Abstract
BACKGROUND:
Low-density lipoprotein cholesterol (LDL-C) levels below 50 mg/dL may suggest familial hypobetalipoproteinemia, particularly in patients with hepatic steatosis. The prevalence of hypobetalipoproteinemia in cohorts with nonalcoholic fatty liver disease (NAFLD) is not known, and it is not clear whether the severity of liver disease of these patients is different. The objective of this study was to address these questions in a large pediatric NAFLD cohort.
METHODS:
Retrospective study of children followed at the Steatohepatitis Center of a tertiary care center from August 2010 to October 2017. Patients with secondary causes of hepatic steatosis and those on statins were excluded.
RESULTS:
Of the 740 patients included, 58 (8%) had hypobetalipoproteinemia. These patients were younger (P = .04), had a lower body mass index (P < .01) and waist circumference (P = .01), and were less likely to be on metformin (P = .01). In spite of that, serum aminotransferase levels were not different between those with low, normal, and high LDL-C levels. Of the 222 patients who had both lipid and histology data available, the steatosis score was higher in those with low LDL-C compared to those with normal or elevated LDL-C, a result that trended toward significance (P = .06). The severity of inflammation and fibrosis did not differ between the groups. When all patients with hypertriglyceridemia were excluded, steatosis severity was higher in those with low LDL-C (P = .04).
CONCLUSION:
Hypobetalipoproteinemia is common among patients with NAFLD and is associated with similar liver disease severity in spite of a leaner phenotype and a more favorable metabolic profile.
Keywords: Nonalcoholic, steatohepatitis, Fibrosis, Children, Diagnosis
Lay summary:
Approximately 1-in-10 patients with fatty liver disease have extremely low levels of LDL cholesterol (LDL-C), otherwise known as the bad cholesterol. This protects them from a heart disease standpoint, but it is not known if their liver disease is any different from that of patients with normal or high LDL-C levels. Here, we show that the liver disease of patients with extremely low LDL-C levels is as serious as that of patients with normal or high LDL-C levels, even though they tend to be younger and thinner.
Background
Dyslipidemia is a common comorbidity of adults and children with nonalcoholic fatty liver disease (NAFLD). Dyslipidemia is concerning because of its association with cardiovascular disease,1 which in adult patients with NAFLD is a prominent, if not the leading, cause of death.2–6 Elevated low-density lipoprotein cholesterol (LDL-C) affects 14 to 21% of children and adolescents with NAFLD.7–10 However, hypobetalipoproteinemia (low LDL-C levels) can also be seen in patients with NAFLD.11 Because low LDL is not associated with increased cardiovascular risk, hypobetalipoproteinemia has not been rigorously studied in this context.11,12 Yet, very low LDL levels may reflect specific disorders of lipid metabolism and alternate mechanisms of developing hepatic steatosis, such as dysregulated export of lipids from the liver, rather than insulin resistance.11 Therefore, it is important to determine the prevalence of low LDL-C levels among children with NAFLD and to discern whether this finding is associated with a specific fatty liver phenotype. If the latter is true, it may suggest that these children will exhibit a different natural history or respond differently to treatment approaches, than those with NAFLD and elevated triglycerides and LDL-C levels.
Familial hypobetalipoproteinemia ([FHBL]; OMIM #615558 and #605019) is an autosomal codominant genetic condition that in heterozygous states is associated with asymptomatic hepatic steatosis and has been reported among cohorts of children with NAFLD.11,13 Although FHBL can be the result of various genetic mutations that affect lipoprotein synthesis or clearance, the best described cases to date result from truncated apolipoprotein B synthesis, which in turn leads to inefficient triglyceride export from hepatocytes in the form of very low-density lipoproteins (VLDL).14,15 As a result, patients with FHBL typically have low serum levels of triglycerides and total cholesterol, particularly LDL-C, whereas HDL-C levels may be low, normal, or elevated depending on the mutation. Specifically, the LDL-C of patients with heterozygous FHBL is typically below the 5th centile for age (≤50 mg/dL).16 Although the low cholesterol seen in the context of FHBL is thought to exert a protective cardiovascular effect over time, the natural history of the liver disease associated with FHBL is unknown. There are reports of cirrhosis developing in these patients,17 but the prevalence of FHBL among children with NAFLD, as well as the phenotype, and natural history of the liver disease seen in this context have not been well described to date.
