Abstract
A multiple-primer PCR was used to identify genes encoding aminoglycoside-modifying enzymes in 381 clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). The technique used three sets of primers delineating specific DNA fragments of the aph(3′)-III, ant(4′)-I, and aac(6′)-aph(2") genes, which influence the MICs of gentamicin, tobramycin, and lividomycin. Isolates with none of the three genes detected were susceptible to all three agents. Isolates with the aph(3′)-III gene showed resistance to lividomycin (MIC > 1,024 μg/ml), and those with the ant(4′)-I gene were resistant to tobramycin (MIC ≥ 8 μg/ml). Isolates with only the aac(6′)-aph(2") gene were resistant to gentamicin (MIC ≥ 8 μg/ml) and tobramycin in decreasing order; those with both the ant(4′)-I and aac(6′)-aph(2") genes also were resistant to gentamicin and tobramycin, but in increasing order. Susceptibility testing, then, could detect specific genes. In 381 Japanese MRSA isolates, the ant(4′)-I, aac(6′)-aph(2"), and aph(3′)-III genes were prevalent in 84.5, 61.7, and 8.9%, respectively. Isolates with only the ant(4′)-I gene had coagulase type II or III, but isolates with both the ant(4′)-I and aac(6′)-aph(2") genes included all coagulase types. Most isolates with coagulase type IV or VII carried the aac(6′)-aph(2") gene. Of the MRSA isolates with ant(4′)-I and/or aac(6′)-aph(2") genes, 97% were resistant to aminoglycosides in clinical use, but a new aminoglycoside, arbekacin, had excellent activity against these isolates.
Enzymatic modification of aminoglycosides is a common mechanism of resistance to these antibiotics shown by clinical bacterial isolates. Among gram-positive cocci such as staphylococci, streptococci, and enterococci, five kinds of aminoglycoside-modifying enzymes (AME) occur: aminoglycoside-6-O-nucleotidyltransferase I [ANT(6)-I] (21), aminoglycoside-9-O-nucleotidyltransferase I [ANT(9)-I] (16), aminoglycoside-3′-O-phosphoryltransferase III [APH(3′)-III] (7), aminoglycoside-4′-O-phosphoryltransferase I [ANT(4′)-I] (14) and aminoglycoside-6′-N-acetyltransferase/2"-O-phosphoryltransferase [AAC(6′)/APH(2")] (6, 24). APH(3′)-III, ANT(4′)-I, and AAC(6′)/APH(2") are of particular significance because they modify aminoglycosides of therapeutic importance, including kanamycin, tobramycin, and gentamicin, respectively. These modifying enzymes can be plasmid or chromosome encoded and often are encoded on transposable elements (3).
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of nosocomial infection (10), and these bacteria have acquired multiple resistance to a wide range of antibiotics including aminoglycosides (9, 10, 37). AME produced by MRSA isolates can be determined by identifying the corresponding genes. Susceptibility profiles to selected aminoglycosides previously have been used to detect specific aminoglycoside resistance mechanisms. However, characterizing strains containing several AME genes solely on the basis of aminoglycoside resistance profiles can be difficult, since one resistance profile is often partially duplicated thereby masking the presence of an additional profile. DNA hybridization and PCR amplification are sensitive and specific methods for the detection of genes including those encoding AME (34, 36, 38). However, such special techniques and the necessary equipment are not practical for the routine clinical laboratory, unlike conventional susceptibility tests.
In the present study, we compared aminoglycoside resistance profiles to PCR data to determine whether susceptibility tests could reproducibly detect specific AME in MRSA. We then used the results to study the epidemiology of AME in Japanese MRSA isolates.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The reference strains used in this study were three transductants, pMS18, pMS91, and pMS555. Each of these was transduced by S2 phage in S. aureus MS353. The pMS18 transductant is known to carry two genes, ant(6)-I and aph(3′)-III; the pMS91 transductant is known to carry three genes, ant(6)-I, aph(3′)-III, and aac(6′)-aph(2"); and the pMS555 transductant carries one gene, ant(4′)-I (27).
A total of 381 MRSA strains were collected from various medical settings in different parts of Japan. MRSA strains were identified by growth on plates containing culture medium supplemented with 6 μg of oxacillin (Sigma, St. Louis, Mo.) per ml and 4% NaCl.
Antibiotics and chemicals.
Reference samples of various aminoglycosides and other antimicrobial agents of known potency were kindly supplied as powders by the manufactures, as follows: kanamycin, streptomycin, and arbekacin were from Meiji Seika Kaisha, Tokyo, Japan; gentamicin was from Schering-Plough Japan, Osaka, Japan; and tobramycin was from Shionogi Pharmaceutical, Osaka, Japan. Lividomycin was obtained commercially (Sigma).
Determination of MICs.
