Abstract
Mesenchymal progenitor cells are broadly distributed across perivascular niches – an observation conserved between species. One common histologic zone with a high frequency of mesenchymal progenitor cells within mammalian tissues is the tunica adventitia, the outer layer of blood vessel walls populated by cells with a fibroblastic morphology. The diversity and functions of (re)generative cells present in this outermost perivascular niche are under intense investigation; we have reviewed herein our current knowledge of adventitial cell potential with a somewhat narrow focus on bone formation. Antigens of interest to functionally segregate adventicytes are discussed, including CD10, CD107a, ALDH isoforms, and CD140a among others. Purified adventicytes (such as CD10+, CD107alow, and CD140a+ cells) have stronger osteogenic potential and promote bone formation in vivo. Recent bone tissue engineering applications of adventitial cells are also presented. A better understanding of perivascular progenitor cell subsets may represent a beneficial advance for future efforts in tissue repair and bioengineering.
Keywords: perivascular stem cell, adipose stem cell, mesenchymal stem cell, mesenchymal stromal cell, tunica adventitia, adipogenesis, osteogenesis, CD107a, LAMP1, CD10, ALDH, CD140a, exocytosis
Graphical Abstract
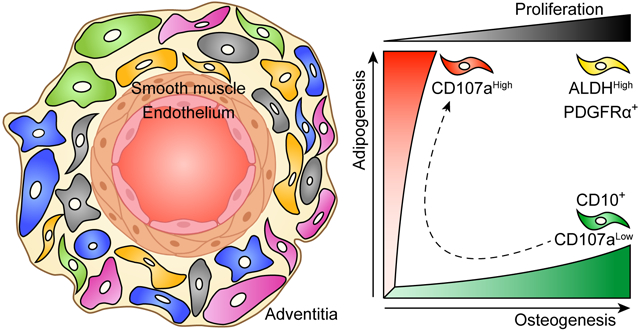
Mesenchymal progenitor cells in the tunica adventitia have a hierarchy of differentiation and proliferation potential. On top of the hierarchy, PDGFRα+ and ALDHHigh cells show a bi-potent differentiation potential into osteogenic and adipogenic cell lineages with high proliferative rate. Conversely, CD10 and CD107a expression separate osteogenic progenitors from adipogenic progenitors, respectively. Moreover, osteoprogenitors can transition into adipogenic phenotype.
Introduction
From her observations of chicken blastoderms within her anatomy laboratory at Johns Hopkins University, Florence Sabin concluded in 1917 that “Angioblasts and later endothelial cells give rise to red blood cells” [1]. This visionary insight into hematopoietic stem cell biology, more than 40 years before Till & McCulloch’s discovery of blood cell progenitors, received dazzling experimental confirmations from the 1970s [2], to result in recent years in the thorough anatomic, molecular, and developmental characterization of the “hemogenic endothelium” [3]. Much later in life, endothelial cells contribute to scarring in the infarcted myocardium by transdifferentiating into fibroblasts [4] in a reversible manner [5]. Moreover, the embryonic dorsal aorta and other adult blood vessels host potent skeletal myogenic progenitors [6], further illustrating the developmental flexibility of some vascular cells. In a teleological perspective, physical association of progenitor cells with blood vessels should permit the ubiquitous dissemination of tissue regenerative potentials. Such a tentative correlation between anatomy and function guided the search for the native origin of mesenchymal stem cells (MSCs), the culture derived multi-lineage mesodermal progenitors that can be extracted from all vascularized tissues [7]. Markers expressed by perivascular cells had been detected on cells from the human uterus that give rise to MSC like progenitors [8]. Then, some of us observed that pericytes, the mural cells that ensheath capillaries and microvessels [9], purified by flow cytometry from all human organs tested produce MSCs in culture [10], establishing a perivascular distribution for the forerunners of these multipotent cells. Purified pericytes have been used experimentally to engineer blood vessels [11], and regenerate lung [12], skeletal muscle [13], cartilage [14], ischemic limbs [15], tendon [16], and uterus [17]. Besides, pericytes naturally contribute to regenerating Leydig cells in the testis [18], satellite cells and myofibers in skeletal muscle [19], white adipocytes [20], follicular dendritic cells [21], dental cells [22], and fibroblasts in multiple tissues [23,24]. Mesodermal lineage potential is also present in the tunica media and tunica adventitia of larger arteries and veins, where it has been studied in the context of pathologic vascular remodeling [25,26]. In agreement, presumptive MSCs have been described in the human [27] and murine vascular adventitia [28], allowing to conclude that blood vessels of all sizes are repositories for mesodermal progenitor cells. Quantitatively, the tunica adventitia represents a substantial reserve of primitive mesodermal progenitors [29] of undisputable pathophysiologic relevance and possible therapeutic significance. The diversity and functions of (re)generative cells present in this outermost perivascular niche are under intense investigation; we have reviewed herein our current knowledge of adventitial cell potential with respect to bone formation, in culture and in vivo.
