Abstract
Alzheimer’s Disease (AD) is a neuroinflammatory disease characterized partly by the inability to clear, and subsequent build-up, of amyloid-beta (Aβ). AD has a bi-directional relationship with circadian disruption (CD) with sleep disturbances starting years before disease onset. However, the molecular mechanism underlying the relationship of CD and AD has not been elucidated. Myeloid-based phagocytosis, a key component in the metabolism of Aβ, is circadianly-regulated, presenting a potential link between CD and AD. In this work, we revealed that the phagocytosis of Aβ42 undergoes a daily circadian oscillation. We found the circadian timing of global heparan sulfate proteoglycan (HSPG) biosynthesis was the molecular timer for the clock-controlled phagocytosis of Aβ and that both HSPG binding and aggregation may play a role in this oscillation. These data highlight that circadian regulation in immune cells may play a role in the intricate relationship between the circadian clock and AD.
Author summary
Disruption of the 24-hour endogenous cycle that controls human behavior and cellular physiology, our circadian rhythms, has been correlated with an increase in both the rate and severity of Alzheimer’s disease (AD), the currently incurable, costly, and steadily growing neurodegenerative disorder that affects millions of elderly patients. AD is a neurodegenerative, neuroinflammatory disease that is caused, in part, by the buildup of Amyloid-beta 42 (Aβ42) plaques in the brain. To understand the mechanistic link between circadian disruption and AD, we studied the immune cells responsible for the clearance of Aβ42 over circadian time. We found there was a daily oscillation in Aβ42 clearance and that this oscillation was lost in cells without a circadian rhythm. We established the underlying cause of this oscillation was the circadian control of cell surface molecules, heparan sulfate proteoglycans, which have previously been shown to play a role in the regulation of Aβ42 clearance. We further showed that this regulation was specific to Aβ42, demonstrating a mechanistic link underlying the connection between the disruption of circadian rhythms and AD.
Introduction
Alzheimer’s Disease (AD) and Alzheimer’s related dementias affect millions of people every year, are a leading cause of death in the U.S., and have associated care costs estimated at US$818 billion globally [1,2]. AD is a neurodegenerative, neuroinflammatory disease that is characterized by extracellular β-amyloid (Aβ) plaques, intracellular hyperphosphorylated tau fibrils, and increased neuroinflammation [3]. Though targeting Aβ as a therapeutic strategy has met limited success, Aβ accumulation is still regarded as a crucial step in AD pathogenesis due to strong evidence from human genetics of familial AD and Down syndrome [4,5]. Thus, understanding the metabolism of Aβ is essential to understand AD mechanisms and develop AD therapies.
A physiological consequence (and perhaps causative factor) of AD is the disruption of the circadian clock, the 24-hour endogenous rhythm that tunes physiology to the day/night cycle [6–10] (S1 Fig). Correlated with this, there is a daily oscillation in the abundance of Aβ42 in cerebrospinal fluid in healthy adults and this oscillation is ablated in patients with AD [6]. A key factor in the clearance of Aβ are the resident microglia and, in the later stages of AD, peripheral macrophages [7,11–13]. Increasing neuroinflammation due to the accumulation of Aβ42 plaques leads to elevated levels of macrophage markers, activating microglia and increasing peripheral macrophage migration across the blood brain barrier (BBB), where peripheral macrophages more efficiently clear Aβ42 [7,11–17]. A double-edged sword, increased microglial activation and peripheral macrophage migration enhances the already high levels of neuroinflammation, leading to heightened cell death and exacerbating disease phenotypes [18–25]. The circadian clock also exerts extensive influence over macrophage/microglial behavior and disruption of circadian regulation affects the ability of macrophages to phagocytize target particles [26–28] (S1 Fig). In total, the concordance of these factors points to a relationship between the circadian regulation of the immune system and AD through the metabolism of Aβ42. Despite the correlation between circadian Aβ42 abundance and circadian control of macrophage/microglial phagocytosis, the link between AD and the clock via the circadian timing of Aβ42 phagocytosis has not been examined.
In this report, we utilized bone-marrow derived macrophages (BMDM’s) as a proxy for cells from the monocyte lineage to demonstrate that Aβ42 phagocytosis is under circadian control. Transcriptomic and proteomic data from macrophages identified few components that are circadianly regulated in the classical phagocytosis pathways but highlighted oscillations in the enzymes of the biosynthesis pathways of cell surface proteoglycans (PGs), which are known to negatively regulate Aβ42 clearance [28–30]. We validated that PG levels oscillated over circadian time in macrophages in vitro, with PG levels reaching their zenith antiphase to the zenith of Aβ42 phagocytosis [29–35]. Chemically reducing PG levels ablated the circadian oscillation of Aβ42 phagocytosis by enhancing Aβ42 phagocytosis at its nadir. These data suggested that the presence of PGs suppresses the phagocytosis of Aβ42 and our investigation into the mechanism behind this suppression showed that aggregation and PG binding were essential to the circadian regulation of Aβ42 phagocytosis. Overall, our data suggests a role for myeloid cells in the circadian timing of the clearance of Aβ42 and an avenue through which the disruption of circadian rhythms can lead to enhanced AD pathogenesis.
Results
Phagocytosis of Aβ42 by Bone marrow derived macrophages is timed by the circadian clock
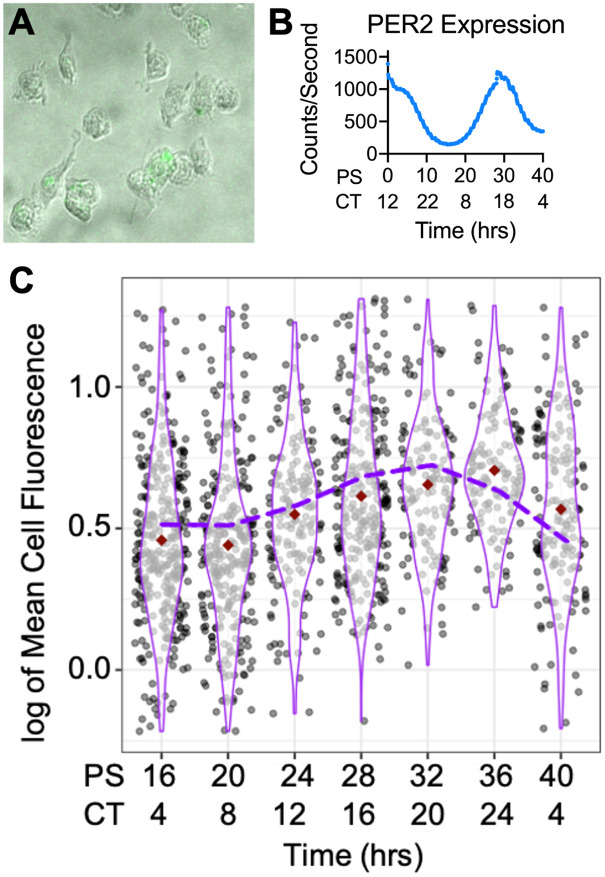
Aβ42 abundance oscillates with a circadian period, microglia and macrophages have been shown to phagocytize Aβ42, and phagocytosis by macrophages is under circadian regulation, leading us to hypothesize that oscillations in the metabolism of Aβ42 may stem from the circadian regulation of phagocytosis in cells from myeloid lineages [12,14,28]. To validate this theory, we first needed to demonstrate oscillations in the phagocytosis of Aβ42. To do so, we modified a previously-employed BMDM phagocytosis assay (as BMDMs are both models for activated microglia and are known to migrate into the brain in late-stage AD), to use fluorescently labeled Aβ42 to determine if the phagocytosis of Aβ42 is controlled by the circadian clock [28,36–38]. In brief, BMDMs were derived from bone marrow progenitor cells extracted from Per2::Luc C57BL/6J mice and differentiated with recombinant Macrophage Colony Stimulating Factor (M-CSF), with flow cytometry confirming complete differentiation into naïve peripheral macrophages [39,40]. These BMDMs were then serum-shock synchronized and luminescence traces confirmed that our protocol resulted in reliable, ∼24-h, PER2 oscillations [28]. 16 h after synchronization, confluent dishes of these BMDMs were treated HiLyte 488 labeled Aβ42 (Anaspec), in triplicate, every 4 h over a 24 h circadian period. BMDMs treated with labeled Aβ42 were harvested two hours after Aβ42 treatment and fixed with formalin. Total cellular fluorescence levels were analyzed with fluorescent confocal microscopy and quantified using a custom cell measurement MATLAB script (Fig 1A, S2 Fig, and S1 Table).
Fig 1. Aβ42 phagocytosis oscillated with a circadian period.
(A) Cropped composite image of fluorescent microscopy of the Aβ42 treated macrophages. (B) PER2::LUC expression from untreated BMDMs, determined via bioluminescence using a lumicycle, reported in post shock (PS) and circadian time (CT). (C) Violin plot of Aβ42 phagocytosis (n = 3) represented in logged mean cell fluorescence values plotted against post shock (PS) and circadian time (CT). Grey dots represent a single cell measurement, purple violin plot lines represent the range of the data, and red diamonds indicate the mean measurement of each time point. The purple dashed line depicts the ECHO fitted model of Aβ42 phagocytosis. CT is in reference to the comparison of PER2 expression from Keller et al., 2009 [41] compared to PER2 expression from our cells. n = number of replicates.
Total cellular fluorescence levels demonstrated that the amount of Aβ42 phagocytized by macrophages underwent a daily oscillation, with the nadir occurring between 16 and 20 h post serum shock (PS) (CT8 in relation to the zenith of PER2-LUC) and the zenith at PS32 (CT20) [41] (Fig 1B and 1C, S1 Fig). The extended harmonic circadian oscillator model (ECHO version 4.1) was used to analyze the average of the three replicates from each timepoint and predicted that Aβ42 total cellular florescence, and therefore the phagocytosis of Aβ42, oscillated with a circadian period (~26 h, ECHO p-value = 1.61 x 10−6) [42]. Additionally, when compared with PER2 levels extrapolated based on the lumicycle data of the macrophages sampled, we saw that total cellular fluorescence was highest after the zenith of PER2 levels [40] (Fig 1B and 1C). The Aβ42 phagocytosis experiment was then repeated using PER1-/-/PER2-/- knockout (KO) mice to confirm the circadian influence of this relationship. BMDMs from the knockout mice were extracted, derived into naïve macrophages, and synchronized as described above. KO macrophages were treated with fluorescently labeled Aβ42 for 2 h, in triplicate, every 4 h for 24 h starting at PS16. Total cellular fluorescence was quantified and showed an ablation of the zenith of fluorescence at PS32 (CT20), confirming that the clock regulates Aβ42 phagocytosis (S3 Fig).
Proteoglycan levels in macrophages undergo daily oscillations
We next used the ECHO program to probe our previously published, highly sampled/replicated, murine macrophage dataset, which tracked the transcriptome and proteome of BMDMs over two circadian days, to determine mechanisms that underlie the oscillation of Aβ42 phagocytosis [28,42]. Many known pathways essential for Aβ42 phagocytosis oscillated with a circadian period at the transcript level, but we found no oscillation in the proteome associated with these pathways except for the low-density lipoprotein receptor (LDLR) (zenith at CT13) and the low-density lipoprotein receptor-related protein isoform 6 (LRP6) (zenith at CT1) [43,44] (S3 Table). However, neither of these proteins reached their zenith in-phase with the oscillation of Aβ42 phagocytosis.
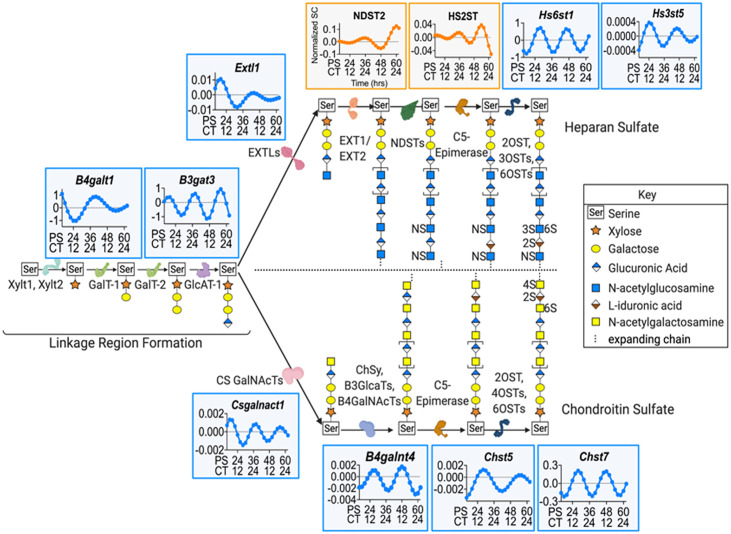
We then probed the two most enriched categories in the BMDM proteome according to Panther, binding and catalysis, and found several proteins related to proteoglycan (PG) synthesis and maintenance [45]. PGs are structurally diverse macromolecules that participate in a wide range of biological functions such as modulation of inflammation, extracellular matrix (ECM) assembly and remodeling, tissue repair, and ligand-receptor interactions [46,47]. PGs like heparan sulfate proteoglycans (HSPGs) and chondroitin sulfate proteoglycans (CSPGs) are involved in the clearance of Aβ42, associate with Aβ42 to foster the formation of plaques, and reduce Aβ plaque load upon the knockout of a key enzyme in the HSPG biosynthesis pathway [29,30,31,33–35,48–51,52,53]. Using the published PG synthesis pathway from Maeda, 2015 [54], the HSPG synthesis pathway from Kreuger and Kjellen, 2012 [55], and the CSPG synthesis pathway from Ly et al., 2011 [56], we cross-referenced the PG synthesis pathway enzymes to the mouse genome using Mouse Genome Informatics (MGI) to establish the homologues for PG synthesis in mice [57]. Once potential PG biosynthesizing genes were identified, we mapped each protein (or transcript if the protein was not found in our dataset) in these pathways to the information from our circadian transcriptomic and proteomics data sets and found that some HSPG and CSPG biosynthesis enzymes undergo a daily oscillation at almost every step of PG biosynthesis in macrophages, from the formation of the linkage region to chain modification (Fig 2).