MICs were determined by the twofold agar dilution method in Sensitivity Disk Agar N (Nissui, Tokyo, Japan). The bacteria were grown overnight in Sensitivity Test broth (Nissui) at 35°C. The culture was diluted to a final concentration of 106 CFU/ml with buffered saline containing gelatin. The bacterial suspensions were delivered by an inoculator (Sakuma Seisaku, Tokyo, Japan) with an inoculum size of 104 CFU/spot on agar plates. Inoculated plates were incubated for 18 h at 35°C. The MIC was defined as the lowest concentration of the compound that prevented visible growth.
DNA isolation.
Each strain was subcultured overnight at 35°C on brain heart infusion agar (Nissui). Bacteria grown on plates were suspended in 100 μl of lysing solution (20 mM Tris-HCl, 140 mM NaCl, 5 mM EDTA [pH 8.0]) containing 250 μg of lysostaphin (Sigma) and incubated at 37°C for 30 min. After the suspensions were cooled on ice, 200 μl of distilled water was added to each, and they were heated at 65°C for 5 min. Subsequently, phenol-chloroform extraction and ethanol precipitation were performed as described by Okamoto et al. (20). The pellet was dried briefly in a vacuum desiccator and dissolved in 100 μl of distilled water. A 10-μl volume of a 1:10 dilution of the total DNA solution was used for PCR.
PCR experiments.
Heat-stable Taq polymerase, the four deoxynucleoside triphosphates, and PCR buffer were purchased from Takara Shuzo (Otsu, Japan). As primers for PCR (see below), 20-mer oligonucleotides were used; these were purchased from Takara Shuzo. Cell lysates as processed above (10 μl) were added to a PCR mixture containing each primer at 0.1 μM, 10 μL of a 10-fold concentrate of PCR buffer, deoxynucleoside triphosphates (each at 200 μM), and 2.5 U of Taq polymerase in a final volume of 90 μl of distilled water. To prevent evaporation, 2 drops of mineral oil (Sigma) was added to each mixture.
A thermal cycler (Perkin-Elmer Cetus, Emeryville, Calif.) was used for amplification of DNA. The cycling program included 30 cycles of a denaturing step at 94°C for 1 min, an annealing step at 57°C for 2 min, and an extension step at 72°C for 30 s. Then 5-μl volumes of the samples were taken for analysis by electrophoresis on 2% agarose gels (FMC BioProducts, Rockland, Maine) in Tris-borate-EDTA buffer. The PCR products were detected by ethidium bromide staining under UV illumination.
Design of primers for PCR.
Three sets of primers were designed to detect the three different genes encoding AME in a single test. All primer sequences were chosen from a site within the nucleotide sequence of the AME gene region known to be specific for an enzyme. The primers for detection of the aph(3′)-III gene were based on the nucleotide sequences reported by Gray and Fitch (7). The 5′ primer was CGATGTGGATTGCGAAAACT; the 3′ primer was CACCGAAATAACTAGAACCC. Primers for detection of the aac(6′)-aph(2") gene were based on the nucleotide sequences reported by Ferretti et al. (6) and Rouch et al. (24). The 5′ primer was CATTATACAGAGCCTTGGGA; the 3′ primer was AGGTTCTCGTTATTCCCGTA. Primers for detection of the ant(4′)-I gene were based on the nucleotide sequences reported by Matsumura et al. (14). The 5′ primer was ATGGCTCTCTTGGTCGTCAG; the 3′ primer was TAAGCACACGTTCCTGGCTG. The primers did not interact with one another or with genes encoding other AME.
Coagulase typing.
Coagulase types were discerned by inactivation of coagulase activity type-specific antisera (33). The specific antisera and normal rabbit plasma for coagulase typing were purchased from Denka Seiken (Tokyo, Japan). Clinical isolates were grown overnight in 5 ml of brain heart infusion agar at 35°C. After a 30-min centrifugation at 1,600 × g 0.1 ml of supernatant was aliquotted into each of nine tubes. Type-specific antiserum (0.1 ml) or control serum was added to each tube, and the mixtures were incubated at 37°C for 1 h. Finally, 0.2 ml of normal rabbit plasma was added to all tubes. Coagulase types were determined by inhibition of clotting after incubation at 37°C for 1 to 48 h.
RESULTS
Primer specificity.
