Abstract
Background:
Wearable transdermal alcohol concentration (TAC) sensors allow passive monitoring of alcohol concentration in natural settings and measurement of multiple features from drinking episodes, including peak intoxication level, speed of intoxication (absorption rate) and elimination, and duration. These passively collected features extend commonly used self-reported drink counts and may facilitate prediction of alcohol-related consequences in natural settings, aiding risk stratification and prevention efforts.
Method:
222 regularly heavy drinking young adults aged 21-29 (M age=22.3, 64% female, 79% non-Hispanic white, 84% undergraduates) participated in a five-day study including ecological momentary assessment (EMA) of alcohol consumption (daily morning reports and participant-initiated episodic EMA sequences) and TAC sensors (SCRAM-CAM anklets). The analytic sample contained 218 participants and 1274 days (including 554 self-reported drinking days). Five features including area under the curve (AUC), peak TAC, rise rate (rate of absorption), fall rate (rate of elimination), and duration – were extracted from TAC-positive trajectories for each drinking day. Day- and person-level associations of TAC features with drink counts (morning and episodic EMA) and alcohol-related consequences were tested using multilevel modeling.
Results:
TAC features were strongly associated with morning drink reports (r=0.6-0.7) but moderately associated with episodic EMA drink counts (r=0.3-0.5) at both day- and person-levels. Higher peaks, larger AUCs, faster rise rates, and faster fall rates were significantly predictive of day-level alcohol-related consequences after adjusting for both morning and episodic EMA drink counts in separate models. Person-means of TAC features added little to alcohol-related consequence prediction above daily scores.
Conclusions:
Results support the utility of TAC sensors in studies of young-adult alcohol misuse in natural settings and outline the specific TAC features that contribute to the day-level prediction of alcohol-related consequences. TAC sensors provide a passive option for obtaining valid and unique information predictive of drinking risk in natural settings.
Keywords: Transdermal alcohol concentration (TAC) sensors, Ecological momentary assessments, Young adults, self-reported drinking, alcohol-related consequences
INTRODUCTION
Young-adult alcohol misuse is a public health problem. Thirty-seven percent of college and 29% of non-college young adults ages 18-25 currently engage in binge drinking, defined as 5+ drinks for men and 4+ for women on a single occasion (National Survey on Drug Use and Health, 2018). Drinking levels for young adults often exceed these thresholds (Esser et al., 2012, Naimi et al., 2010, Schulenberg et al., 2019). One in 20 young adults report consuming 10+ drinks, and 1 in 33 report 15+ drinks, on a single occasion at least once in the past two weeks (Schulenberg et al., 2019). Among college students, alcohol is involved in 23,000 physical assaults, 3,200 alcohol-related sexual assaults or date rapes, 730 hospitalizations, and 50 deaths each weekend of the academic year (Hingson et al., 2005, Hingson et al., 2017, Hingson et al., 2009). Although rates are higher among college young adults, the past-year prevalence of negative alcohol-related consequences remains high (43%) for non-college young adults (versus 56% of college young adults; Patrick et al., 2020).
Many negative alcohol-related consequences occur during or closely after the event (Clapp et al., 2018, Clapp et al., 2017) and long-term consequences of alcohol misuse occur partly as a result of frequent alcohol misuse days (Clapp et al., 2018, Holder, 2006, Rehm et al., 2009). Most day-level research has used self-reported number of drinks as the primary predictor (e.g., Bravo et al., 2017, Cronce et al., 2015, Pearson, 2013). The wide usage of drink counts is supported by their straightforwardness in assessment and consistent prediction of consequences (Del Boca and Darkes, 2003, Greenfield et al., 2014, Greenfield and Kerr, 2008). Enhanced ecological momentary assessment (EMA) capabilities brought by handheld smart devices allow collection of self-reports during drinking events, reducing potential for recall biases that may exist even in daily reports (Piasecki, 2019). However, sole reliance on self-reports may limit characterization of drinking events and prediction of negative alcohol-related consequences. Some argue that alternative measures are needed (Andreasson, 2016) because number of drinks is an imperfect proxy for biological alcohol concentration, the more proximal measure of alcohol-related risk (Andreasson, 2016, Bond et al., 2014, Greenfield et al., 2014, Pearson et al., 2016). Across individuals, equivalent number of drinks does not mean equivalent levels of blood alcohol concentration (BAC). For example, 6 drinks in two hours will result in a different BAC level for a 250-pound male versus a 125-pound female (Andreasson, 2016, Pearson et al., 2016). The BAC discrepancy between these two individuals will widen if drink counts are inaccurate and if differences in alcohol by volume within beverage types are not considered (Andreasson, 2016, Greenfield et al., 2014).