Fig 2. Enzyme levels in the HSPG and CSPG biosynthesis pathways varied over the circadian day.
A diagrammatic representation of the pathways in HSPG and CSPG biosynthesis with the enzymes involved in each pathway that are under circadian regulation mapped to their location in the pathway. Pathways were adapted from Maeda, 2015 [54]; Kreuger and Kjellen, 2012 [55]; and Ly et al., 2011 [56]. The plotted expression graphs depict the ECHO fitted model of the gene expression for either RNA (blue) or protein (orange) over PS and CT. Representative HSPG and CSPG chains are shown.
According to the current understanding of PG biosynthesis, creation of both HSPGs and CSPGs start with the formation of the linkage region by xylosyltransferases, XYLT1 and XYLT2, which add a xylose to a serine. Following this, Galactosyltransferases, GALT-1 and GALT-2 (B4galt1 zenith at CT4.35), sequentially facilitate the addition of two galactose residues, followed by the glucuronosyltransferase, GLCAT-1 (Bgat3 zenith at CT7.1), catalyzed addition of glucuronic acid. The HSPG and CSPG biosynthesis pathways then diverge and both branch-point enzymes exostosin-like glycosyltransferase, EXTLs (Extl1 zenith at CT8.44), in the HSPG pathway and chondroitin sulfate N-acetylgalactosaminyltransferase, CS GALNACT (Csgalnact1 zenith at CT7.01), in the CSPG pathway are under the regulation of the circadian clock. HSPG chains undergo elongation through the alternating additions of glucuronic acid and N-acetylglucosamine by exostosin glycosyltransferases, EXT1 and EXT2, modification by N-sulfotransferases, NDSTs (NDST2 zenith at CT22.61), the conversion of D-glucuronic acid into L-iduronic acid by C5-epimerase, and the addition of sulfate groups by sulfotransferases (Hs6st1 zenith at CT15.53; Hs3st5 zenith at CT12.72; Hs2st zenith at CT4.37). In the CSPG pathway, CSPG elongation is catalyzed by chondroitin sulfate synthase, CHSY, along with glucuronosyl transferases, B3GLACTs, and N-acetyl galactosaminyl transferases, B4GALNACTs (B4Galnt4 zenith at CT14.87). The chain is then modified by C5-Epimerase, and various sulfotransferases (Chst5 zenith at CT16.71 and Chst7 zenith at CT16.11) add sulfate groups (Fig 2 and S2 Table). Clearly, many enzymes involved in the synthesis pathway for both HSPGs and CSPGs are tightly timed by the circadian clock and this could lead to an oscillation in HSPG and CSPG levels.
To confirm that the oscillations we noted in PG synthesizing enzymes lead to oscillations of PG levels in macrophages, we extracted bone marrow from Per2::Luc mice and differentiated the extracted monocytes into BMDMs. We then grew these BMDMs to confluency, synchronized using serum shock, and harvested the BMDMs in quadruplicate every 4 h for 24 h, beginning at 16 h PS. Additionally, spent media and extracellular matrix (ECM) scrapings were sampled at four timepoints over 24 h (S4A Fig). These samples were then analyzed using Liquid Chromatography Tandem Mass-Spectrometry (LC-MS/MS) for HSPG and CSPG disaccharides in various sulfation states (Fig 3). LC-MS/MS results were normalized by employing the CyQUANT cell proliferation assay at each time point to determine the total number of cells per pellet (S4B Fig).
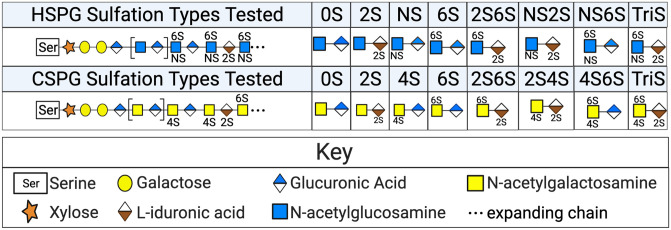
Fig 3. PG sulfation states quantified by LC-MS/MS.
A schematic representation of the sulfation states tested in the cell samples, spent media, and ECM LC-MS/MS macrophage analysis. HSPGs investigated (top row) included 0S (no sulfation), 2S (sulfation on carbon 2), NS (sulfation on the amide), 6S (sulfation on carbon 6), 2S6S (sulfation on carbons 2 &6), NS2S (sulfation on the amide group and on carbon 2), NS6S (sulfation on the amide group and carbon 6), and TriS (sulfation on carbons 2 & 6 and on the amide group). CSPGs investigated (bottom row) include 0S (no sulfation), 2S (sulfation on carbon 2), 4S (sulfation on carbon 4), 6S (sulfation on carbon 6), 2S6S (sulfation on carbons 2 & 6), 2S4S (sulfation on carbon 2 & 4), 4S6S (sulfation on carbons 4 & 6), and TriS (sulfation on carbons 2, 4, and 6).
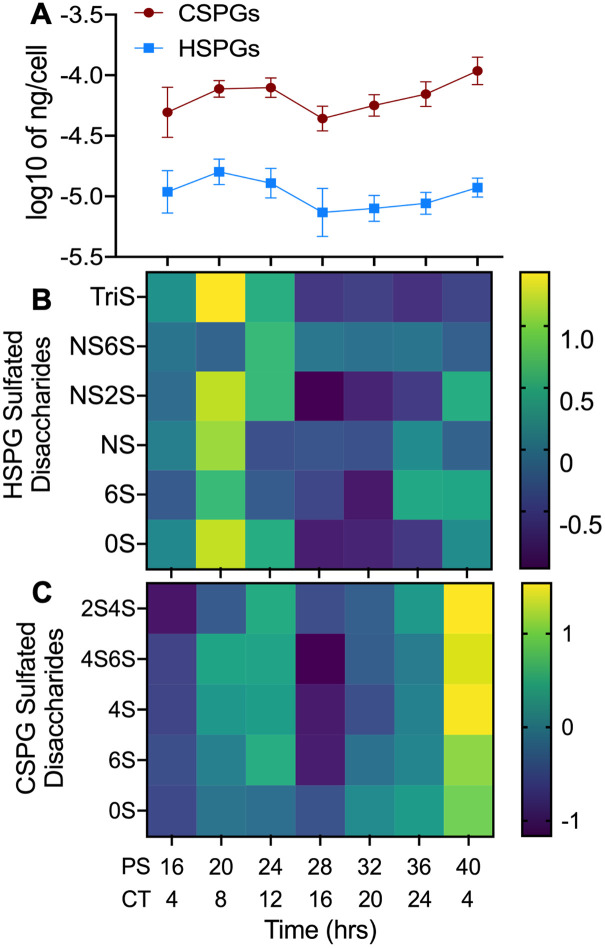
Our LC-MS/MS analysis of the macrophage cell pellet samples over circadian time demonstrated that, overall, the levels of both HSPGs and CSPGs oscillated with a circadian period (ECHO p-values = 1.30 x 10−7 and 1.62 x 10−7 for HSPGs and CSPGs, respectively) (Fig 4A). Overall, circadian oscillations in the levels of PGs synthesis and expression reached their zenith at or near PS20/CT8. Interestingly, the oscillation of Aβ42 phagocytosis occurred antiphase to HSPG and CSPG levels, suggesting the presence of PGs inhibited Aβ42 phagocytosis (compare Figs 1 to 4). As the phase of the transcripts and proteins that regulate the creation of HSPGs and CSPGs are discordant, it is clear the circadian timing of this process is highly complex and post-transcriptionally regulated (compare Figs 2 to 4).
Fig 4. HSPG and CSPG levels in macrophages oscillated with a circadian period.
(A) The sum of total cellular CSPG (n = 4) and HSPG (n = 4) levels (ng/cell) plotted against post shock (PS) and circadian time (CT). (B) Heat map of changes in cellular concentrations of specific HSPG sulfated disaccharides (n = 4) shown in normalized Z-scores (relative levels) over PS and CT time. (C) Heat map of changes in the concentrations of specific CSPG sulfated disaccharides (n = 4) shown in Z-scores over PS and CT. n = number of replicates, error bars represent the standard deviation of the replicates.
When all detected species of HSPGs identified in the cell pellet (0S, 6S, NS, NS2S, TriS, and NS6S) were analyzed individually, they were all found by ECHO to oscillate with a circadian period (Fig 4B and S5A Fig, and S4 Table). Similarly, when the detected species of CSPGs identified in the cell pellet (2S4S, 4S6S, 4S, 6S, and 0S) were analyzed individually by ECHO (Fig 4C and S5D Fig, and S4 Table), they were all classified as oscillating with a circadian period. In the spent media samples, all detected HSPG and CSPG types (NS6S, NS2S, NS, and 0S for HSPGs and 0S, 4S, and 6S for CSPGs) were not found to be oscillating with a circadian period by ECHO (S5C and S5F Fig).
Finally, fewer HSPG and CSPG species were detected in the ECM scrapings (TriS, NS6S, NS2S, and 0S for HSPGs and 4S for CSPGs) and these species also appeared to have a daily oscillation (S5B and S5E Fig). We noted that in ECM samples the zenith in both HSPG and CSPG levels is at PS28 (S5B and S5E Fig). We also found in our proteomic data that proteins related to cell division such as cyclin dependent kinases, CDK1 and CDK2, CHK2 (a key regulator of cell cycle progress into mitosis), RAD21 (a cohesion complex component), and CDCA5 (Sororin, a chromatin-associated cohesion complex stabilizer), reached their zenith at PS28 [28,58–60] (S3 Table). As HSPGs and CSPGs are known to be involved in ECM remodeling, an essential step in mitosis, this implies that the timing of the zenith of ECM PGs may represent an additional link between cell division and the circadian clock [46,61,62].
Rhythmic phagocytosis of Aβ42 is differentially regulated by high- and low-sulfated HSPGs
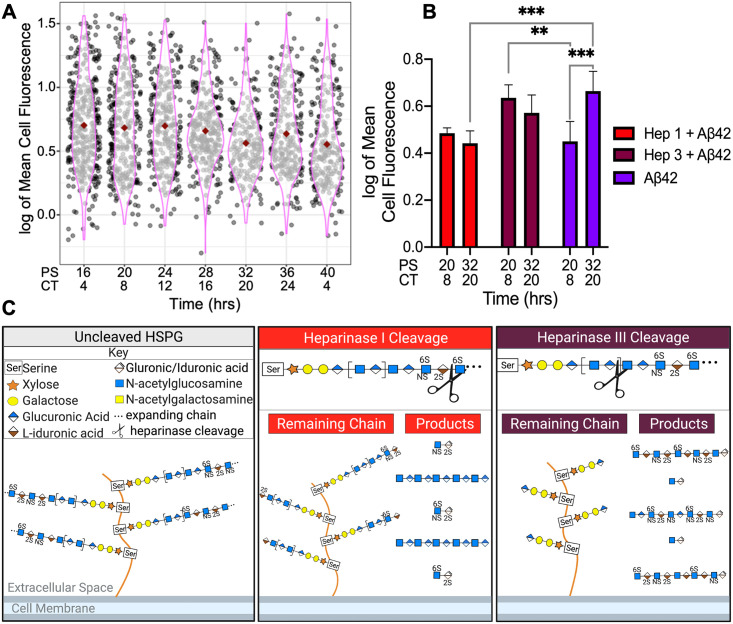
We hypothesized that the rhythmic oscillation of HSPGs may inhibit the phagocytosis of Aβ42 due to the antiphase relationship between Aβ42 phagocytosis and PG levels and the published repressive effect of HSPGs on Aβ42 phagocytosis [29,30] (Figs 1 and 4). To validate this hypothesis, we individually purified Heparinases I, II, and III, removed endotoxins, and confirmed Heparinase enzymatic activity (see Methods) [63–66] (S5 Table). Heparinase I cleaves between D-glucosamine and L-iduronic acid in highly sulfated regions, heparinase III cleaves between N-acetyl-D-glucosamine in low or unsulfated regions, and heparinase II cleaves in both positions [63]. We then extracted, derived, synchronized, and exposed BMDMs to fluorescently-labeled Aβ42 every 4 h over one circadian day. We next added a mixture of Heparinase I, II, and III to cleave all forms of HSPGs, with each biological replicate receiving a decreasing concentration of total heparinases (Replicate 1: 0.9577mg/ml of total heparinases, Replicate 2: 0.5071 mg/ml of total heparinases, Replicate 3: 0.2794 mg/ml of total heparinases) of the mixture of the three heparinases (see S5 Table). Immediately following the addition of the heparinases, we added fluorescently labeled Aβ42 and sampled Aβ42 levels inside the cell 2 hours later. By using fluorescently labeled Aβ42, we controlled for endogenously generated Aβ, which can be affected by HSPG expression through its interaction with BACE1 processing of the amyloid precursor protein [67]. We found that regardless of Heparinase concentration, the addition of Heparinases I, II, and III ablated the oscillation in Aβ42 phagocytosis (average of all data points in Fig 4A, each treatment in S6C Fig). When comparing the relative levels of Aβ42 phagocytosis between the Heparinase treated and untreated macrophages, we found that Heparinase treatment increased phagocytosis when Aβ42 rhythmic phagocytosis reached its nadir (Heparinase Treatment PS20 vs Aβ42 PS20 Welch’s T-test p<2.2x10-16 and Hedges’ g = 0.724791) but did not enhance overall levels of phagocytosis as compared to Aβ42-alone due to the low Hedges g value (Heparinase treatment PS32 vs Aβ42 PS32 Welch’s T-test p = 0.006426 and Hedges’ g = 0.361095) (Fig 5). This data confirmed that circadianly-timed increases in the levels of HSPGs were rhythmically inhibiting the phagocytosis of Aβ42.
Fig 5. Heparinase treatment ablated daily oscillations in Aβ42 phagocytosis in macrophages.
