomitant interferon alpha stimulation and TLR3 activation induces neuronal expression of depression-related genes that are elevated in the brain of suicidal persons. PLoS One 8(12), e83149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T, 2004. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol. Psychiatry 56(7), 490–496. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lee CT, 2007. T-helper 1/T-helper 2 cytokine imbalance and clinical phenotypes of acute-phase major depression. Psychiatry Clin Neurosci 61(4), 415–420. [DOI] [PubMed] [Google Scholar]
- Isung J, Mobarrez F, Nordström P, Asberg M, Jokinen J, 2012. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J Biol Psychiatry 13(6), 468–473. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin Å, Träskman-Bendz L, Brundin L, 2011. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun 25(2), 335–339. [DOI] [PubMed] [Google Scholar]
- Joffre C, Rey C, Layé S, 2019. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front Pharmacol 10, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlović D, Serretti A, Vrkić N, Martinac M, Marčinko D, 2012. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res 198(1), 74–80. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Brodsky B, Ellis SP, Stanley B, John Mann J, 2006. Aggressiveness, not impulsiveness or hostility, distinguishes suicide attempters with major depression. Psychol. Med. 36(12), 1779–1788. [DOI] [PubMed] [Google Scholar]
- Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF, 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135(5), 373–387. [DOI] [PubMed] [Google Scholar]
- Kok Kendirlioglu B, Unalan Ozpercin P, Yuksel Oksuz O, Sozen S, Cihnioglu R, Kalelioglu T, Ilnem MC, Karamustafalioglu N, 2020. Resolvin D1 as a novel anti-inflammatory marker in manic, depressive and euthymic states of bipolar disorder. Nord J Psychiatry 74(2), 83–88. [DOI] [PubMed] [Google Scholar]
- Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, Campanelli F, Natale G, Marino G, Calabrese V, Vedele F, Ghiglieri V, Picconi B, Di Lazzaro G, Schirinzi T, Sancesario G, Casadei N, Riess O, Bernardini S, Pisani A, Calabresi P, Viscomi MT, Serhan CN, Chiurchiù V, D’Amelio M, Mercuri NB, 2019. Author Correction: Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun 10(1), 4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layé S, Nadjar A, Joffre C, Bazinet RP, 2018. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol Rev 70(1), 12–38. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD, 2011. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J Clin Psychiatry 72(12), 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP, 2010. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 68(2), 140–147. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Erhardt S, Traskman-Bendz L, Engstrom G, Brundin L, 2011. CSF biomarkers in suicide attempters--a principal component analysis. Acta Psychiatr. Scand. 124(1), 52–61. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Björkqvist M, Träskman-Bendz L, Brundin L, 2009. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry 66(3), 287–292. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Hezghia A, Shaikh SR, Cenido JF, Stark RE, Mann JJ, Sublette ME, 2018. Regulation of monoamine transporters and receptors by lipid microdomains: implications for depression. Neuropsychopharmacology 43(11), 2165–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Li S, Li H, Mou T, Zhou L, Huang B, Huang M, Xu Y, 2019. Changes In Plasma NPY, IL-1β And Hypocretin In People Who Died By Suicide. Neuropsychiatr Dis Treat 15, 2893–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Carvalho AF, 2018. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol Neurobiol 55(12), 8885–8903. [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Bosmans E, Lin A, Neels H, 2000. In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol. Psychiatry 47(10), 910–920. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Bosmans E, 2007. Why fish oils may not always be adequate treatments for depression or other inflammatory illnesses: docosahexaenoic acid, an omega-3 polyunsaturated fatty acid, induces a Th-1-like immune response. Neuro Endocrinol Lett 28(6), 875–880. [PubMed] [Google Scholar]
- Martinez JM, Garakani A, Yehuda R, Gorman JM, 2012. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress. Anxiety 29(1), 32–38. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW, 2010. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. PLEFA 83(4–6), 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef MA, Munro IA, Garg ML, 2009. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur. J. Clin. Nutr. 63(9), 1154–1156. [DOI] [PubMed] [Google Scholar]
- Miller ES, Sakowicz A, Roy A, Yang A, Sullivan JT, Grobman WA, Wisner KL, 2019. Plasma and cerebrospinal fluid inflammatory cytokines in perinatal depression. Am J Obstet Gynecol 220(3), 271.e271–271.e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfrim X, Gazal M, De Leon PB, Quevedo L, Souza LD, Jansen K, Oses JP, Pinheiro RT, Silva RA, Lara DR, Ghisleni G, Spessato B, Kaster MP, 2014. Immune dysfunction in bipolar disorder and suicide risk: is there an association between peripheral corticotropin-releasing hormone and interleukin-1β? Bipolar Disord 16(7), 741–747. [DOI] [PubMed] [Google Scholar]
- Nassberger L, Traskman-Bendz L, 1993. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta Psychiatr. Scand. 88(1), 48–52. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, O’Farrelly C, Malone KM, 2013. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress. Anxiety 30(4), 307–314. [DOI] [PubMed] [Google Scholar]