Different cell types
The osteoblastogenic potential of perivascular adventitial cells
Adventitial cells (a.k.a. adventicytes), so-named as they lie in the tunica adventitia of blood vessels, have a non-descript fibroblastic morphology and at times appear to be in continuity with fascial connective tissue. Defined as a CD34+CD146−Lin− cell population, we and others have described their multipotency [27,28]. The osteoblastic potential of adventitial cells has been summarized in several recent reviews [30,31]. Perivascular adventitial cells participate directly in bone formation and repair [32,33] as well as indirectly induce bone repair via interaction with native skeletal cells [34,35]. Implanted perivascular cells regenerate bone indirectly via pleiotropic mechanisms, including for example release of extracellular vesicles (EV) [34] as well as non-vesicular paracrine effectors, such as bone morphogenetic proteins [36]. For example, human perivascular EVs induce osteoprogenitor cell proliferation, migration and osteogenic differentiation to induce bone repair [34]. In contrast, human perivascular cells inhibit osteoclast formation and prevent bone graft resorption via non-vesicular paracrine mechanisms [36]. Negative regulators of osteoclast differentiation were enriched within perivascular stem cells (PSCs), including the decoy receptor for RANKL osteoprotegerin (TNRSF11B), the Wnt and RANKL inhibitor secreted frizzled-related protein-1 (SFRP1), and anti-osteoclastic/axonal guidance molecules such as semaphorin 3A (SEMA3A) and slit guidance ligand 3 (SLIT3). The relative roles of human adventitial cells and pericytes in bone repair were described recently by our group [37]. Here, CD34+ adventitial cells have a more prominent synthetic role in the formation of bone matrix, whereas CD146+ pericytes play a supportive role in the induction of blood vessel ingrowth [37]. Other markers that typify adventitial cells have been described in mouse models, including stem cell antigen-1 (Sca-1) [38], Gli1 [28], and platelet-derived growth factor receptor (PDGFR) α [32]. The expression of PDGFRα on most adventitial cells brings to the fore the possible overlap in terminology between fibro-adipoprogenitor cells (FAPs) and adventitial cells. Certainly soft tissue resident FAPs, like adventitial cells, have been described to ossify under appropriate contexts [39,40]. Although adventitial cells have been clearly identified as an osteogenic precursor, the heterogeneity within this cell population has been increasingly documented.
Roles in pathophysiologic processes
Cellular heterogeneity within the tunica adventitia of mice
The functional study of subsets of adventitial cells has been possible by the generation of different mouse models that helped track the origin and contribution of these cells during injury and disease as well as tissue homeostasis. Indeed, different groups have identified subsets of adventitial cells involved in fibrosis, calcification and regeneration. Earlier studies implicated Sca-1- and PDGFRβ-expressing adventitial cells enriched for sonic hedgehog (Shh) signaling activity as cells with a stem-like identity [41]. For instance, Gli1+ adventitial cells are myofibroblast progenitors and contribute to fibrosis in different organs [42,43]. Moreover, this same population of adventitial cells expressing Gli1 can migrate to the intima, become osteoblast-like cells and contribute to vessel calcification during chronic kidney disease (CKD) [28], and in the bone marrow Gli1+ mesenchymal cells contribute to bone marrow fibrosis (BMF) and dysregulation of hematopoietic stem cells [43]. In this context, Gli1 appears to be a pan-marker of fibrotic cells in different organs making it a potential therapeutic target. Nonetheless, the adventitia shows high heterogeneity and other markers have been described.
Understanding of the mechanisms by which perivascular cells contribute during the regeneration process is crucial to develop new strategies to treat diseases such as fibrosis. In specific, the identification of functional subsets is important to either inhibit or promote a given cell fate and improve tissue regeneration. For example, Rafael Kramann’s group has recently reported a cell atlas of both human and mouse kidney in which they identified subpopulations of mesenchymal cells including perivascular cells as likely contributors to kidney fibrosis and furthermore described Naked Cuticle Homolog 2 (Nkd2) as a specific myofibroblast target [44].
PDGFRα and PDGFRβ play key roles in mesenchymal biology. Both of these receptors are involved in cellular proliferation, migration and differentiation [45]. Moreover, subsets of cells expressing PDGFRα, PDGFRβ, or both have divergent functions in regeneration. For example, PDGFRα+PDGFRβ+ perivascular cells within skeletal muscle have been observed to have fibroadipogenic properties, whereas PDGFRβ+PDGFRα− perivascular cells have regenerative / myogenic features [32]. In skeletal muscle and cardiac tissue, αv integrins on PDGFRβ+ perivascular cells promote the formation of fibrotic tissue [24]. In adipose tissue, PDGFRα/PDGFRβ regulate cell differentiation into white or brown adipocytes as well as transition into myofibroblasts [46,47].