(A) A violin plot showing the phagocytosis of Aβ42 in the presence of a mixture of heparinases I, II, and III in logged mean fluorescence values plotted against PS and CT. Each cell sampled per time point is represented by a black or gray dot with the mean shown as a red diamond. The pink outline represents the range of values per timepoint. Note that data points from all the decreasing heparinase treatments were averaged to prepare this violin plot. (B) A bar graph comparing Aβ42 phagocytosis when heparinases I or III were added at PS20 (CT8) and PS32 (CT20). Red bars denote Heparinase 1 + Aβ42, maroon bars denote Heparinase 3 + Aβ42, purple bars denote Aβ42 without heparinases. All data shown was performed in triplicate (n>100 cells per replicate) and the error bars represent the standard deviation of the averaged replicates. *** = p < 2.2 x 10−16 and Hedge’s G > 0.5 (C) Images depicting where individual heparinase cleave representative HSPG chains.
Notably, our data demonstrated that 0S HSPG was the predominant HSPG disaccharide in macrophages, indicating that macrophage HSPGs may have large unsulfated regions, which could be a factor in the circadian inhibition of Aβ42 phagocytosis. To determine if our theory was correct, we next analyzed the phagocytosis of Aβ42 at the zenith and nadir of Aβ42 phagocytosis (PS20 and PS32) in the presence of either Heparinase III or Heparinase I only (based on Fig 4 and S4 Table). Isolated BMDMs, at PS20 and PS32 were treated in triplicate with HiLyte-488 labeled Aβ42 and actively-equal amounts of either Heparinase I or Heparinase III and analyzed as described above (S5 Table). Heparinase I specifically cleaves between L-iduronic acid and N-acetylglucosamine on highly sulfated chains in HSPGs, leaving lower sulfated chains bound to the cell surface. Heparinase III specifically cleaves between glucuronic acid and N-acetylglucosamine on lower-sulfated chains in HSPGs, leaving fragments of highly-sulfated heparan sulfate chains in the solution (Fig 5C).
We found that in macrophages that had been treated with Heparinase I, there was a loss of daily oscillation compared to untreated (Aβ42-Hep 1 PS20 vs PS32 Welch’s T-test p = 0.08295 and Hedges’ g = 0.101721; Aβ42-alone PS20 vs PS32 Welch’s T-test p = 8.979 x 10−15 and Hedges’ g = 0.764648) and overall levels of phagocytosis significantly decreased at the circadian zenith, but not the nadir, as compared to Aβ42 phagocytosis in untreated macrophages (zenith: Aβ42-Hep 1 PS32 vs Aβ42-alone PS32 Welch’s T-test p = 2.2 x 10−16 and Hedges’ g = 0.635008; nadir: Aβ42-Hep 1 PS20 vs Aβ42-alone PS20 Welch’s T-test p = 0.06002 and Hedges’ g = 0.132791) (Fig 5 and S6A Fig). When macrophages were treated with Heparinase III, oscillations in Aβ42 phagocytosis were also ablated as compared to untreated (Aβ42-Hep 3 PS20 vs PS32 Welch’s T-test p = 0.001997 and Hedges’ g = 0.1663366). Conversely, phagocytosis increased at the nadir but not the zenith, as compared to Aβ42 phagocytosis in untreated macrophages (nadir: Aβ42-Hep 3 PS20 vs Aβ42-alone PS20 Welch’s T-test p<2.2 x 10−16 and Hedges g = 0.5408882; zenith: Aβ42-Hep 3 PS32 vs Aβ42-alone PS32 Welch’s T-test p = 0.0007397 and Hedges’ g = 0.21997) (Fig 5 and S6B Fig). Based on the previous study of the resultant cleavage products of Heparinase I and Heparinase III treatment of HSPGs, the differentially ablated circadian oscillations of Aβ42 phagocytosis upon heparinase I and heparinase III treatment suggests that the proper ratio of cell-surface sulfation states of HSPGs regulated the circadian timing of Aβ42 phagocytosis [63,68–70]. However, additional experiments to determine the degradation products afforded upon treatment with heparinase I and heparinase III would be required to confirm this supposition.
Binding of HSPGs to Aβ peptides is a repressive factor in the circadian phagocytosis of Aβ42
Highly sulfated HSPGs, like perlecan, and CSPGs and dermatan sulfate PGs, having chondroitin-4S and dermatan sulfate GAG chains, have been shown to directly interact with Aβ [48,50,71]. PGs electrostatically bind a wide range of ligands and prefer basic amino acid residues with the highest preference for arginine, followed by lysine, and then to a much smaller extent, histidine.[72,73] The interaction between Aβ42 and heparan sulfate is known to occur through electrostatics from the dense negative charge of heparan sulfate and positively charged residues of Aβ42, particularly the HHQK cluster on Aβ42 (amino acids 13–16), which is included in the known heparin binding motif of Aβ XBBXBX where X is a hydropathic residue and B is a basic residue [73–79]. One important characteristic of Aβ42 binding to PGs is that upon binding, it changes its conformation and adopts a beta sheet structure, allowing it to rapidly aggregate [80–83]. Mouse Aβ42 (mAβ42) is nearly identical to human Aβ42, with only three amino acid substitutions (R5G, Y10F, and H13R). However, these differences all reside within the known binding region to heparin, particularly the substitution of an arginine at the histidine residue, reside in or near the HHQK cluster and affects the interaction between HSPGs and mAβ42 as compared to human Aβ42 [73,77–79,84,85] (Fig 6C). This difference is important as while mice naturally produce mAβ42, mAβ42 plaques do not form and there is no natural occurrence of AD in mice, suggesting binding between HSPGs and Aβ42 may play a role in the development of AD disease phenotypes [86–88].
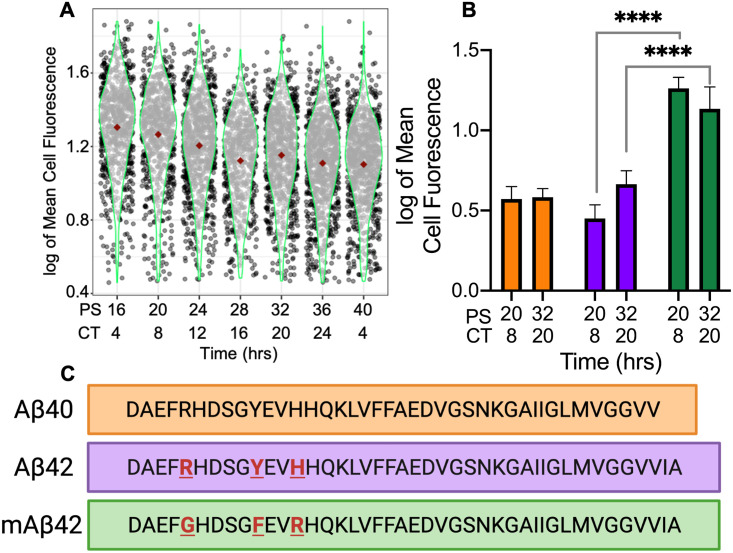
Fig 6. HSPG-regulated circadian macrophage phagocytosis was contingent upon HSPG-Aβ binding and aggregation.
(A) Violin plot of mAβ42 phagocytosis represented in logged mean cell fluorescence values plotted against PS and CT. Grey dots represent a single cell measurement, green violin plot lines represent the range of the data, and red diamonds indicate the mean measurement of each time point. (B) A bar chart comparing Aβ40 phagocytosis (orange) to Aβ42 phagocytosis (purple) and mAβ42 phagocytosis (green) at PS20/CT8 and PS32/CT20. All data shown was performed in triplicate and the error bars represent the standard deviation of the replicates. **** = p<2.2 x 10−16 and Hedge’s g>2.0 (C) An amino acid sequence comparison to show the differences between the Aβ42, Aβ40, and mAβ42 sequences.
This led us to hypothesize that binding between HSPGs and Aβ42 at the cell surface may play a mechanistic role in the circadian phagocytosis of Aβ42. We therefore repeated our phagocytosis experiment using mouse HiLyte 488 labeled mAβ42 (Anaspec). Bone marrow derived from Per2::Luc mice was used to generate synchronized macrophages and, in triplicate, mAβ42 phagocytosis was assayed an analyzed as described above every 4 h for 24 h. mAβ42 phagocytosis did not have a circadian oscillation according to ECHO, suggesting that HSPG binding and subsequent conformational changes leading to rapid aggregation are essential for HSPG to impart circadian timing of Aβ42 phagocytosis in macrophages [42,80–83] (Fig 6A). Aligned with our hypothesis, and with the data suggesting that HSPGs inhibit the phagocytosis of human but not mouse Aβ42, the resulting data showed a significant increase in mAβ42 phagocytosis at the zenith and nadir of human Aβ42 phagocytosis (mAβ42 PS20 vs Aβ42 PS20 Welches T test p<2.2 x 10−16 and Hedges’ g = 3.031588; mAβ42 PS32 vs Aβ42 PS32 Welch’s T test p<2.2 x 10−16 and Hedges g = 2.020813) as well as increased phagocytosis compared to human Aβ42 phagocytosis at all sampled times [30] (Fig 6B). This data, correlated with the zenith in HSPG levels occurring at the nadir in Aβ42 phagocytosis (Figs 1 and 4), suggested the circadian timing of increased cell-surface HSPG levels, and therefore interactions, negatively regulates the phagocytosis of Aβ42.
Circadian phagocytosis of Aβ42 is correlated with Aβ42 plaque formation
In addition to binding Aβ42, HSPGs regulate the formation of Aβ42 plaques, with highly-sulfated HSPGs accumulating in the plaques [48,50,71,84,89]. Aβ peptides exist in two forms (Aβ40 or Aβ42) based on the cleavage of the amyloid precursor protein (APP) by β- and γ-secretase [90]. Previous studies show differential phagocytosis of Aβ42 and Aβ40, yet the key amino acids essential to binding are present in Aβ40 (Fig 6C) (13–16, HHQK) [29,91]. However, the anionic bridge between lysine 28 and alanine 42, which is broken by HSPGs to allow the formation of Aβ42 aggregates by rapidly changing its conformation from an alpha helix to a beta-sheet, does not occur immediately with Aβ40, and thus HSPGs do not have an effect on Aβ40 aggregation during short periods of interaction (< 1 day) [77,81,92]. Correlated with this, the Aβ42 peptide shows higher aggregation kinetics and higher toxicity than Aβ40 both as a free peptide and when bound to HSPGs [81,93].
Paired with our data showing an increase in phagocytosis when free highly-sulfated HSPG fragments are present (Fig 5), we hypothesized that if Aβ42 aggregation was a key factor in HSPG timing of circadian phagocytosis, then there should be no oscillation in the phagocytosis of Aβ40. We utilized our above-described phagocytosis assay to analyze the phagocytosis of Aβ40 at the zenith and nadir times of Aβ42 phagocytosis (PS20 and PS32) to determine if there was an oscillation in the phagocytosis of Aβ40. We found no difference in Aβ40 phagocytosis at PS20 and PS32 (Aβ40 PS20 vs PS32 Welch’s T-test p = 0.2362 and Hedges’ g = 0.067337) confirming that there is no oscillation in Aβ40 phagocytosis, and when we compared total phagocytosis of Aβ40 at PS20 and PS32 to total phagocytosis of Aβ42 at PS20 and PS32, we found no difference in overall phagocytosis due to the low Hedges’ g value (Aβ40 vs Aβ42 Welch’s T-test p = 1.278 x 10−05 and Hedges’ g = 0.275235). Overall, our results confirmed our hypothesis that the HSPG-regulated circadian-timing of Aβ42 phagocytosis is also dependent upon Aβ42 plaque formation (Fig 6B).
Discussion
The clearance of Aβ42 is essential for a healthy neuronal microenvironment and accumulation of Aβ42 is known to accelerate the development of the symptoms associated with AD [6,94]. The accumulation of Aβ42 may in part be due to the impairment of the phagocytosis of Aβ42 in Alzheimer’s Disease model macrophages. As metabolism of Aβ42 is controlled by the circadian clock in vivo, and aging and other cellular stresses are known to modulate the output of the circadian clock, it is logical to assume that disruption of oscillations in Aβ42 metabolism due to AD-induced clock dysregulation could affect the accumulation of Aβ42 [6,19,95,96]. This connection between the clock and Aβ42 metabolism could provide a possible mechanism for the positive correlation between AD and circadian dysregulation [8,9,94]. To add to this connection, our results demonstrate circadian control of the phagocytosis of Aβ42 in murine macrophages (Fig 1). As peripheral macrophages are models for microglia and also migrate to the brain in the later stages of AD, our findings suggest the disruption of the circadian timing of macrophage/microglia phagocytosis may be a vital component in Aβ42 metabolism, highlighting a potential causative factor in the increase in accumulation of Aβ42 in patients with clock/sleep disturbances [8,13,20,22,25,94,97].
Our analysis showed that a key factor in the circadian timing of Aβ42 phagocytosis was the presence of cell-surface HSPGs (Fig 7). LC-MS/MS analysis of macrophages over circadian time showed a distinct rhythm in HSPG levels (Figs 2 and 4). The zenith and nadirs of the HSPG oscillation were antiphase to those of Aβ42 phagocytosis, suggesting that HSPGs play an inhibitory role in the phagocytosis of Aβ42 (Fig 7). The removal of all HSPG chains by treating synchronized macrophages with Heparinase I, II and III ablated the rhythm in the oscillation of Aβ42 phagocytosis by increasing phagocytosis at the nadir, cementing the importance of HSPGs in the circadian timing of Aβ42 clearance (Fig 5). Previous studies have shown differential effects of HSPG species on Aβ42, and our data paralleled this as we found that it is the presence of HSPGs or lack thereof is essential for the circadian timing of phagocytosis, though the complexity of the resultant HSPG fragments after individual Heparinase treatment made determining these roles difficult [50,52,53,98–100] (Fig 5). While HSPGs have previously been implicated in multiple pathways of Aβ clearance, cellular toxicity, and plaque generation, our work is the first to demonstrate a link between the circadian timing of HSPGs and Aβ42 clearance [48,50,67,100–102].