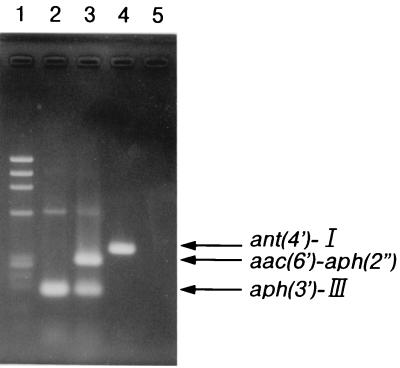
An agarose gel separation of DNA fragments amplified from total DNA isolated from reference strains is shown in Fig. 1. The primers for the aph(3′)-III gene yielded a fragment of 175 bp. This DNA fragment was amplified from total DNA isolated from S. aureus MS353(pMS18) and S. aureus MS353(pMS91). The primers for the aac(6′)-aph(2") gene yielded a fragment of 279 bp. This DNA fragment was amplified only from total DNA isolated from MS353(pMS91). The primers for the ant(4′)-I gene yielded a fragment of 367 bp. This DNA fragment was amplified only from total DNA isolated from S. aureus MS353(pMS555). Then different primers for the three genes were mixed and used to test the specificity of these primers with mixed DNA isolated from MS353(pMS18), MS353(pMS91), and MS353(pMS555). As expected, three different sizes of amplified DNA, of 175, 279, and 367 bp, were detected. These results indicated that the PCR products following amplification and the aminoglycoside resistance profiles were in correct agreement. Therefore, the specificity of the primers selected for this study was confirmed, as well as the specificity and sensitivity of the method for detection of these three genes encoding AME.
FIG. 1.
Agarose gel electrophoresis of amplified DNA fragments from reference strains. Lanes: 1, maker DNA (φX174 HaeIII digest); 2, S. aureus MS353(pMS18); 3, S. aureus MS353(pMS91); 4, S. aureus MS353(pMS555); 5, S. aureus MS353 as a negative control.
PCR identification of genes encoding AME in clinical isolates.
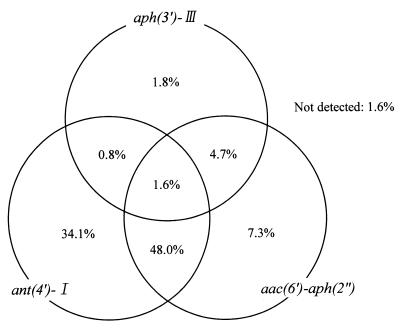
The genes encoding AME were subjected to PCR amplification and to agarose gel electrophoresis. The frequencies of the genes encoding AME detected by PCR are shown in Fig. 2 for the 381 isolates. PCR products were amplified from 375 of the 381 isolates but not from the remaining 6 isolates (1.6%). The ant(4′)-I gene was encountered most frequently (84.5%), and 59.6% of isolates carried this gene in combination with one or both of the others. The aph(3′)-III and aac(6′)-aph(2") genes were present in 8.9 and 61.7% of isolates, respectively. The most frequent combination of genes was ant(4′)-I with aac(6′)-aph(2") (48%). The aph(3′)-III gene was present in combination with either the aac(6′)-aph(2") gene or the ant(4′)-I gene in 4.7 and 0.8% of isolates respectively. The triple combination of aph(3′)-III, ant(4′)-I, and aac(6′)-aph(2") was present in 1.6% of isolates.
FIG. 2.
Distribution of genes encoding AME as determined by PCR detection in 381 isolates of MRSA.
Correlation of aminoglycoside susceptibilities and the presence of AME genes.
The MICs of three aminoglycosides, gentamicin, tobramycin, and lividomycin, for the 381 isolates characterized above by PCR are shown in Table 1. All 235 isolates with the aac(6′)-aph(2") gene were resistant to gentamicin (≥8 μg/ml), and most of them were also resistant to tobramycin (≥8 μg/ml). A total of 322 isolates with the ant(4′)-I gene were highly resistant to tobramycin (≥128 μg/ml); 34 isolates with the aph(3′)-III gene were highly resistant to lividomycin (≥1,024 μg/ml); and 6 isolates with none of the three genes were susceptible to gentamicin (≤1 μg/ml), tobramycin (≤1 μg/ml), and lividomycin (≤8 μg/ml).
TABLE 1.