- Oliver E, McGillicuddy FC, Harford KA, Reynolds CM, Phillips CM, Ferguson JF, Roche HM, 2012. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between LC n-3 PUFA. The Journal of nutritional biochemistry 23(9), 1192–1200. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ, 2004. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am. J. Psychiatry 161(8), 1433–1441. [DOI] [PubMed] [Google Scholar]
- Ovaskainen Y, Koponen H, Jokelainen J, Keinänen-Kiukaanniemi S, Kumpusalo E, Vanhala M, 2009. Depressive symptomatology is associated with decreased interleukin-1 beta and increased interleukin-1 receptor antagonist levels in males. Psychiatry Res 167(1–2), 73–79. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y, 2014. Toll-like receptors in the depressed and suicide brain. J. Psychiatr. Res. 53, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y, 2012. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J. Psychiatr. Res. 46(1), 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X, 2018. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J Psychiatry Neurosci 43(6), 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschoal VA, Vinolo MA, Crisma AR, Magdalon J, Curi R, 2013. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid differentially modulate rat neutrophil function in vitro. Lipids 48(2), 93–103. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH, 2010. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry 15(4), 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey C, Nadjar A, Buaud B, Vaysse C, Aubert A, Pallet V, Layé S, Joffre C, 2016. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun 55, 249–259. [DOI] [PubMed] [Google Scholar]
- Serhan CN, 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510(7503), 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki P, Aminoshariae A, Jin G, Montagnese TA, Mickel A, 2013. The effect of docosahexaenoic acid (DHA) on expression of IL-1ß, IL-6, IL-8, and TNF-α in normal and lipopolysaccharide (LPS)-stimulated macrophages. Quintessence Int 44(6), 393. [DOI] [PubMed] [Google Scholar]
- Suarez EC, 2003. Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosom. Med. 65(4), 523–527. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, Young KH, 2004. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology 29(9), 1119–1128. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Kuhn C, 2002. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain. Behav. Immun. 16(6), 675–684. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT, 2011a. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain. Behav. Immun. 25(6), 1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ, 2006. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am. J. Psychiatry 163(6), 1100–1102. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Segal-Isaacson CJ, Cooper TB, Fekri S, Vanegas N, Galfalvy HC, Oquendo MA, Mann JJ, 2011b. Validation of a food frequency questionnaire to assess intake of n-3 polyunsaturated fatty acids in subjects with and without major depressive disorder. J Am Diet Assoc 111(1), 117–123.e111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Sullenbarger B, Prakash R, McDaniel JC, 2018. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids 132, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR, 1992. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. J. Immunol. Methods 153(1–2), 115–124. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Möller HJ, Chen HH, Postolache TT, 2008. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand. 117(3), 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N, 2014. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain. Behav. Immun. 42, 50–59. [DOI] [PubMed] [Google Scholar]
- van Heeringen K, Mann JJ, 2014. The neurobiology of suicide. The lancet. Psychiatry 1(1), 63–72. [DOI] [PubMed] [Google Scholar]
- Ventro GJ, Yang Y, Chen M, Harmon CM, 2017. The molecular impact of omega 3 fatty acids on hepatic pro-inflammatory cytokine signaling. J Pediatr Surg 52(6), 1020–1025. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP, 2005. IL-6 and its circadian secretion in humans. Neuroimmunomodulation 12(3), 131–140. [DOI] [PubMed] [Google Scholar]
- Virkkunen ME, Horrobin DF, Jenkins DK, Manku MS, 1987. Plasma phospholipid essential fatty acids and prostaglandins in alcoholic, habitually violent, and impulsive offenders. Biol. Psychiatry 22(9), 1087–1096. [DOI] [PubMed] [Google Scholar]
- Wang AK, Miller BJ, 2018. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr Bull 44(1), 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM, 2007. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. The Journal of nutritional biochemistry 18(4), 250–258. [DOI] [PubMed] [Google Scholar]
- WHO, 2020. Suicide Prevention. https://www.who.int/mental_health/prevention/suicide/suicideprevent/en/. 2020).
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH, 2009. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 284(40), 27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Song Y, Guo A, 2019. Anti-Apoptotic Effects of Docosahexaenoic Acid in IL-1β-Induced Human Chondrosarcoma Cell Death through Involvement of the MAPK Signaling Pathway. Cytogenet Genome Res 158(1), 17–24. [DOI] [PubMed] [Google Scholar]
- Zaalberg A, Wielders J, Bulten E, van der Staak C, Wouters A, Nijman H, 2016. Relationships of diet-related blood parameters and blood lead levels with psychopathology and aggression in forensic psychiatric inpatients. Crim Behav Ment Health 26(3), 196–211. [DOI] [PubMed] [Google Scholar]
- Zhou X, Fragala MS, McElhaney JE, Kuchel GA, 2010. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care 13(5), 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.