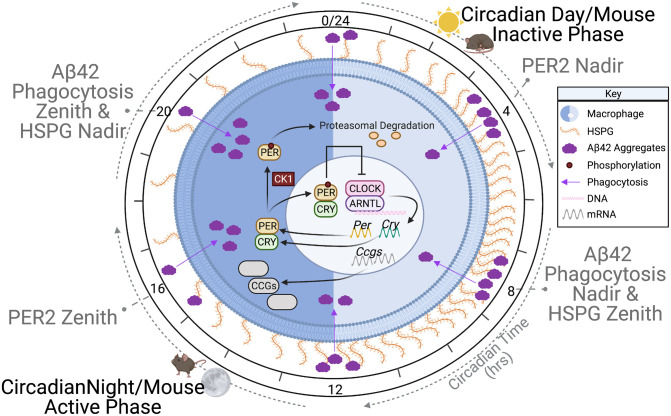
Fig 7. Circadian regulation of cell surface HSPGs time amyloid-beta phagocytosis.
A diagrammatic representation of the model demonstrating how cell surface HSPGs regulate the phagocytosis of amyloid-beta. As time progresses from CT0, HSPGs (orange) increase on the cell surface of a macrophage (blue) and decrease the phagocytosis of amyloid-beta (purple), reaching a nadir in phagocytosis at CT8. Near CT12, HSPG levels decrease, and amyloid-beta phagocytosis rises, with the zenith in phagocytosis at CT20. Superimposed on the macrophage is a simplified version of the transcription/translation negative feedback loop that drives the oscillation of HSPG levels. The phases of mouse activity are shown in parallel with the HSPG levels.
The relationship between Aβ42 clearance and HSPG binding has been shown, with HSPGs, perlecan, and syndecans binding Aβ42, regulating Aβ42 toxicity [49,84,102,103]. Highly sulfated HSPGs bind Aβ42 both extracellularly or at the cell surface to affect phagocytosis [71,100,104]. Additionally, HSPGs play a role in other Aβ42 clearance pathways, i.e., receptor mediated endocytosis, in neurons and glia cells [105,106]. We predict that the circadianly-timed increase in HSPGs on the cell surface could affect HSPG/Aβ42 binding and this binding could be the mechanistic cause of the temporal inhibition in Aβ42 phagocytosis. Our data showing that the overall phagocytosis of mAβ42, which contains mutations in the binding region specific to HSPGs, was higher and had no oscillation compared to human Aβ42, supports this theory [77,84,85] (Fig 6). We theorize the HSPG-correlated inhibition of Aβ42 phagocytosis is due to HSPG-induced Aβ42 aggregation on the cell surface, which can inhibit protease degradation of Aβ42 and/or receptor mediated endocytosis of Aβ42 [81,107]. Correlated with this, microglial phagocytosis and sequestration of Aβ in lysosomes has been shown to promote plaque formation [108] (Fig 1). Therefore, the suppression of phagocytosis at non-advantageous circadian times through the binding of Aβ42 to cell surface HSPGs may be important to preventing intracellular plaque formation, which is known to lead to cellular toxicity [109].
Congruently, Aβ42 plaque formation has been positively correlated to the presence of HSPGs [53,71,100]. Previous studies have shown that low-sulfated HSPGs did not enhance Aβ42 plaque formation and lack of N-sulfated heparin reduced Aβ phagocytosis [50]. Our work correlated with this, as the phagocytosis of Aβ40, which forms far fewer plaques than Aβ42, has no circadian oscillation in phagocytosis, suggesting that plaque formation is also correlated with circadian phagocytosis of Aβ42 [90] (Fig 6). Aβ40 had similar levels of overall phagocytosis as compared to Aβ42, though this is possibly due to solubility differences as microglia readily clear soluble Aβ, or due to energy independent endocytosis of Aβ40, which has been demonstrated in neurons [110–113]. Moreover, when we released HSPGs from the cell surface using Heparinase III, making long sulfated HSPG chain fragments readily available for the formation of aggregates off of the cell surface, we saw an increase in phagocytosis at the nadir, supporting this hypothesis [89] (Fig 5). Furthermore, the source of HSPGs involved in plaque formation is hypothesized to be from microglia and macrophages, providing a further potential connection between AD, the circadian clock, and the immune system [114–116].
Beyond the discovery of the circadian timing of Aβ42 phagocytosis by HSPGs, there is a striking amount of circadian regulation devoted to timing oscillations in the levels of all PGs (Fig 4). We found that the circadian clock controlled many elements of the pathways that regulate the production of HSPGs and CSPGs, with 16.67% of identified proteins and 38.33% of identified RNAs in the pathway oscillating with a circadian rhythm (Fig 2). Much of this regulation was differential between the transcript and protein levels, in parallel with the findings from Collins et al 2021, suggesting that circadian post-transcriptional regulation is key to this process [28]. As PGs are key regulators for cell surface interactions in the inflammatory response, the circadian regulation of PGs in macrophages has strong implications for inflammation and immunity beyond its affect in AD [117]. Macrophage polarization, which is also controlled by the circadian clock, results in differently expressed HSPGs, with less 2S and 6S sulfation when pushed into the M1 or inflammatory state [114,118,119]. This implies that the circadian regulation of HSPG and CSPG expression could be critical to preventing a hyper inflamed state in inflammatory diseases such as AD. Moreover, as PGs play critical roles in development and cellular processes, it is likely that PGs fall under the regulation of the circadian clock in other tissue types, making it a promising target for future circadian studies [117,120,121].
Methods
Ethics statement
The experiments conducted with mice were done in accordance with the guidelines set by the National Institutes of Health Office of Intramural Research and were approved and supervised by the Rensselaer Polytechnic Institute Animal Care and Use Committee (protocol number HUR-001-18).
Animals
PER2::LUC C57BL/6J male mice 3–6 months of age bred from Jackson Labs stock strains (Accession # 006852) were used for all WT experiments and were euthanized using CO2 gas and cervical dislocation [40]. Per2::Luc C57BL/6J mice were kept on a strict lighting schedule of 12L:12D to maintain synchronized circadian rhythms and fed standard rodent chow ad libitum. PER1-/-/PER2-/- knockout male mice 3–6 months in age were used in all KO experiments and were euthanized using CO2 gas and cervical dislocation [122]. PER1-/-/PER2-/- mice were kept on a strict 12L:12D lighting schedule and were fed antibiotic rodent chow ad libitum due to a Helicobacter infection in this mouse line.
Reagents
Amyloid-beta 42 labeled with HiLyte 488 (AS-60479-01), Amyloid-beta 40 labeled with HiLyte 488 (AS-60491-01), and custom Mouse amyloid-beta 42 labeled with HiLyte 488 (SQ-ANCPXXXX) were all purchased from Anaspec (Fremont, CA, USA). ACK lysing buffer (10-548E) was manufactured by Lonza.
Media
For macrophage differentiation media, DMEM media (Corning 10-013-CV) was used and supplemented with fetal bovine serum (FBS) (Gibco 10437028) at a 1:10 dilution, 100x Penstrep at a 1:100 dilution (Corning MT30002CI), 200mM L-glutamine at a 1:100 dilution (Corning 25-005-CI), Beta-mercaptoethanol (BME) at a 1:1000 dilution (Gibco 21985–023), and 50 mg/ml Gentamicin at a 1:1000 dilution (Gibco 15750–060).
For macrophage assay medium (after serum shock), Leibovitz media (Gibco 21083–027) was used and supplemented with fetal bovine serum (FBS) at a 1:10 dilution (Gibco 10437028), 100x Penstrep at a 1:100 dilution (Corning MT30002CI), 200mM L-glutamine at a 1:100 dilution (Corning 25-005-CI), Beta-mercaptoethanol (BME) at a 1:1000 dilution (Gibco 21985–023), 1 M HEPES at a 1:100 dilution (Gibco 15630–080), 50 mg/ml Gentamicin at a 1:1000 dilution (Gibco 15750–060), and Luciferin at a 1:100 ratio supplied by (Gold Biotechnology LUCK-1G) when appropriate.
For macrophage serum starvation assay media, Leibovitz media (Gibco 21083–027) was used and supplemented with 100x Penstrep at a 1:100 dilution (Corning MT30002CI), 200 mM L-glutamine at a 1:100 dilution (Corning 25-005-CI), Beta-mercaptoethanol (BME) at a 1:1000 dilution (Gibco 21985–023), 1 M HEPES at a 1:100 dilution (Gibco 15630–080), and 50 mg/ml Gentamicin at a 1:1000 dilution (Gibco 15750–060).
For macrophage serum shock media, Leibovitz media (Gibco 21083–027) was used and supplemented with FBS at a 50% dilution (Gibco 10437028), 100x Penstrep at a 1:100 dilution (Corning MT30002CI), 200 mM L-glutamine at a 1:100 dilution (Corning 25-005-CI), Beta-mercaptoethanol (BME) at a 1:1000 dilution (Gibco 21985–023), 1M HEPES at a 1:100 dilution (Gibco 15630–080), and 50mg/ml Gentamicin at a 1:1000 dilution (Gibco 15750–060).
All cell culture medias contained Macrophage Colony Stimulating Factor (M-CSF) at a 1:1000 dilution (Prospec cyt-439-c). Phosphate buffered saline (PBS) (Corning 21-040-CV) was used to wash the cells in between media changes. Cell Stripper (Corning 25-056-CI) was used to remove the adherent macrophages from cell culture dishes for harvesting. Buffered formalin phosphate (Fisher Chemical SFL004) was used for fixing harvested cells.
Mass spectrometry standards
Unsaturated disaccharide standards of CS (0SCS-0: ΔUA-GalNAc; 4SCS-A: ΔUA-GalNAc4S; 6SCS-C: ΔUA-GalNAc6S; 2SCS: ΔUA2S-GalNAc; 2S4SCS-B: ΔUA2S-Gal-NAc4S; 2S6SCS-D: ΔUA2S-GalNAc6S; 4S6SCS-E: ΔUA-GalNAc4S6S; TriSCS: ΔUA2S-GalNAc4S6S), (Iduron, CD001, CD002, CD003, CD004, CD005, CD006, CD007 and CD008, respectively), unsaturated disaccharide standards of HS (0SHS: ΔUA-GlcNAc; NSHS: ΔUA-GlcNS; 6SHS: ΔUA-GlcNAc6S; 2SHS: ΔUA2S-GlcNAc; 2SNSHS: ΔUA2S-GlcNS; NS6SHS: ΔUA-GlcNS6S; 2S6SHS: ΔUA2S-GlcNAc6S; TriSHS: ΔUA2S-GlcNS6S), and unsaturated 1,3-linked disaccharide standard of HA (0SHA: ΔUA-GlcNAc), (Iduron, HD006, HD005, HD008, HD007, HD002, HD004, HD003, HD001 and HA02, respectively) were purchased from Iduron, UK, where ΔUA is 4-deoxy-α-L-threo-hex-4-enopyranosyluronic acid.
Enzymes
Chondroitin lyase ABC from Proteus vulgaris was expressed in the Linhardt laboratory (see below). Recombinant Flavobacterial heparin lyases I, II, and III were expressed in the Linhardt laboratory using Escherichia coli strains provided by Jian Liu (College of Pharmacy, University of North Carolina).
Chemicals
2-Aminoacridone (AMAC) (Sigma-Aldrich 06627) and sodium cyanoborohydride (NaCNBH3) (Sigma-Aldrich 156159) Calcium chloride (449709), DMSO (Sigma-Aldrich 94563), acetic acid (Sigma-Aldrich A6283), ammonium acetate (Sigma-Aldrich 73594) and methanol (Sigma-Aldrich 646377) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Bone marrow derived macrophage extraction and synchronization
Bone marrow was harvested from the femurs and tibias of one 3–6-month-old male mouse using a 26 g needle and syringe filled with DMEM supplemented media. ACK lysing buffer (Lonza 10-548E) was used to lyse red blood cells in order to prevent erythrocyte contamination. Filtered bone marrow cells were counted using a BioRad TC20 automated cell counter and plated on 35 mm cell culture plates at a density of 1 x 106 in macrophage differentiation media. After three days of incubation at 37°C with 5% CO2, fresh macrophage differentiation media was added. After another 3 days of incubation, the cells were washed with 2 ml warm PBS per plate, and replaced with macrophage assay media, and the cultures were incubated for 24 h. Macrophage cultures were then washed with 2 ml warm PBS, macrophage serum starvation media was added, and the plates were incubated in the starve media for 24 h. Next, macrophage serum starvation media was removed, and macrophage serum shock media was added, and cultures were incubated for 2 h [123]. Macrophage cultures were then washed with 2 ml warmed PBS each and macrophage media Luciferin was added. Cultures were sealed with grease and glass cover slips and placed in a LumiCycle 32 luminometer (Actimetrics) to confirm synchronization of circadian rhythmicity. Assays began 16 h post serum shock to allow the synchronized cells to return to homeostasis.
Amyloid-beta reconstitution
All amyloid-beta isoforms were reconstituted 24 h or less before experimentation in water and PBS (1:1) and were dissolved using sonication for approximately 30 min. The reconstituted Aβ was pooled from individual vials before aliquoting for each experimental replicate. The aliquots were then flash frozen and stored at -80°C until 20 min before use.
Phagocytosis assay
Using synchronized macrophages (as described above) starting at PS16 macrophages were treated every 4 h for 24 h, in triplicate, with 115 μl (0.25 mg/ml) of fluorescently labeled amyloid-beta (PS16, PS20, PS24, PS28, PS32, PS36, and PS40) and heparinases at the reported activity levels (S5 Table). The treated macrophages were then incubated at 37°C for 2 h. Following the two-hour incubation, the media was aspirated, and the macrophages were washed with 2ml of PBS three times before Cell Stripper (Corning 25-056-CI) was added to the cultures, which were then incubated at 37°C for 3 min. Detached macrophages were next centrifuged at 400 g for 5 min, the supernatant was aspirated, and the macrophage cell pellets were resuspended in formalin and incubated in the dark at room temperature for 30 min. Macrophages were then centrifuged for 5 min at 400 g, the supernatant was aspirated, and the macrophages were resuspended in 250 μL of PBS and kept at 4°C. The fixed macrophages were imaged using fluorescent microscopy within a week of the experiment. All fluorescent images from the phagocytosis experiments are available on Mendeley Data (doi: 10.17632/zrmgjyggmr.1).