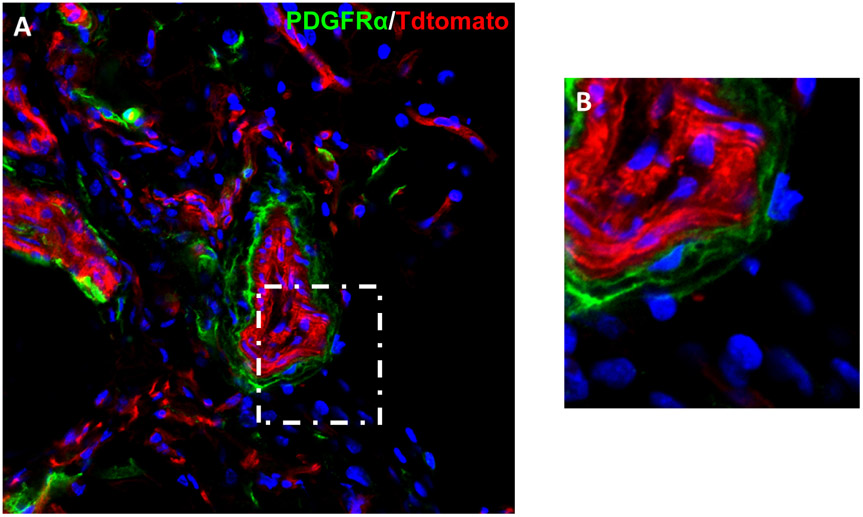
The use of PDGFRα reporter activity within mouse white adipose tissue to differentiate subsets of adventitial cells has been recently described [32] (Fig. 1). PDGFRα reporter cells are located predominantly in the inner layer of the adventitia, while the cell surface marker CD34 highlights the majority of this layer [32]. Using fluorescence-activated cell sorting (FACS) isolation of adventitial cell subsets, PDGFRα and CD34 co-expressing adventicytes showed greater osteogenic potential than PDGFRα+ only or CD34+PDGFRα− cells [32]. Indeed PDGFRα+ perivascular cells demonstrated more stem cell features than other cell fractions. In addition to higher proliferation rate, PDGFRα+ cells re-populated the tunica adventitia more effectively than PDGFRα− perivascular cells upon isolation and re-transplantation. However, the stem-like identity of PDGFRα-expressing adventitial cells was restricted to bone and adipose lineages. For example, long-term lineage tracing failed to reveal any contribution of PDGFRα-expressing adventicytes to the smooth muscle medial layer in homeostatic conditions [32]. When implanted with bone graft material, PDGFRα+ cells participated in osteoblastogenesis to a greater degree than PDGFRα− perivascular cells. Finally, upon stimulation with BMP2, endogenous PDGFRα+ reporter cells and their cellular descendants became both osteoblasts, adipocytes and new perivascular cells within new-formed ossicles [32]. These results parallel experimental studies in skeletal muscle where a large portion of PDGFRα-expressing cells give rise to dystrophic calcification and ossification during heterotopic bone formation [41].
Figure 1. PDGFRα marks a population of cells within the tunica adventitia.
(A) PDGFRαmT/mG reporter mice contain green PDGFRα + cells within the tunica adventitia in the inguinal fat pad . All other cells are red. Nuclear counterstain appears in blue. (B) High magnification of the tunica adventitia.
Cellular heterogeneity of the human adventitia
Less is known about the mechanism regulating vascular stem cells in the human adventitia, and whether the subsets described in mice have analogous counterparts in the human vasculature. In vitro studies of FACS sorted perivascular cells, transcriptomic analysis and immunohistochemistry on tissue samples from healthy and diseased individuals can help us understand the mechanisms by which these cells contribute to regeneration and link findings from mouse models to human pathobiology. For example, Kramann et al. extended their findings on vascular calcification during mouse CKD by performing Gli1 immunohistochemistry on human arteries obtained from dialysis-dependent and non-CKD subjects. Expression of Gli1 in non-CKD patients was mainly found in the adventitial layer, whereas in dialysis-dependent patients Gli1 expression was present in the calcified media and atherosclerotic plaque [28]. This suggests that Gli1 has similar functions in human and mouse arteries, making it a possible therapeutic target in vascular calcification. We have identified cell subsets in the human adventitia expressing CD10 or CD107a, and distinct differentiation potentials [33,48]. Transcriptomic analysis of human adventitial cells also revealed that high aldehyde dehydrogenase (ALDH) activity marks stem cell-like cells [29]. In this section, we will discuss in detail these novel markers of the human adventitia.
ALDH activity has been used as a marker of stem cells: hematopoietic and neural stem and progenitor cells exhibit high ALDH activity [49], also reported in adipose tissue [50] and myogenic progenitors [51]. On the other hand, high ALDH activity has been linked, in various cancers [52], to stem cell features such as tumor initiation, clonogenic growth, self-renewal and drug resistance [53,54]. Hardy et al. analyzed gene expression in single human pericytes and adventitial cells further separated according to ALDH activity and revealed the existence of a developmental hierarchy of human perivascular cells, ranging from ALDH high adventicytes (most primitive) to ALDH low pericytes (least primitive). Adventitial cells show a distribution of cells ranging from low to high ALDH activity, whereas pericytes exhibit mostly low ALDH activity, suggesting that adventitial cells contain more stem cell-like cells than pericytes do. Therefore, the tunica adventitia and more specifically adventitial cells with high ALDH activity may contain cells with stem cell properties [29]. The stem cell properties of this subset of adventitial cells may be related to the involvement of ALDH isoforms in the retinoic acid pathway. For instance, the ALDH1 family of enzymes regulate cell proliferation and differentiation by converting oxidase retinaldehyde (retinal) to retinoic acid (RA), which subsequently interacts with nuclear receptors to promote gene transcription [28,55]. Lastly, unpublished data from our group indicate the existence of a specific isoform from the ALDH1 family expressed in adventitial cells (Gomez-Salazar et al., in preparation).
