Abstract
Purpose:
Lung cancer causes the highest number of cancer-related deaths in the United States. Lung cancer incidence rates, mortality rates, and rates of advanced stage disease are higher among those who live in rural areas. Known disparities in lung cancer outcomes between rural and non-rural populations may, in part, be due to barriers faced by rural populations. We tested the hypothesis that among Veterans who receive initial lung cancer screening, rural Veterans would be less likely to complete annual repeat screening than non-rural Veterans.
Methods:
We performed a retrospective cohort study of 10 Veterans Affairs Medical Centers from 2015-2019. We identified rural and non-rural Veterans having lung cancer screening. Rural status was defined by the Rural-Urban Commuting Areas (RUCA). The primary outcome was annual repeat lung cancer screening in the 9-15 month window (primary analysis) and 31 day-18 month window (sensitivity analysis) following the first documented lung cancer screening. To examine rurality as a predictor of annual repeat lung cancer screening, we used multivariable logistic regression models.
Results:
In the final analytic sample of 11,402 Veterans, annual repeat lung cancer screening occurred in 27.7% (641/2,316) of rural Veterans versus 31.8% (2,891/9,086) of non-rural Veterans [aOR 0.86, CI 0.73-1.03]. Similar results were seen in the sensitivity analysis with 41.6% (963/2,316) of rural Veterans versus 45.2% (4,110/9,086) of non-rural Veterans, [aOR 0.88, CI 0.73-1.04] having annual repeat screening in the expanded 31 day - 18 month window.
Conclusion:
Among a national cohort of Veterans, rural residence was associated with numerically lower odds of annual repeat lung cancer screening than non-rural residence. Continued, intentional outreach efforts to increase annual repeat lung cancer screening amongst rural Veterans may offer an opportunity to decrease deaths from lung cancer.
Introduction:
With over 130,000 estimated deaths from lung cancer in 2021, lung cancer causes the highest number of cancer-related deaths in the United States (US).[1, 2] Lung cancer incidence rates, mortality rates, and rates of advanced stage disease are higher among those who live in rural areas.[3-5] Detection of early-stage lung cancer with low-dose computed tomography (LDCT) screening offers an opportunity to improve survival for individuals at high risk for lung cancer.[6, 7] While lung cancer screening with a single baseline LDCT can identify early stage cancers, the life-saving benefit occurs from consistent annual repeat screening.[8-10]
Known disparities in lung cancer outcomes between rural and non-rural populations may, in part, be due to barriers that rural populations face in obtaining cancer care and to the higher proportion of smokers in rural areas.[11] Barriers to cancer care for rural populations include lower socioeconomic status, lack of geographic access to screening services and specialty care, and inadequate provider-patient communication.[5, 12-15] Veterans face additional barriers to care such as limited access to affordable and convenient healthcare, concern about social exclusion and stigma, and unmet basic needs for self and family that may prevent them from pursuing health-seeking behavior such as lung cancer screening.[16] While US policies are supportive of annual screening for individuals at high risk for lung cancer, these barriers may reduce appropriate repeat lung cancer screening examinations for rural dwellers.[17-20]
Based on the above-described barriers to cancer care and known lung cancer mortality disparities for individuals living in rural areas, we tested the hypothesis that among Veterans who receive an initial lung cancer screening LDCT, rural Veterans would be less likely to complete an annual repeat lung cancer screening LDCT than non-rural Veterans.
Methods:
Study design and Data sources
We assembled a retrospective cohort using the national Veterans Health Administration (VHA) Observational Medical Outcomes Partnership (OMOP) dataset that contains information from Veterans’ electronic health records (EHR) and claims, the Veterans Affairs (VA) Corporate Data Warehouse (CDW), and the VA Informatics and Computing Infrastructure (VINCI) data warehouse. From these sources, we extracted Veteran-level data including demographics, death dates, and diagnostic and procedure information related to inpatient and outpatient encounters. The cohort included Veterans who underwent LDCT for lung cancer screening at Veterans Affairs Medical Centers (VAMCs) actively enrolled in the Veterans Affairs Partnership to increase access to Lung Screening (VA-PALS), an enterprise-wide initiative to implement lung cancer screening programs at VAMCs, between January 1, 2015 and March 31, 2019.[21] The complete study time period extended through September 30, 2020 to allow for the necessary 18 months of follow-up.