PCR detection of genes encoding AME and MIC distributions in clinical MRSA isolates
| Detection by PCR
|
Total no. of strains | antibiotic | No. of strains for which the MIC (μg/ml) is:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aac(6′)-aph(2") | ant(4′)-I | aph(3′)-III | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1,024 | >1,024 | ||
| Gentamicin | 1 | 2 | 3 | |||||||||||||||
| + | + | + | 6 | Tobramycin | 2 | 1 | 3 | |||||||||||
| Lividomycin | 6 | |||||||||||||||||
| Gentamicin | 4 | 3 | 2 | 45 | 86 | 16 | 14 | 8 | 5 | |||||||||
| + | + | − | 183 | Tobramycin | 7 | 28 | 92 | 44 | 12 | |||||||||
| Lividomycin | 7 | 92 | 77 | 6 | 1 | |||||||||||||
| Gentamicin | 1 | 2 | 3 | 5 | 4 | 2 | 1 | |||||||||||
| + | − | + | 18 | Tobramycin | 1 | 2 | 4 | 5 | 5 | 1 | ||||||||
| Lividomycin | 18 | |||||||||||||||||
| Gentamicin | 5 | 6 | 1 | 6 | 5 | 2 | 2 | 1 | ||||||||||
| + | − | − | 28 | Tobramycin | 5 | 6 | 1 | 8 | 5 | 1 | 2 | |||||||
| Lividomycin | 5 | 22 | 1 | |||||||||||||||
| Gentamicin | 3 | |||||||||||||||||
| − | + | + | 3 | Tobramycin | 3 | |||||||||||||
| Lividomycin | 3 | |||||||||||||||||
| Gentamicin | 6 | 104 | 20 | |||||||||||||||
| − | + | − | 130 | Tobramycin | 7 | 54 | 63 | 6 | ||||||||||
| Lividomycin | 4 | 74 | 49 | 3 | ||||||||||||||
| Gentamicin | 6 | 1 | ||||||||||||||||
| − | − | + | 7 | Tobramycin | 6 | 1 | ||||||||||||
| Lividomycin | 7 | |||||||||||||||||
| Gentamicin | 5 | 1 | ||||||||||||||||
| − | − | − | 6 | Tobramycin | 5 | 1 | ||||||||||||
| Lividomycin | 3 | 3 | ||||||||||||||||
The gentamicin resistance in MRSA isolates was associated only with the aac(6′)-aph(2") gene, and the cutoff MIC of gentamicin between susceptible and resistant isolates was 8 μg/ml. The lividomycin resistance in MRSA isolates was associated with the aph(3′)-III and ant(4′)-I genes. All isolates with the aph(3′)-III gene were highly resistant to lividomycin (≥1,024 μg/ml); on the other hand, the isolates with the ant(4′)-I gene but without the aph(3′)-III gene were only mildly resistant to lividomycin (8 to 128 μg/ml). The tobramycin resistance in MRSA isolates was subjected to the genes carrying ant(4′)-I and aac(6′)-aph(2"). However, from the determination of the MIC of tobramycin, it was difficult to identify these genes in tobramycin-resistant isolates.
Relationship between the MICs of gentamicin and tobramycin in MRSA isolates with the aac(6′)-aph(2") gene.
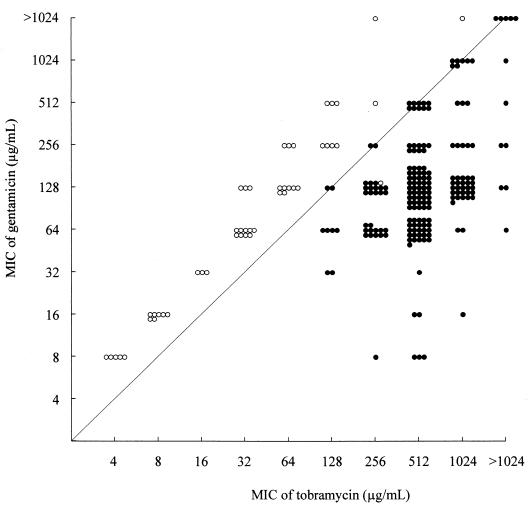
As mentioned above, although all 235 isolates with the aac(6′)-aph(2") gene were resistant to gentamicin, it has not been clarified in susceptibility tests using a kind of aminoglycoside whether they also contained the ant(4′)-I gene. However, determining whether the aac(6′)-aph(2") gene was combined with the ant(4′)-I gene required a comparison of the MICs of gentamicin and tobramycin (Fig. 3). For most isolates (45 of 46) with the aac(6′)-aph(2") gene and without the ant(4′)-I gene, the MIC of gentamicin was higher than that of tobramycin. All 189 isolates with both the ant(4′)-I and aac(6′)-aph(2") genes were resistant to tobramycin and gentamicin, but for these bacteria the MIC of tobramycin was either higher than or equivalent to that of gentamicin. Using the above results, susceptibility tests for lividomycin, tobramycin, and gentamicin could reproducibly detect specific AME in MRSA.
FIG. 3.
Correlation between the MICs of gentamicin and tobramycin for 235 MRSA isolates with the aac(6′)-aph(2") gene. Each circle indicates one of strains. The solid circles indicate the isolates with both the aac(6′)-aph(2") and ant(4′)-I genes, and the open circles indicate the isolates with the aac(6′)-aph(2") gene but not the ant(4′)-I gene.
Coagulase typing and AME.
The coagulase types of 350 of the 381 tested strains were successfully determined using specific antisera against eight different types of coagulase in S. aureus. Type II predominated (83.7% of 381 isolates). In contrast, the isolates with coagulase type III, IV, VII, or I were infrequent (3.7, 2.4, 1.3, and 0.8%, respectively). The coagulase types of 31 isolates were indistinguishable.
The relationship between coagulase type and genes encoding AME in MRSA isolates was examined (Table 2). Isolates with only the ant(4′)-I gene were of coagulase type II or III but not type I, IV, or VII. The isolates carrying the both ant(4′)-I and aac(6′)-aph(2") included all coagulase types. The results showed that isolates with the aac(6′)-aph(2") gene were more frequent among isolates with coagulase type I, IV, or VII than among those with type II or III.
