Abstract
Cerebrovascular disease (CVD) manifests through a broad spectrum of mechanisms that negatively impact brain and cognitive health. Oftentimes, CVD changes (excluding acute stroke) are insufficiently considered in aging and dementia studies which can lead to an incomplete picture of the etiologies contributing to the burden of cognitive impairment. Our goal with this focused review is three-fold. First, we provide a research update on the current magnetic resonance imaging (MRI) methods that can measure CVD lesions as well as early CVD related brain injury specifically related to small vessel disease. Second, we discuss the clinical implications and relevance of these CVD imaging markers for cognitive decline, incident dementia and disease progression in Alzheimer’s disease, and Alzheimer’s related dementias. Finally, we present our perspective on the outlook and challenges that remain in the field. With the increased research interest in this area, we believe that reliable CVD imaging biomarkers for aging and dementia studies are on the horizon.
Cerebrovascular disease (CVD) is a multi-factorial process negatively affecting the structure and function of the cerebral vasculature. While stroke is a clinical syndrome of CVD, a wide spectrum of CVD related disease processes that impact cognition include clinically asymptomatic cerebral infarctions, white matter hyperintensities (WMH), micro-infarctions, and microbleeds as well as more general brain changes such as blood brain barrier dysfunction, impaired interstitial fluid drainage, altered cerebral blood flow, and microstructural myelin injury1, 2. The potential impact of clinically asymptomatic CVD and the vascular etiology of these imaging findings were first identified by Hachinksi and colleagues3, 4 who described the presence of attenuated white matter signals seen on cerebral x-ray computed tomography as “leuko-araiosis”. Coincident to the discovery of leuko-araiosis, Awad and colleagues identified “incidental subcortical lesions” on MRI5 (currently denoted as WMH) and suggested a possible vascular etiology to which they ascribed the state of “état criblé” based on pathology6. These coincident observations heralded a new era in medical research related to the risk and consequences of asymptomatic cerebrovascular brain injury as evaluated by various non-invasive neuroimaging methods. The advancements and versatility of magnetic resonance imaging (MRI) have made it an indispensable tool for capturing a variety of mechanisms through which CVD impacts the brain. The multiplicity of the markers and CVD related brain changes, however, has added confusion to the field as to which marker(s) should be considered as measures of CVD risk for cognitive impairment and dementia.
Our aim for this review is to focus on MRI which as a versatile imaging modality that aids in capturing a variety of mechanisms through which CVD can impact the brain7. We largely focus on the aspect of small vessel disease because it contributes to about 50% of dementias worldwide1 and do not expand on acute stroke related changes. With this approach, we have three goals in mind. First, we present key imaging markers for measuring CVD starting with traditional lesion markers followed by methods available for measuring early CVD related brain injury. Next, we discuss clinical implications and relevance of these CVD imaging markers for cognitive decline, incident dementia and disease progression in Alzheimer’s disease (AD), and Alzheimer’s related dementias. Finally, and most importantly, we present our perspective on the outlook of research in this area.
CVD Imaging Features: Lesions
The most traditional markers of CVD related injury noted on MRI are WMH, microbleeds, microinfarcts, cortical superficial siderosis, and large infarcts (Figure 1). The population prevalence of these MRI findings are generally less than 10% at 50–59 years but increase to over 70% for those older than 80 years of age8. These changes are due to multiple different pathological processes. While genetics is a prominent driver of these changes in familial CVD, hypertension and cerebral amyloid angiopathy play a major mechanistic role in sporadic CVD that can be exacerbated by APOE4 genotype.
Figure 1:
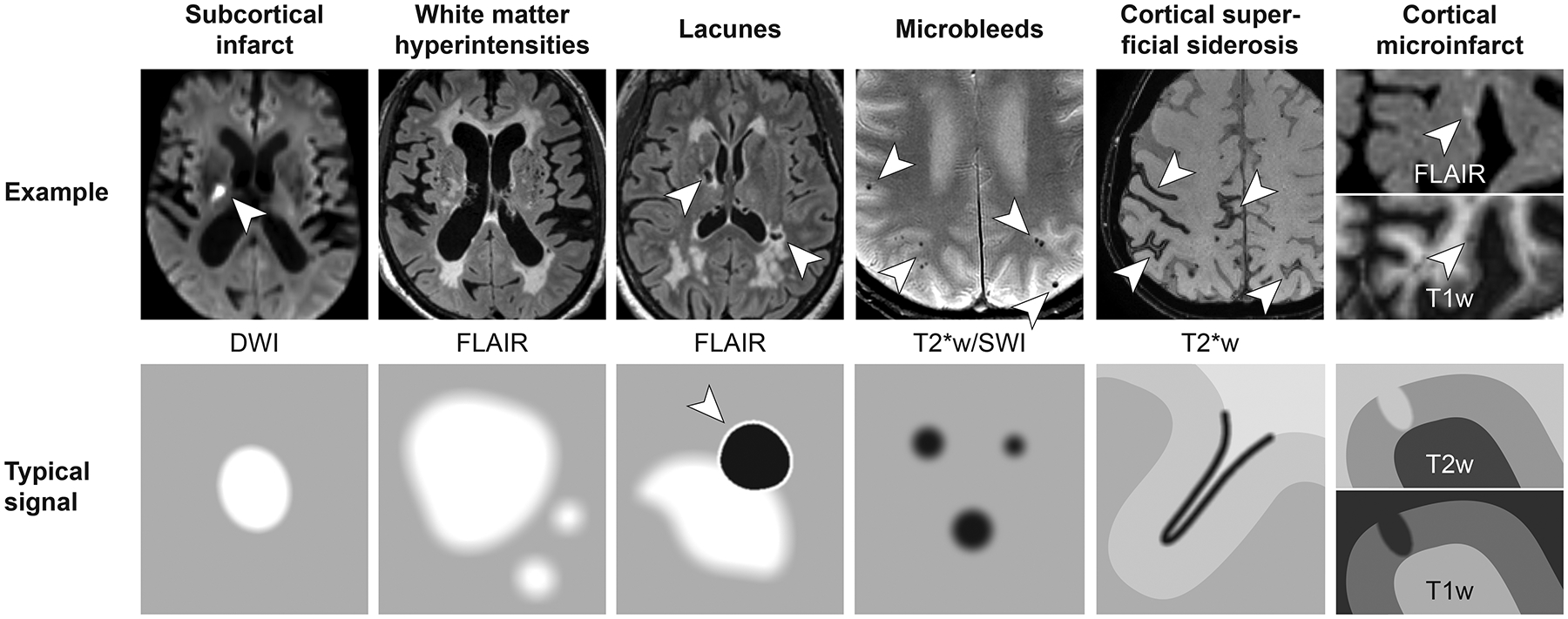
Common CVD lesions and the contrast seen on MRI. DWI=diffusion weighted imaging; FLAIR= Fluid Attenuated Inversion Recovery; SWI=Susceptibility weighted imaging.