VA Central IRB and the VA Tennessee Valley Healthcare System’s Research and Development Committee approved the study with a waiver of informed consent.
Study Population
Per 2013 US Preventive Services Taskforce (USPSTF) lung cancer screening recommendations, we identified all Veterans age 55 to 80 who underwent LDCT for lung cancer screening during the defined study time period in the 10 VA-PALS VAMCs.[20] The VA-PALS VAMCs were Atlanta, Cleveland, Chicago Hines, Denver, Indianapolis, Milwaukee, Nashville, Philadelphia, Phoenix, and St. Louis. These sites were selected because of their location and ability to implement resources such as a screening navigator that would have the ability to reach out and enroll rural Veterans in the lung cancer screening program.
We identified lung cancer screening LDCTs by selecting records that had current procedural terminology (CPT) codes G0297 or 71250 and contained a description phrase of “screening,” “lung cancer screening,” “LCS”, “low-dose,” “LDCT” or “VCAR ” (see Supplemental Table 1), using methods previously described.[22] This previously described algorithm has a positive predictive value (PPV) of 92% (95% confidence intervals (CI) 0.81.0.97) to detect LDCT performed for lung cancer screening.[22] We excluded LDCTs missing patient identification numbers, date of birth, or address information; performed on Veterans under 55 years old or over 80 years old; associated with a diagnosis code for lung cancer prior to screening; or conducted within 31 days of the previous exam. After applying exclusion criteria, the first documented lung cancer screening LDCT during the defined time period of this study was defined as the Veteran’s initial lung cancer screening LDCT. To avoid possible bias due to differential death rates between rural and non-rural Veterans, we excluded any Veteran who had a documented date of death during the follow-up time frame.
Predictor Variable: Rurality Status
Rural status, defined by the Rural-Urban Commuting Areas (RUCA) which characterize Veterans as residing in highly rural, rural, or urban locations, was extracted from the CDW.[23] RUCA was developed by the US Department of Agriculture (USDA) and the Department of Health and Human Services (HHS) and takes into consideration population density and how closely a community is linked socio-economically to larger urban centers. In our analysis, we defined rurality as a binary variable with those residing in highly rural and rural areas defined as “rural” and those residing in urban areas defined as “non-rural”.
Outcomes: Subsequent LDCT examination
The primary outcome was a one-year annual repeat LDCT following the initial lung cancer screening LDCT, defined as documentation of a lung cancer screening LDCT between 9 and 15 months after the initial lung cancer screening LDCT. This window was based on existing literature for annual repeat lung cancer screening [24] and allows for logistical challenges for Veterans due to scheduling, transportation or other issues.[25, 26] A sensitivity analysis expanded the window for repeat lung cancer screening LDCTs to between 31 days and 18 months following initial lung cancer screening LDCT, which is also used by investigators.[27]
Statistical Methods:
Veteran characteristics and distribution of lung cancer screening LDCTs by year and VAMC were described using means, standard deviations, and frequencies. We anonymized the VAMCs for reporting of lung cancer screening LDCTs.
To examine rurality as a predictor of annual repeat lung cancer screening, we used multivariable logistic regression models and report adjusted odds ratios (aOR) and 95% confidence intervals (CI). The models adjusted for the following covariates: screening date and age at initial screening (both using 3-knot restricted cubic splines to allow greater flexibility), sex (male/female), 4-level race/ethnicity (Hispanic; Black, non-Hispanic; White, non-Hispanic; other or unknown), and the presence or absence of three comorbidities at initial screening (chronic obstructive pulmonary disease, coronary artery disease, and diagnosis of tobacco use in medical record - defined in Supplemental Table 2). Additionally, to account for variation among and correlation within VAMCs, we adjusted for VAMC and utilized robust standard errors clustered at the VAMC level.
To allow for the possibility of a differential burden of rurality on outcome by race, we performed a secondary analysis that added an interaction between rurality and race/ethnic group to the model used for the primary analysis. We also performed subgroup analyses among Veterans age less than 65 and Veterans age 65 and older to examine potential differences in screening due to additional Medicare coverage benefits.