Direct measurement of biological alcohol concentration in the field would provide an effective complement to drinking self-reports. Blood and breath alcohol concentration (BAC and BrAC, respectively) are too invasive and/or burdensome to be practical for field use (Campbell et al., 2018, Fairbairn et al., 2019, van Egmond et al., 2020). Wearable transdermal alcohol concentration (TAC) sensors offer a viable option. TAC sensors are worn on the wrist or ankle and passively measure alcohol concentration through the skin (Barnett, 2015, Leffingwell et al., 2013, Piasecki, 2019). Approximately 1% of ethanol consumed is eliminated transdermally (Sakai et al., 2006, Swift, 2000), and ethanol concentration in sweat is approximately equal to that of blood (Nyman and Palmlöv, 1936). Differences in alcohol secretion dynamics through the skin versus breath (Hill-Kapturczak et al., 2015, Karns-Wright et al., 2017, Norman et al., 2020, Sakai et al., 2006, Swift, 2003, Wang et al., 2021) mean that (a) TAC will not be equivalent to BrAC at any given timepoint and (b) BrAC will be a more accurate measure of a biological alcohol concentration than TAC at a single point in time. However, TAC provides a more comprehensive summary of alcohol use in the field compared to BrAC due to its continuous, passive, and unobtrusive collection (Norman et al., 2020). Evidence also shows that peak and area under the curve (AUC) of TAC values correlate strongly with those of BrAC in lab settings (e.g., 0.8-0.9; Sakai et al., 2006), establishing the validity of the TAC metric.
Figure 1 shows an example of a TAC-detected event using actual participant data. TAC event trajectories can be summarized as a single number such as the AUC, which is the total geometric area under the TAC data points and can be thought of as the total amount of biological alcohol “exposure” seen during the drinking event (Barnett et al., 2011, Sakai et al., 2006, Simons et al., 2015). AUC has shown predictive validity for alcohol-related consequences above self-reports (e.g., Simons et al., 2015), but it is difficult to interpret because AUC blends the height of the curve with the length of the event in a single number. More elementary features displayed in Figure 1 aid in characterization of the event. Peak TAC is the maximum value of the TAC trajectory. Higher peak TAC values represent higher levels of intoxication. Absorption or rise rate reflects the speed of TAC increase; elimination or fall rates reflect the speed of TAC decrease. Larger rise rates reflect faster intoxication rates; larger fall rates reflect faster alcohol elimination. Duration reflects the number of hours the participant spent biologically “exposed” to alcohol; it is the number of hours TAC was positive (> 0). Each of these has been previously proposed (e.g., Barnett et al., 2011, Clapp et al., 2017, Simons et al., 2015), but no studies to our knowledge have derived all of these features and tested their associations with self-reported drinking and alcohol-related consequences in natural settings. Knowing which TAC features are most strongly associated with alcohol misuse and consequences would inform future research with TAC sensors and development of novel self-report instruments.
Figure 1.