T2/FLAIR MRI for measuring WMH and infarctions
Increasing WMH burden seen on T2 weighted or Fluid Attenuated Inversion Recovery (FLAIR) MRI is a common consequence of the aging process9, 10, exacerbated by vascular risk factors, particularly hypertension11 and generally increases over time12, 13 as individuals age. This process, however, may evolve over decades as recent studies indicate white matter injury associated with emerging WMH can begin during middle life14, 15. Numerous observations support the clinical relevance of WMH7. Not only is WMH burden associated with cognitive impairment in cross-sectional studies16, but evolution of WMH are associated with declines in both memory and executive function17. Furthermore, extensive WMH predicts incident MCI, stroke and dementia7. Consequently, the sum of existing evidence has led to WMH being recognized as the hallmark neuroimaging measure of small vessel vascular disease and associated vascular contributions to cognitive impairment and dementia (VCID).
Evidence for silent cerebral infarction was first described by Case et al. in 198918 through a comprehensive survey of stroke patients in the Framingham Heart Study. Nearly 10 years later, however, Bryan et al. identified “stroke-like lesions” on MRI of asymptomatic individuals in the Cardiovascular Health19 and the Atherosclerosis Risk in Communities studies20 and showed strong age-related increase in prevalence reaching >30% amongst the oldest participants. Subsequent large community-based studies using multi-modal MRI imaging to more accurately detect MRI infarcts21, 22 have found similar age-related differences9, 22, 23. Silent MRI infarctions share the same vascular risk factors as clinically apparent infarcts24, 25 confirming the causal association. While diffusion weighted imaging is used for reliable detection of recent infarctions, T1 imaging and FLAIR MRI are typically used for detection and quantification of silent infarction in dementia related research studies2. Multiple studies show that the MRI infarction is associated with poorer cognitive performance26–28 and predicts future MCI, dementia and death29.
The location of infarctions and WMH on T2/FLAIR vary and have been linked to heterogeneity of the underlying etiology. While lacunar strokes are likely due to hypertensive arteriopathy, cortical strokes are due to large vessel disease. There is increasing understanding that the etiology of WMH as well as progression is complex (with also some evidence of WMH reversibility30). In addition, there is evidence that there are likely non-vascular contributions to WMH in addition to the primary CVD contribution to WMH31. Research in familial AD patients, where CVD contribution to WMH is much lower, and pathology studies have provided initial evidence for the role of cerebral amyloid angiopathy (CAA) markers and cortical tau deposition in the emergence of WMH32–35. While there is evidence for greater CAA and AD related posterior WMH, further studies are required to understand the dynamics of WMH evolution with concurrent measurement of vascular health, amyloid, and tau pathologies.
T2* GRE/SWI for measuring microbleeds and superficial siderosis
T2* Gradient Recalled Echo (GRE) or Susceptibility Weighted (SWI) MR pulse sequences are most useful for measuring hemorrhagic manifestations, i.e. microbleeds and cortical superficial siderosis. Microbleeds or microhemorrhages are microscopic (~200μm) areas of blood leakage from injured vessels. Following the leakage of blood from a damaged vessel, hemosiderin is deposited in macrophages and these deposits are visible as hypointense lesions <10mm in size on T2* GRE and SWI36. SWI offers greater reliability and sensitivity in detecting microbleeds in comparison to T2* GRE37. Microbleeds can occur in either subcortical or lobar cortical brain regions. Subcortical microbleeds are associated with small vessel disease pathologies and vascular risk factors38, whereas lobar cortical microbleeds are associated mostly with cerebral amyloid angiopathy (CAA)39 where amyloid deposits in the vessel wall lead to injury and subsequent blood leakage. Focal tracer uptake in the areas of cerebral microbleeds40–43 as seen on amyloid PET support the causal relationship between amyloid and microbleeds. The presence of microbleeds are clinically relevant as meta-analyses consistently show associations with cognitive dysfunction7, 44 but further work is still warranted to understand the impact of the location of the microbleeds (lobar vs. deep) on cognitive performance (including specific domains impacted). It also remains unclear to what extent the association between microbleeds and cognition is independent of co-existing injuries such as microinfarcts or AD pathology.
Cortical superficial siderosis is a distinct pattern of iron deposits in superficial cortical layers, observed by a dark rim along the gyri on T2* GRE or SWI images45. This lesion is relatively rare in the general population and sporadic forms of small vessel disease, but more common among patients with CAA46. In CAA, cortical superficial siderosis is the strongest predictor for future intracerebral hemorrhage and poor functional outcome47.
T1 and T2 images for detection of Microinfarcts
With the emergence of increased imaging resolution of 7 Tesla ultra-high field MRI, small cortical lesions (small cavitation’s) termed as cortical microinfarcts48 can now be detected, although most microinfarcts detected on histopathological examination are even smaller, and escape high-resolution scans49. High-quality 3 T MRI can also be applied to detect small cortical lesions using standardized rating procedures, but rating is labor-intense and rater reliability is typically modest48. Cortical microinfarcts have an independent effect on cognitive impairment and dementia after accounting for traditional CVD markers such as WMH, microbleeds, and infarctions50. Small cortical lesions can also be seen with diffusion-weighted imaging, presumably resembling small acute or subacute cortical ischemia51. These cortical lesions on diffusion-weighted imaging mostly disappear without any trace on follow-up scanning,52, suggesting that the remnants of most acute cortical infarcts are too small to be detected by MRI. This is a clear limitation to current technology, but an area of future research as this technology continues to evolve.
CVD Imaging Features: Early brain changes
While lesions are traditionally used to indicate vascular brain injury, vascular risk factors specifically hypertension, causes both functional and structural alterations to the brain prior to evidence of the typical lesions noted above. New MRI measures of brain microstructural and functional changes now enable identification of more subtle brain injury due to CVD. There are four recognized methods for measuring the spectrum of early CVD changes: 1) Diffusion MRI for measuring white matter microstructural injury, 2) Arterial spin labeling and cerebrovascular reactivity for measuring cerebral blood flow, 3) T2 images for measuring dilated perivascular spaces, and 4) Volumetric methods for measuring regional brain atrophy. An image with several of these early CVD related changes is shown in Figure 2. We have placed greater focus on diffusion MRI due to the extensive evidence available in the literature indicating that it may be a specific and a sensitive biomarker to CVD.