TABLE 2.
Relationship between AME Genes and coagulase type in MRSA isolates
| AME genes present
|
Total no. of strains | No. of strains with coagulase type of:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| aac(6′)-aph(2") | ant(4′)-I | aph(3′)-III | I | II | III | IV | VII | NDa | |
| + | + | + | 6 | 2 | 1 | 3 | |||
| + | + | − | 183 | 1 | 147 | 7 | 1 | 4 | 23 |
| + | − | + | 18 | 13 | 3 | 2 | |||
| + | − | − | 28 | 2 | 21 | 1 | 3 | 1 | |
| − | + | + | 3 | 3 | |||||
| − | + | − | 130 | 123 | 5 | 2 | |||
| − | − | + | 7 | 5 | 1 | 1 | |||
| − | − | − | 6 | 5 | 1 | ||||
| Total | 381 | 3 | 319 | 14 | 9 | 5 | 31 | ||
ND, not determined.
Drug resistance and AME.
The relationship between genes encoding AME and aminoglycoside resistance in MRSA isolates was examined (Table 3). The interpretive categories of gentamicin, tobramycin, and kanamycin resistance were recommended by National Committee for Clinical Laboratory Standards (NCCLS), and their cutoff MIC were 8, 8, and 32 μg/ml, respectively (18). For other aminoglycosides, such as streptomycin, lividomycin, and arbekacin, the interpretive categories were not listed in the NCCLS publication. Therefore, their interpretive categories were provisionally established as follows: streptomycin, 32 μg/ml; lividomycin, 256 μg/ml; and arbekacin, 8 μg/ml. The MRSA isolates with at least one of three genes were resistant to kanamycin. Of 381 isolates, 54 (14.2%) were resistant to streptomycin. All 34 isolates with the aph(3′)-III gene were resistant to streptomycin, whereas only 5.8% of the isolates without this gene were resistant to streptomycin. Twenty-four isolates (6.3%) were resistant to arbekacin, and they were found only in isolates with the aac(6′)-aph(2") gene and showed high resistance (≥512 μg/ml) to gentamicin (data not shown).
TABLE 3.
AME and aminoglycoside resistance
| AME gene present
|
Total no. of strains | % of isolates resistant toa:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| aac(6′)-aph(2") | ant(4′)-I | aph(3′)-III | Gm (8) | Tob (8) | Lvdm (256) | Sm (32) | Km (32) | Abk (8) | |
| + | + | + | 6 | 100 | 100 | 100 | 100 | 100 | 0 |
| + | + | − | 183 | 100 | 100 | 0 | 8.2 | 100 | 11.5 |
| + | − | + | 18 | 100 | 100 | 100 | 100 | 100 | 5.6 |
| + | − | − | 28 | 100 | 82.1 | 0 | 0 | 100 | 7.1 |
| − | + | + | 3 | 0 | 100 | 100 | 100 | 100 | 0 |
| − | + | − | 130 | 0 | 100 | 0 | 3.8 | 100 | 0 |
| − | − | + | 7 | 0 | 0 | 100 | 100 | 100 | 0 |
| − | − | − | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 381 | 61.7 | 95.3 | 8.9 | 14.2 | 98.4 | 6.3 | ||
Gm, gentamicin; Tob, tobramycin; Lvdm, lividomycin; Sm, streptomycin; Km, kanamycin; Abk, arbekacin; the cutoff MIC (in micrograms per milliliter) is given in parentheses.
DISCUSSION
Beginning in the late 1970s and continuing for the last 20 years, MRSA have been isolated in connection with outbreaks of nosocomial infection in many countries around the world (2, 13). In 1982 and 1983, MRSA began to increase in prevalence throughout Japan (10). MRSA typically are resistant to various antimicrobial agents such as penicillins, cephalosporins, macrolides, aminoglycosides, tetracyclines, and fluoroquinolones (9). Because of this multidrug resistance and tendency to spread in hospital populations, MRSA have a special clinical significance, requiring epidemiologic monitoring as a measure for control of nosocomial infection. Conventional methods commonly used in the clinical laboratory for typing of S. aureus, including phage typing (22), coagulase typing (33), and antibiotyping (19), often prove useless as epidemiologic tools since of most MRSA isolated in Japan have nonsensitivity to phage production of type II coagulase and resistance to many kinds of antibiotics (9, 10). In contrast to conventional methods of MRSA typing, genetic analyses such as pulsed field gel electrophoresis (23), the DNA hybridization techniques (15, 28), and the PCR technique (35) are sensitive and versatile tools. Vanhoof et al. (36) have reported that defining the genetic determinants of AME by PCR was useful for epidemiologic surveillance of MRSA. In the present work, a relationship was found between the PCR detection of genes encoding AME and aminoglycoside resistance patterns in clinically isolated MRSA; the distribution of AME, coagulase types, and antibiotic susceptibility patterns was studied in MRSA isolated from 30 hospitals widely distributed throughout Japan.