The objective of this study was to determine the prevalence of very low LDL-C levels, potentially suggestive of FHBL in a large cohort of children and adolescents with presumed (elevated transaminases and/or imaging suggestive of steatosis in the context of obesity with a negative work up for other liver diseases) or histologically confirmed NAFLD and to assess whether the liver disease severity of patients with hypobetalipoproteinemia is different than that of their peers with normal or elevated LDL-C levels.
Methods
This was a retrospective study performed at Cincinnati Children’s Hospital Medical Center. Patients with presumed or confirmed NAFLD followed at the Steatohepatitis Center from August 2010 to October 2017 were included. All patients had negative screening for hepatitis B or C, autoimmune hepatitis, alpha 1 antitrypsin, Wilson disease, hemochromatosis, medication-induced toxicity, or celiac disease as other causes for chronic hepatitis. Additional exclusion criteria were secondary causes of hepatic steatosis (eg, lysosomal acid lipase deficiency), as well as evidence of thyroid dysfunction or use of statins at the time of the laboratory investigations. Approval by the Institutional Research Ethics Board was obtained before data collection and informed consent was waived.
Data collected included baseline characteristics (age, ethnicity, sex, anthropometrics, medications etc.), results of laboratory investigations (hepatic profile, lipid profile, hemoglobin A1c) obtained at the first clinic visit, as well as within 3 months of the first available liver biopsy, and liver histology. Patients were categorized using standard pediatric definitions as overweight (BMI in the 85–95th percentile), class I obesity (BMI between 95th and 1.2 ☓ 95th percentile), class II obesity (BMI between 1.2 ☓ 95th percentile and 1.4 ☓ 95th percentile), or class III obesity (BMI>1.4 ☓ 95th percentile).18 Waist circumference (WC) to height ratio was calculated as a measure of abdominal adiposity and an indicator of metabolic risk in a subset of patients.19,20 Histologic liver disease severity was determined using the NASH Clinical Research Network criteria reported by Kleiner et al.21 The NAFLD activity score was calculated by adding the individual scores for steatosis (0-3), lobular inflammation (0-3), and ballooning degeneration (0-2). Lobular inflammation and ballooning reflect hepatocellular injury and cell death that develops in the context of steatohepatitis and may precede the development of fibrosis.
Hypobetalipoproteinemia was defined as a serum LDL-C level below 50 mg/dL, as this threshold is at or below the 5th percentile for LDL-C in the general pediatric population using the National Health and Nutrition Examination Survey (NHANES) III.16
Statistical analyses
Stata MP 13.0 (College Station, TX) was used for the statistical analyses. Descriptive statistics were used, and the data were presented as means with standard deviation (SD) or medians with ranges. Kruskal Wallis was used to compare nonparametric variables between groups, and chi-square testing was used to compare proportions. Analysis of variance (with Bonferroni for multiple comparisons) was used to compare normally distributed continuous variables between groups.
Results
Of the 797 children followed in the steatohepatitis clinics during the study period, lipid profile data were available on 740 patients who also met inclusion/exclusion criteria. Of those, 58 (8%) patients had an LDL-C<50 mg/dL, 608 (82%) had an LDL-C 51-130 mg/dL, and 74 (10%) had an LDL-C>130 mg/dL. The clinical and laboratory characteristics of these patients are shown in Table 1.
Table 1.