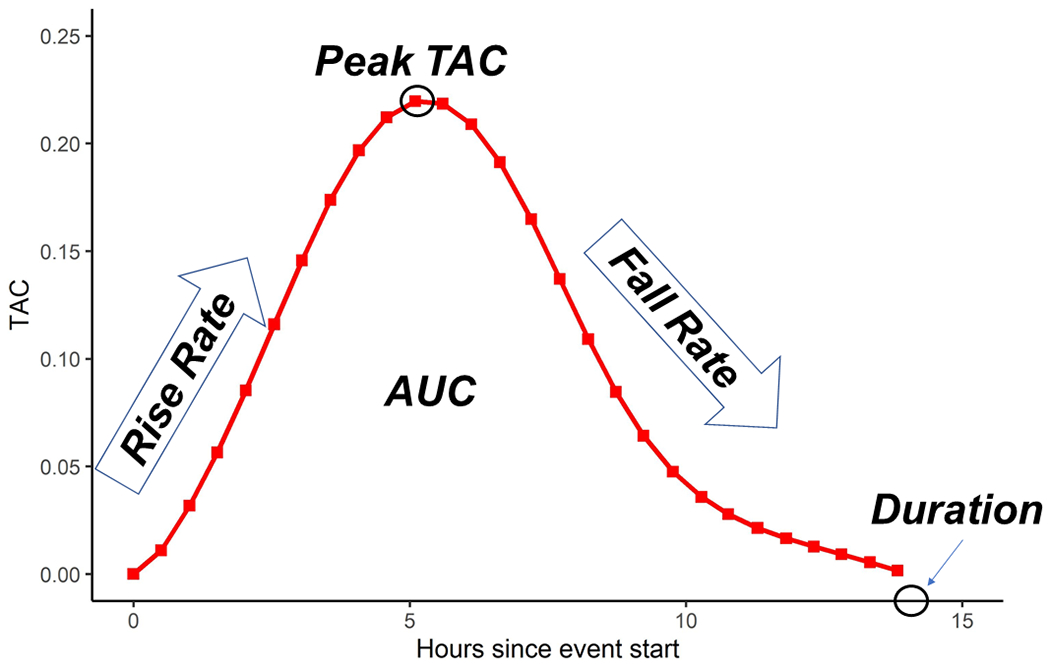
TAC trajectory from a single episode of actual participant data, illustrating TAC features. Peak is the maximum TAC value. Rise rate is the average point-to-point ascending slope, capturing rate of ethanol absorption. Fall rate is the average point-to-point descending slope, capturing rate of ethanol elimination. Duration is the length of the TAC trajectory, capturing the number of hours under the alcohol concentration curve. Area under the curve (AUC) is the total area under the TAC trajectory, representing estimated cumulative biological alcohol exposure. Drinking reports for this participant included both EMA and next morning retrospective reports: 6 standard drinks were reported as consumed in the episodic EMA for this drinking day, whereas 3 standard drinks were reported in the retrospective morning report.
Among the studies that have derived TAC features, many have used TAC features only to characterize drinking events or to test whether TAC sensor classification of drinking versus non-drinking days corresponds with classifications of self-reports or BrAC (Barnett et al., 2014, Barnett et al., 2011, Bond et al., 2014, Fairbairn et al., 2018, Fairbairn et al., 2019, Sakai et al., 2006). Some studies have tested correlations between TAC AUC and number of drinks, including known drinks in the lab (e.g., Dougherty et al., 2015) and self-reported drinks in the field (e.g., Barnett et al., 2014, Bond et al., 2014, Karns-Wright et al., 2018, Simons et al., 2015). These studies provide evidence of convergent validity of TAC sensors with self-reports by showing moderate-to-high correspondence between sensor-detected and self-reported drinking in natural settings. Other studies have begun to test select TAC features (e.g., AUC, peak, and rise rate) as predictors of alcohol-related consequences and acute dependence symptoms between persons, either through averages of these features across events or by comparing individuals on single events (e.g., Clapp et al., 2017, Simons et al., 2015). Most work has compared TAC sensors to daily retrospective self-reports; few studies have examined correspondence of TAC sensors with prospective episodic EMA (but see Clapp et al., 2017, Norman et al., 2020, Simons et al., 2015 for exceptions), which may provide a closer comparison due to its prospective nature. The current study extends previous work by (a) deriving an array of TAC features describing multiple aspects of alcohol use events, (b) obtaining self-reported number of drinks both daily (using daily retrospective reports) and during ongoing drinking episodes, and (c) testing which TAC features are most strongly associated with self-reported number of drinks and most predictive of negative alcohol-related consequences after adjusting for self-reported number of drinks (daily and episodic EMA) across multiple drinking days in young adults’ natural settings. The current study is the first to combine these elements in a large sample and test an array of TAC features as predictors of alcohol-related consequences.