As a preliminary exploration, we evaluated the average annual proportion of eligible Veterans who received initial lung cancer screening LDCTs by rurality status. This exploratory analysis required an estimation of the denominator of eligible Veterans by rurality status. First, we calculated the average number of unique Veterans between the ages of 55 and 80 seen annually at each of the VAMCs using summary data provided by VINCI. We then estimated the number of these Veterans meeting 2013 USPSTF lung cancer screening criteria as 32% of the total based on previous estimates.[28] To establish average annual counts of rural and non-rural Veterans, we used rural population estimates for each VAMC provided by the VHA Office of Rural Health (see Supplemental Table 3). All analyses were conducted using R version 4.0.4.
Role of the Funding Source:
This study was supported in part by the VHA Office of Rural Health with resources and use of facilities at VA Tennessee Valley Healthcare System, Nashville TN. The study was also supported in part by the Vanderbilt-Ingram Cancer Center Support Grant CA68485, Vanderbilt Scholars in T4 Translational Research (VSTTaR) K12 Program, funded by the National Heart, Lung, and Blood Institute K12HL137943.
The Office of Rural Health had the opportunity to review the manuscript prior to submission but did not participate in study design, data collection, data analysis, data interpretation or the decision to publish results. Study results and the decision to publish are solely the responsibility of the authors and do not necessarily represent official views of the funder.
Results:
Study Cohort and Characteristics of Veterans with Initial Lung Cancer Screening:
We identified 13,142 unique Veterans undergoing lung cancer screening LDCT at 9 VAMCs during the defined time period (Figure 1) as one VAMC did not perform any lung cancer screening LDCTs during the defined time period. Of these, 11,951 Veterans had a lung cancer screening LDCT who met inclusion criteria. After exclusion of 549 Veterans who died during the follow-up period, our final analytic sample included 11,402 Veterans from 9 VAMCs. Characteristics of Veterans included in the cohort and distribution of lung cancer screening LDCTs by year and VAMC can be seen in Table 1.
Figure 1.
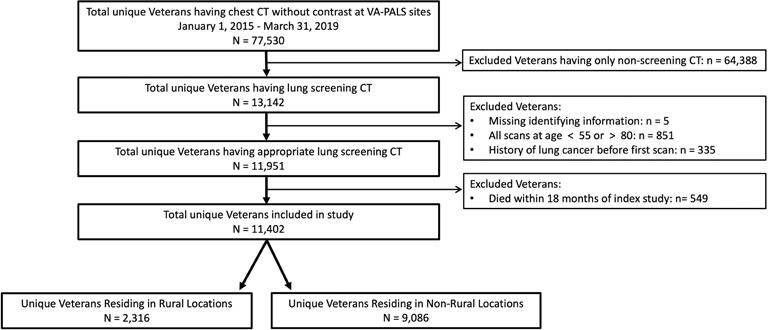
Study Cohort.
Table 1.
Veteran Characteristics and Distribution of lung cancer screening LDCTs by Year and VAMC
| Characteristic | Rural N (%) |
Non-Rural N (%) |
|---|---|---|
| Total | 2,316 | 9,086 |
| Patient Characteristics | ||
| Age, mean (SD) | 66.6 (5.4) | 66.1 (5.5) |
| Sex | ||
| Male | 2,219 (95.8) | 8,668 (95.4) |
| Female | 97 (4.2) | 418 (4.6) |
| Race | ||
| White | 2,028 (87.6) | 5,820 (64.1) |
| Black or African American | 152 (6.6) | 2,897 (31.9) |
| Asian | 0 (0) | 11 (0.1) |
| American Indian or Alaska Native | 13 (0.6) | 19 (0.2) |
| Native Hawaiian or other Pacific Islander | 7 (0.3) | 61 (0.7) |
| Unknown | 116 (5.0) | 278 (3.1) |
| Ethnicity | ||
| Hispanic or Latino | 21 (0.9) | 226 (2.5) |
| Not Hispanic or Latino | 2,250 (97.2) | 8,711 (95.9) |
| Unknown | 45 (1.9) | 149 (1.6) |
| Comorbidities | ||
| Chronic obstructive pulmonary disease | 1,059 (45.7) | 3,185 (35.1) |
| Coronary artery disease | 455 (19.6) | 1,461 (16.1) |
| Documentation of tobacco use | 1,195 (51.6) | 4,928 (54.2) |