Clinical and laboratory characteristics of patients with low, normal, and elevated low-density lipoprotein levels (entire cohort, n = 740)
| Variables | LDL< 50 mg/dL | LDL 51–130 mg/dL | LDL>130 mg/dL | P value |
|---|---|---|---|---|
| N (% of total) | 58 (8%) | 608 (82%) | 74 (10%) | N/A |
| Clinical data | ||||
| Age (y) | 12 ± 3 | 13 ± 3 | 14 ± 3 | .04 |
| Sex (N, %male) | 34 (59%) | 377 (62%) | 47% (64%) | .84 |
| Ethnicity | ||||
| N (%) Hispanic | 11 (19%) | 128 (21%) | 5 (7%) | .02 |
| N (%) Non-Hispanic | 47 (81%) | 480 (79%) | 69 (93%) | |
| BMI (kg/m2) | 31.9 ± 6.0 | 34.9 ± 7.4 | 36.7 ± 7.0 | <.01 |
| BMI category (n; %) | ||||
| Overweight | 2 (3%) | 21 (3%) | 3 (4%) | .97 |
| Class I obesity | 16 (28%) | 134 (22%) | 10 (14%) | .13 |
| Class II obesity | 26 (45%) | 221 (36%) | 24 (32%) | .32 |
| Class III obesity | 13 (22%) | 229 (38%) | 37 (50%) | <.01 |
| WC (cm) | 97 ± 13 | 110 ± 18 | 111 ± 13 | .01 |
| WC to height ratio | 0.63 ± 0.07 | 0.68 ± 0.08 | 0.69 ± 0.06 | .05 |
| Laboratory values | ||||
| ALT (U/L) | 69 ± 41 | 75 ± 56 | 97 ± 77 | <.01 |
| AST (U/L) | 39 ± 21 | 44 ± 31 | 62 ± 62 | <.01 |
| GGT (U/L) | 34 ± 19 | 42 ± 40 | 73 ± 78 | <.01 |
| ALP (U/L) | 220 ± 105 | 208 ± 113 | 189 ± 103 | .26 |
| Total Cholesterol (mg/dL) | 106 ± 22 | 156 ± 25 | 222 ± 26 | <.01 |
| LDL (mg/dL) | 37 ± 10 | 89 ± 20 | 152 ± 20 | <.01 |
| HDL (mg/dL) | 40 ± 13 | 39 ± 9 | 38 ± 11 | .52 |
| Triglycerides (mg/dL) | 139 ± 106 | 145 ± 93 | 176 ± 106 | .02 |
| HbA1c% | 5.2 ± 0.4 | 5.3 ± 0.7 | 5.4 ± 1.1 | .26 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WC, waist circumference.
The results are presented as means ± standard deviation, unless otherwise indicated.
Those with an LDL-C <50 mg/dL had lower mean age (P = .04) and WC (available for n = 266 patients) compared to those with a normal or elevated LDL-C. Although the frequency of overweight, class I, and class II obesity was not significantly different between the groups, class III obesity was least frequent in those with hypobetalipoproteinemia. The proportion of patients with a WC to height ratio >0.6 was significantly lower in those with hypobetalipoproteinemia than those with normal or high LDL levels (29% vs 87% vs 93%, respectively; P < .01). Although serum HbA1c levels were similar among the groups, metformin use for prediabetes (pre-T2DM) or type 2 diabetes mellitus (T2DM) was significantly less common in those with hypobetalipoproteinemia vs normal or high LDL-C levels (7% vs 18% vs 27%, respectively; P = .01). With the exception of alkaline phosphatase, biochemical markers of liver injury (ALT, AST, GGT) were higher in the elevated LDL-C group compared to the other two but were not different between those with low vs normal LDL-C levels. HDL-C levels did not vary by group.
Histology data were available in 227 patients, of whom 222 also had LDL-C data available (6% had low, 77% had normal, and 17% had elevated LDL-C), and are summarized in Table 2. The proportion of patients with low LDL-C was not different in those who had undergone a liver biopsy vs the original cohort of 740 patients (6% vs 8%, P = .447); however, the proportion of patients with high LDL-C was higher in the group with histological data (17% vs 10%, P = .022). The subcohort with available biopsy data had a mean age of 13 ± 2 years, was 65% male, and was 18% Hispanic. These characteristics were not different from those without liver biopsy.