We had two hypotheses. First, we hypothesized that TAC features would correlate strongly with self-reported number of drinks. Although we expected each TAC feature to be reflective of alcohol consumption, we hypothesized that AUC, peak, and rise would be more strongly controlled by alcohol consumption than fall rate and duration, which were expected to be more strongly influenced by biological processes controlling alcohol elimination. Second, we hypothesized that each TAC feature would be significantly predictive of alcohol-related consequences, and that these associations would remain after adjusting for self-reported drink counts. We expected each TAC feature to predict alcohol-related consequences even after adjusting for self-reported number of drinks per the following rationale. First, evidence shows that the accuracy of drinking self-reports decreases the more a person drinks (Northcote and Livingston, 2011). Second, recent sophisticated work deriving the level and pace of alcohol consumption from EMA episodes has proven valuable (e.g., Carpenter and Merrill, 2020). However, episodic EMA tends to terminate only when the person stops drinking, leaving the elimination process unassessed and preventing assessment of the full alcohol concentration curve (Norman et al., 2020, Piasecki, 2019). Because the wearable TAC sensor is device-based and passive, its assessment mechanism is not impaired by increasing alcohol consumption and it unobtrusively collects alcohol concentration data before, during, and after consumption periods. These characteristics (a) allow measurement of elimination rate, duration of alcohol concentration, and area under the curve in addition to what could be assessed by estimated BAC from self-report (peak and absorption rate), (b) avoid inaccuracies that may arise in self-reports during periods of high intoxication, and (c) reduce participant burden. These characteristics of TAC sensor measurement facilitate the capture of alcohol-use dimensions that self-reports cannot easily obtain, and we hypothesize that this information will prove valuable in the prediction of alcohol-related consequences. It was expected that larger AUCs, higher peaks, faster rise rates, slower fall rates, and longer durations would be predictive of greater alcohol-consequence risk.
MATERIALS AND METHODS
Participants and Procedure
Participants included 222 young adults aged 21-29 who regularly engaged in binge drinking, defined as 4+/5+ drinks on a single occasion for females/males. Table 1 shows demographic information and alcohol history (collected during screening). Alcohol Use Disorder Identification Test (AUDIT; Babor et al., 2001) scores collected at baseline (M=12.4, SD=5.0) showed that 85% of participants scored ≥ 8, indicating high risk for alcohol use disorder.
Table 1.
Participant demographics and alcohol history
| Gender |
n
|
% |
| Female | 141 | 63.5% |
| Male | 81 | 36.5% |
|
M
|
SD
|
|
| Age | 22.3 | 1.3 |
| Bodyweight (in pounds) | 157.7 | 33.5 |
| Race, non-Hispanic ethnicity |
n
|
% |
| White | 175 | 78.8% |
| Asian | 15 | 6.8% |
| Black | 8 | 3.6% |
| Native American | 0 | 0.0% |
| Mixed | 8 | 3.6% |
| Race, Hispanic ethnicity |
n
|
% |
| White | 11 | 5.0% |
| Asian | 0 | 0.0% |
| Black | 0 | 0.0% |
| Native American | 1 | 0.5% |
| Mixed | 3 | 1.4% |
| Student Status |
n
|
% |
| Undergraduate student | 187 | 84.2% |
| Graduate, professional, or other student | 14 | 6.4% |
| Non-student | 21 | 9.5% |
| Past 12-month alcohol use frequency |
n
|
% |
| 2-3 days a month | 15 | 6.8% |
| 1-2 days a week | 114 | 51.4% |
| 3-5 days a week | 91 | 41.0% |
| Every day or almost every day | 2 | 0.9% |
| Past 12-month binge drinking frequency |
n
|
% |
| 1-2 days | 2 | 0.9% |
| Once a month or less | 17 | 7.7% |
| 2-3 days a month | 56 | 25.3% |
| 1-2 days a week | 104 | 47.1% |
| 3-5 days a week | 40 | 18.1% |
| Every day or almost every day | 2 | 0.9% |
| Typical binge drinking frequency during academic year (Sept-May) |
n
|
% |
| 1 day per week | 87 | 39.4% |
| 2 days per week | 71 | 32.1% |
| 3 days per week | 54 | 24.4% |
| 4 or more days per week | 9 | 4.1% |
| Past 2-week binge drinking frequency |
n
|
% |
| 0 times | 5 | 2.3% |
| 1 time | 34 | 15.3% |
| 2 times | 56 | 25.2% |
| 3-5 times | 89 | 40.1% |
| 6-9 times | 31 | 14.0% |
| 10 or more times | 7 | 3.2% |
Recruitment and screening.