| Year of initial screening LDCT | ||
| 2015 | 219 (9.5) | 1,225 (13.5) |
| 2016 | 484 (20.9) | 2,415 (26.6) |
| 2017 | 839 (36.2) | 3,210 (35.3) |
| 2018 | 679 (29.3) | 1,959 (21.6) |
| 2019 | 95 (4.1) | 277 (3.0) |
| VAMC | ||
| 1 | 273 (11.8) | 2,928 (32.2) |
| 2 | 174 (7.5) | 538 (5.9) |
| 3 | 115 (5.0) | 522 (5.7) |
| 4 | 7 (0.3) | 38 (0.4) |
| 5 | 292 (12.6) | 1,189 (13.1) |
| 6 | 927 (40.0) | 554 (6.1) |
| 7 | 131 (5.7) | 328 (3.6) |
| 8 | 332 (14.3) | 1,275 (14.0) |
| 9 | 65 (2.8) | 1,714 (18.9) |
LDCT: low-dose computed tomography; VAMC: Veterans Affairs Medical Center; SD: standard deviation
Rurality associated with Annual Repeat Lung Cancer Screening LDCT:
For the primary outcome of annual repeat lung cancer screening, 27.7% (641/2,316) of rural Veterans vs 31.8% (2,891/9,086) of non-rural Veterans obtained a repeat LDCT within the 9 - 15 month timeframe. We found a numerically lower odds of annual repeat lung cancer screening with wide confidence intervals [aOR 0.86, CI 0.73-1.03] amongst Veterans residing in rural locations when compared to Veterans residing in non-rural locations (Table 2). Results of the complete multivariable analysis output can be found in Supplemental Table 4. Similarly, a numerically lower odds [aOR 0.88, CI 0.73-1.04] for annual repeat lung cancer screening amongst rural Veterans was seen in the sensitivity analysis which extended the follow-up time period to range from 31 days - 18 months. Using this expanded time frame for repeat lung cancer screening, 41.6% (963/2,316) of rural Veterans vs 45.2% (4,110/9,086) of non-rural Veterans obtained annual repeat LDCT.
Table 2.
Initial and annual repeat lung cancer screening LDCT by Facility and Rural Status
| Study Analysis Group | Rural | Non-Rural |
|---|---|---|
| Primary Analysis: Annual repeat LDCT within 9-15 months | ||
| Initial screening LDCT eligible for annual repeat screening, n | 2,316 | 9,086 |
| Annual repeat LDCT performed, n | 641 | 2,891 |
| Odds of annual repeat LDCT, aOR (95% CI) | 0.86 (0.73, 1.03) | Ref |
| Sensitivity Analysis: Annual repeat LDCT within 1-18 months | ||
| Initial screening LDCT eligible for annual repeat screening, n | 2,316 | 9,086 |
| Annual repeat LDCT performed, n | 963 | 4,110 |
| Odds of annual repeat LDCT, aOR (95% CI) | 0.88 (0.73, 1.04) | Ref |
| Subgroup for annual repeat LDCT 9-15 months: Age 65 + | ||
| Initial screening LDCT eligible for annual repeat screening, n | 1,484 | 5,546 |
| Annual repeat LDCT performed, n | 410 | 1,809 |
| Odds of annual repeat LDCT, aOR (95% CI) | 0.83 (0.71, 0.98) | Ref |
| Subgroup for annual repeat LDCT 9-15 months: Age <65 | ||
| Initial screening LDCT eligible for annual repeat screening, n | 832 | 3,630 |
| Annual repeat LDCT performed, n | 231 | 1,082 |
| Odds of annual repeat LDCT, aOR (95% CI) | 0.93 (0.77, 1.12) | Ref |
LDCT: low-dose computed tomography; aOR: adjusted odds ratio; CI: confidence interval
The multivariable logistic regression models adjusted for the following covariates: screening date and age at initial screening (both using 3-knot restricted cubic splines to allow greater flexibility), sex (male/female), 4-level race/ethnicity (Hispanic; Black, non-Hispanic; White, non-Hispanic; other or unknown), and the presence or absence of three comorbidities at initial screening (chronic obstructive pulmonary disease, coronary artery disease, and diagnosis of tobacco use).