Table 2.
Clinical, laboratory, and histology data of patients who had undergone a diagnostic liver biopsy (histology cohort, n = 222) grouped according to low-density lipoprotein levels
| Variables | LDL < 50 mg/dL | LDL 51–130 mg/dL | LDL>130 mg/dL | P value |
|---|---|---|---|---|
| N (% of total) | 14 (6%) | 171 (77%) | 37 (17%) | N/A |
| Clinical data | ||||
| Age (y) | 12 ± 3 | 13 ± 3 | 14 ± 3 | .25 |
| Sex, N (%male) | 7, 50% | 115, 67% | 24, 65% | .45 |
| Ethnicity | ||||
| N (%) Hispanic | 3 (21%) | 40 (23%) | 3 (8%) | .12 |
| N (%) Non-Hispanic | 11 (79%) | 131 (77%) | 34 (92%) | |
| BMI (kg/m2) | 32.1 ± 5.1 | 35.5 ± 7.5 | 37.3 ± 6.9 | .05 |
| Laboratory values | ||||
| ALT (U/L) | 91 ± 47 | 96 ± 70 | 110 ± 64 | .22 |
| AST (U/L) | 52 ± 24 | 55 ± 35 | 64 ± 35 | .17 |
| GGT (U/L) | 55 ± 22 | 54 ± 56 | 81 ± 88 | <.01 |
| ALP (U/L) | 207 ± 66 | 213 ± 110 | 195 ± 117 | .45 |
| HbA1c% | 5.1 ± 0.4 | 5.3 ± 0.7 | 5.4 ± .1.0 | .81 |
| Histology data | ||||
| Steatosis | 2.4 ± 0.9 | 1.8 ± 1.0 | 2.0 ± 0.9 | .06 |
| Lobular inflammation | 1.3 ± 0.6 | 1.2 ± 0.7 | 1.0 ± 0.7 | .38 |
| Portal inflammation | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.6 | .88 |
| NAFLD activity score | 4.5 ± 1.3 | 3.7 ± 1.6 | 3.7 ± 1.6 | .14 |
| Fibrosis score | 1.0 ± 1.1 | 0.8 ± 1.0 | 0.8 ± 0.9 | .80 |
The results are shown as means ± standard deviation, unless otherwise indicated.
Patients with low LDL-C had higher steatosis score that trended toward statistical significance (Table 2). NAFLD activity score, lobular inflammation, portal inflammation, and fibrosis scores were not different between the groups. The proportion of patients with and without fibrosis was also not different between the groups (any fibrosis present in 65%, 52%, and 60% of patients with low, normal, and elevated serum LDL-C levels, respectively; P = .54). Similarly, the proportion of patients with advanced fibrosis (stage 2 and above) was not different between the groups (21%, 21%, and 14%, respectively, P = .59).
Considering the known effect of hypertriglyceridemia on LDL-C levels,22 we subsequently excluded patients with triglyceride levels above 200 mg/dL from the histology cohort (n = 42 were excluded; n = 2 from the low LDL-C group, n = 30 from the normal LDL-C group, and n = 10 from the high LDL-C group) and repeated the aforementioned analyses. In this subcohort, the steatosis score was higher in patients with low serum LDL-C levels compared to those with normal levels (mean steatosis score 2.6 ± 0.7, 1.8 ± 1.0, and 2.0 ± 0.9, in those with low, normal, and high LDL-C levels, respectively; P = .042, Fig. 1). The remaining histological findings were not different between the groups.
Figure 1.