Figure 2:
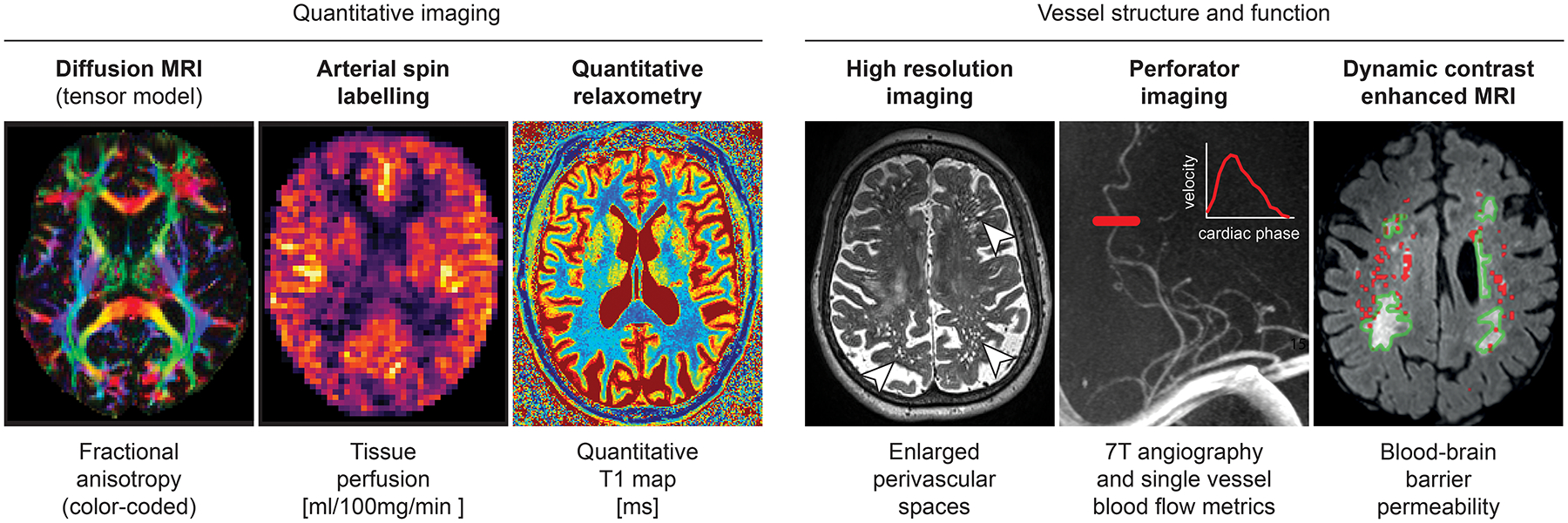
More novel MRI markers used to capture early and subtle CVD related changes through quantitative imaging or by more directly probing vessel structure and function. Perforator image was unpublished and included courtesy of Tine Arts, Jaco Zwannenburg and Geert Jan Biessels. Permeability map was adapted from Huisa et. al. 2015 with permission. Copyright 2015, the American Heart Association.
Diffusion MRI for measuring microstructural changes
Diffusion MRI has emerged as a key method for quantifying cerebrovascular burden53–56. It has been found to be more sensitive than conventional, lesion-based markers in detecting disease-related white matter alterations, even in regions appearing normal on conventional MRI53, 57. Diffusion MRI assesses brain microstructure indirectly by characterizing water mobility in the tissue. Quantification is achieved by fitting models on top of raw diffusion weighted data with multiple diffusion-encoding directions. The most used model is diffusing tensor imaging. Various metrics can be derived from the tensor including Fractional Anisotropy (FA) that specifies the directedness of diffusion (decreased with pathology) and Mean Diffusivity (MD) which summarizes the extent of diffusion (increased with pathology). Since the imaging method is based on water motion, two important sources need to be considered and controlled: patients head motion and contamination by freely diffusing water in CSF space through partial volume effects, especially in brains with atrophy.
Diffusion MRI-based metrics have shown strong associations with CVD-related clinical deficits, such as cognitive and gait impairment, typically outperforming conventional lesion markers in this regard53, 58–60. Furthermore, longitudinal diffusion MRI measures can track small vessel disease progression with high accuracy61, 62. Beyond small vessel disease, diffusion MRI can also be used to measure secondary degeneration of white matter microstructure after stroke63, 64. While other etiologies, developmental differences, and abnormalities due to inflammation, oxidative stress can cause diffusion changes, the effect of CVD has been suggested to be the most dominant effect65. Regional analysis further suggests that AD pathology causes mostly posterior white matter injury, whereas CVD is periventricular, consistent with the differing pathophysiology of these processes66, 67.
In addition to various diffusion MRI metrics, there are multiple analytical approaches, such as whole-brain or region-of-interest-based analysis of diffusion metrics, voxel-wise analysis, or structural connectomics using tractography, brain parcellations and graph theory network metrics68. By restricting analyses to the center of major white matter tracts to avoid CSF contamination, peak width of skeletonized mean diffusivity (www.psmd-marker.com) has been proposed and validated in multiple CVD samples53, 69. As more diffusion methods are proposed and more accurate biophysical models can now be applied based on newer acquisition technologies70, there is a need to thoroughly validate and compare across various approaches.
Arterial Spin Labeling and Cerebrovascular reactivity for measuring Cerebral Blood Flow
Arterial Spin Labeling (ASL) was developed in the 1990’s71 based on the idea of using water in the arterial blood as an endogenous tracer. The signal relies on blood flow and the time it takes for the labeled spins to travel from the labeling plane to the imaged voxel in the capillary bed where there is exchange of water and nutrients in the tissue. Altered perfusion is of particular interest in CVD where there is compromise of blood flow in cerebral vasculature and of the neurovascular unit is suspected72. Though ASL is considered a quantitative measure of CVD, three sources of variability reduce the utility of ASL as a marker of CVD: 1) low signal to noise of the image acquisition modality; 2) in populations with vascular pathology, prolonged arterial transit time to tissue can cause artifacts, i.e. increased signal in vascular regions, and spuriously reduced perfusion in tissue73; and 3) reduced perfusion due to neurodegeneration unrelated to CVD72. In a systematic review by Shi et. al.74, the association between cerebral blood flow and WMH was significantly attenuated when demented individuals were excluded suggesting that the effect sizes of perfusion deficits in relationship to small vessel disease especially in early disease may be small.
Cerebrovascular reactivity, CVR as is generally referred to as, measures the change in relative blood flow due to vasodilation-- most commonly after CO2 inhalation. Altered cerebrovascular reactivity is highly predictive of stroke75 but also occurs with dementia76. While cerebrovascular reactivity is an attractive methodology to measure inefficient cerebrovascular function, further systematic validation across large populations is needed.
T2 images for measuring perivascular spaces
Space surrounding the vasculature is important for the clearance of fluid and metabolic waste77. Enlarged perivascular spaces (PVS) or Virchow-Robin spaces are visible on high resolution MR imaging (hyperintensity on T2-weighted images in conjunction with hypointensity on T1-weighted images) and are identified as dilated spaces along the blood vessels. PVS are very common with aging, neurodegenerative diseases, and vascular risk factors78. Due to the importance of increased PVS in mechanisms involving several aging related disorders78 and the potential to significantly impact cognition79, there is tremendous interest in measuring these changes. The location of PVS’s are likely related to different etiologies. So far, the literature on PVS has not been consistent and there is a need for robust and reliable PVS measurements to test their clinical usefulness consistently across studies.