Results of the secondary analysis that included the interaction between rurality and race/ethnic group can be seen in Table 3. The confidence intervals of the four race/ethnic groups were too wide to allow for a meaningful conclusion to be drawn.
Table 3.
Summary Results from Model with Interaction between Rural Status and Race/Ethnic Group
| Specified Group | aOR* (95% CI) |
|---|---|
| Black, Non-Hispanic | 1.20 (0.97, 1.48) |
| White, Non-Hispanic | 0.85 (0.70, 1.03) |
| Hispanic | 0.68 (0.18, 2.54) |
| Other/Unknown | 0.67 (0.52, 0.87) |
| Overall p-value for the interaction (3 d.f.) | 0.025 |
aOR = adjusted odds ratio of one-year repeat low-dose computed tomography (LDCT) for rural vs. non-rural individuals. The models adjusted for rural status, race/ethnic group, and their interaction, in addition to all covariates included in the main model: screening date and age at initial screening (both using 3-knot restricted cubic splines to allow greater flexibility), sex (male/female), and the presence or absence of three comorbidities at initial screening (chronic obstructive pulmonary disease, coronary artery disease, and diagnosis of tobacco use). CI = confidence interval. d.f. = degrees of freedom
The results of the subset analyses for Veterans age less than 65 and those 65 and older are similar to those of the primary and sensitivity analyses but cannot be directly compared as the study samples are different. Veterans age 65 and older residing in rural locations had lower odds of annual repeat lung cancer screening than those residing in non-rural locations [aOR 0.83, CI 0.71-0.98]. Veterans age less than 65 residing in rural locations had numerically lower odds of annual repeat lung cancer screening than those residing in non-rural locations [aOR 0.93, CI 0.77-1.12].
In the exploratory analysis which evaluated the association of rurality with initial lung cancer screening, we found that while only 0.6% (545/91,786) of eligible rural Veterans obtained an initial lung cancer screening, 3.5% (2,138/61,937) of non-rural Veterans did (Table 4). Rural Veterans eligible for lung cancer screening had 80% lower odds [OR 0.17, CI 0.15-0.18] of obtaining an initial lung cancer screening LDCT than non-rural Veterans.
Table 4.
Initial Lung Cancer Screening LDCT’s in Eligible Veterans by Rural Status
| Rural | Non-Rural | |
|---|---|---|
| Initial screening | ||
| Veterans annually eligible for lung cancer screening, n (estimated)* | 91,786 | 61,937 |
| Average number of initial LDCTs performed per year, n | 545 | 2,138 |
| Odds of initial screening LDCT, OR (95% CI) | 0.17 (0.15, 0.18) | Ref |
OR: odds ratio; CI: confidence interval
estimated as the average across actual inpatient and outpatient visits at all sites and using estimated rural population estimates at each site
Discussion:
We found that among Veterans who received an initial lung cancer screening LDCT, rural Veterans were less likely to complete annual repeat lung cancer screening LDCT than non-rural Veterans. Lower odds of annual repeat lung cancer screening were seen in the primary analysis (with follow-up time period of 9-15 months), the sensitivity analysis (with follow-up time period of 31 days - 18 months), and subgroup analyses of Veterans age 65 and older and Veterans age less than 65. Our exploratory analysis also found a lower odds of initial screening amongst rural Veterans than non-rural Veterans.
Randomized controlled trials of lung cancer screening, such as the National Lung Screening Trial (NLST), have found adherence rates of more than 90% for follow-up scans.[7, 8] In the Veterans Affairs Lung Cancer Screening Demonstration Project, a VHA program that provided resources in a real world setting to evaluate lung cancer screening, lung cancer screening follow-up adherence (with a broader follow-up time period of 1 day – 18 months) was found to be 82.2%.[29] Adherence rates to annual repeat lung screening in the real-world setting are reported between 59% and 85.7%.[24, 30, 31] A pooled meta-analysis found 55% adherence across all follow-up periods (ranging from 12-36 months).[32] In our study, we found an annual repeat lung cancer screening rate of 27.7% for rural Veterans and 31.8% for non-rural Veterans, with lower odds of annual repeat screening among rural Veterans. The lower proportion of annual repeat screening identified amongst all Veterans, but in particular rural Veterans, might be explained by the barriers to care many populations face in receiving follow-up lung cancer screening.