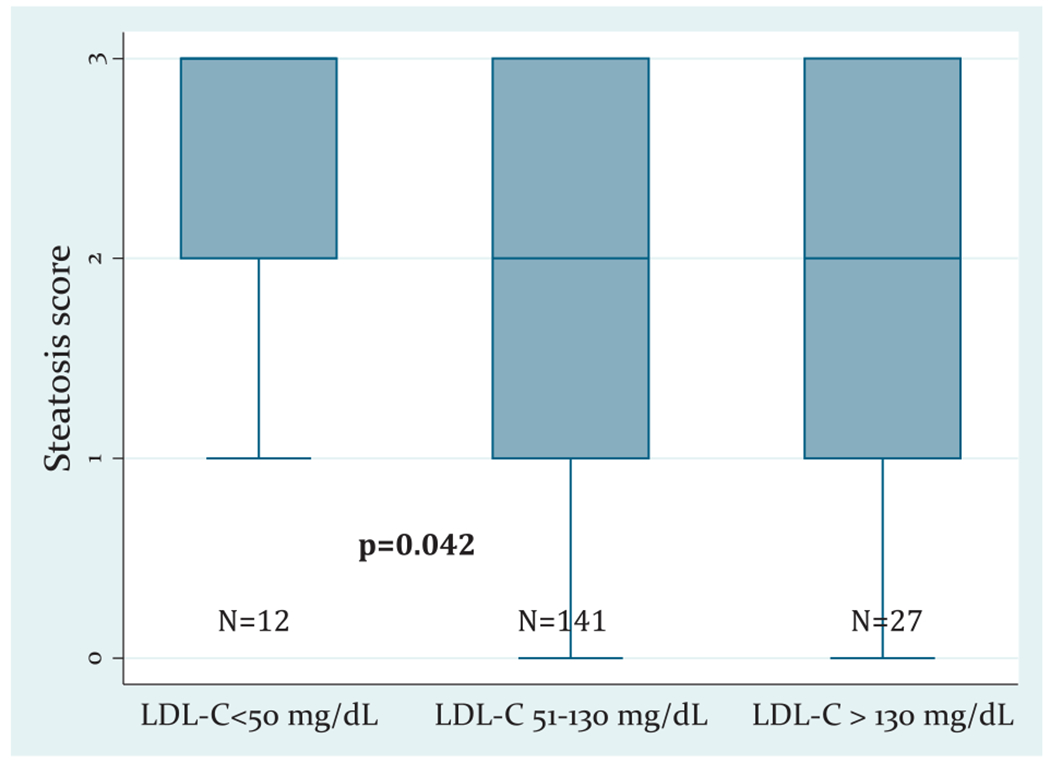
Differences in the steatosis score of nonhypertriglyceridemic patients grouped by LDL-C levels. The boxplots depict the median value (thick line inside the box), the first and third quartiles (lower and upper limit of the box), and the range (tip of lines) of the steatosis score.
Discussion
The results of this study show that, in a large pediatric cohort of predominantly overweight/obese children with presumed or confirmed NAFLD, almost 1 of 10 had LDL-C levels below 50 mg/dL, which is at or below the 5th percentile for age and most consistent with presumed FHBL. These patients had less significant abdominal adiposity and were less likely to need treatment for pre-T2DM/T2DM. This group also had lower triglyceride levels, whereas HDL-C was not different between the groups. In spite of the favorable anthropometrics, insulin sensitivity and lipid profiles, patients with low LDL-C levels had increased steatosis severity. Inflammation and fibrosis were not different between the LDL-C groups.
The most likely condition leading to a combination of hepatic steatosis and hypobetalipoproteinemia in the context of low total cholesterol and triglyceride levels but normal HDL-C levels is heterozygous FHBL.12 Deficient export of VLDL from hepatocytes in patients with FHBL contributes to steatosis. In addition, decreased hepatic VLDL export and limited chylomicron synthesis in the enterocytes lead to lower serum triglyceride and, subsequently, LDL-C levels.