Volumetric MRI for measuring atrophy
While the impact of CVD is generally assumed to be associated with MRI measures such as WMH and infarctions, multiple studies show that CVD can cause cerebral and hippocampal atrophy9, 67, 80. The etiology of gray matter loss associated with CVD has received limited attention, despite studies suggesting that it may result from metabolic dysregulation81, 82, inflammation associated with atherosclerosis83 or secondary neurodegeneration63. The non-specific nature of MRI volumetric changes due to CVD, however, does not make for wide use as a CVD marker.
Clinical Implications of CVD features for Dementia
Recognizing that vascular brain injury can lead to cognitive decline and incident dementia, prompts discussion regarding the impact of CVD on dementia incidence within the general population, where AD pathology is common and co-occurrence of these two pathologies is likely84. In this section, we discuss potential mechanisms by which these two processes combine to result in incident dementia.
CVD as an essential Dementia Risk Factor
Two widely recognized AD risk factor scales include common vascular risk factors85, 86 supporting the role of vascular disease in dementia incidence even when the clinical phenotype is AD dementia. In this regard, CVD—due to increased prevalence earlier in life87 and known subtle brain injury in middle age15, 88—would lead to increased risk for late-life dementia89.
CVD and AD as additive pathologies
Pathological studies of individuals with dementia as well as combined MRI and PET imaging studies of non-demented individuals indicate an additive effect of CVD and AD pathologies90–93. This evidence is particularly strong for neuroimaging studies that find no significant association between amyloid deposition and MRI vascular makers92, 94. Moreover, in each of these studies, individuals show faster decline with both AD and CVD pathologies95, 96 as compared to either in isolation.
Synergistic effects of CVD and AD and common features
Several synergistic mechanistic hypotheses have been proposed in the literature but to date have been inconclusive and need further validation. One hypothesis proposes changes in blood flow and blood brain barrier dysfunction as an earliest marker of all neurodegenerative disorders97. Another suggests that hypoperfusion and hypoxia caused by atherosclerosis of cerebral vessels may enhance the production of Aβ, which in turn, may promote formation of atherosclerotic lesions through vascular oxidative stress and endothelial dysfunction leading to additional vascular damage38. A third hypothesizes CVD leads to poor clearance of extracellular Aβ through the glymphatic system98. Finally, there is evidence that CVD may interact with the AD process (ATN biomarker cascade99) through worsening systemic vascular health that exacerbates both amyloid100 and tau deposition101, 102. In addition, some of the CVD features are a direct consequence of AD pathophysiology. For example, it has been suggested that some of the WMH are seen due to Wallerian degeneration triggered by AD pathology103 104. However, there is no clear consensus on the mechanisms and evolution of these proposed inter-relationships.
Open Questions and Exciting Research on the Horizon
While tremendous progress towards improving the acquisition protocols, measuring these CVD changes, understanding risk factors, prevalence, and the impact of these changes on cognition has been made, biological questions remain, and exciting research avenues are on the horizon. Some of the current impediments are related to measuring the reliability of the markers across studies, need for greater precision in measurements, better acquisition and quantification of CVD changes, and lack of understanding of the pathological underpinnings and are discussed here.
1. Repeatable and reproducible marker(s) across different populations
A wide variety of measures of CVD exist including several discussed in this review. Reliability, however, is a crucial aspect for widespread application of an MRI marker in research as well as clinical care. To be reliable, MRI markers should be both repeatable (when scanning a patient twice) and reproducible (when scanning a patient on two different MRI scanners)105, 106. Unfortunately, there have been few technical validation studies, which assess the reliability of CVD measures in the target populations. Among more advanced measures, reliability is best assessed for diffusion MRI. In cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephathy or CADASIL patients, reproducibility of diffusion measures across two scanners without harmonized MRI protocol was improved by using histogram analysis53. Another study with a harmonized acquisition protocol found almost perfect reproducibility of tensor and kurtosis metrics and excellent repeatability using high-frequency serial imaging data70, 107.
Biological/clinical validity is another important issue. For example, evidence suggests that diffusion MRI has greater predictive value in comparison to traditional WMH measures70 108. There has also been literature on the need to combine several measures to create a composite CVD measure for use in clinical and research studies109–111. Yet, none of these metrics have widely been validated.
Recently, the Mark VCID consortium, supported by the National Institute on Neurological Disorders and Stroke, an institute within the NIH, was created to develop and validate methods to identify and longitudinally assess VCID (https://markvcid.partners.org/). This consortium consists of five sites and a coordinating center that has developed a series of novel “kits” using various imaging tools, blood and CSF based biomarkers to further refine methods developed for use in clinical trials specifically aimed at treatment of VCID. While this study is still ongoing, near future expectations for reliable, validated biomarkers of CVD are high.
Generalizability is another limitation of current work in CVD neuroimaging. There is a compelling need for expanding demographic diversity in studies of CVD cognitive decline. Dementia112 and vascular disease prevalence87 are both increased among diverse individuals and it is hypothesized that vascular disease may have a greater impact on the cognitive health of these individuals. Evidence for this comes from epidemiologic studies showing that APOE4 genotype conveys less risk for dementia among non-white populations113 and that vascular risk factors are associated with cognitive decline in both Black and Hispanic populations114–118. These studies are consistent with neuropathological findings that CVD is more prevalent among demented Black and Hispanic individuals119. To be relevant to the changing demographics of our aging population120, therefore, these markers need to be assessed and validated in diverse populations.
2. Development of Quantitative Imaging based measures
In a lesion-centric approach, tissue is typically labelled in a binary fashion as either being pathological or normal appearing. However, especially studies using diffusion tensor imaging have already shown that CVD causes gradual damage, which established the concept of the WMH penumbra57. Binarization is therefore artificial. Further, it can be regarded as ill-posed to quantify disease burden based on image contrast developed for visual rating, not for quantification. It is not straightforward to simply quantify arbitrary values on T1 weighted or T2 weighted scans, which do not correspond to a physical measure. Quantitative relaxometry, on the other hand, determines relaxation times (measured in ms) as a physical property of tissue, providing the basis for quantifying gradual signal alterations using quantitative T1, T2 and T2* maps121. These techniques are not new, but – except for T2* – rarely applied in patients because of long acquisition times. Recent developments in acquisition acceleration, e.g. using k-space under sampling, might propel a renaissance of quantitative MRI122. Of particular interest is magnetic resonance fingerprinting, a dictionary-based technique to obtain multi-parametric quantitative MRI data from unique patterns of signal evolution during a single, short image acquisition123.