Previously identified barriers to returning for annual lung cancer screening include lack of transportation, financial cost, lack of communication, and lack of current symptoms at time screening was due.[31, 33] Beyond these barriers faced by the general population, individuals residing in rural locations face additional barriers to lung cancer screening including lack of geographic access to screening and specialty care, lack of access to lung cancer screening centers of excellence, and inadequate provider-patient communication.[5, 34] Nationwide efforts, such as those of VA-PALS, the GO2 Foundation, and the LuCa National Training Network to increase access to high-quality lung cancer screening represent an opportunity to address existing lung cancer mortality disparities for rural Veterans.[21, 35, 36] The VHA Office of Rural Health has established programming, such as VA-PALS, to improve care for rural Veterans by addressing systemic healthcare and access challenges.[37] Programming efforts through the Office of Rural Health include offering telehealth, improving transportation services, adding social workers, and providing rural health training and education. Continued intentional outreach to rural populations using novel approaches may be needed to address the identified disparity in lung cancer screening.
Our study is one of the first to evaluate the association of rurality with annual repeat lung cancer screening and entry into the lung cancer screening pathway. The study has several limitations. The study represents the Veteran population and also consists of primarily white men. This limitation should be considered when generalizing study results to other populations. Further, Veterans have the opportunity to choose where they receive their healthcare and may not receive all of their care at VHA facilities, and some follow-up examinations may not be accounted for. While this may cause an undercounting of some LDCT exams, we do not think this would have occurred differentially between rural and urban dwellers. Further, misclassification of CTs as lung cancer screening examinations may have occurred, although a previous study documented a 92% positive predictive value (PPV) for identifying lung cancer screening CTs with this algorithm.[22] The study also utilizes administrative data which may be imperfect. This administrative data also does not include detail on the assessments (e.g. Lung CT Screening & Reporting Data System (LungRADS) or International Early Lung Cancer Action Program (IELCAP) scores) and follow-up recommendations for individual lung cancer screening examinations.[38-40] Additionally, we classified Veterans as living in rural or non-rural locations at the time the data was extracted. Some Veterans may have resided in different locations at time of initial screening or follow-up screening. While the preliminary exploratory analysis of the association between rurality and initial screening provides important hypothesis-forming data, it is based on several estimations including the percentage Veterans meeting smoking eligibility criteria and the proportion of rural versus non-rural Veterans at each VAMC.
Increasing uptake of annual repeat lung cancer screening offers an opportunity to decrease the number of deaths from lung cancer, the leading cause of cancer deaths in the US. Rural Veterans have lower odds of both receiving initial lung cancer screening and pursuing annual repeat lung cancer screening than their non-rural counterparts. Future interventions should specifically focus on gathering data on barriers to lung cancer screening for Veterans through collection of qualitative data and developing strategies to increase rural Veterans’ uptake of initial and annual repeat lung cancer screening.
Supplementary Material
SUMMARY SENTENCE:
Rural Veterans have lower odds of pursuing annual repeat lung cancer screening than non-rural Veterans. Continued, intentional outreach efforts to increase annual repeat lung cancer screening amongst rural Veterans may offer an opportunity to decrease deaths from lung cancer.
TAKE HOME POINTS:
Lung cancer incidence rates, mortality rates, and rates of advanced stage disease are higher among those who live in rural areas. While lung cancer screening with a single baseline LDCT can identify early stage cancers, the life-saving benefit occurs from consistent annual repeat screening.
Rural Veterans have lower odds of pursuing annual repeat lung cancer screening than non-rural Veterans.
Continued, intentional outreach efforts to increase annual repeat lung cancer screening amongst rural Veterans may offer an opportunity to decrease deaths from lung cancer.
Acknowledgments:
This study was supported in part by the VA Office of Rural Health (LBS, JAL, LRS, CLR), Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program (JAL, CCL, RD, CLR) with resources and use of facilities at VA Tennessee Valley Healthcare System, Nashville TN (LBS, JAL, LRS, CCL, MEM, JD, JAR, RSD, PPM, CLR). The study was also supported in part by the Vanderbilt-Ingram Cancer Center Support Grant CA68485 (LBS, JAL, PPM), Vanderbilt Scholars in T4 Translational Research (VSTTaR) K12 Program, funded by the National Heart, Lung, and Blood Institute K12HL137943 (JAL and CLR).