Beyond FHBL, other conditions associated with low LDL-C levels, such as PCSK9 deficiency, chylomicron retention disease, hyperlipoproteinemia III, and apolipoprotein A-I are unlikely in this context.13 Mutations in the PCSK9 gene (OMIM#607786) lead to low LDL-C due to increased clearance of LDL from the circulation, which is driven by an increased number of LDL receptors on the surface of hepatocytes. Patients with this condition typically do not have hepatic steatosis, nor do they have lower triglyceride levels. However, genetic polymorphisms in genes that increase the risk of NAFLD can occur in patients carrying mutations in PCSK9 leading to the development of NAFLD.23 Chylomicron retention disease is typically associated with failure to thrive and hypobetalipoproteinemia in the context of normal triglycerides, so it is unlikely to be the cause of low LDL-C in obese children with NAFLD. Hyperlipoproteinemia III (OMIM#617347) is another condition characterized by low LDL-C levels but in that context total cholesterol and triglycerides are significantly elevated, which was not the case in our study. Similarly, other conditions leading to hypobetalipoproteinemia are characterized by different lipid profiles (eg, low HDL-C levels) than those seen in our cohort and as such are also not likely.13 In summary, even though genetic testing or serum apolipoprotein B levels were not available for our patients with NAFLD and low LDL-C levels, a predominant etiology for this phenotype is heterozygous HBL.
It should be noted that low LDL-C levels occurring in the context of moderate to severe elevations in serum triglyceride levels may be reflective of increased LDL-C clearance, rather than decreased LDL-C synthesis.22 In our study, 42 patients (19%) of the histology cohort had triglyceride levels above 200 mg/dL. When these patients were excluded from the analyses, the differences in steatosis severity between the LDL-C groups were even more evident, as depicted in Figure 1. This further supports the hypothesis that the hypobetalipoproteinemia seen in these patients is suggestive of an underlying genetic disorder that impairs lipid export from the hepatocytes leading to more severe steatosis.
An important reason to distinguish subphenotypes of pediatric NAFLD, such as that associated with low LDL-C and presumed FHBL, is to determine whether the long-term outcomes of these patients are different. From a cardiovascular perspective, patients with low LDL-C levels are protected long term.24 Liver-related outcomes among patients with FHBL are not known. Among the children who were biopsied, liver histology was comparable between children with hypobetalipoproteinemia and those with normal or elevated LDL-C levels, other than a trend toward increased steatosis in patients with low LDL-C levels. This implies similar long-term liver-related morbidity in those with low LDL-C levels, as in patients with normal or high LDL-C levels. However, the transaminases of the subcohort with biopsy data were not different between the LDL-C groups (similarly to other previously reported small cohorts11), which may have influenced the decision to perform liver biopsy. Proportionally, 24% of those with hypobetalipoproteinemia, 28% of those with normal to mildly elevated LDL-C, and 50% of those with elevated LDL-C levels underwent liver biopsy. In our larger, unselected patient cohort (n = 740), children with hypobetalipoproteinemia had lower transaminases than patients with elevated LDL-C and may have been less likely to undergo a liver biopsy for this reason. Nonetheless, our findings show that NAFLD severity is not necessarily milder in children with very low LDL-C levels and rising transaminase levels should warrant concern in this group.