Participants were recruited on and around the campus of a large northeastern US university using flyers. When participants emailed, research staff responded with a brief study description and a screening survey link. To be eligible, participants needed to: (1) be between the ages of 21-29, (2) have engaged in binge drinking at least weekly on average during the past year or typically engage in binge drinking at least weekly during the academic year, and (3) be sufficiently proficient in written English to complete study procedures. 531 individuals completed the screening survey and 419 (78.9%) were eligible. Eligible versus ineligible participants were more likely to be male (35% versus 26%, p=0.066), non-Hispanic white (83% versus 72%, p=0.01), and undergraduates (93% versus 73%, p <0.001). Of the 419 eligible participants, 343 were invited to participate. Time and resource limitations prevented invitations to all eligible participants; invitations were sent in the order in which screening surveys were received. Of the 343 invited, 222 completed the study. No evidence of bias was observed when comparing those who completed versus those who did not on dimensions of sex, race/ethnicity, student status, or past-two-week binge drinking frequency (all p ≥.10).
Procedure.
The study spanned six consecutive days (Wednesday to Monday) and consisted of five 24-hour periods. The study included baseline and endpoint assessments, three-times daily EMA, participant-initiated drinking-episodic EMA sequences, and transdermal sensor wear. Participants began by attending the baseline appointment in our research laboratory. Participants were consented before privately completing a thirty-minute questionnaire containing measures of background/demographics and alcohol history using the AUDIT (Babor et al., 2001). Participants were then fitted for the TAC sensor and received training on EMA surveys.
After baseline, participants began the field protocol. Participants wore a TAC monitor on their ankle (the Secure Continuous Remote Alcohol Monitoring-Continuous Alcohol Monitor or SCRAM-CAM) and carried a mobile phone on which they completed EMA surveys. Participants were compensated up to $110: $10 for completing the baseline, up to $45 for completing the EMA portion of the study (receiving $10 upon consenting and $2 per morning, afternoon, or evening survey they completed along with a $5 bonus for achieving compliance ≥ 90%), $45 for wearing the SCRAM-CAM anklet ($9 for each 24-hour period), and $10 for completing an endpoint survey. All procedures were approved by the university institutional review board.
SCRAM-CAM anklet.
Participants wore the SCRAM-CAM anklet during wake and sleep hours. SCRAM-CAM uses self-generated air flow to capture transdermal ethanol evaporation. TAC is determined using fuel-cell technology (Fairbairn and Kang, 2019, van Egmond et al., 2020). After study completion, SCRAM-CAM data were uploaded to the company’s online server (SCRAMNet), which houses TAC data, records TAC “positives”, and tracks compliance with device wear through skin temperature and sensor quality (infrared voltage) readings. SCRAMNet algorithms are designed for forensic use and are inherently conservative, missing a high percentage of light-to-moderate drinking days (Barnett et al., 2014, Roache et al., 2019). We instead applied validated research algorithms more sensitive to light-to-moderate drinking (described below; see Roache et al., 2019). SCRAMNet also uses a proprietary algorithm to flag instances of device removal or interference – referred to hereafter as “non-compliance” – from infrared and temperature sensor data streams native to the SCRAM-CAM device. Compliance was high. Only 2.0% of TAC data points we collected showed any evidence of device removal or interference, and these data points were clustered within a minority of individuals (n=24; 11.0%). Among these individuals, a median of 10% of their study hours were affected by non-compliance (IQR=7%, 16%), with only five participants showing non-compliance across 20% or more of their study hours (median=59%, IQR=39%, 73%). Compliance was not associated with study demographics (gender, age, race/ethnicity, student status) or AUDIT scores (ps >.05).
EMA protocol.
Participants were provided with an Android-based smartphone equipped with a custom “app” designed by specialist EMA survey programmers at the Survey Research Center of the authors’ academic institution. The app contained two survey types. The first was a three-times daily scheduled EMA report, which prompted participants to complete surveys at fixed times in the morning (scheduled for 10 AM), afternoon (4 PM), and evening (9 PM). Participants could choose to have these prompts occur one hour later (11 AM, 5 PM, 10 PM; 26% chose this option). Compliance was high; 94% of scheduled morning, afternoon, and evening reports were completed.