Funding Source:
The study was supported in part by the Veterans Health Administration Office of Rural Health, Vanderbilt-Ingram Cancer Center Support Grant CA68485 and the Vanderbilt Scholars in T4 Translational Research (VSTTaR) K12 Program, funded by the National Heart, Lung, and Blood Institute K12HL137943.
Footnotes
Data access/integrity: The author(s) declare(s) that they had full access to all of the data in this study and the author(s) take(s) complete responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest:
LBS, JAL, and CCL serve on the Steering Committee for this VAMC lung screening program. JAL and CCL are clinical co-directors of the clinical lung cancer screening program. Neither receive financial compensation for these roles.
DFY is a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are non-exclusively licensed to General Electric. As an inventor of these patents, DFY is entitled to a share of any compensation which CRF may receive from its commercialization of these patents. He is also an equity owner in Accumetra, a privately held technology company committed to improving the science and practice of image-based decision making. DFY also serves on the advisory board of GRAIL.
CIH is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation. She receives no compensation from the Foundation. The Foundation is established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases. CIH is also a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest which are owned by Cornell Research Foundation (CRF). Since 2009, CIH does not accept any financial benefit from these patents including royalties and any other proceeds related to the patents or patent applications owned by CRF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.American Cancer Society. Cancer Facts & Figures 2021. Atlanta: American Cancer Society; 2021. [Google Scholar]
- 2.Moghanaki D, Williams C. Lung Cancer in the VA at a National Level. Fed Pract.Cancer Data Trends:S26–8. [Google Scholar]
- 3.Zahnd WE, James AS, Jenkins WD, Izadi SR, Fogleman AJ, Steward DE, et al. Rural-Urban Differences in Cancer Incidence and Trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC. Invasive Cancer Incidence, 2004-2013, and Deaths, 2006-2015, in Nonmetropolitan and Metropolitan Counties - United States. MMWR Surveill Summ. 2017;66(14):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odahowski CL, Zahnd WE, Eberth JM. Challenges and Opportunities for Lung Cancer Screening in Rural America. J Am Coll Radiol. 2019;16(4 Pt B):590–5. [DOI] [PubMed] [Google Scholar]
- 6.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503–13. [DOI] [PubMed] [Google Scholar]
- 7.National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369(10):920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tendler S, Holmqvist M, Wagenius G, Lewensohn R, Lambe M, De Petris L. Educational level, management and outcomes in small-cell lung cancer (SCLC): A population-based cohort study. Lung Cancer. 2020;139:111–7. [DOI] [PubMed] [Google Scholar]
- 10.Henschke CI, Yip R, Shaham D, Zulueta JJ, Aguayo SM, Reeves AP, et al. The Regimen of Computed Tomography Screening for Lung Cancer: Lessons Learned Over 25 Years From the International Early Lung Cancer Action Program. J Thorac Imaging. 2021;36(1):6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doogan NJ, Roberts ME, Wewers ME, Stanton CA, Keith DR, Gaalema DE, et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev Med. 2017;104:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow CJ, Al-Refaie WB, Abraham A, Markin A, Zhong W, Rothenberger DA, et al. Does patient rurality predict quality colon cancer care?: A population-based study. Dis Colon Rectum. 2015;58(4):415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: issues and challenges. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. J Cancer Epidemiol. 2011;2011:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashibe M, Kirchhoff AC, Kepka D, Kim J, Millar M, Sweeney C, et al. Disparities in cancer survival and incidence by metropolitan versus rural residence in Utah. Cancer Med. 2018;7(4):1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misra-Hebert AD, Santurri L, DeChant R, Watts B, Rothberg M, Sehgal AR, et al. Understanding the Health Needs and Barriers to Seeking Health Care of Veteran Students in the Community. South Med J. 2015;108(8):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8. [DOI] [PubMed] [Google Scholar]