CD10, also known as neprilysin, or membrane metalloendopeptidase, is a zinc-dependent metalloendoprotease involved in peptide signaling. CD10 regulates the extracellular concentration of various peptides, changing the availability for receptor binding and therefore regulating biological processes [48]. Expression of CD10 plays key roles in the regulation of stem cells by cleaving peptides which are then either activated or inhibited to continue or stop the signaling cascade [56]. For example, CD10 regulates cell migration and angiogenesis through Akt, Rho, and FGF signaling [57,58]. CD10 is also highly expressed in leukemia and in solid childhood tumors including nephroblastoma and neuroblastoma [59], and is used as a marker of good prognosis in certain types of leukemia [60]. On the other hand, CD10 expression also identifies normal stem cells in different tissues including hematopoietic (lymphoid) progenitors, as well as other organ systems [61,62]. In the context of vascular biology, our group identified a novel CD10+ adventitial progenitor cell type with higher proliferation rate and osteogenic differentiation potential compared to the negative population, suggesting pathological functions during vessel remodeling [48]. Ding et al., showed that expression of CD10 by adventitial cells is regulated through SHH/Gli1, which is interesting since Gli1 is involved in vessel calcification. CD10+ adventitial cells express genes related to stem cell potential, such as SRY-box transcription factor 2 (SOX2) and NANOG, as well as the cell proliferation related gene cell cycle G1/S specific cyclin D2 (CCND2). Moreover, CD10+ adventitial cells strongly express neural epidermal growth factor-like 1 (NELL1), which is a promoter of bone development. Whether bone regeneration or vascular calcification directly involve CD10, or whether this is a mere marker of a functional cell subset within the adventitia, is not known yet.
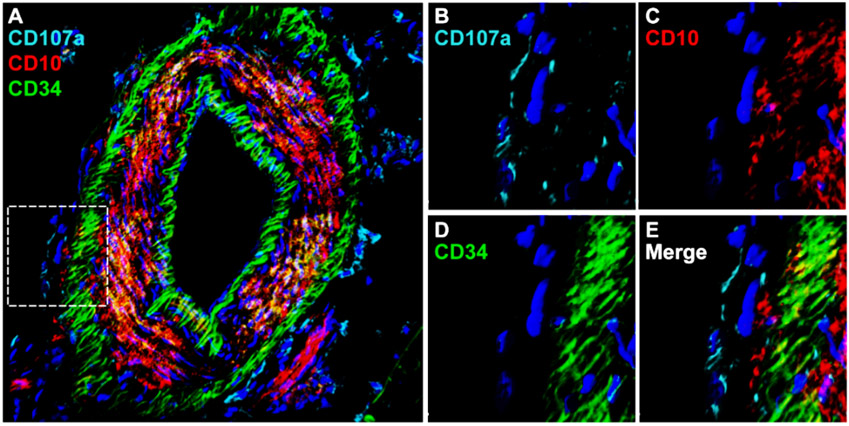
CD107a, also known as lysosome-associated membrane protein-1 (LAMP1), is a type 1 membrane protein highly expressed in lysosomes and other intracellular vesicles [63]. While CD107a is ubiquitously expressed intracellularly, only a fraction of mammalian cells display detectable surface CD107a. Our group recently identified surface CD107a as a marker to segregate functionally relevant cells within the human adventitial cell niche [33]. CD107a immunoreactivity is found most frequently within the outermost layers of blood vessels, and more common in the outer tunica adventitia. FACS-derived CD107alow and CD107ahigh stromal cells from human white adipose tissue have opposite differentiation potentials. The CD107alow stromal component contains a precursor cell population with high osteoblastogenic potential, while CD107ahigh cells represent an adipocyte precursor cell. Transcriptomic analysis demonstrates that genes associated with adipogenic differentiation, such as FABP4 (fatty acid binding protein 4), LPL (lipoprotein lipase), PPARGC1A (PPARG coactivator 1 α), and CEBPA (CCAAT enhancer binding protein α), are highly expressed among CD107ahigh stromal cells. Conversely, negative regulators of adipogenesis, such as KLF2 (Krüppel-like Factor 2), KLF3, SIRT1 (sirtuin 1), and DDIT3 (DNA damage inducible transcript 3), are increased among CD107alow stromal cells. In addition, CD107alow stromal cells are enriched for signaling pathways associated with bone formation and cellular respiration and metabolism, including Wnt/β-catenin signaling, oxidative phosphorylation, and glutathione metabolism. CD107alow cells also drive higher osteogenic differentiation in vivo. Xenotransplantation confirmed significant quantitative differences in bone generation among CD107a cellular subsets. Briefly, an accumulation of new bone at 8 weeks was observed after intramuscular implantation in NOD-SCID mice of CD107alow rather than CD107ahigh sorted cells. Human CD107alow cells also increased posterolateral lumbar spine fusion in athymic rats. Analyses performed after 8 weeks demonstrated 62.5% spine fusion among CD107alow cell treated animals, whereas CD107ahigh cell transplanted animals only showed 37.5% fusion. In summary, these studies pointed to CD107alow mesenchymal cells as a cell subset with higher osteogenic potential. Interestingly, and as expected from functional differences, zones of expression of CD10 and CD107a within the tunica adventitia of vessels are distinct (Fig. 2).