The PCR technique used three sets of primers delineating specific DNA fragments, aph(3′)-III, ant(4′)-I, and aac(6′)-aph(2"), defined as detecting and identificating AME genes in reference strains. PCR was performed in 381 clinical isolates to identify AME genes. The 34 isolates with the aph(3′)-III gene showed high resistance to lividomycin (≥1,024 μg/ml), which has been reported to be a specific substrate of the enzyme APH(3′)-III (26). Therefore, APH(3′)-III production was determined by testing the susceptibility of strains to lividomycin. In this study, ANT(4′)-I-producing strains showed low resistance to lividomycin (8 to 128 μg/ml). Although it has not been reported that lividomycin was inactivated by ANT(4′)-I, this result suggested that it was inactivated only weakly by this enzyme. All isolates carrying the aac(6′)-aph(2") gene were resistant to gentamicin (≥8 μg/ml); therefore, it was possible to detect AAC(6′)/APH(2") production by susceptibility testing with gentamicin. However, since most aminoglycosides are substrates of this enzyme, the additional production of ANT(4′)-I is difficult to identify on the basis of antibiotic resistance patterns. Interestingly, the PCR results were almost always related to the MICs of gentamicin and tobramycin. In most isolates (45 of 46) with the aac(6′)-aph(2") gene but without the ant(4′)-I gene, the MIC of gentamicin was higher than that of tobramycin; in the isolates with both the aac(6′)-aph(2") and ant(4′)-I genes, the MIC of tobramycin was higher than or similar to that of gentamicin (Fig. 3). Ubukata et al. (30) have reported that AAC(6′)/APH(2") inactivates gentamicin more effectively than it inactivates tobramycin, and our results are compatible with and explained by this observation. Only 1 of the 235 isolates with the aac(6′)-aph(2") gene was exceptional in that the relationship between the PCR result and susceptibility testing did not show the same tendency. The reason for the discrepancy between PCR and MIC in this strain is not clear; it is possible that a mutation of the aac(6′)-aph(2") gene is incriminated in the change of substrate specificity. We should almost always be able to determine AME production in clinical isolates of MRSA by testing their susceptibility to lividomycin, gentamicin, and tobramycin, because the agreement between this method and the PCR method was 99.7% (380 of 381). Therefore, we recommend the use of this method in clinical laboratories in the epidemiologic study of MRSA.
The frequencies of genes encoding AME was studied in 381 Japanese isolates. The ant(4′)-I, aac(6′)-aph(2"), and aph(3′)-III genes were evident in 84.5, 61.7, and 8.9% of isolates, respectively. One of the reasons why the ant(4′)-I gene is the most frequent is that it adjoins the mecA gene (5, 31). In contrast, isolates with coagulase type I, IV, or VII did not carry the ant(4′)-I gene as frequently as did those with coagulase type II (Table 2). These results suggested that mec DNA regions differed between coagulase types and were compatible with other observations (M. Kurazono and T. Ida, unpublished data). AAC(6′)/APH(2") has been the enzyme most frequently found among MRSA isolated in Europe (25, 36). In contrast, gentamicin-resistant MRSA carrying the aac(6′)-aph(2") gene were encountered less frequently among isolates from Japan. The aac(6′)-aph(2") gene is encoded by transposon Tn4001 or Tn4001-like elements (11, 12, 24), and those have been detected in large plasmids in S. aureus (1, 29). The gentamicin resistance plasmids in S. aureus vary in conjugational transfer and have been isolated from different geographic areas (17). The reasons for the prevalence of the AAC(6′)/APH(2") enzyme in Japan may be more closely related to the spread of isolates with coagulase type II than to gentamicin resistance plasmids being conjugative or nonconjugative. The isolates carrying the aph(3′)-III gene were not frequent among isolates from Japan. In 27 of 34 isolates with the aph(3′)-III gene, this gene was combined with the aac(6′)-aph(2") gene and/or the ant(4′)-I gene. Since AAC(6′)/APH(2") and ANT(4′)-I are capable of inactivation for kanamycin, the aph(3′)-III gene does not appear to be necessary for these isolates. However, all isolates with the aph(3′)-III gene showed resistance to streptomycin, which is inactivated only by ANT(6)-I. The aph(3′)-III and ant(6)-I genes are carried on transposon Tn3854 on the staphylococcal plasmid and chromosome (32). For these isolates, it may be more important to produce ANT(6)-I rather than APH(3′)-III.