Fluorescent image analysis
Macrophage cells containing fluorescent Aβ were imaged on a Zeiss LSM 510 Laser scanning confocal microscope using the 40x objective and an argon laser at 488nm excitation wavelength. Macrophages were viewed in two channels, the light channel and the fluorescent channel and microscope settings were saved and reused for consistency between experiments. Images were taken until 100+ macrophages were sampled. To analyze these images, we used a custom MATLAB script. This script uses defined cell size ratios and edge detection to pick out single cells and objects (up to 4 cells clumped together) and measures the average pixel intensity, max pixel intensity, and area of each object. This script thresholds out the background and subtracts this measurement from the average cellular pixel intensities. This script is available on GitHub at https://github.com/gclark304/fluorescent_image_analysis.
Statistical analysis of cellular pixel intensities
Average cellular pixel intensities were used as a proxy for of Aβ phagocytosis. At least 100 cells measured per time point were analyzed using the above-described software. All measurements below the background signal were considered below the threshold and removed. The average pixel intensities were then log10 transformed and the interquartile range (IQR) was used to remove outliers. ECHO, Welch’s two-sample T-tests, and Hedges’ g were used to determine the statistical significance of the differences between the average cellular pixel intensities [42]. We ran a Hedges’ g since it is an appropriate statistical analysis to measure effect with samples of varying sample size. A Hedges’ g value above 0.2 shows a small effect, above 0.5 shows a medium effect, and above 0.8 shows a large effect; therefore, a large Hedges’ g confirms significance whereas a small Hedges’ g negates significance. All raw phagocytosis data of cellular pixel intensities are available on Mendeley Data (doi: 10.17632/zrmgjyggmr.1).
ECHO Analysis
ECHO version 4.1 was used to analyze all transcriptome, proteome, PG concentrations, and phagocytosis data that had samples gathered over circadian time [42]. All data were free run with the smooth data and linear detrend data options selected. In order to be considered circadian, the ECHO model must predict a period of 18–30 hours, an AC coefficient of ±0.15, and all p-values including BH and BY adjust p-values to be less than 0.05. Transcriptome data is reported in normalized transcripts per million and the proteome data is reported in normalized spectral counts. Transcriptome and Proteome data analyzed in ECHO has an x-axis of 0 to 46 corresponding to the number of samples taken in chronological order, which when added to 16 equals the post shock time of each sample. In order to calculate zeniths for the transcriptome and proteome data, the hours shifted from the ECHO analysis was added to 16 to get the post shock time, which was then converted to CT time using Keller et al., 2009 [41]. PG level analysis included all four replicates per time point. For the phagocytosis data, the average of the replicates was analyzed at each time point.
Liquid chromatography-tandem mass spectrometry
Using synchronized macrophages (as described above) starting at PS16 every 4 h for 24 h (PS16, PS20, PS24, PS28, PS32, PS36, and PS40), macrophages, in quadruplicate, were washed once with PBS, and removed from the plate using Cell Stripper (Corning 25-056-CI). Macrophages were centrifuged at 400 g for three minutes, the supernatant was aspirated, the cells were resuspended in 250 μL PBS, and flash frozen. Additionally, at PS16, PS20, PS28, and PS36, spent media from each culture dish was centrifuged at 400 x g for three minutes to remove any particulates and flash frozen. Finally, after macrophage removal, at PS16, PS20, PS28, and PS36, the culture dish was scraped using a sterile rubber policeman, the dish was flushed with 1 ml of PBS, and the resultant sample was flash frozen to sample the ECM.
To analyze total cellular levels of PGs using LC-MS/MS, macrophage pellets were treated with 100 μl BugBuster 10× protein extraction reagent (MilliporeSigma) and transferred into 1 mL microcentrifuge tubes, which were then sonicated at room temperature for 20 min. After that, 300 μl digestion buffer (50 mM ammonium acetate, 2 mM calcium chloride) was added. To analyze total supernatant and ECM levels of PGs using LC-MS/MS, 400 μl of clarified supernatant/ECM scrapings were loaded onto a 3 KDa spin column (Millipore Sigma UFC500396), washed with distilled water, and then the upper solution was aspirated and mixed with 300 μl digestion buffer. In all cases, recombinant heparin lyase I, II, III and chondroitin lyase ABC (10 mU each) (prepared by the Linhardt lab) were added to the 300 μl of digestion buffer and incubated at 37°C overnight. Digestion reactions were terminated by passing the sample through a 3 KDa MWCO spin column to eliminate the lyases. The 3 KDa MWCO spin columns were washed twice with 300 μl distilled water and the filtrate was lyophilized. Lyophilized samples were AMAC-labeled by incubating them at room temperature for 10 min with 10 μl of 0.1 M AMAC in DMSO/acetic acid (17/3,V/V), after which 10 μl of 1 M aqueous NaBH3CN was added and the mixture was incubated for 1 h at 45°C. The resultant samples were centrifuged at 13,200 rpm for 10 min. Finally, each supernatant was collected and stored in a light resistant container at room temperature until LC-MS/MS analysis.
LC was performed on an Agilent 1200 LC system at 45°C using an Agilent Poroshell 120 ECC18 (2.7 μm, 3.0 × 50 mm) column. Mobile phase A (MPA) was a 50 mM ammonium acetate aqueous solution, and mobile phase B (MPB) was methanol. The mobile phase passed through the column at a flow rate of 300 μl/min. The gradient was 0–10 min, 5–45% B; 10–10.2 min, 45–100%B; 10.2–14 min, 100%B; 14–22 min, 100–5%B. A triple quadrupole mass spectrometry system equipped with an ESI source (Thermo Fisher Scientific, San Jose, CA) was used as a detector. The online MS analysis was in the multiple reaction monitoring (MRM) mode. MS parameters: negative ionization mode with a spray voltage of 3,000 V, a vaporizer temperature of 300°C, and a capillary temperature of 270°C. The conditions and collision energies for all of the disaccharides MRM transitions are listed in S6 Table. Sample measurements for the cell pellet were normalized in nanograms of PGs per cell (ng/cell) using a CyQUANT Assay to quantify the number of cells used per pellet as described in the Methods. Sample measurements from the ECM and spent media samples were calculated in nanograms per milliliter (ng/ml). All HSPG and CSPG raw data generated from this experiment are available on Mendeley Data (doi: 10.17632/zrmgjyggmr.1).
CyQUANT assay
Due to the varying amount of protein present in a cell over time, we normalized the HSPG and CSPG cell pellet findings to the number of cells present in each sample. The cells were quantified using a CyQUANT Cell Proliferation assay (Invitrogen C7026). This kit was used to lyse 100 μl of the cells stripped from each plate and stain the nucleic acids with a fluorescently labeled dye (included in kit cited above), which was then read by a plate reader at an excitation wavelength of 480 nm and emission wavelength of 520 nm. The concentration of cells was then back calculated using a standard curve made by measuring known amounts of the primary murine macrophages. This standard curve was constructed per the manufacturer’s instructions (Invitrogen C7026 manual). The cells were diluted to a known amount using a BioRad TC20 automated cell counter, lysed, dyed, and read on a Tecan Infinite M1000 Pro plate reader at an excitation wavelength of 480 nm and emission wavelength at 520 nm. The number of cells from each sample was first calculated using the equation of the standard curve (R2 = 0.9938) and then multiplied by the dilution factor to calculate the total number of cells in each cell pellet.
Production of heparinases
Heparinases were prepared as has been previously reported [63–65]. All heparinases used were purified away from endotoxins using an Endotoxin Removal Kit from GenScript (L00338) [66].
Supporting information
The macrophage circadian rhythm is imposed by a conserved, core molecular clock that imparts control of key gene and protein expression through a transcription/translation negative feedback loop (TTFL). In murine macrophages, pictured as the blue cell above, the positive arm of the clock, in the core circadian timekeeper (Loop 1), is made up of ARNTL and CLOCK, which binds to the E-box promoter to turn on the creation of the negative arm protein (PER, CRY), auxiliary loop proteins (NR1D1, DBP, ROR), and clock-controlled genes (ccgs). The negative arm proteins then dimerize in the cytoplasm and are temporally phosphorylated by CK1 (also known as Csnk1a1 in mice), until they migrate back into the nucleus and inhibit their own transcription by inhibiting ARNTL and CLOCK, the negative arm is then ubiquinated and degraded, restarting the cycle. Two auxiliary loops to the circadian clock, Loop 2 and Loop 3, impart further fine-tuning of circadian regulation. In Loop 2, the cycle starts when ROR binds to the ROR promoter sequences to turn on expression of arntl, nfil3, and other ccgs. This loop is inhibited when the positive arm activates transcription of NR1D1, which inhibits transcription in Loop 2. Loop 3 is activated via the DBP binding to the D-box promoter and turns on expression of PER and CCGs. This expression is in turn inhibited by the expression of NFIL3 from Loop 2. The surrounding gene expression graphs show the timing of expression of key clock proteins in Post Shock (PS) time and Circadian Time (CT) using transcriptome data from Collins et al., 2020 [S1] [28]. The murine clock pathway was adapted from Curtis et al., 2014 [S2] [124].
(TIF)
Our procedure began with the extraction of bone marrow monocytes from PER2::LUC mice. Extracted bone marrow monocytes were treated with mCSF with fresh media (10% FBS) added every 3 days and these bone marrow derived macrophages were grown to confluency. After seven days, macrophages were transferred to media without serum (0%FBS) for 24 hours before being transferred to serum shock media (50% FBS) for 2 hours, in order to synchronize their clocks. Synchronized macrophages were transferred into fresh media (10% FBS) and, starting at 16 hours post shock (PS 16), samples were treated in triplicate with Aβ (for 2 hours) every four hours for 24 hours. Macrophages were then harvested, fixed using formalin, and imaged using fluorescence microscopy. PS hours as represented in yellow for the active and grey for the inactive phase of the mice as reported previously [S1].
(TIF)
A) The log of mean cell fluorescence of Aβ42 phagocytosis in PER2::LUC cells (purple circles), and in PER1-/-PER2-/- knockout cells (brown squares) plotted over time. Error bars represent the standard deviation. B) Violin plot of Aβ42 phagocytosis in PER1-/-PER2-/- knockout cells represented in logged mean cell fluorescence values plotted against post shock (PS) and circadian time (CT). Grey dots represent a single cell measurement, brown violin plot lines represent the range of the data, and red diamonds indicate the mean measurement of each time point. CT is in reference to the comparison of PER2 expression from Keller et al., 2009 [S3] [41] compared to PER2 expression from our cells. All data shown was performed in triplicate.
(TIF)
(A) BMDMs were collected at PS 16, 20, 24, 28, 32, and 36, in quadruplicate. Spent media from these cell pellets was collected at PS 16, 20, 28, and 36, in triplicate. Concurrently, ECM samples were collected in duplicate at post shock times 16, 20, 28, and 36. (B) The above samples were analyzed by LC-MS/MS and normalized using data from a CyQUANT assay that calculated total cellular DNA by UV-VIS spectroscopy and an interpolated standard curve (standard curve in inset from actual macrophage data).
(TIF)
(A) The level of each HSPG sulfation type in macrophage cells (n = 4) plotted against PS and CT time. (B) The level of each HSPG sulfation type in macrophage ECM scrapings (n = 2) plotted against PS and CT time. (C) The level of each HSPG sulfation type in macrophage spent media cultures (n = 3) plotted against PS and CT time. (D) The level of each CSPG sulfation type in macrophage cells (n = 4) plotted against PS and CT time. (E) The level of each CSPG sulfation type in macrophage ECM scrapings (n = 2) plotted against PS and CT time. (F) The level of each CSPG sulfation type in macrophage spent media cultures (n = 3) plotted against PS and CT time. n = number of replicates, error bars are represented by the standard deviation of the replicates.
(TIF)
Violin plot of Aβ42 phagocytosis in the presence of A) Heparinase I and B) Heparinase III represented in logged mean cell fluorescence values plotted against PS and CT. Grey dots represent a single cell measurement, A) red and B) maroon violin plot lines represent the range of the data, and red diamonds indicate the mean measurement of each time point. CT is in reference to the comparison of PER2 expression from Keller et al., 2009 [S3] [41] compare to PER2 expression from our cells. C) Phagocytosis of Aβ42 when heparinases I, II, and III are added at decreasing concentrations (shown in key), represented in the log of the mean cell fluorescence over PS and CT time.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We would like to acknowledge Emily Collins for her transcriptome and proteome datasets, Lufeng Yu for performing heparinase activity assays, Ke Xia for providing editing on the LC-MS/MS section, and the PER1-/-/PER2-/- knockout mice shared by the labs of Jay Dunlap and Jennifer Loros. Additionally, we would like to acknowledge the Analytical Biochemistry Core at RPI, Sergey Pryshchep of the Microscopy core at RPI for his training and guidance, and Antigone McKenna and the BioResearch core staff at RPI for their excellent care of our mouse lines. We would like to thank BioRender.com for support in figure creation.
Data Availability
All data related to this manuscript has been placed on Mendeley Data (doi: 10.17632/zrmgjyggmr.1) and GitHub (https://github.com/gclark304/fluorescent_image_analysis).