Figure 2. CD10 and CD107a mark distinct subpopulations within the tunica adventitia.
(A-E) Immunofluorescent staining for CD107a, CD10, and CD34 in an artery within human subcutaneous white adipose tissue. (A) Whole vessel in cross-section showing CD34 expression in the endothelial and adventitial layers. CD10 and CD107a expression are seen on different subsets in the inner and outer adventitia. (B-E) High magnification of the tunica adventitia.
Clinical application and perspectives
Despite the high number of pre-clinical studies showing positive results with the use of mesenchymal progenitor cells, their use in the clinical setting is limited [64]. Among the factors affecting efficiency is the use of total cell preparations containing subsets that may hinder the efficacy of regeneration, resulting in inconsistent clinical outcomes. Moreover, clonal selection within total cell preparations may further reduce numbers of highly regenerative progenitor cells. Our group specially has focused on elucidating functional heterogeneity of perivascular cells that may contribute to standardizing cell preparations and improving clinical outcomes. For instance, we have shown that CD10+ cell preparations have increased osteogenic potential, which will likely enhance regeneration in skeletal injuries. Tailoring of cell therapies for specific pathologies may represent a step forwards in realizing the potential of multipotent progenitor cells for tissue engineering [7].
Not discussed here, the vehicle or scaffold for cell deployment is also vitally important for efforts in skeletal tissue regeneration. Progenitor cells are highly influenced by their microenvironment, and the physical and molecular characteristics of a given scaffold will result in shifts in cell phenotype and functional outcomes in terms of tissue formed. One such example using human perivascular cells was recently reported, where tunable supramolecular hydrogels along with different stiffnesses exert changes in pericyte differentiation toward osteogenic and chondrogenic lineages [65].
Conclusion
Despite its relatively unremarkable histologic appearance, the tunica adventitia houses a wealth of cell types – some of which have mesenchymal progenitor cell attributes. This brief review covered only some of the established and emerging markers in mouse and human tissues that resolve functionally relevant subsets of perivascular cells. In addition to harboring progenitor cells, the adventitial layer is a major site of accumulation of immune cells including macrophages, lymphocytes, mast cells and dendritic cells that carry out important surveillance and innate immune functions in response to foreign antigens and play a role in vascular pathologies including atherosclerosis and tissue fibrosis [66]. Whether a specific subset of mesenchymal progenitor cells in this perivascular niche is involved in immune regulation and subsequent tissue remodeling is yet to be investigated. A critical point is that many markers used to purify cells within the tunica adventitia are also present in minor degrees in other cellular locations, such as the perineural tissues or fascia of white adipose tissue. The fascia is a framework of connective tissue that envelops and separates organs and tissues [67]. In adipose tissue, the fascia contains pre-adipocytes with high differentiation potential [68]. Cells in the fascia express markers shared with perivascular cells such as CD34 and CD44 [69]. In a similar manner, perineural cells express markers also found in mesenchymal cells such as vimentin, CD34 and α-SMA [70,71]. Moreover, during development in zebrafish and mouse, Schwann cell precursors give rise to mesenchymal progenitors that subsequently differentiate into chondrocytes and osteocytes, describing a common developmental origin that may explain why they share similar expression patterns with adventitial cells [72]. All this exemplifies the complexity of purifying and studying perivascular progenitor cells. Until we have more specific markers for adventitial cells, purification of perivascular progenitors will be prone to contain a fraction of other cell types. Importantly, the inherent regenerative potential of specific subsets of adventitial cells will further improve efficiency and consistency when used in bioengineering approaches.
This review focused on adipose tissue perivascular cells, but similar cells within the bone marrow are also well characterized as multipotent progenitors, termed CXC chemokine ligand (CXCL)12-abundant reticular (CAR) cells or leptin-receptor-positive (LepR+) stromal cells. LepR+ cells are the major source of bone and adipocytes in adult bone marrow [73]. Short-term ablation of CAR cells in vivo impairs osteogenesis from marrow cells [74]. Furthermore, CAR cells and LepR+ stromal cells have been implicated in maintaining the quiescent hematopoietic stem cell (HSC) pool and appear to be a key component of HSC niches [75,76]. Mechanistically, Foxc1, expressed in CAR cells, is essential for HSC maintenance and promotes CAR cell development by upregulating CXCL12 and stem cell factor expression [77]. In addition, the transcription factor early B-cell factor 3 (Ebf3) is preferentially expressed in CAR/LepR+ cells, required to create HSC niches and maintain spaces for HSCs [78]. In contrast to adipose tissue as discussed above, bone marrow perivascular cells are primarily housed within microvessels and have a perivascular position consistent with pericytes. To our knowledge, adventitial cells have not been isolated or characterized from skeletal sources.