3. Advanced diffusion models and their application to CVD
While the tensor model has frequently been used to study white matter microstructure alterations in CVD, there are other, more advanced diffusion models which potentially allow better characterization of the complex brain microstructure and thus additional insight. Free water imaging is a bi-tensor model that allows separating the contribution of extracellular free water and tissue microstructure on the diffusion signal. Studies using free water imaging have shown that diffusion alterations in CVD are largely driven by alterations in free water content.124 Free water itself has been used as a sensitive marker for clinical deficits,107, 125 and strong predictor of cognitive and functional trajectories among older individuals, as well as for unraveling pathophysiological cascades of events triggered by elevated blood pressure126. These findings led to the hypothesis that increased extracellular water due to blood brain barrier dysfunction may precede microstructural white matter changes and WMH development127.
Even more advanced models, such as diffusion kurtosis imaging or biophysical models, e.g. neurite orientation dispersion and density imaging, typically require a more elaborate diffusion acquisition scheme, with multiple and stronger diffusion weighting (multi-shell acquisition)128, 129. This leads to a prolonged acquisition time and comes with higher requirements regarding scanner hardware. So far, only one study systematically assessed the added benefit of these advanced, more demanding models and found a benefit of diffusion kurtosis imaging in assessing associations with cognitive deficits in early-stage small vessel disease70.
4. Pathological and multimodal imaging studies to understand early CVD changes
There is paucity in the number of antemortem imaging and postmortem validation studies for confirming the pathologic basis of early CVD markers. For example, diffusion alterations have been suggested to be largely driven by increased free water130 not by the degeneration of white matter tracts124, indicating a perturbed blood-brain barrier mechanism due to vascular pathology. On the other hand, Colgan et. al. showed greater sensitivity of neurite density measures from advanced diffusion models to histological measures of tau pathology131. However, unless there are systematic histopathological studies mapping the CVD features to pathological changes (e.g. leaky vessels, demyelination), we will be unable to discern the source of variability in our biomarkers.
5. Newer imaging techniques for detecting small vessel function and pathology
To date, most CVD MRI markers do not measure the small vessels, but downstream tissue alterations because of vessel pathology. MRI metrics of vessel function or integrity can be obtained, e.g. cerebrovascular reactivity using blood oxygenation level dependent-based MRI after CO2 stimulus or blood-brain-barrier function using dynamic contrast enhanced MRI132, 133. Neither of these methods are generally preferred across common dementia studies, since they are time-consuming, suffer from low signal-to-noise ratio, and require additional equipment or contrast agent application.
Instead, there is a need to develop robust methods to measure small vessel function/pathology, impaired blood-brain barrier, and endothelial dysfunction to test the mechanistic hypothesis by which CVD impacts cognitive aging and dementia. Examples of this type in development include 7 Tesla ultra-high field MRI can image single perforating arteries in vivo134 and also measure flow characteristics, such as vessel stiffness measured by pulsatility135. Similarly, non-contrast mapping of water exchange across the blood-brain barrier by diffusion-prepared perfusion also has been proposed for measuring impaired blood-brain barrier dysfunction in CVD patients imaging136. As newer methodologies emerge, however, there will be need for rapid validation and clinical translation of the techniques.
6. Better lesion detection and quantification tools
MRI acquisitions now allow for high resolution 3D acquisitions that offer better delineation and detection of CVD lesions. While manual or semi-manual detection and editing of CVD lesions have been the most common initial approaches, they are extremely labor intensive to implement given the much higher frequency of CVD lesions detected through the current acquisition strategies. There is a need, therefore, for robust computational methodologies that are widely available and can be employed to efficiently measure obvious CVD lesions as well as early brain injury for the general population.
Conclusions
MRI enables detection of a broad spectrum of CVD changes that significantly impact brain and cognitive health and newer technologies are beginning to explore causal mechanisms at the level of small vessel and BBB integrity. Moreover, newer concepts of dementia pathophysiology that encompass disease heterogeneity has led to tremendous progress in understanding the clinical implications of CVD on the burden of dementia. While several open questions and challenges remain, ongoing work on development and validation of reliable CVD imaging markers will bring us closer to identification and implementation of CVD imaging biomarkers for use in observational and interventional studies of aging and dementia.
Sources of Funding
Dr. Vemuri receives funding from National Institute of Health (R01 NS097495, R01 AG056366). Dr. Decarli receives funding from National Institute of Health (P30 AG010129, R01 AG047827, R01 AG 031563, UH3 NS100608, U19 NS 120384). Dr. Duering is an employee of MIAC AG, Basel, Switzerland.
Non-standard Abbreviations and Acronyms:
- CVD
Cerebrovascular disease
- MRI
Magnetic resonance imaging
- WMH
White matter hyperintensities
- AD
Alzheimer’s disease
- FLAIR
Fluid Attenuated Inversion Recovery
- GRE
Gradient Recalled Echo
- SWI
Susceptibility Weighted Imaging
- CAA
Cerebral amyloid angiopathy
- PVS
Perivascular spaces
Footnotes
Disclosures
Dr. Vemuri received speaker compensation from Miller Medical Communications LLC. Dr. Decarli is a consultant for Novartis regarding a safety trial in heart failure. Dr. Duering reports honoraria for lectures from Pfizer, Bayer and Sanofi Genzyme, and is a consultant for Roche Pharma and Hovid Berhad.