Most isolates in this study produced ANT(4′)-I and/or AAC(6′)/APH(2") and were resistant to aminoglycosides used in clinical therapy. However, arbekacin, a derivative of dibekacin, showed excellent antibacterial activity against tobramycin- and gentamicin-resistant MRSA (Table 3) (8, 9), because arbekacin is modified very little by ANT(4′)-I and/or AAC(6′)/APH(2") (30). In Japan, arbekacin has been approved for clinical use in MRSA infection since 1990, and no increase in the prevalence of arbekacin-resistant MRSA has been reported (4); nonetheless, a nosocomial infection caused by arbekacin-resistant MRSA has been reported at one hospital (9). Therefore, clinical laboratories should monitor the spread of arbekacin-resistant MRSA and the genes encoding AME. The susceptibility-based technique we describe and recommend here should facilitate the detection and characterization of AME.
ACKNOWLEDGMENTS
We thank the Working Group for collecting isolates of MRSA. We also thank Toshiko Hashizume and Mizuyo Kurazono for technical assistance.
This study was supported in part by the All Kitasato Project Study, Kitasato University, and the Working Group for MRSA cooperated by Meiji Seika Kaisha, Ltd., Tokyo, Japan.
REFERENCES
- 1.Archer G L, Dietrick D R, Johnston J L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985;151:243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Ayliffe G A. The progressive intercontinental spread of methicillin-resistant Sthapylococcus aureus. Clin Infect Dis. 1997;24:74–79. doi: 10.1093/clinids/24.supplement_1.s74. [DOI] [PubMed] [Google Scholar]
- 3.Brown N M, Reeves D S. Mechanisms and epidemiology of aminoglycoside resistance. J Med Microbiol. 1992;36:11–14. [Google Scholar]
- 4.Deguchi K, Suzuki Y, Ishihara R, Ishii Y, Nakazawa A. Antimicrobial activities of arbekacin against methiillin-resistant Staphylococcus aureus. Jpn J Antibiot. 1997;50:1–11. [PubMed] [Google Scholar]
- 5.Dubin D T, Matthews P R, Chikramane S G, Stewart P R. Physical mapping of the mec region of an American methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1991;35:1661–1665. doi: 10.1128/aac.35.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferretti J J, Gilmore K S, Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6′-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986;167:631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray G S, Fitch W M. Evolution of antibiotic resistance genes: the DNA sequence of a kanamycin resistance gene from Staphylococcus aureus. Mol Biol Evol. 1984;1:57–66. doi: 10.1093/oxfordjournals.molbev.a040298. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton-Miller J M T, Shah S. Activity of semi-synthetic kanamycin B derivative, arbekacin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1995;35:865–868. doi: 10.1093/jac/35.6.865. [DOI] [PubMed] [Google Scholar]
- 9.Inoue M, Nonoyama M, Okamoto R, Ida T. Antimicrobial activity of arbekacin, a new aminoglycoside antibiotic, against methicillin-resistance Staphylococcus aureus. Drugs Exp Clin Res. 1994;20:233–240. [PubMed] [Google Scholar]
- 10.Konno M. Nosocomial infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Infect Chemother. 1995;1:30–39. [Google Scholar]
- 11.Lyon B R, May J W, Skurray R A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193:554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- 12.Lyon B R, Gillespie M T, Byrne M E, May J W, Skurray R A. Plasmid-mediated resistance to gentamicin in S. aureus: the involvement of a transposon. J Med Microbiol. 1987;23:101–110. doi: 10.1099/00222615-23-2-101. [DOI] [PubMed] [Google Scholar]
- 13.Maple P A, Hamilton-Miller J M, Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Sthapylococcus aureus. Lancet. 1989;i:537–540. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura M, Katakura Y, Imanaka T, Aiba S. Enzymatic and nucleotide sequence studies of a kanamycin-inactivating enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J Bacteriol. 1984;160:413–420. doi: 10.1128/jb.160.1.413-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meugnier H, Fernandez M P, Bes M, Brun Y, Bornstein N, Freney J, Fleurette J. rRNA gene restriction patterns as an epidemiological marker in nosocomial outbreaks of Stahpylococcus aureus infections. Res Microbiol. 1993;144:25–33. doi: 10.1016/0923-2508(93)90212-k. [DOI] [PubMed] [Google Scholar]