Several unanswered questions regarding these recent findings are most notable. For example, distribution of novel markers such as CD10 and CD107a suggests a microarchitectural spatial organization of the tunica adventitia within fat tissue that had been previously underrecognized. Yet, the broader spatial relations between adventitial cells, and whether these are conserved across organ systems remain unknown. Most obviously, the hunt for a definitive stem cell within the tunica adventitia – one with self-renewal potential – is a matter of considerable interest. Certainly, identification of more primitive / progenitor cell types within vessel walls has broad implications for vascular biology, but also usefulness in the field of tissue engineering and regenerative medicine.
Table 1.
Phenotypes and functionalities of arterial adventitial cells in man and mice.
| Markers | Organisms | Function | Reference |
|---|---|---|---|
| ALDHhigh | Human | Osteogenic and adipogenic potential (predicted) | Hardy WR, et al. Stem Cells 2017 29 |
| PDGFRα+ | Mice | Osteogenic and adipogenic potential | Wang Y, et al. Stem Cells 2020 32 |
| CD107ahigh | Human | Adipogenic potential | Xu J, et al. Elife 2020 33 |
| CD10+ | Human | Osteogenic potential | Ding L, et al. Stem Cells 2020 48 |
Acknowledgments
A.W.J. is funded by NIH/NIAMS (R01 AR070773), NIH/NIDCR (R21 DE027922), USAMRAA through the Peer Reviewed Medical Research Program (W81XWH-18-1-0121, W81XWH-18-1-0336), Broad Agency Announcement (W81XWH-18-10613), Peer Reviewed Orthopaedic Research Program (W81XWH-20-1-0795), American Cancer Society (Research Scholar Grant, RSG-18-027-01-CSM), and the Maryland Stem Cell Research Foundation.
Footnotes
Conflicts
A.W.J. is a paid consultant for Novadip LLC and Lifesprout LLC. This arrangement has been reviewed and approved by the JHU in accordance with its conflict of interest polices. A.W.J. receives funding for unrelated research from MTF Biologics and Novadip, and is on the editorial board of American Journal of Pathology and Bone Research. A.W.J. is the inventor of methods to purify CD107a progenitor cells held by the Johns Hopkins University. B.P. is the inventor of perivascular stem cell related patents held by the UC Regents and is on the editorial board of Stem Cells.
All of the other authors declared no potential conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. J Hematother Stem Cell Res 2002;11(1):5–7. [DOI] [PubMed] [Google Scholar]
- 2.Dieterlen-Lievre F On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol 1975;33(3):607–619. [PubMed] [Google Scholar]
- 3.Gritz E, Hirschi KK. Specification and function of hemogenic endothelium during embryogenesis. Cell Mol Life Sci 2016;73(8):1547–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeisberg EM, Tarnavski O, Zeisberg M et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007;13(8):952–961. [DOI] [PubMed] [Google Scholar]
- 5.Ubil E, Duan J, Pillai IC et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 2014;514(7524):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng B, Cao B, Crisan M et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol 2007;25(9):1025–1034. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Discher DE, Peault BM et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 2007;22(11):2903–2911. [DOI] [PubMed] [Google Scholar]
- 9.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011;21(2):193–215. [DOI] [PubMed] [Google Scholar]
- 10.Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3(3):301–313. [DOI] [PubMed] [Google Scholar]
- 11.He W, Nieponice A, Soletti L et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials 2010;31(32):8235–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montemurro T, Andriolo G, Montelatici E et al. Differentiation and migration properties of human foetal umbilical cord perivascular cells: potential for lung repair. J Cell Mol Med 2011;15(4):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park TS, Gavina M, Chen CW et al. Placental perivascular cells for human muscle regeneration. Stem Cells Dev 2011;20(3):451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alakpa EV, Jayawarna V, Burgess KEV et al. Improving cartilage phenotype from differentiated pericytes in tunable peptide hydrogels. Sci Rep 2017;7(1):6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dar A, Domev H, Ben-Yosef O et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 2012;125(1):87–99. [DOI] [PubMed] [Google Scholar]
- 16.Devana SK, Kelley BV, McBride OJ et al. Adipose-derived Human Perivascular Stem Cells May Improve Achilles Tendon Healing in Rats. Clin Orthop Relat Res 2018;476(10):2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Yan G, Diao Q et al. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell Res Ther 2019;10(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curley M, Gonzalez ZN, Milne L et al. Human Adipose-derived Pericytes Display Steroidogenic Lineage Potential in Vitro and Influence Leydig Cell Regeneration in Vivo in Rats. Sci Rep 2019;9(1):15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellavalle A, Maroli G, Covarello D et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2011;2:499. [DOI] [PubMed] [Google Scholar]