REFERENCES
- 1.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: Mechanisms and clinical implications. The Lancet. Neurology 2019;18:684–696 [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet neurology. 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hachinski VC, Potter P, Merskey H. Leuko-araiosis: An ancient term for a new problem. Can J Neurol Sci. 1986;13:533–534 [DOI] [PubMed] [Google Scholar]
- 4.Janota I, Mirsen TR, Hachinski VC, Lee DH, Merskey H. Neuropathologic correlates of leuko-araiosis [published erratum appears in arch neurol 1990 mar;47(3):281]. Archives of Neurology. 1989;46:1124–1128 [DOI] [PubMed] [Google Scholar]
- 5.Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–1089 [DOI] [PubMed] [Google Scholar]
- 6.Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. Ii. Postmortem pathological correlations. Stroke. 1986;17:1090–1097 [DOI] [PubMed] [Google Scholar]
- 7.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: A systematic review and meta-analysis. JAMA neurology. 2019;76:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graff-Radford J, Aakre JA, Knopman DS, Schwarz CG, Flemming KD, Rabinstein AA, et al. Prevalence and heterogeneity of cerebrovascular disease imaging lesions. Mayo Clinic proceedings. 2020;95:1195–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiology of aging. 2005;26:491–510 [DOI] [PubMed] [Google Scholar]
- 10.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The rotterdam scan study. J Neurol Neurosurg Psychiatry. 2001;70:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: The framingham study. Stroke. 2004;35:1857–1861 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt R, Fazekas F, Enzinger C, Ropele S, Kapeller P, Schmidt H. Risk factors and progression of small vessel disease-related cerebral abnormalities. J Neural Transm Suppl. 2002:47–52 [DOI] [PubMed] [Google Scholar]
- 13.Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, et al. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the framingham heart study: A cross-sectional study. The Lancet. Neurology 2012;11:1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: The framingham heart study. Arch Neurol. 2006;63:246–250 [DOI] [PubMed] [Google Scholar]
- 17.Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79:442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kase CS, Wolf PA, Chodosh EH, Zacker HB, Kelly-Hayes M, Kannel WB, et al. Prevalence of silent stroke in patients presenting with initial stroke: The framingham study. Stroke. 1989;20:850–852 [DOI] [PubMed] [Google Scholar]
- 19.Bryan RN, Wells SW, Miller TJ, Elster AD, Jungreis CA, Poirier VC, et al. Infarctlike lesions in the brain: Prevalence and anatomic characteristics at mr imaging of the elderly--data from the cardiovascular health study [see comments]. Radiology. 1997;202:47–54 [DOI] [PubMed] [Google Scholar]
- 20.Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, et al. Prevalence and anatomic characteristics of infarct-like lesions on mr images of middle-aged adults: The atherosclerosis risk in communities study. AJNR Am J Neuroradiol. 1999;20:1273–1280 [PMC free article] [PubMed] [Google Scholar]
- 21.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241 [DOI] [PubMed] [Google Scholar]
- 22.van Dijk EJ, Prins ND, Vermeer SE, Koudstaal PJ, Breteler MM. Frequency of white matter lesions and silent lacunar infarcts. J Neural Transm Suppl. 2002:25–39 [DOI] [PubMed] [Google Scholar]
- 23.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2002;33:21–25 [DOI] [PubMed] [Google Scholar]
- 24.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, et al. Prevalence and correlates of silent cerebral infarcts in the framingham offspring study. Stroke. 2008;39:2929–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatemi F, Kantarci K, Graff-Radford J, Preboske GM, Weigand SD, Przybelski SA, et al. Sex differences in cerebrovascular pathologies on flair in cognitively unimpaired elderly. Neurology. 2018;90:e466–e473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 27.Blum S, Luchsinger JA, Manly JJ, Schupf N, Stern Y, Brown TR, et al. Memory after silent stroke: Hippocampus and infarcts both matter. Neurology. 2012;78:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, et al. Mri predictors of cognition in subcortical ischemic vascular disease and alzheimer’s disease. Neurology. 2001;57:2229–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wardlaw JM, Chappell FM, Valdés Hernández MDC, Makin SDJ, Staals J, Shuler K, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology. 2017;89:1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (vcid): Knowledge gaps and opportunities. Alzheimer’s & dementia (New York, N. Y.) 2019;5:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAleese KE, Firbank M, Dey M, Colloby SJ, Walker L, Johnson M, et al. Cortical tau load is associated with white matter hyperintensities. Acta Neuropathol Commun. 2015;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, Ni J, Ayres A, Reed A, et al. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology. 2014;83:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan NS, Biessels G-J, Kim L, Nicholas JM, Barber PA, Walsh P, et al. Genetic determinants of white matter hyperintensities and amyloid angiopathy in familial alzheimer’s disease. Neurobiology of aging. 2015;36:3140–3151 [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TL, et al. White matter hyperintensities are a core feature of alzheimer’s disease: Evidence from the dominantly inherited alzheimer network. Annals of neurology. 2016;79:929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrag M, McAuley G, Pomakian J, Jiffry A, Tung S, Mueller C, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: A postmortem mri study. Acta neuropathologica. 2010;119:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, et al. Susceptibility-weighted imaging is more reliable than t2*-weighted gradient-recalled echo mri for detecting microbleeds. Stroke. 2013;44:2782–2786 [DOI] [PubMed] [Google Scholar]
- 38.Iadecola C The pathobiology of vascular dementia. Neuron. 2013;80:844–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: A review of clinical, genetic, and neuroimaging associations. Frontiers in neurology. 2014;4:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg SM, Grabowski T, Gurol ME, Skehan ME, Nandigam RN, Becker JA, et al. Detection of isolated cerebrovascular beta-amyloid with pittsburgh compound b. Annals of neurology. 2008;64:587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantarci K, Gunter JL, Tosakulwong N, Weigand SD, Senjem MS, Petersen RC, et al. Focal hemosiderin deposits and beta-amyloid load in the adni cohort. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9:S116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, et al. Microbleed topography, leukoaraiosis, and cognition in probable alzheimer disease from the sunnybrook dementia study. Arch Neurol. 2008;65:790–795 [DOI] [PubMed] [Google Scholar]
- 43.Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Annals of neurology. 2007;62:229–234 [DOI] [PubMed] [Google Scholar]
- 44.Wu R, Feng C, Zhao Y, Jin A-P, Fang M, Liu X. A meta-analysis of association between cerebral microbleeds and cognitive impairment. Med Sci Monit. 2014;20:2189–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charidimou A, Perosa V, Frosch MP, Scherlek AA, Greenberg SM, van Veluw SJ. Neuropathological correlates of cortical superficial siderosis in cerebral amyloid angiopathy. Brain : a journal of neurology. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wollenweber FA, Baykara E, Zedde M, Gesierich B, Achmüller M, Jouvent E, et al. Cortical superficial siderosis in different types of cerebral small vessel disease. Stroke. 2017;48:1404–1407 [DOI] [PubMed] [Google Scholar]
- 47.Wollenweber FA, Opherk C, Zedde M, Catak C, Malik R, Duering M, et al. Prognostic relevance of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. 2019;92:e792–e801 [DOI] [PubMed] [Google Scholar]
- 48.van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. The Lancet. Neurology 2017;16:730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: The invisible lesions. The Lancet. Neurology 2012;11:272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilal S, Sikking E, Shaik MA, Chan QL, van Veluw SJ, Vrooman H, et al. Cortical cerebral microinfarcts on 3t mri: A novel marker of cerebrovascular disease. Neurology. 2016;87:1583–1590 [DOI] [PubMed] [Google Scholar]