The second survey type was the episodic EMA, which consisted of a participant-initiated survey sequence with timed follow-ups occurring every 30 minutes during ongoing drinking events (Piasecki, 2019). Participants began the episodic EMA sequence by initiating a “first drink” survey while consuming their first drink of the event. Participants were then triggered by the device every 30 minutes to report the number of drinks they consumed since the last survey. Prompts continued every 30 minutes until (a) participants reported that they had not consumed alcohol since the last survey and that they were finished drinking for the next 2-3 hours or (b) three consecutive prompts were missed. Episodic EMA surveys were very brief (median completion time=1 minute, IQR=0.67, 2). Episodic EMA data were grouped into episodes using first drink surveys to mark the start of a new episode. If two reports within the same episode were separated by more than 3 hours (n obs=31, 0.7% of total), episodes were divided. We obtained 582 EMA drinking episodes containing 3,656 drink reports. Ninety-two percent of participants provided EMA drinking episode data; these participants provided a median of 3 days with EMA drinking episode data (IQR=2, 3). Days with EMA drinking episode data contained a median of 6 drink reports (IQR=4, 9).
Initial TAC data processing.
Drinking episodes were identified and coded using validated research guidelines shown to (a) increase sensitivity of detection for low- to moderate-level drinking with little sacrifice of specificity and (b) remove events and observations that indicate environmental alcohol contamination (e.g., spilled drinks near the device, fumes from cleaning products; Roache et al., 2019). We began with 52,726 TAC observations collected from 218 individuals; data from 4 participants were lost due to device failure. We retained 608 TAC drinking events containing 16,385 data points among 195 participants (87.8% of sample) across 694 person-days. These 195 participants provided a median of 4 days with TAC event data. Days with TAC event data contained a median of 21 TAC-positive data points (IQR: 11, 31). Prior to analysis, TAC data were smoothed to remove noise and facilitate feature extraction using penalized b-spline smoothing (Eilers and Marx, 1996) in SAS PROC TRANSREG. A separate smoothing parameter was optimized for each drinking episode.
Segmenting TAC and episodic EMA data into “social days”.
Morning report drinking and consequence assessments were retrospective, querying participants about drinking “yesterday or last night”. Because drinking often extends past midnight, we assumed that a participant’s report of the previous day’s drinking included the hours between midnight and the morning report. As in previous EMA and TAC sensing studies of alcohol use (e.g., Karns-Wright et al., 2018, Mun et al., 2021, Russell et al., 2020), we therefore segmented TAC and episodic EMA data into “social days”. We used 10 AM as the social day boundary, rather than midnight, because 10 AM was the modal time of the morning report. If the morning report was provided before 10 AM but TAC or episodic EMA data were present in the interim between 5 and 10 AM, the day boundary was reset so that the time of the morning report marked the start of the new day (104 TAC and 7 episodic EMA observations were shifted).
Measures
TAC features.
Five TAC features were extracted from each day with TAC-positive episode data. TAC episode functions showed a diversity of shapes (see Figure 2). Each feature was calculated by the 10 AM-10 AM day to allow correlations with morning drink reports and consequences. If a TAC episode spanned multiple days, features were calculated separately for each day (e.g., id 1038, days 0 and 1 in Figure 2). If a day contained multiple TAC events, features were calculated using all data for the day (e.g., id 1101 day 2). Peak TAC was the maximum TAC value (n days=694). Rise rate was the average of all ascending point-to-point TAC rates (; n days=634) and is interpreted as the day’s average rate of TAC increase per hour. Fall rate was the average rate of all descending point-to-point TAC rates (; n days=690) and is interpreted as the day’s average rate of TAC decrease per hour. Point-to-point rates during TAC events that were equal to 0 (n=116, 0.7% of TAC event data points) were not included in rate calculation. Rise rates could not be estimated on 60 TAC-positive days because these did not include positive point-to-point rates (e.g., id 1187 day 3 in Figure 2). Similarly, fall rates could not be calculated for four TAC-positive days containing only ascending TAC (e.g., id 1038 day 0). Duration captured the number of hours a person spent under the alcohol concentration curve that day. Duration was calculated as the sum of all TAC-positive point-to-point time differences during TAC-positive events () from the first positive TAC reading of the day to the last positive for the day (n days=692). Area under the curve (AUC) was used to represent the cumulative alcohol concentration experienced by the person that day. AUC was calculated during TAC-positive windows using . (n days=692). Days with no duration or AUC (n days=2) were days with a single TAC datapoint marking the start of a TAC episode that carried into the next day (e.g., id 1183 day 0 in Figure 2). For days with no TAC-positive events (n days=611), all features were coded as 0 if no evidence of non-compliance (removal or interference) was observed that day (n days=587) as these days were valid indicators of non-detection. Days with no TAC-positive events were set to missing if evidence of non-compliance was present, as these non-positive readings could have been due to sensor non-wear (n days=24). Combining the 587 TAC-negative days with the 694 TAC-positive days left 1281 days. For all TAC features, outliers were defined as values above the 99th percentile. These were removed, leaving 1274 days of TAC data across 218 persons for analysis.