Funding Statement
This work was supported by an NIH-National Institute of Biomedical Imaging and Bioengineering Grant U01EB022546 (to J.M.H) (https://www.nibib.nih.gov/), an NIH-National Institute of General Medical Sciences Grant R35GM128687 (to J.M.H.) (https://www.nigms.nih.gov/), an National Science Foundation CAREER Award 2045674 (to J.M.H.) (https://www.nsf.gov/), National Institutes of Health grants 1RF1AG069039 (to C.W.), DK111958 and CA231074 (to R.J.L.) (https://www.nih.gov/), Rensselaer Polytechnic Startup funds (to J.M.H.) (https://www.rpi.edu/), a gift from the Warren Alpert Foundation (to J.M.H.) (https://warrenalpert.org/), and a NIH-National Institute of Aging T32 Fellowship AG057464 (to G.T.C.) (https://www.nia.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement [Internet]. 2019. Mar 1;15(3):321–87. Available from: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 2.El-Hayek YH, Wiley RE, Khoury CP, Daya RP, Ballard C, Evans AR, et al. Tip of the iceberg: Assessing the global socioeconomic costs of Alzheimer’s Disease and related dementias and strategic implications for stakeholders. J Alzheimer’s Dis [Internet]. 2019. Jul 23;70(2):323–41. Available from: https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD-190426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince M. A, Wimo A, Guerchet M, Gemma-Claire Ali M, Wu Y-T, Prina M, et al. World Alzheimer report 2015 the global impact of dementia an analysis of prevalence, incidence, cost and trends [Internet]. 2015. www.alz.co.uk/worldreport2015corrections.
- 4.Barage SH, Sonawane KD. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides [Internet]. 2015. Aug 1;52:1–18. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0143417915000657 [DOI] [PubMed] [Google Scholar]
- 5.Uddin MS, Kabir MT, Rahman MS, Behl T, Jeandet P, Ashraf GM, et al. Revisiting the amyloid cascade hypothesis: From anti-aβ therapeutics to auspicious new ways for alzheimer’s disease. Int J Mol Sci [Internet]. 2020. Aug 2;21(16):1–33. Available from: /pmc/articles/PMC7461598/. doi: 10.3390/ijms21165858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y. Effects of Age and Amyloid Deposition on Aβ Dynamics in the Human Central Nervous System. Arch Neurol [Internet]. 2012. Jan 1;69(1):51. Available from: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneurol.2011.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Miller RG, Madison C, Jin X, Honrada R, Harris W, et al. Systemic immune system alterations in early stages of Alzheimer’s disease. J Neuroimmunol [Internet]. 2013. Mar;256(1–2):38–42. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165572813000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer Disease. Exp & Mol Med [Internet]. 2015. Mar 13;47:e148. Available from: doi: 10.1038/emm.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju Y-ES. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol [Internet]. 2018. May 1;75(5):582. Available from: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/jamaneurol.2017.4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghmousavi S, Eskian M, Rahmani F, Rezaei N. The effect of insomnia on development of Alzheimer’s disease [Internet]. Vol. 17, Journal of Neuroinflammation. BioMed Central Ltd; 2020. /pmc/articles/PMC7542374/. doi: 10.1186/s12974-020-01960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, et al. Amyloid-β Induces Chemokine Secretion and Monocyte Migration across a Human Blood-Brain Barrier Model. Mol Med [Internet]. 1998. Jul 1;4(7):480–9. Available from: https://molmed.biomedcentral.com/articles/10.1007/BF03401753. [PMC free article] [PubMed] [Google Scholar]
- 12.Simard AR, Rivest S. Neuroprotective properties of the innate immune system and bone marrow stem cells in Alzheimer’s disease [Internet]. Vol. 11, Molecular Psychiatry. Mol Psychiatry; 2006. p. 327–35. https://pubmed.ncbi.nlm.nih.gov/16491130/. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K, Tian L, Liu L, Feng Y, Dong Y Bin, Li B, et al. CXCL1 contributes to β-amyloid-induced transendothelial migration of monocytes in Alzheimer’s Disease. PLoS One [Internet]. 2013. Aug 14;8(8):e72744. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23967336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, et al. Distinct and Non-Redundant Roles of Microglia and Myeloid Subsets in Mouse Models of Alzheimer’s Disease. J Neurosci [Internet]. 2011. Aug 3;31(31):11159–71. Available from: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.6209-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Möhle L, Israel N, Paarmann K, Krohn M, Pietkiewicz S, Müller A, et al. Chronic Toxoplasma gondii infection enhances β-amyloid phagocytosis and clearance by recruited monocytes. Acta Neuropathol Commun [Internet]. 2016. Mar 16;4:25. Available from: https://link.springer.com/articles/10.1186/s40478-016-0293-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang HS, Onos KD, Choi K, Keezer KJ, Skelly DA, Carter GW, et al. Natural genetic variation determines microglia heterogeneity in wild-derived mouse models of Alzheimer’s disease. Cell Rep [Internet]. 2021. Feb 9;34(6):108739. Available from: 10.1016/j.celrep.2021.108739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman SE, Allison EK, Khoury J El. Microglial dysfunction and defective-amyloid clearance pathways in aging Alzheimer’s Disease mice. Neurobiol Dis [Internet]. 2008;28(33):8354–60. Available from: www.jneurosci.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grammas P. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging [Internet]. 2001. Dec 1;22(6):837–42. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0197458001002767 [DOI] [PubMed] [Google Scholar]
- 19.Zaghi J, Goldenson B, Inayathullah M, Lossinsky AS, Masoumi A, Avagyan H, et al. Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol [Internet]. 2009. Feb 13;117(2):111–24. Available from: http://link.springer.com/10.1007/s00401-008-0481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh SE, Abud EM, Lakatos A, Karimzadeh A, Yeung ST, Davtyan H, et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc Natl Acad Sci [Internet]. 2016. Mar 1;113(9):E1316–25. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1525466113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jevtic S, Sengar AS, Salter MW, McLaurin JA. The role of the immune system in Alzheimer disease: Etiology and treatment [Internet]. Vol. 40, Ageing Research Reviews. Elsevier Ireland Ltd; 2017. p. 84–94. http://www.ncbi.nlm.nih.gov/pubmed/28941639. [DOI] [PubMed] [Google Scholar]
- 22.Le Page A, Dupuis G, Frost EH, Larbi A, Pawelec G, Witkowski JM, et al. Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp Gerontol [Internet]. 2018. Jul;107:59–66. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0531556517305417 [DOI] [PubMed] [Google Scholar]
- 23.Jairani PS, Aswathy PM, Krishnan D, Menon RN, Verghese J, Mathuranath PS, et al. Apolipoprotein E polymorphism and oxidative stress in peripheral blood-derived macrophage-mediated amyloid-beta phagocytosis in Alzheimer’s Disease patients. Cell Mol Neurobiol [Internet]. 2019;39(3):355–69. Available from: 10.1007/s10571-019-00651-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brás JP, Bravo J, Freitas J, Barbosa MA, Santos SG, Summavielle T, et al. TNF-alpha-induced microglia activation requires miR-342: impact on NF-kB signaling and neurotoxicity. Cell Death Dis [Internet]. 2020. Jun 2;11(6):415. Available from: http://www.nature.com/articles/s41419-020-2626-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Hayden EY, Garcia VJ, Fuchs D-T, Sheyn J, Daley DA, et al. Activated Bone Marrow-Derived Macrophages Eradicate Alzheimer’s-Related Aβ42 Oligomers and Protect Synapses. Front Immunol [Internet]. 2020. Jan 31;11. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2020.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labrecque N, Cermakian N. Circadian clocks in the immune system [Internet]. Vol. 30, Journal of Biological Rhythms. SAGE Publications Inc.; 2015. p. 277–90. http://www.ncbi.nlm.nih.gov/pubmed/25900041. [DOI] [PubMed] [Google Scholar]
- 27.Hergenhan S, Holtkamp S, Scheiermann C. Molecular interactions between components of the circadian clock and the immune system. J Mol Biol [Internet]. 2020;432(12):3700–13. Available from: http://www.sciencedirect.com/science/article/pii/S0022283620300280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins EJ, Cervantes-Silva MP, Timmons GA, O’Siorain JR, Curtis AM, Hurley JM. Post-transcriptional circadian regulation in macrophages organizes temporally distinct immunometabolic states. Genome Res [Internet]. 2021. Feb 1;31(2):171–85. Available from: https://genome.cshlp.org/content/31/2/171.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Callaghan P, Sandwall E, Li, Yu H, Ravid R, Guan Z-Z, et al. Heparan sulfate accumulation with Abeta deposits in Alzheimer’s disease and Tg2576 mice is contributed by glial cells. Brain Pathol [Internet]. 2008/04/11. 2008. Oct;18(4):548–61. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18422760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C-C, Zhao N, Yamaguchi Y, Cirrito JR, Kanekiyo T, Holtzman DM, et al. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-β clearance and aggregation in Alzheimer’s disease. Sci Transl Med [Internet]. 2016. Mar 30;8(332). Available from: https://www.ncbi.nlm.nih.gov/pubmed/27030596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser PE, Darabie AA, McLaurin JA. Amyloid-β interactions with chondroitin sulfate-derived monosaccharides and disaccharides: Implications for drug development. J Biol Chem [Internet]. 2001. Mar 2;276(9):6412–9. Available from: https://pubmed.ncbi.nlm.nih.gov/11106653/ [DOI] [PubMed] [Google Scholar]
- 32.Esko JD, Selleck SB. ORDER OUT OF CHAOS: Assembly of Ligand Binding Sites in Heparan Sulfate 1. 2002;71:435–71. Available from: www.annualreviews.org. [DOI] [PubMed] [Google Scholar]
- 33.Sandwall E, O’Callaghan P, Zhang X, Lindahl U, Lannfelt L, Li J-P. Heparan sulfate mediates amyloid-beta internalization and cytotoxicity. Glycobiology [Internet]. 2010. May 1;20(5):533–41. Available from: doi: 10.1093/glycob/cwp205 [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Li J-P. Heparan sulfate proteoglycans in amyloidosis. In: Zhang LBT, editor. Glycosaminoglycans in Development, Health and Disease [Internet]. Academic Press; 2010. p. 309–34. http://www.sciencedirect.com/science/article/pii/S1877117310930135. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Wang B, O’Callaghan P, Hjertström E, Jia J, Gong F, et al. Heparanase overexpression impairs inflammatory response and macrophage-mediated clearance of amyloid-β in murine brain. Acta Neuropathol [Internet]. 2012. Oct;124(4):465–78. Available from: https://pubmed.ncbi.nlm.nih.gov/22692572/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marques F, Sousa JC, Sousa N, Palha JA. Blood-brain-barriers in aging and in Alzheimer’s disease [Internet]. Vol. 8, Molecular Neurodegeneration. BioMed Central; 2013. p. 1–9. https://link.springer.com/articles/10.1186/1750-1326-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trouplin V, Boucherit N, Gorvel L, Conti F, Mottola G, Ghigo E. Bone marrow-derived macrophage production. J Vis Exp [Internet]. 2013;(81):50966. Available from: /pmc/articles/PMC3991821/. doi: 10.3791/50966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco R, Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system [Internet]. Vol. 131, Progress in Neurobiology. Elsevier Ltd; 2015. p. 65–86. doi: 10.1016/j.pneurobio.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages [Internet]. Vol. Chapter 14, Current Protocols in Immunology. Curr Protoc Immunol; 2008. https://pubmed.ncbi.nlm.nih.gov/19016445/. [DOI] [PMC free article] [PubMed]
- 40.Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci [Internet]. 2004. Apr 13;101(15):5339–46. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.0308709101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk H-D, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci [Internet]. 2009. Dec 15;106(50):21407 LP–21412. Available from: http://www.pnas.org/content/106/50/21407.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De los Santos H, Collins EJ, Mann C, Sagan AW, Jankowski MS, Bennett KP, et al. ECHO: an application for detection and analysis of oscillators identifies metabolic regulation on genome-wide circadian output. Bioinformatics [Internet]. 2020. Feb 1;36(3):773–81. Available from: https://academic.oup.com/bioinformatics/article/36/3/773/5544107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellano JM, Deane R, Gottesdiener AJ, Verghese PB, Stewart FR, West T, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc Natl Acad Sci U S A [Internet]. 2012. Sep 18;109(38):15502–7. Available from: https://www.pnas.org/content/109/38/15502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyman BT, Strickland D, Rebeck GW. Role of the low-density lipoprotein receptor-related protein in β- amyloid metabolism and Alzheimer disease. Arch Neurol [Internet]. 2000. May 1;57(5):646–50. Available from: https://jamanetwork.com/ [DOI] [PubMed] [Google Scholar]
- 45.Mi H, Ebert D, Muruganujan A, Mills C, Albou L-P, Mushayamaha T, et al. PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res [Internet]. 2021. Jan 8;49(D1):D394–403. Available from: https://academic.oup.com/nar/article/49/D1/D394/6027812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ariga T, Miyatake T, Yu RK. Role of proteoglycans and glycosaminoglycans in the pathogenesis of Alzheimer’s disease and related disorders: Amyloidogenesis and therapeutic strategies—A review. J Neurosci Res [Internet]. 2010. Aug 15;88(11):2303–15. Available from: https://pubmed.ncbi.nlm.nih.gov/20623617/ [DOI] [PubMed] [Google Scholar]
- 47.Wu F, Zhou C, Zhou D, Ou S, Liu Z, Huang H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr Polym [Internet]. 2018. Oct 15;198:611–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0144861718307252 [DOI] [PubMed] [Google Scholar]