- 51.Ter Telgte A, Scherlek AA, Reijmer YD, van der Kouwe AJ, van Harten T, Duering M, et al. Histopathology of diffusion-weighted imaging-positive lesions in cerebral amyloid angiopathy. Acta Neuropathol. 2020;139:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ter Telgte A, Wiegertjes K, Gesierich B, Baskaran BS, Marques JP, Kuijf HJ, et al. Temporal dynamics of cortical microinfarcts in cerebral small vessel disease. JAMA Neurol. 2020;77:643–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baykara E, Gesierich B, Adam R, Tuladhar AM, Biesbroek JM, Koek HL, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Annals of neurology. 2016;80:581–592 [DOI] [PubMed] [Google Scholar]
- 54.Nitkunan A, Barrick TR, Charlton RA, Clark CA, Markus HS. Multimodal mri in cerebral small vessel disease: Its relationship with cognition and sensitivity to change over time. Stroke. 2008;39:1999–2005 [DOI] [PubMed] [Google Scholar]
- 55.Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. International Journal of Stroke. 2011;6:47–59 [DOI] [PubMed] [Google Scholar]
- 56.Zeestraten EA, Benjamin P, Lambert C, Lawrence AJ, Williams OA, Morris RG, et al. Application of diffusion tensor imaging parameters to detect change in longitudinal studies in cerebral small vessel disease. PloS one. 2016;11:e0147836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, Mungas D, et al. White matter hyperintensity penumbra. Stroke. 2011;42:1917–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuladhar AM, van Dijk E, Zwiers MP, van Norden AG, de Laat KF, Shumskaya E, et al. Structural network connectivity and cognition in cerebral small vessel disease. Human brain mapping. 2016;37:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reijmer YD, Fotiadis P, Martinez-Ramirez S, Salat DH, Schultz A, Shoamanesh A, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain : a journal of neurology. 2015;138:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain : a journal of neurology. 2011;134:73–83 [DOI] [PubMed] [Google Scholar]
- 61.Zeestraten EA, Lawrence AJ, Lambert C, Benjamin P, Brookes RL, Mackinnon AD, et al. Change in multimodal mri markers predicts dementia risk in cerebral small vessel disease. Neurology. 2017;89:1869–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benjamin P, Zeestraten E, Lambert C, Ster IC, Williams OA, Lawrence AJ, et al. Progression of mri markers in cerebral small vessel disease: Sample size considerations for clinical trials. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:228–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology. 2015;84:1685–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, et al. Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018;91:e1338–e1347 [DOI] [PubMed] [Google Scholar]
- 65.Finsterwalder S, Vlegels N, Gesierich B, Araque Caballero MA, Weaver NA, Franzmeier N, et al. Small vessel disease more than alzheimer’s disease determines diffusion mri alterations in memory clinic patients. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vemuri P, Lesnick TG, Knopman DS, Przybelski SA, Reid RI, Mielke MM, et al. Amyloid, vascular, and resilience pathways associated with cognitive aging. Annals of neurology. 2019;86:866–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vemuri P, Lesnick TG, Przybelski SA, Graff-Radford J, Reid RI, Lowe VJ, et al. Development of a cerebrovascular magnetic resonance imaging biomarker for cognitive aging. Annals of neurology. 2018;84:705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: Jacc scientific expert panel. J Am Coll Cardiol. 2019;73:3326–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCreary CR, Beaudin AE, Subotic A, Zwiers AM, Alvarez A, Charlton A, et al. Cross-sectional and longitudinal differences in peak skeletonized white matter mean diffusivity in cerebral amyloid angiopathy. Neuroimage Clin. 2020;27:102280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Konieczny MJ, Dewenter A, Telgte AT, Gesierich B, Wiegertjes K, Finsterwalder S, et al. Multi-shell diffusion mri models for white matter characterization in cerebral small vessel disease. Neurology. 2020 [DOI] [PubMed] [Google Scholar]
- 71.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist’s guide to arterial spin labeling mri in clinical practice. Neuroradiology. 2015;57:1181–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mutsaerts HJ, Petr J, Vaclavu L, van Dalen JW, Robertson AD, Caan MW, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37:3184–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJM, et al. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. Journal of Cerebral Blood Flow & Metabolism. 2016;36:1653–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and tia risk in patients with carotid artery stenosis and occlusion. Brain : a journal of neurology. 2001;124:457–467 [DOI] [PubMed] [Google Scholar]
- 76.Glodzik L, Randall C, Rusinek H, de Leon MJ. Cerebrovascular reactivity to carbon dioxide in alzheimer’s disease. Journal of Alzheimer’s Disease. 2013;35:427–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: A beginner’s guide. Neurochemical research. 2015;40:2583–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nature Reviews Neurology. 2020;16:137–153 [DOI] [PubMed] [Google Scholar]
- 79.Passiak BS, Liu D, Kresge HA, Cambronero FE, Pechman KR, Osborn KE, et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. Neurology. 2019;92:e1309–e1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villeneuve S, Reed BR, Madison CM, Wirth M, Marchant NL, Kriger S, et al. Vascular risk and abeta interact to reduce cortical thickness in ad vulnerable brain regions. Neurology. 2014;83:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, et al. Inflammatory biomarkers are associated with total brain volume: The framingham heart study. Neurology. 2007;68:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: The framingham offspring study. Diabetes care. 2011;34:1766–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altendahl M, Maillard P, Harvey D, Cotter D, Walters S, Wolf A, et al. An il-18-centered inflammatory network as a biomarker for cerebral white matter injury. PloS one. 2020;15:e0227835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta neuropathologica. 2017;134:171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014;10:562–570 [DOI] [PubMed] [Google Scholar]
- 86.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. The Lancet. Neurology 2006;5:735–741 [DOI] [PubMed] [Google Scholar]
- 87.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e66 [DOI] [PubMed] [Google Scholar]
- 88.Weinstein G, Maillard P, Himali JJ, Beiser AS, Au R, Wolf PA, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology. 2015;84:2329–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeCarli C Clinically asymptomatic vascular brain injury: A potent cause of cognitive impairment among older individuals. Journal of Alzheimer’s disease : JAD. 2013;33 Suppl 1:S417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from alzheimer disease pathology. Neurology. 2004;62:1148–1155 [DOI] [PubMed] [Google Scholar]
- 91.Chui HC, Zheng L, Reed BR, Vinters HV, Mack WJ. Vascular risk factors and alzheimer’s disease: Are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimer’s research & therapy. 2012;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marchant NL, Reed BR, DeCarli CS, Madison CM, Weiner MW, Chui HC, et al. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiology of aging. 2012;33:1006 e1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. Ad brain pathology: Vascular origins? Results from the haas autopsy study. Neurobiology of aging. 2008;29:1587–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han JW, Maillard P, Harvey D, Fletcher E, Martinez O, Johnson DK, et al. Association of vascular brain injury, neurodegeneration, amyloid and cognitive trajectory. Neurology. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain : a journal of neurology. 2015;138:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han JW, Maillard P, Harvey D, Fletcher E, Martinez O, Johnson DK, et al. Association of vascular brain injury, neurodegeneration, amyloid, and cognitive trajectory. 2020;95:e2622–e2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Annals of neurology. 2014;76:845–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/t/n: An unbiased descriptive classification scheme for alzheimer disease biomarkers. Neurology. 2016;87:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (aric) cohort. JAMA neurology. 2017;74:1246–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Lowe VJ, Graff-Radford J, et al. Age, vascular health, and alzheimer disease biomarkers in an elderly sample. Annals of neurology. 2017;82:706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim HJ, Park S, Cho H, Jang YK, San Lee J, Jang H, et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18f-av1451 positron emission tomography imaging. JAMA Neurol. 2018;75:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McAleese KE, Walker L, Colloby SJ, Taylor JP, Thomas AJ, DeCarli C, et al. Cortical tau pathology: A major player in fibre-specific white matter reductions in alzheimer’s disease? Brain : a journal of neurology. 2018;141:e44. [DOI] [PubMed] [Google Scholar]