Figure 2.
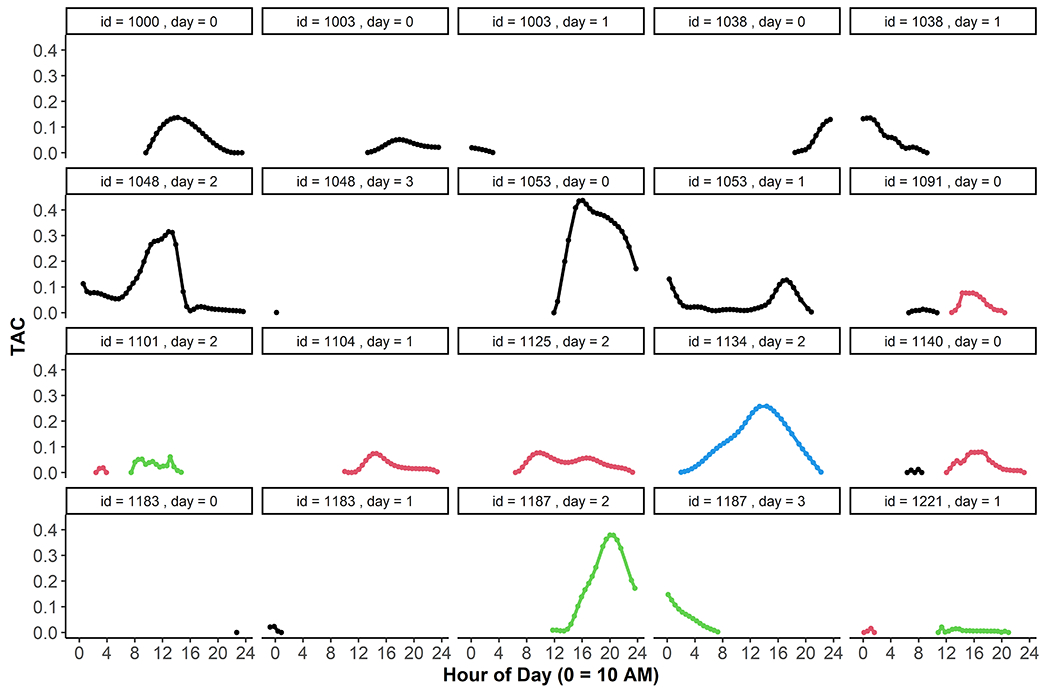
Single days with TAC event data. Multiple day types are displayed. Some days contained a single TAC event that began and ended within the same day (e.g., id=1000, day=0; id=1104, day=1). Some TAC events spanned more than one day, as indicated by the break across two panels (e.g., id=1038, day=0 and day=1). Some days contained more than one event within the same day, as noted by two distinctly colored curves within the same panel (e.g., id=1091, day=0; id=1101, day=2).
Episodic EMA drinks.