- 18.Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Centers for Medicare &Medicaid Services website. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed February 13, 2021. [Google Scholar]
- 19.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69(3):184–210. [DOI] [PubMed] [Google Scholar]
- 20.US Preventive Services Taskforce. 2013 Lung Cancer Screening Recommendations. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening. February 14, 2021. [Google Scholar]
- 21.Lewis JA, Spalluto LB, Henschke CI, Yankelevitz DF, Aguayo SM, Morales P, et al. Protocol to evaluate an enterprise-wide initiative to increase access to lung cancer screening in the Veterans Health Administration. Clin Imaging. 2020;73:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JA, Samuels LR, Denton J, Edwards GC, Matheny ME, Maiga A, et al. National Lung Cancer Screening Utilization Trends in the Veterans Health Administration. JNCI Cancer Spectr. 2020;4(5):pkaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Veterans Affairs. Office of Rural Health. Rural Veterans. Available from: https://www.ruralhealth.va.gov/aboutus/ruralvets.asp#def. April 7, 2021. [Google Scholar]
- 24.Alshora S, McKee BJ, Regis SM, Borondy Kitts AK, Bolus CC, McKee AB, et al. Adherence to Radiology Recommendations in a Clinical CT Lung Screening Program. J Am Coll Radiol. 2018;15(2):282–6. [DOI] [PubMed] [Google Scholar]
- 25.Hubbard RA, O'Meara ES, Henderson LM, Hill D, Braithwaite D, Haas JS, et al. Multilevel factors associated with long-term adherence to screening mammography in older women in the U.S. Prev Med. 2016;89:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gierisch JM, Earp JA, Brewer NT, Rimer BK. Longitudinal predictors of nonadherence to maintenance of mammography. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wildstein KA, Faustini Y, Yip R, Henschke CI, Ostroff JS. Longitudinal predictors of adherence to annual follow-up in a lung cancer screening programme. J Med Screen. 2011;18(3):154–9. [DOI] [PubMed] [Google Scholar]
- 28.Kinsinger LS, Anderson C, Kim J, Larson M, Chan SH, King HA, et al. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399–406. [DOI] [PubMed] [Google Scholar]
- 29.Tanner NT, Brasher PB, Wojciechowski B, Ward R, Slatore C, Gebregziabher M, et al. Screening Adherence in the Veterans Administration Lung Cancer Screening Demonstration Project. Chest. 2020;158(4):1742–52. [DOI] [PubMed] [Google Scholar]
- 30.Brasher P, Tanner N, Yeager D, Silvestri G. Adherence to Annual Lung Cancer Screening within the Veterans Health Administration Lung Cancer Screening Demonstration Project. Chest. 2018;154(4):636A–7A. [DOI] [PubMed] [Google Scholar]
- 31.Spalluto LB, Lewis JA, LaBaze S, Sandler KL, Paulson AB, Callaway-Lane C, et al. Association of a Lung Screening Program Coordinator With Adherence to Annual CT Lung Screening at a Large Academic Institution. J Am Coll Radiol. 2020;17(2):208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Olivo MA, Maki KG, Choi NJ, Hoffman RM, Shih YT, Lowenstein LM, et al. Patient Adherence to Screening for Lung Cancer in the US: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(11):e2025102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang GX, Narayan AK, Park ER, Lehman CD, Gorenstein JT, Flores EJ. Screening Mammography Visits as Opportunities to Engage Smokers With Tobacco Cessation Services and Lung Cancer Screening. J Am Coll Radiol. 2020;17(5):606–12. [DOI] [PubMed] [Google Scholar]
- 34.Boudreau JH, Miller DR, Qian S, Nunez ER, Caverly TJ, Wiener RS. Access to lung cancer screening in the Veterans Health Administration: Does geographic distribution match need in the population? Chest. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GO2 Foundation. Available from: https://go2foundation.org. March 30, 2021. [Google Scholar]
- 36.LuCa National Training Network. Available from: https://lucatraining.org. April 9, 2021. [Google Scholar]
- 37.US Department of Veterans Affairs. Office of Rural Health Programs. Available from: https://www.ruralhealth.va.gov/aboutus/programs.asp. April 8, 2021. [Google Scholar]
- 38.American College of Radiology. LungRADS. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. June 29, 2021. [Google Scholar]
- 39.IELCAP Protocols. Available from: http://www.ielcap.org/protocols. June 29, 2021. [Google Scholar]
- 40.Henschke CI, Li K, Yip R, Salvatore M, Yankelevitz DF. The importance of the regimen of screening in maximizing the benefit and minimizing the harms. Ann Transl Med. 2016;4(8):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