- 20.Tang W, Zeve D, Suh JM et al. White fat progenitor cells reside in the adipose vasculature. Science 2008;322(5901):583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krautler NJ, Kana V, Kranich J et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 2012;150(1):194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H, Feng J, Seidel K et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014;14(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulauroy S, Di Carlo SE, Langa F et al. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 2012;18(8):1262–1270. [DOI] [PubMed] [Google Scholar]
- 24.Murray IR, Gonzalez ZN, Baily J et al. alphav integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat Commun 2017;8(1):1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passman JN, Dong XR, Wu SP et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A 2008;105(27):9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker AH, Peault B. A Gli(1)ttering Role for Perivascular Stem Cells in Blood Vessel Remodeling. Cell Stem Cell 2016;19(5):563–565. [DOI] [PubMed] [Google Scholar]
- 27.Corselli M, Chen CW, Sun B et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 2012;21(8):1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramann R, Goettsch C, Wongboonsin J et al. Adventitial MSC-like Cells Are Progenitors of Vascular Smooth Muscle Cells and Drive Vascular Calcification in Chronic Kidney Disease. Cell Stem Cell 2016;19(5):628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy WR, Moldovan NI, Moldovan L et al. Transcriptional Networks in Single Perivascular Cells Sorted from Human Adipose Tissue Reveal a Hierarchy of Mesenchymal Stem Cells. Stem Cells 2017;35(5):1273–1289. [DOI] [PubMed] [Google Scholar]
- 30.James AW, Peault B. Perivascular Mesenchymal Progenitors for Bone Regeneration. J Orthop Res 2019;37(6):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crisan M, Corselli M, Chen WC et al. Perivascular cells for regenerative medicine. J Cell Mol Med 2012;16(12):2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Xu J, Meyers CA et al. PDGFRalpha marks distinct perivascular populations with different osteogenic potential within adipose tissue. Stem Cells 2020;38(2):276–290. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Wang Y, Hsu CY et al. Lysosomal protein surface expression discriminates fat- from bone-forming human mesenchymal precursor cells. Elife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Wang Y, Hsu CY et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung CG, James AW, Asatrian G et al. Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl Med 2014;3(10):1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negri S, Wang Y, Sono T et al. Human perivascular stem cells prevent bone graft resorption in osteoporotic contexts by inhibiting osteoclast formation. Stem Cells Transl Med 2020;9(12):1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Xu J, Chang L et al. Relative contributions of adipose-resident CD146(+) pericytes and CD34(+) adventitial progenitor cells in bone tissue engineering. NPJ Regen Med 2019;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psaltis PJ, Simari RD. Vascular Wall Progenitor Cells in Health and Disease [in English]. Circulation Research 2015;116(8):1392–1412. [DOI] [PubMed] [Google Scholar]
- 39.Wosczyna MN, Biswas AA, Cogswell CA et al. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res 2012;27(5):1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lees-Shepard JB, Yamamoto M, Biswas AA et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun 2018;9(1):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisner C, Cummings M, Johnston G et al. Murine Tissue-Resident PDGFRalpha+ Fibro-Adipogenic Progenitors Spontaneously Acquire Osteogenic Phenotype in an Altered Inflammatory Environment. J Bone Miner Res 2020;35(8):1525–1534. [DOI] [PubMed] [Google Scholar]
- 42.Kramann R, Schneider RK, DiRocco DP et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015;16(1):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider RK, Mullally A, Dugourd A et al. Gli1(+) Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell 2017;20(6):785–800 e788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuppe C, Ibrahim MM, Kranz J et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen PH, Chen X, He X. Platelet-derived growth factors and their receptors: structural and functional perspectives. Biochim Biophys Acta 2013;1834(10):2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dani C, Pfeifer A. The complexity of PDGFR signaling: regulation of adipose progenitor maintenance and adipocyte-myofibroblast transition. Stem Cell Investig 2017;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao Z, Daquinag AC, Su F et al. PDGFRalpha/PDGFRbeta signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development 2018;145(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding L, Vezzani B, Khan N et al. CD10 expression identifies a subset of human perivascular progenitor cells with high proliferation and calcification potentials. Stem Cells 2020;38(2):261–275. [DOI] [PubMed] [Google Scholar]
- 49.Corti S, Locatelli F, Papadimitriou D et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells 2006;24(4):975–985. [DOI] [PubMed] [Google Scholar]