- 16.Murphy E. Nucleotide sequence of a spectinomycin adenyltransfrase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3")(9) Mol Gen Genet. 1985;200:33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- 17.Murphy E. Transposable elements in gram-positive bacteria. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: ASM Press; 1989. pp. 269–288. [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 19.Noble W C, Howell S A. Labile antibiotic resistance in Staphylococcus aureus. J Hosp Infect. 1995;31:135–141. doi: 10.1016/0195-6701(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto R, Okubo T, Inoue M. Detection of genes regulating β-lactamase production in Enterococcus faecalis and Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:2550–2554. doi: 10.1128/aac.40.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ounissi H, Courvalin P. Appendix B. Nucleotide sequences of streptococcal genes. In: Ferretti J J, Curtiss III R, editors. Streptococcal genetics. Washington, D.C.: American Society for Microbiology; 1987. p. 275. [Google Scholar]
- 22.Parker M T. The significance of phage-typing patterns in Staphylococcus aureus. In: Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. Vol. 1. London, United Kingdom: Academic Press, Ltd.; 1983. pp. 33–62. [Google Scholar]
- 23.Prevost G, Pottecher B, Dahlet M, Bientz M, Mantz J M, Piemont Y. Pulsed-field gel electrophoresis as a new epidemiological tool for monitoring methicillin-resistant Staphylococcus aureus in an intensive care unit. J Hosp Infect. 1991;17:255–269. doi: 10.1016/0195-6701(91)90270-i. [DOI] [PubMed] [Google Scholar]
- 24.Rouch D A, Byrne M E, Kong Y C, Skurray R A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987;133:3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz F J, Fluit A C, Gondolf M G, Beyrau R, Lindenlauf E, Verhoef J, Heinz H P, Jones M E. The prevalence of aminoglycoside resistance and corresponding resistance genes in clinical isolates of staphylococci from 19 European hospitals. J Antimicrob Chemother. 1999;43:253–259. [PubMed] [Google Scholar]
- 26.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki T, Fujita K, Nagamachi Y, Okubo T. Emergence of arbekacin resistant strains among methicillin-resistant Sthapylococcus aureus. Jpn J Antibiot. 1994;47:634–639. [PubMed] [Google Scholar]
- 28.Tenover F C, Arbeit R, Archer G, Biddle J, Byrne S, Goering R, Hancock G, Hebert G A, Hill B, Hoollis R, Jarvis W R, Kreiswirth B, Eisner W, Maslow J, McDougal L K, Miller J M, Mulligan M, Pfaller M A. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J Clin Microbiol. 1994;32:407–415. doi: 10.1128/jcm.32.2.407-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend D E, Ashdown N, Greed L C, Grubb W B. Analysis of plasmids mediating gentamicin resistance in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1984;13:347–352. doi: 10.1093/jac/13.4.347. [DOI] [PubMed] [Google Scholar]
- 30.Ubukata K, Yamashita N, Gotoh A, Konno M. Purification and characterization of aminoglycoside-modifying enzymes from Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1984;25:754–759. doi: 10.1128/aac.25.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubukata K, Nonoguchi R, Matsuhashi M, Song M D, Konno M. Restriction maps of the regions coding for methicillin and tobramycin resistances in chromosomal DNA in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1989;33:1624–1626. doi: 10.1128/aac.33.9.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udo E E, Grubb W B. Transposition of genes encoding kanamycin, neomycin and streptomycin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1991;27:713–720. doi: 10.1093/jac/27.6.713. [DOI] [PubMed] [Google Scholar]
- 33.Ushioda H, Terayama T, Sakai S, Zen-Yoji H, Nishiwaki M, Hidano A. Coagulase typing of Staphylococcus aureus and its application in routine work. In: Jeljaszewicz J, editor. Staphylococci and staphylococcal infections. Stuttgart, Germany: Gustav Fischer Verlag; 1981. pp. 77–83. [Google Scholar]
- 34.van Asselt G J, Vliegenthart J S, Petit P L C, van de Klundert J A M, Mouton R P. High-level aminoglycoside resistance among enterococci and group A streptococci. J Antimicrob Chemother. 1992;30:651–659. doi: 10.1093/jac/30.5.651. [DOI] [PubMed] [Google Scholar]
- 35.van Belkum A, Bax R, Peerbooms P, Goessens W H F, van Leeuwen N, Quint W G V. Comparison of phage typing and DNA fingerprinting by polymerase chain reaction for discrimination of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1993;31:798–803. doi: 10.1128/jcm.31.4.798-803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanhoof R, Godard C, Content J, Nyssen H J, Hannecart-Pokorni E the Belgian Study Group of Hospital Infections (GDEPIH/GOSPIZ) Detection by polymerase chain reaction of genes encoding aminoglycoside-modifying enzymes in methicillin-resistant Staphylococcus aureus isolates of epidemic phage types. J Med Microbiol. 1994;41:282–290. doi: 10.1099/00222615-41-4-282. [DOI] [PubMed] [Google Scholar]
- 37.Warsa C W, Okubo T, Okamoto R. Antimicrobial susceptibilities and phage typing of Staphylococcus aureus clinical isolates in Indonesia. J Infect Chemother. 1996;2:29–33. [Google Scholar]
- 38.Weems J J, Lowrance J H, Baddour L M, Simpson W A. Molecular epidemiology of nosocomial, multiply aminoglycoside resistant Enterococcus faecalis. J Antimicrob Chemother. 1989;24:121–130. doi: 10.1093/jac/24.2.121. [DOI] [PubMed] [Google Scholar]