- 48.Snow AD, Sekiguchi R, Nochlin D, Fraser P, Kimata K, Mizutani A, et al. An important role of heparan sulfate proteoglycan (perlecan) in a model system for the deposition and persistence of fibrillar aβ-amyloid in rat brain. Neuron [Internet]. 1994. Jan 1;12(1):219–34. Available from: https://linkinghub.elsevier.com/retrieve/pii/0896627394901651 [DOI] [PubMed] [Google Scholar]
- 49.Snow AD, Kinsella MG, Parks E, Sekiguchi RT, Miller JD, Kimata K, et al. Differential binding of vascular cell-derived proteoglycans (perlecan, biglycan, decorin, and versican) to the beta-amyloid protein of Alzhiemer’s Disease [Internet]. Archives of Biochemistry and Biophysics; 1995. p. 84–95. https://pubmed.ncbi.nlm.nih.gov/7793988/. [DOI] [PubMed] [Google Scholar]
- 50.Castillo GM, Lukito W, Wight TN, Snow AD. The sulfate moieties of glycosaminoglycans are critical for the enhancement of β-amyloid protein fibril formation. J Neurochem [Internet]. 1999. Apr 1;72(4):1681–7. Available from: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1471-4159.1999.721681.x [DOI] [PubMed] [Google Scholar]
- 51.Li J-P, Galvis MLE, Gong F, Zhang X, Zcharia E, Metzger S, et al. In vivo fragmentation of heparan sulfate by heparanase overexpression renders mice resistant to amyloid protein A amyloidosis. Proc Natl Acad Sci [Internet]. 2005. May 3;102(18):6473–7. Available from: https://www.pnas.org/content/102/18/6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruinsma IB, te Riet L, Gevers T, ten Dam GB, van Kuppevelt TH, David G, et al. Sulfation of heparan sulfate associated with amyloid-β plaques in patients with Alzheimer’s disease. Acta Neuropathol [Internet]. 2010. Feb 28;119(2):211–20. Available from: http://link.springer.com/10.1007/s00401-009-0577-1 [DOI] [PubMed] [Google Scholar]
- 53.Wall JS, Richey T, Stuckey A, Donnell R, Oosterhof A, van Kuppevelt TH, et al. SPECT imaging of peripheral amyloid in mice by targeting hyper-sulfated heparan sulfate proteoglycans with specific scFv antibodies. Nucl Med Biol [Internet]. 2012;39(1):65–75. Available from: 10.1016/j.nucmedbio.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maeda N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front Neurosci [Internet]. 2015. Mar 23;9:98. Available from: http://www.frontiersin.org/Neurogenesis/10.3389/fnins.2015.00098/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreuger J, Kjellén L. Heparan sulfate biosynthesis: regulation and variability. J Histochem Cytochem [Internet]. 2012;60(12):898–907. Available from: https://pubmed.ncbi.nlm.nih.gov/23042481/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ly M, Leach FE, Laremore TN, Toida T, Amster IJ, Linhardt RJ. The proteoglycan bikunin has a defined sequence. Nat Chem Biol [Internet]. 2011;7(11):827–33. Available from: /pmc/articles/PMC3197799/?report=abstract. doi: 10.1038/nchembio.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eppig JT. Mouse genome informatics (MGI) resource: Genetic, genomic, and biological knowledgebase for the laboratory mouse. ILAR J [Internet]. 2017. Jul 1;58(1):17–41. Available from: https://academic.oup.com/ilarjournal/article/58/1/17/3867191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castedo M, Perfettini J-L, Roumier T, Yakushijin K, Horne D, Medema R, et al. The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene [Internet]. 2004. May 29;23(25):4353–61. Available from: http://www.nature.com/articles/1207573 [DOI] [PubMed] [Google Scholar]
- 59.Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase- promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell [Internet]. 2005. Apr 15;18(2):185–200. Available from: https://pubmed.ncbi.nlm.nih.gov/15837422/ [DOI] [PubMed] [Google Scholar]
- 60.Hanse EA, Nelsen CJ, Goggin MM, Anttila CK, Mullany LK, Berthet C, et al. Cdk2 plays a critical role in hepatocyte cell cycle progression and survival in the setting of cyclin D1 expression in vivo. Cell Cycle [Internet]. 2009. Sep 28;8(17):2802–9. Available from: http://www.tandfonline.com/doi/abs/10.4161/cc.8.17.9465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ughy B, Schmidthoffer I, Szilak L. Heparan sulfate proteoglycan (HSPG) can take part in cell division: inside and outside. Cell Mol Life Sci [Internet]. 2019. Mar 15;76(5):865–71. Available from: doi: 10.1007/s00018-018-2964-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong CI, Zamborszky J, Baek M, Labiscsak L, Ju K, Lee H, et al. Circadian rhythms synchronize mitosis in Neurospora crassa. Proc Natl Acad Sci [Internet]. 2014. Jan 28;111(4):1397–402. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1319399111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang VC, Linhardt RJ, Bernstein H, Cooney CL, Langer R. Purification and characterization of heparinase from Flavobacterium heparinum. J Biol Chem [Internet]. 1985. Feb;260(3):1849–57. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021925818896715. [PubMed] [Google Scholar]
- 64.Ernst S, Venkataraman G, Winkler S, Godavarti R, Langer R, Cooney CL, et al. Expression in Escherichia coli, purification and characterization of heparinase I from Flavobacterium heparinum. Biochem J [Internet]. 1996. Apr 15;315(2):589–97. Available from: https://pubmed.ncbi.nlm.nih.gov/8615834/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Godavarti R, Davis M, Venkataraman G, Cooney C, Langer R, Sasisekharan R. Heparinase III from Flavobacterium heparinum: cloning and recombinant expression in Escherichia coli. Biochem Biophys Res Commun [Internet]. 1996. Aug 23;225(3):751–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006291X96912462 [DOI] [PubMed] [Google Scholar]
- 66.Chen N, Wan X-L, Huang C-X, Wang W-M, Liu H, Wang H-L. Study on the immune response to recombinant Hsp70 protein from Megalobrama amblycephala. Immunobiology [Internet]. 2014. Nov 1;219(11):850–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0171298514001259 [DOI] [PubMed] [Google Scholar]
- 67.Scholefield Z, Yates EA, Wayne G, Amour A, McDowell W, Turnbull JE. Heparan sulfate regulates amyloid precursor protein processing by BACE1, the Alzheimer’s β-secretase. J Cell Biol [Internet]. 2003. Oct 13;163(1):97–107. Available from: http://www.jcb.org/cgi/doi/10.1083/jcb.200303059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva ME, Dietrich CP. Structure of heparin. Characterization of the products formed from heparin by the action of a heparinase and a heparitinase from Flavobacterium heparinum. J Biol Chem [Internet]. 1975. Sep 10;250(17):6841–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021925819410089. [PubMed] [Google Scholar]
- 69.Linhardt RJ, Grant A, Cooney CL, Langer R. Differential anticoagulant activity of heparin fragments prepared using microbial heparinase. J Biol Chem [Internet]. 1982. Jul 10;257(13):7310–3. Available from: http://www.jbc.org/article/S0021925818343771/fulltext. [PubMed] [Google Scholar]
- 70.Hausser H, Kresse H. Decorin endocytosis: Structural features of heparin and heparan sulphate oligosaccharides interfering with receptor binding and endocytosis. Biochem J [Internet]. 1999. Dec 15;344(3):827–35. Available from: /pmc/articles/PMC1220705/?report=abstract. [PMC free article] [PubMed] [Google Scholar]
- 71.van Horssen J, Wesseling P, van den Heuvel LP, de Waal RM, Verbeek MM. Heparan sulphate proteoglycans in Alzheimer’s disease and amyloid-related disorders. Lancet Neurol [Internet]. 2003. Aug 1;2(8):482–92. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1474442203004848 [DOI] [PubMed] [Google Scholar]
- 72.Fromm J, Hileman R, Caldwell E, Weiler J, Linhardt R. Pattern and spacing of basic amino acids in heparin binding sites. Arch Biochem Biophys [Internet]. 1997. Jul 1;343(1):92–100. Available from: https://pubmed.ncbi.nlm.nih.gov/9210650/ [DOI] [PubMed] [Google Scholar]
- 73.Iannuzzi C, Irace G, Sirangelo I. The effect of glycosaminoglycans (GAGs) on amyloid aggregation and toxicity. Molecules [Internet]. 2015. Feb 2;20(2):2510–28. Available from: https://www.mdpi.com/1420-3049/20/2/2510/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fromm JR, Hileman RE, Caldwell EEO, Weiler JM, Linhardt RJ. Differences in the interaction of heparin with arginine and lysine and the importance of these basic amino acids in the binding of heparin to acidic fibroblast growth factor. Arch Biochem Biophys [Internet]. 1995. Nov 10;323(2):279–87. Available from: https://pubmed.ncbi.nlm.nih.gov/7487089/ [DOI] [PubMed] [Google Scholar]
- 75.Caldwell EEO, Nadkarni VD, Fromm JR, Linhardt RJ, Weiler JM. Importance of specific amino acids in protein binding sites for heparin and heparan sulfate. Int J Biochem Cell Biol [Internet]. 1996;28(2):203–16. Available from: https://pubmed.ncbi.nlm.nih.gov/8729007/ [DOI] [PubMed] [Google Scholar]
- 76.Capila I, Linhardt RJ. Heparin—Protein interactions. Angew Chem Int Ed Engl [Internet]. 2002. Feb 1;41(3):390–412. Available from: https://pubmed.ncbi.nlm.nih.gov/12491369/ [DOI] [PubMed] [Google Scholar]
- 77.Nguyen K, Rabenstein DL. Interaction of the heparinbBinding consensus sequence of β-amyloid peptides with heparin and heparin-derived oligosaccharides. J Phys Chem B [Internet]. 2016. Mar 17;120(9):2187–97. Available from: https://pubmed.ncbi.nlm.nih.gov/26872053/ [DOI] [PubMed] [Google Scholar]
- 78.Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arterioscler An Off J Am Hear Assoc Inc [Internet]. 1989;9(1):21–32. Available from: https://www.ahajournals.org/doi/abs/10.1161/01.ATV.9.1.21 [DOI] [PubMed] [Google Scholar]
- 79.Madine J, Pandya MJ, Hicks MR, Rodger A, Yates EA, Radford SE, et al. Site-specific identification of an Aβ fibril–heparin interaction site by using solid-state NMR spectroscopy. Angew Chemie [Internet]. 2012. Dec 21;124(52):13317–20. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ange.201204459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fraser PE, Nguyen JT, Chin DT, Kirschner DA. Effects of sulfate ions on Alzheimer β/A4 peptide assemblies: Implications for amyloid fibril-proteoglycan interactions. J Neurochem [Internet]. 1992. Oct;59(4):1531–40. Available from: http://doi.wiley.com/10.1111/j.1471-4159.1992.tb08470.x [DOI] [PubMed] [Google Scholar]
- 81.McLaurin J, Franklin T, Zhang X, Deng J, Fraser PE. Interactions of Alzheimer amyloid-β peptides with glycosaminoglycans. Eur J Biochem [Internet]. 1999. Dec 15;266(3):1101–10. Available from: http://doi.wiley.com/10.1046/j.1432-1327.1999.00957.x [DOI] [PubMed] [Google Scholar]
- 82.McLaurin J, Fraser PE. Effect of amino-acid substitutions on Alzheimer’s amyloid-beta peptide-glycosaminoglycan interactions. Eur J Biochem [Internet]. 2000;267(21):6353–61. Available from: https://pubmed.ncbi.nlm.nih.gov/11029577/ [DOI] [PubMed] [Google Scholar]
- 83.Calamai M, Kumita JR, Mifsud J, Parrini C, Ramazzotti M, Ramponi G, et al. Nature and significance of the interactions between amyloid fibrils and biological polyelectrolytes. Biochemistry [Internet]. 2006. Oct 24;45(42):12806–15. Available from: https://pubs.acs.org/doi/full/10.1021/bi0610653 [DOI] [PubMed] [Google Scholar]
- 84.Zhang GL, Zhang X, Wang XM, Li JP. Towards understanding the roles of heparan sulfate proteoglycans in Alzheimer’s Disease. Biomed Res Int [Internet]. 2014;2014. Available from: https://pubmed.ncbi.nlm.nih.gov/25157361/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giulian D, Haverkamp LJ, Yu JH, Karshin W, Tom D, Li J, et al. Specific domains of b-Amyloid from Alzheimer plaque elicit neuron killing in human microglia. 1996;16(19):6021–37. Available from: https://pubmed.ncbi.nlm.nih.gov/8815885/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Güntert A, Döbeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience [Internet]. 2006. Dec 1;143(2):461–75. Available from: https://www.sciencedirect.com/science/article/pii/S0306452206010839?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 87.Sarasa M, Pesini P. Natural non-trasgenic animal models for research in Alzheimers Disease. Curr Alzheimer Res [Internet]. 2009. Mar 6;6(2):171–8. Available from: /pmc/articles/PMC2825666/. doi: 10.2174/156720509787602834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edrey YH, Medina DX, Gaczynska M, Osmulski PA, Oddo S, Caccamo A, et al. Amyloid beta and the longest-lived rodent: The naked mole-rat as a model for natural protection from Alzheimer’s Disease. Neurobiol Aging [Internet]. 2013. Oct 1;34(10):2352–60. Available from: https://pubmed.ncbi.nlm.nih.gov/23618870/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindahl B, Westling C, Giménez-Gallego G, Lindahl U, Salmivirta M. Common binding sites for β-amyloid fibrils and fibroblast growth factor-2 in heparan sulfate from human cerebral cortex. J Biol Chem [Internet]. 1999. Oct 22;274(43):30631–5. Available from: https://pubmed.ncbi.nlm.nih.gov/10521448/ [DOI] [PubMed] [Google Scholar]
- 90.Gu L, Guo Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J Neurochem [Internet]. 2013. Aug 1;126(3):305–11. Available from: http://doi.wiley.com/10.1111/jnc.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wesén E, Gallud A, Paul A, Lindberg DJ, Malmberg P, Esbjörner EK. Cell surface proteoglycan-mediated uptake and accumulation of the Alzheimer’s disease peptide Aβ(1–42). Biochim Biophys Acta—Biomembr [Internet]. 2018. Nov 1;1860(11):2204–14. Available from: doi: 10.1016/j.bbamem.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez RA, Chen LY, Plascencia-Villa G, Perry G. Elongation affinity, activation barrier, and stability of Aβ42 oligomers/fibrils in physiological saline. Biochem Biophys Res Commun [Internet]. 2017. May 27;487(2):444–9. Available from: https://pubmed.ncbi.nlm.nih.gov/28427941/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bitan GM, Kirkitadze D, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A [Internet]. 2003. Jan 7;100(1):330–5. Available from: www.pnas.orgcgidoi10.1073pnas.222681699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.You JC, Jones E, Cross DE, Lyon AC, Kang H, Newberg AB, et al. Association of β-amyloid burden with sleep dysfunction and cognitive impairment in elderly individuals with cognitive disorders. JAMA Netw open [Internet]. 2019. Oct 2;2(10):e1913383. Available from: /pmc/articles/PMC6806437/. doi: 10.1001/jamanetworkopen.2019.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Solanas G, Peixoto FO, Perdiguero E, Jardí M, Ruiz-Bonilla V, Datta D, et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell [Internet]. 2017. Aug 10;170(4):678–692.e20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28802040 [DOI] [PubMed] [Google Scholar]