Apart from liver disease severity, categorizing patients with NAFLD by LDL-C levels may be of relevance in terms of therapeutic approaches. If indeed those with fatty liver and LDL-C levels below the 5th percentile for age have heterozygous FHBL, which limits their ability to export the excess hepatocellular fat in the form of VLDL, they may be more resistant to treatment with lifestyle interventions. Interestingly, however, Schonfeld et al. reported that even in those with hypobetalipoproteinemia (n = 21), hepatic steatosis measured with magnetic resonance spectroscopy was positively correlated with WC.14 This does suggest that lifestyle interventions aimed at decreasing visceral adiposity (including eliminating the consumption of sugar-sweetened beverages, which are directly linked to hepatic steatosis25,26) have the potential to improve the hepatic steatosis of those with FHBL. Nonetheless, it is possible that compared to those without hypobetalipoproteinemia, more significant weight loss is needed to obtain the same results.27 This is supported by our finding that hepatic steatosis was more significant in patients with low LDL-C levels even though they were less likely to have class III obesity, and their WC to height ratio was lower than that of the other two groups. Apart from lifestyle changes, vitamin E is currently the only available treatment for nondiabetic patients with NAFLD.28,29 Vitamin E supplementation may not be beneficial in those with heterozygous FHBL, as studies have failed to show evidence of oxidative stress in this context.30 Furthermore, delineating the liver disease severity and outcomes of patients with NAFLD with hypobetalipoproteinemia may allow for a more personalized approach to treatment.
Limitations of our study include its retrospective nature and the fact that while our cohort was large, a smaller proportion of patients had undergone a liver biopsy to confirm the diagnosis of NAFLD. In addition our study cohort may have been affected by selection bias as these patients had been referred to specialized clinics of a tertiary care center. Finally, we did not have genetic data to confirm our suspicion regarding heterozygous FHBL as the likely cause of the hypobetalipoproteinemia seen in these patients. However, as discussed, other causes of very low LDL-C levels would be unlikely in this group. These limitations are mitigated by the large cohort size and availability of liver biopsy data in a large sample of patients. To our knowledge, this is the largest cohort of pediatric patients with NAFLD ever to be systematically investigated for evidence of hypobetalipoproteinemia.
To conclude, hypobetalipoproteinemia was found in nearly one tenth of a large cohort of predominantly overweight and obese, and largely non-Hispanic children with NAFLD in spite of less significant abdominal adiposity and less prevalent pre-T2DM/T2DM. Among those undergoing biopsy for persistently elevated liver enzymes, histological severity was comparable among those with suspected FHBL. Further validation in multicenter cohorts and clinical trials is recommended to assess if there are demographic or ethnic variation in suspected FHBL and the degree to which FHBL may influence histological severity of disease, natural history, or response to treatments. Investigation of the exact cause of low LDL-C levels in the context of NAFLD is also warranted, including genetic testing for FHBL. In patients with advanced liver disease, assessment of cholesteryl ester transfer protein activity in sera may also be indicated.31
Acknowledgments
This study was not funded.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose. Dr Mouzaki is acting as the submission’s guarantor.
Contributor Information
Marialena Mouzaki, Division of Gastroenterology, Hepatology and Nutrition, University of Cincinnati School of Medicine, Cincinnati, OH, USA.
Amy Shah, Division of Endocrinology, University of Cincinnati School of Medicine, Cincinnati, OH, USA.
Ana Catalina Arce-Clachar, Division of Gastroenterology, Hepatology and Nutrition, University of Cincinnati School of Medicine, Cincinnati, OH, USA.
Jennifer Hardy, Division of Gastroenterology, Hepatology and Nutrition, University of Cincinnati School of Medicine, Cincinnati, OH, USA.
Kristin Bramlage, Division of Gastroenterology, Hepatology and Nutrition, University of Cincinnati School of Medicine, Cincinnati, OH, USA.
Stavra A. Xanthakos, Division of Gastroenterology, Hepatology and Nutrition, University of Cincinnati School of Medicine, Cincinnati, OH, USA.