- 104.Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, et al. White matter hyperintensities: Relationship to amyloid and tau burden. Brain : a journal of neurology. 2019;142:2483–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Guio F, Jouvent E, Biessels GJ, Black SE, Brayne C, Chen C, et al. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:1319–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith EE, Biessels GJ, De Guio F, de Leeuw FE, Duchesne S, During M, et al. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimer’s & dementia (Amsterdam, Netherlands). 2019;11:191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gesierich B, Tuladhar AM, Ter Telgte A, Wiegertjes K, Konieczny MJ, Finsterwalder S, et al. Alterations and test-retest reliability of functional connectivity network measures in cerebral small vessel disease. Human brain mapping. 2020;41:2629–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raghavan S, Przybelski SA, Reid RI, Graff-Radford J, Lesnick TG, Zuk SM, et al. Reduced fractional anisotropy of the genu of the corpus callosum as a cerebrovascular disease marker and predictor of longitudinal cognition in mci. Neurobiology of aging. 2020;96:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brickman AM, Tosto G, Gutierrez J, Andrews H, Gu Y, Narkhede A, et al. An mri measure of degenerative and cerebrovascular pathology in alzheimer disease. Neurology. 2018;91:e1402–e1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Banerjee G, Jang H, Kim HJ, Kim ST, Kim JS, Lee JH, et al. Total mri small vessel disease burden correlates with cognitive performance, cortical atrophy, and network measures in a memory clinic population. 2018;63:1485–1497 [DOI] [PubMed] [Google Scholar]
- 111.Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total mri brain small-vessel disease burden. Neurology. 2014;83:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein e genotype and alzheimer disease. A meta-analysis. Apoe and alzheimer disease meta analysis consortium. Jama. 1997;278:1349–1356 [PubMed] [Google Scholar]
- 114.Gonzalez HM, Tarraf W, Schneiderman N, Fornage M, Vasquez PM, Zeng D, et al. Prevalence and correlates of mild cognitive impairment among diverse hispanics/latinos: Study of latinos-investigation of neurocognitive aging results. Alzheimers Dement. 2019;15:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lamar M, Durazo-Arvizu RA, Sachdeva S, Pirzada A, Perreira KM, Rundek T, et al. Cardiovascular disease risk factor burden and cognition: Implications of ethnic diversity within the hispanic community health study/study of latinos. PLoS One. 2019;14:e0215378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tarraf W, Kaplan R, Daviglus M, Gallo LC, Schneiderman N, Penedo FJ, et al. Cardiovascular risk and cognitive function in middle-aged and older hispanics/latinos: Results from the hispanic community health study/study of latinos (hchs/sol). Journal of Alzheimer’s disease : JAD. 2020;73:103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, et al. Structural mri predictors of late-life cognition differ across african americans, hispanics, and whites. Current Alzheimer research. 2015;12:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gavett BE, Fletcher E, Harvey D, Farias ST, Olichney J, Beckett L, et al. Ethnoracial differences in brain structure change and cognitive change. Neuropsychology. 2018;32:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Filshtein TJ, Dugger BN, Jin LW, Olichney JM, Farias ST, Carvajal-Carmona L, et al. Neuropathological diagnoses of demented hispanic, black, and non-hispanic white decedents seen at an alzheimer’s disease center. Journal of Alzheimer’s disease : JAD. 2019;68:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ortman JM, Velkoff VA, H. H An aging nation: The older population in the united states. Current Population Reports. 2014:p25–1140 [Google Scholar]
- 121.De Guio F, Vignaud A, Chabriat H, Jouvent E. Different types of white matter hyperintensities in cadasil: Insights from 7-tesla mri. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2018;38:1654–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mussard E, Hilbert T, Meuli R, Thiran J-P, Kober T. Accelerated mp2rage imaging using sparse iterative reconstruction. ISMRM 2016, ISMRM 24rd Annual Meeting & Exhibition, SMRT 25th Annual Meeting. 2016 [Google Scholar]
- 123.Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, et al. Magnetic resonance fingerprinting. Nature. 2013;495:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duering M, Finsterwalder S, Baykara E, Tuladhar AM, Gesierich B, Konieczny MJ, et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2018;14:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maillard P, Fletcher E, Singh B, Martinez O, Johnson DK, Olichney JM, et al. Cerebral white matter free water: A sensitive biomarker of cognition and function. Neurology. 2019;92:e2221–e2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maillard P, Mitchell GF, Himali JJ, Beiser A, Fletcher E, Tsao CW, et al. Aortic stiffness, increased white matter free water, and altered microstructural integrity: A continuum of injury. Stroke. 2017;48:1567–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maillard P, Carmichael O, Harvey D, Fletcher E, Reed B, Mungas D, et al. Flair and diffusion mri signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol. 2013;34:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic resonance in medicine. 2005;53:1432–1440 [DOI] [PubMed] [Google Scholar]
- 129.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. Noddi: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61:1000–1016 [DOI] [PubMed] [Google Scholar]
- 130.Ji F, Pasternak O, Liu S, Loke YM, Choo BL, Hilal S, et al. Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in alzheimer’s disease with and without cerebrovascular disease. Alzheimer’s research & therapy. 2017;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colgan N, Siow B, O’Callaghan JM, Harrison IF, Wells JA, Holmes HE, et al. Application of neurite orientation dispersion and density imaging (noddi) to a tau pathology model of alzheimer’s disease. NeuroImage. 2016;125:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thrippleton MJ, Backes WH, Sourbron S, Ingrisch M, van Osch MJP, Dichgans M, et al. Quantifying blood-brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2019;15:840–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA. Long-term blood-brain barrier permeability changes in binswanger disease. Stroke. 2015;46:2413–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7 t magnetic resonance imaging. Invest Radiol. 2014;49:307–313 [DOI] [PubMed] [Google Scholar]
- 135.Geurts LJ, Zwanenburg JJM, Klijn CJM, Luijten PR, Biessels GJ. Higher pulsatility in cerebral perforating arteries in patients with small vessel disease related stroke, a 7t mri study. Stroke. 2018:STROKEAHA118022516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shao X, Ma SJ, Casey M, D’Orazio L, Ringman JM, Wang DJJ. Mapping water exchange across the blood-brain barrier using 3d diffusion-prepared arterial spin labeled perfusion mri. Magnetic resonance in medicine. 2019;81:3065–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]