- 50.Estes BT, Wu AW, Storms RW et al. Extended passaging, but not aldehyde dehydrogenase activity, increases the chondrogenic potential of human adipose-derived adult stem cells. J Cell Physiol 2006;209(3):987–995. [DOI] [PubMed] [Google Scholar]
- 51.Jean E, Laoudj-Chenivesse D, Notarnicola C et al. Aldehyde dehydrogenase activity promotes survival of human muscle precursor cells. J Cell Mol Med 2011;15(1):119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginestier C, Hur MH, Charafe-Jauffret E et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1(5):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Awad O, Yustein JT, Shah P et al. High ALDH activity identifies chemotherapy-resistant Ewing's sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PLoS One 2010;5(11):e13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Hoogen C, van der Horst G, Cheung H et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res 2010;70(12):5163–5173. [DOI] [PubMed] [Google Scholar]
- 55.Tomita H, Tanaka K, Tanaka T et al. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016;7(10):11018–11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kenny AJ, O'Hare MJ, Gusterson BA. Cell-surface peptidases as modulators of growth and differentiation. Lancet 1989;2(8666):785–787. [DOI] [PubMed] [Google Scholar]
- 57.Sumitomo M, Shen R, Nanus DM. Involvement of neutral endopeptidase in neoplastic progression. Biochim Biophys Acta 2005;1751(1):52–59. [DOI] [PubMed] [Google Scholar]
- 58.Goodman OB Jr., Febbraio M, Simantov R et al. Neprilysin inhibits angiogenesis via proteolysis of fibroblast growth factor-2. J Biol Chem 2006;281(44):33597–33605. [DOI] [PubMed] [Google Scholar]
- 59.Pilkington GR, Pallesen G. Phenotypic characterization of non-haemopoietic small cell tumours of childhood with monoclonal antibodies to leucocytes, epithelial cells and cytoskeletal proteins. Histopathology 1989;14(4):347–357. [DOI] [PubMed] [Google Scholar]
- 60.Dakka N, Bellaoui H, Bouzid N et al. CD10 AND CD34 expression in childhood acute lymphoblastic leukemia in Morocco: clinical relevance and outcome. Pediatr Hematol Oncol 2009;26(4):216–231. [DOI] [PubMed] [Google Scholar]
- 61.Buhring HJ, Battula VL, Treml S et al. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci 2007;1106:262–271. [DOI] [PubMed] [Google Scholar]
- 62.Bachelard-Cascales E, Chapellier M, Delay E et al. The CD10 enzyme is a key player to identify and regulate human mammary stem cells. Stem Cells 2010;28(6):1081–1088. [DOI] [PubMed] [Google Scholar]
- 63.Defays A, David A, de Gassart A et al. BAD-LAMP is a novel biomarker of nonactivated human plasmacytoid dendritic cells. Blood 2011;118(3):609–617. [DOI] [PubMed] [Google Scholar]
- 64.Gomez-Salazar M, Gonzalez-Galofre ZN, Casamitjana J et al. Five Decades Later, Are Mesenchymal Stem Cells Still Relevant? Front Bioeng Biotechnol 2020;8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enateri V. Alakpa, Vineetha Jayawarna, Ayala Lampel et al. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem 2016;1(2):298–319. [Google Scholar]
- 66.Zhu X, Zhang HW, Chen HN et al. Perivascular adipose tissue dysfunction aggravates adventitial remodeling in obese mini pigs via NLRP3 inflammasome/IL-1 signaling pathway. Acta Pharmacol Sin 2019;40(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schleip R, Jager H, Klingler W. What is 'fascia'? A review of different nomenclatures. J Bodyw Mov Ther 2012;16(4):496–502. [DOI] [PubMed] [Google Scholar]
- 68.Su X, Lyu Y, Wang W et al. Fascia Origin of Adipose Cells. Stem Cells 2016;34(5):1407–1419. [DOI] [PubMed] [Google Scholar]
- 69.Correa-Gallegos D, Jiang D, Christ S et al. Patch repair of deep wounds by mobilized fascia. Nature 2019;576(7786):287–292. [DOI] [PubMed] [Google Scholar]
- 70.Triolo D, Dina G, Taveggia C et al. Vimentin regulates peripheral nerve myelination. Development 2012;139(7):1359–1367. [DOI] [PubMed] [Google Scholar]
- 71.Cizkova D, Soukup T, Mokry J. Expression of nestin, desmin and vimentin in intact and regenerating muscle spindles of rat hind limb skeletal muscles. Histochem Cell Biol 2009;131(2):197–206. [DOI] [PubMed] [Google Scholar]
- 72.Xie M, Kamenev D, Kaucka M et al. Schwann cell precursors contribute to skeletal formation during embryonic development in mice and zebrafish. Proc Natl Acad Sci U S A 2019;116(30):15068–15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou BO, Yue R, Murphy MM et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014;15(2):154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omatsu Y, Sugiyama T, Kohara H et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 2010;33(3):387–399. [DOI] [PubMed] [Google Scholar]
- 75.Sugiyama T, Kohara H, Noda M et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006;25(6):977–988. [DOI] [PubMed] [Google Scholar]
- 76.Ding L, Saunders TL, Enikolopov G et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012;481(7382):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omatsu Y, Seike M, Sugiyama T et al. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature 2014;508(7497):536–540. [DOI] [PubMed] [Google Scholar]
- 78.Seike M, Omatsu Y, Watanabe H et al. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev 2018;32(5-6):359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.