References
- 1.Domanski JP, Park SJ, Harrison SA. Cardiovascular disease and nonalcoholic fatty liver disease: does histologic severity matter? J Clin Gastroenterol. 2012;46(5):427–430. [DOI] [PubMed] [Google Scholar]
- 2.Patil R, Sood GK. Non-alcoholic fatty liver disease and cardiovascular risk. World J Gastrointest Pathophysiol 2017;8(2):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. [DOI] [PubMed] [Google Scholar]
- 4.Allen AM, Terry TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51(2):595–602. [DOI] [PubMed] [Google Scholar]
- 6.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7(2):234–238. [DOI] [PubMed] [Google Scholar]
- 7.Newton KP, Hou J, Crimmins NA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170(10):e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Zepeda A, Newton KP, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9(11):e112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harlow KE, Africa JA, Wells A, et al. Clinically actionable hypercholesterolemia and hypertriglyceridemia in children with nonalcoholic fatty liver disease. J Pediatr. 2018;198:76–83.e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corey KE, Vuppalanchi R, Vos M, et al. Improvement in liver histology is associated with reduction in dyslipidemia in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2015; 60(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Della Corte C, Fintini D, Giordano U, et al. Fatty liver and insulin resistance in children with hypobetalipoproteinemia: the importance of aetiology. Clin Endocrinol. 2013;79(1):49–54. [DOI] [PubMed] [Google Scholar]
- 12.Non-alcoholic Fatty Liver Disease Study G, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47(12):997–1006. [DOI] [PubMed] [Google Scholar]
- 13.Peretti N, Sassolas A, Roy CC, et al. Guidelines for the diagnosis and management of chylomicron retention disease based on a review of the literature and the experience of two centers. Orphanet J Rare Dis. 2010;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonfeld G, Patterson BW, Yablonskiy DA, et al. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res. 2003; 44(3):470–478. [DOI] [PubMed] [Google Scholar]
- 15.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45(5):941–947. [DOI] [PubMed] [Google Scholar]
- 16.Hickman TB, Briefel RR, Carroll MD, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27(6): 879–890. [DOI] [PubMed] [Google Scholar]
- 17.Heeks LV, Hooper AJ, Adams LA, et al. Non-alcoholic steatohepatitis-related cirrhosis in a patient with APOB L343V familial hypobetalipo-proteinaemia. Clin Chim Acta. 2013;421:121–125. [DOI] [PubMed] [Google Scholar]
- 18.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. [DOI] [PubMed] [Google Scholar]
- 19.Brambilla P, Bedogni G, Heo M, Pietrobelli A. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. Int J Obes. 2013;37(7):943–946. [DOI] [PubMed] [Google Scholar]
- 20.Khoury M, Manlhiot C, McCrindle BW. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013;62(8):742–751. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 22.Sigurdsson G, Nicoll A, Lewis B. The metabolism of low density lipoprotein in endogenous hypertriglyceridaemia. Eur J Clin Invest. 1976;6(2):151–158. [DOI] [PubMed] [Google Scholar]
- 23.Di Filippo M, Vokaer B, Seidah NG. A case of hypocholesterolemia and steatosis in a carrier of a PCSK9 loss-of-function mutation and polymorphisms predisposing to nonalcoholic fatty liver disease. J Clin Lipidol. 2017;11(4):1101–1105. [DOI] [PubMed] [Google Scholar]
- 24.Sankatsing RR, Fouchier SW, de Haan S, et al. Hepatic and cardiovascular consequences of familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol. 2005;25(9):1979–1984. [DOI] [PubMed] [Google Scholar]
- 25.Mager DR, Iniguez IR, Gilmour S, Yap J. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). JPEN J Parenter Enteral Nutr. 2015; 39(1):73–84. [DOI] [PubMed] [Google Scholar]
- 26.Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57(6):2525–2531. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues J Non-alcoholic fatty liver disease associated with hypobe-talipoproteinemia: report of three cases and a novel mutation in APOB gene. Nascer e Crecer. 2016;25(2):104–107. [Google Scholar]
- 28.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 29.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke MW, Hooper AJ, Headlam HA, Wu JH, Croft KD, Burnett JR. Assessment of tocopherol metabolism and oxidative stress in familial hypobetalipoproteinemia. Clin Chem. 2006;52(7):1339–1345. [DOI] [PubMed] [Google Scholar]
- 31.Trieb M, Horvath A, Birner-Gruenberger R, et al. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim Biophys Acta. 2016;1861(7):630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]