- 96.Yin Z, Raj D, Saiepour N, Van Dam D, Brouwer N, Holtman IR, et al. Immune hyperreactivity of Aβ plaque-associated microglia in Alzheimer’s disease. Neurobiol Aging [Internet]. 2017;55:115–22. Available from: http://www.sciencedirect.com/science/article/pii/S0197458017300970 [DOI] [PubMed] [Google Scholar]
- 97.Tansey KE, Cameron D, Hill MJ. Genetic risk for Alzheimer’s disease is concentrated in specific macrophage and microglial transcriptional networks. Genome Med [Internet]. 2018. Feb 26;10(1):14. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29482603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jastrebova N, Vanwildemeersch M, Rapraeger AC, Gimé Nez-Gallego G, Lindahl U, Spillmann D. Heparan sulfate-related oligosaccharides in ternary complex formation with fibroblast growth factors 1 and 2 and their receptors. J Biol Chem [Internet]. 2006;281(37):26884–92. Available from: https://pubmed.ncbi.nlm.nih.gov/16807244/ [DOI] [PubMed] [Google Scholar]
- 99.Kreuger J, Spillmann D, Li J, Lindahl U. Interactions between heparan sulfate and proteins: the concept of specificity. J Cell Biol [Internet]. 2006. Jul 31;174(3):323–7. Available from: 10.1083/jcb.200604035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stopschinski BE, Holmes BB, Miller GM, Manon VA, Vaquer-Alicea J, Prueitt WL, et al. Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus -synuclein and -amyloid aggregates. J Biol Chem [Internet]. 2018;293(27):10826–40. Available from: 10.1074/jbc.RA117.000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Narindrasorasak S, Lowery D, Gonzalez-DeWhitt P, Poorman RA, Greenberg B, Kisilevsky R. High affinity interactions between the Alzheimer’s beta-amyloid precursor proteins and the basement membrane form of heparan sulfate proteoglycan. J Biol Chem [Internet]. 1991;266(20):12878–83. Available from: http://www.sciencedirect.com/science/article/pii/S0021925818987764. [PubMed] [Google Scholar]
- 102.Schulz JG. Evidence that glypican is a receptor mediating β-amyloid neurotoxicity in PC12 cells. Eur J Neurosci [Internet]. 1998;10(6):2085–93. Available from: https://pubmed.ncbi.nlm.nih.gov/9753095/ [DOI] [PubMed] [Google Scholar]
- 103.Fuki I V., Iozzo R V., Williams KJ. Perlecan heparan sulfate proteoglycan: A novel receptor that mediates a distinct pathway for ligand catabolism. J Biol Chem [Internet]. 2000;275(33):25742–50. Available from: 10.1074/jbc.M909173199 [DOI] [PubMed] [Google Scholar]
- 104.Yanagishita M. Cellular catabolism of heparan sulfate proteoglycans. Trends Glycosci Glycotechnol [Internet]. 1998;10(52):57–63. Available from: http://www.jstage.jst.go.jp/article/tigg1989/10/52/10_52_57/_article/-char/ja/. [Google Scholar]
- 105.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat Med [Internet]. 2003. Apr 1;9(4):453–7. Available from: http://www.nature.com/naturemedicine [DOI] [PubMed] [Google Scholar]
- 106.Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-β uptake. J Neurosci [Internet]. 2011;31(5):1644–51. Available from: https://www.jneurosci.org/content/31/5/1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology [Internet]. Vol. 446, Nature. Nature Publishing Group; 2007. p. 1030–7. https://www.nature.com/articles/nature05817. [DOI] [PubMed] [Google Scholar]
- 108.Huang Y, Happonen KE, Burrola PG, O’Connor C, Hah N, Huang L, et al. Microglia use TAM receptors to detect and engulf amyloid β plaques. Nat Immunol [Internet]. 2021. Apr 15;22(5):1–9. Available from: http://www.nature.com/articles/s41590-021-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baik SH, Kang S, Son SM, Mook-Jung I. Microglia contributes to plaque growth by cell death due to uptake of amyloid β in the brain of Alzheimer’s disease mouse model. Glia [Internet]. 2016. Dec 1;64(12):2274–90. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/glia.23074 [DOI] [PubMed] [Google Scholar]
- 110.Weldon DT, Rogers SD, Ghilardi JR, Finke MP, Cleary JP, O’Hare E, et al. Fibrillar β-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci [Internet]. 1998. Mar 15;18(6):2161–73. Available from: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.18-06-02161.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kandimalla KK, Scott OG, Fulzele S, Davidson MW, Poduslo JF. Mechanism of neuronal versus endothelial cell uptake of Alzheimer’s Disease amyloid β protein. Cookson MR, editor. PLoS One [Internet]. 2009. Feb 27;4(2):e4627. Available from: https://dx.plos.org/10.1371/journal.pone.0004627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Omtri RS, Davidson MW, Arumugam B, Poduslo JF, Kandimalla KK. Difference in the cellular uptake and intracellular itineraries of amyloid beta proteins 40 and 42; ramifications for the Alzheimer’s drug discovery. Bone [Internet]. 2012;9(7):1887–97. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3858471/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wesén E, Jeffries GDM, Matson Dzebo M, Esbjörner EK. Endocytic uptake of monomeric amyloid-β peptides is clathrin- and dynamin-independent and results in selective accumulation of Aβ(1–42) compared to Aβ(1–40). Sci Rep [Internet]. 2017. Dec 17;7(1):2021. Available from: http://www.nature.com/articles/s41598-017-02227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards IJ, Xu H, Obunike JC, Goldberg IJ, Wagner WD. Differentiated macrophages synthesize a heparan sulfate proteoglycan and an oversulfated chondroitin sulfate proteoglycan that bind lipoprotein lipase. Arterioscler Thromb Vasc Biol [Internet]. 1995. Mar;15(3):400–9. Available from: https://www.ahajournals.org/doi/10.1161/01.ATV.15.3.400 [DOI] [PubMed] [Google Scholar]
- 115.Miller JD, Cummings J, Maresh GA, Walker DG, Castillo GM, Ngo C, et al. Localization of perlecan (or a perlecan-related macromolecule) to isolated microglia in vitro and to microglia/macrophages following infusion of beta-amyloid protein into rodent hippocampus. Glia [Internet]. 1997. Oct 1;21(2):228–43. Available from: [DOI] [PubMed] [Google Scholar]
- 116.Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, et al. Regulation of amyloid-β dynamics and pathology by the circadian clock. J Exp Med [Internet]. 2018. Apr 1;215(4):1059–68. Available from: /pmc/articles/PMC5881473/. doi: 10.1084/jem.20172347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ori A, Wilkinson MC, Fernig DG. A systems biology approach for the investigation of the heparin/heparan sulfate interactome. J Biol Chem [Internet]. 2011;286(22):19892–904. Available from: http://www.sciencedirect.com/science/article/pii/S0021925820510648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swart M, Troeberg L. Effect of polarization and chronic inflammation on macrophage expression of heparan sulfate proteoglycans and biosynthesis enzymes. J Histochem Cytochem [Internet]. 2019. Jan 1;67(1):9–27. Available from: 10.1369/0022155418798770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen S, Fuller KK, Dunlap JC, Loros JJ. A Pro- and Anti-inflammatory axis modulates the macrophage circadian clock. Front Immunol [Internet]. 2020. May 14;11:867. Available from: /pmc/articles/PMC7240016/. doi: 10.3389/fimmu.2020.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindahl U, Li J. Chapter 3 Interactions between heparan sulfate and proteins—Design and functional implications. In: International Review of Cell and Molecular Biology [Internet]. Academic Press; 2009. p. 105–59. https://linkinghub.elsevier.com/retrieve/pii/S1937644809760034 [DOI] [PubMed] [Google Scholar]
- 121.Zhang X, Wang B, Li J-P. Implications of heparan sulfate and heparanase in neuroinflammation. Matrix Biol [Internet]. 2014. Apr;35:174–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0945053X13001777 [DOI] [PubMed] [Google Scholar]
- 122.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell [Internet]. 2001. Jun 1;105(5):683–94. Available from: https://pubmed.ncbi.nlm.nih.gov/11389837/ [DOI] [PubMed] [Google Scholar]
- 123.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell [Internet]. 1998. Jun 12;93(6):929–37. Available from: http://www.cell.com/article/S009286740081199X/fulltext [DOI] [PubMed] [Google Scholar]
- 124.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LAJ. Circadian clock proteins and immunity. Immunity [Internet]. 2014;40(2):178–86. Available from: http://www.sciencedirect.com/science/article/pii/S1074761314000405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The macrophage circadian rhythm is imposed by a conserved, core molecular clock that imparts control of key gene and protein expression through a transcription/translation negative feedback loop (TTFL). In murine macrophages, pictured as the blue cell above, the positive arm of the clock, in the core circadian timekeeper (Loop 1), is made up of ARNTL and CLOCK, which binds to the E-box promoter to turn on the creation of the negative arm protein (PER, CRY), auxiliary loop proteins (NR1D1, DBP, ROR), and clock-controlled genes (ccgs). The negative arm proteins then dimerize in the cytoplasm and are temporally phosphorylated by CK1 (also known as Csnk1a1 in mice), until they migrate back into the nucleus and inhibit their own transcription by inhibiting ARNTL and CLOCK, the negative arm is then ubiquinated and degraded, restarting the cycle. Two auxiliary loops to the circadian clock, Loop 2 and Loop 3, impart further fine-tuning of circadian regulation. In Loop 2, the cycle starts when ROR binds to the ROR promoter sequences to turn on expression of arntl, nfil3, and other ccgs. This loop is inhibited when the positive arm activates transcription of NR1D1, which inhibits transcription in Loop 2. Loop 3 is activated via the DBP binding to the D-box promoter and turns on expression of PER and CCGs. This expression is in turn inhibited by the expression of NFIL3 from Loop 2. The surrounding gene expression graphs show the timing of expression of key clock proteins in Post Shock (PS) time and Circadian Time (CT) using transcriptome data from Collins et al., 2020 [S1] [28]. The murine clock pathway was adapted from Curtis et al., 2014 [S2] [124].
(TIF)
Our procedure began with the extraction of bone marrow monocytes from PER2::LUC mice. Extracted bone marrow monocytes were treated with mCSF with fresh media (10% FBS) added every 3 days and these bone marrow derived macrophages were grown to confluency. After seven days, macrophages were transferred to media without serum (0%FBS) for 24 hours before being transferred to serum shock media (50% FBS) for 2 hours, in order to synchronize their clocks. Synchronized macrophages were transferred into fresh media (10% FBS) and, starting at 16 hours post shock (PS 16), samples were treated in triplicate with Aβ (for 2 hours) every four hours for 24 hours. Macrophages were then harvested, fixed using formalin, and imaged using fluorescence microscopy. PS hours as represented in yellow for the active and grey for the inactive phase of the mice as reported previously [S1].
(TIF)
A) The log of mean cell fluorescence of Aβ42 phagocytosis in PER2::LUC cells (purple circles), and in PER1-/-PER2-/- knockout cells (brown squares) plotted over time. Error bars represent the standard deviation. B) Violin plot of Aβ42 phagocytosis in PER1-/-PER2-/- knockout cells represented in logged mean cell fluorescence values plotted against post shock (PS) and circadian time (CT). Grey dots represent a single cell measurement, brown violin plot lines represent the range of the data, and red diamonds indicate the mean measurement of each time point. CT is in reference to the comparison of PER2 expression from Keller et al., 2009 [S3] [41] compared to PER2 expression from our cells. All data shown was performed in triplicate.
(TIF)
(A) BMDMs were collected at PS 16, 20, 24, 28, 32, and 36, in quadruplicate. Spent media from these cell pellets was collected at PS 16, 20, 28, and 36, in triplicate. Concurrently, ECM samples were collected in duplicate at post shock times 16, 20, 28, and 36. (B) The above samples were analyzed by LC-MS/MS and normalized using data from a CyQUANT assay that calculated total cellular DNA by UV-VIS spectroscopy and an interpolated standard curve (standard curve in inset from actual macrophage data).
(TIF)
(A) The level of each HSPG sulfation type in macrophage cells (n = 4) plotted against PS and CT time. (B) The level of each HSPG sulfation type in macrophage ECM scrapings (n = 2) plotted against PS and CT time. (C) The level of each HSPG sulfation type in macrophage spent media cultures (n = 3) plotted against PS and CT time. (D) The level of each CSPG sulfation type in macrophage cells (n = 4) plotted against PS and CT time. (E) The level of each CSPG sulfation type in macrophage ECM scrapings (n = 2) plotted against PS and CT time. (F) The level of each CSPG sulfation type in macrophage spent media cultures (n = 3) plotted against PS and CT time. n = number of replicates, error bars are represented by the standard deviation of the replicates.
(TIF)
Violin plot of Aβ42 phagocytosis in the presence of A) Heparinase I and B) Heparinase III represented in logged mean cell fluorescence values plotted against PS and CT. Grey dots represent a single cell measurement, A) red and B) maroon violin plot lines represent the range of the data, and red diamonds indicate the mean measurement of each time point. CT is in reference to the comparison of PER2 expression from Keller et al., 2009 [S3] [41] compare to PER2 expression from our cells. C) Phagocytosis of Aβ42 when heparinases I, II, and III are added at decreasing concentrations (shown in key), represented in the log of the mean cell fluorescence over PS and CT time.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All data related to this manuscript has been placed on Mendeley Data (doi: 10.17632/zrmgjyggmr.1) and GitHub (https://github.com/gclark304/fluorescent_image_analysis).