During episodic EMA survey sequences, participants were shown a figure displaying standard drink sizes for 5% beer (12 oz), 12% wine (5 oz), and 40% liquor (1.5 oz) and reported their current alcohol consumption by providing the number of drinks they had consumed since the last survey. First drink surveys marking the start of an episode were counted as 1 drink. If the participant indicated in their first drink survey that it was not the first drink of the episode (n episodes=89; 15.3% of episodes), the first drink survey was not counted as one drink and participants were instead asked to report the number of drinks consumed since the start of the episode using the standard drink figure described above. The daily total of episodic EMA drinks was used in analyses. Prior to analysis, episodic EMA drink totals above 15 were set equal to 15 (n days = 77). These episodic EMA drink totals ranged from 15-50, with a median of 20 drinks and an interquartile range of 17 to 23.5. Five outlier days exceeded 30 drink reports (range = 35-50). Although capping episodic EMA drink totals at 15 may underestimate consumption among the heaviest drinkers, it was done for two reasons. First, it allowed more direct and meaningful comparison of EMA drink totals to morning-report drink totals, for which response options were capped at “15+”. Second, it aimed to reduce the influence of rare and extreme values on our regression estimates. Analyses using episodic EMA drink totals only included days with a participant-initiated EMA episode (n days=536, n persons=204). Because we could not count the absence of a participant-initiated episodic EMA report as evidence of an episodic EMA non-drinking day, episodic EMA drink counts were set to missing on days with no episodic EMA reports.
Morning drink reports.
In the morning EMA survey, participants were shown the standard drink figure described above and were asked “how many drinks of alcohol did you drink yesterday/last night?” Responses ranged from 0 to 15+, with 15+ coded as 15. Morning reported drinks were collected from 222 participants across 1069 person-days; 554 drinking days reported across 209 individuals were captured. Morning drink counts were “led” one day (shifted up one row in the daily data file) prior to analysis to align day-level retrospective reports with prospectively collected TAC features.
Alcohol-related consequences were assessed on morning-report drinking days only. If participants endorsed drinking in the morning survey, they then reported whether a set of alcohol-related consequences had occurred as a result of drinking yesterday/last night. Consequences were taken from the Importance of Consequences of Drinking-Short Form (Patrick and Maggs, 2011) and the Brief Young Adult Alcohol Consequences Questionnaire (adapted for daily use; Kahler et al., 2005). Single consequences are presented in Supplemental Table 1 along with their frequencies across days and persons. Participants received a score each day representing the number of alcohol-related consequences they experienced. Alcohol-related consequences were also led one day prior to analysis.
Statistical Analysis
Analyses were conducted in R (R Core Team, 2020). Means, between- and within-person SD, and intraclass correlations (ICC) were generated from empty multilevel models using lme4::lmer. Correspondence between morning report drinking days, episodic EMA days, and TAC episode days was examined using generalized estimating equations (GEE) with an independent working correlation matrix and robust standard errors. Logistic GEE was used to generate probabilities of TAC or episodic EMA detection and their 95% confidence intervals (CIs). Modified Poisson GEE was used to estimate prevalence ratios (PR) and their 95% CIs across detected and non-detected days (Zou, 2004). Poisson GEE was used to generate predicted drink counts and test incidence rate ratios (IRRs) representing ratios of drink counts across detected versus non-detected days.
Multilevel models with standardized person-mean centered variables were used to generate correlations between TAC features, morning report drinks, and episodic EMA drink totals at the within-person level. These models tested hypothesis 1: that TAC features would correlate strongly with self-reported number of drinks. Correlations between TAC features and morning report drinks included all available drinking and non-drinking days; correlations with episodic EMA drink totals were limited to days with episodic EMA data. Pearson correlations were calculated at the between-person level using standardized person-means. r effect size cutoffs used for these tests were 0.3, 0.5, and 0.7, representing “weak”, “moderate”, and “strong” convergent validity (Abma et al., 2016).
Negative binomial multilevel models were used to estimate associations between TAC features (predictors) and the count of alcohol-related consequences (outcome) using the glmmTMB R function (family=nbinom1). These models tested hypothesis 2: that each TAC feature would be significantly predictive of alcohol-related consequences, and that these associations would remain significant after adjusting for self-reported drink counts. Consequences were only assessed on days with morning report drinking; these analyses therefore included only morning-report drinking days. IRR effect sizes estimated the proportional difference in the number of alcohol-related consequences with each unit difference in predictors. Separate models were tested for each TAC feature and included the day-level feature score along with its person mean, both grand-mean centered. The coefficient for the day-level TAC feature represents the within-person association between the TAC feature and alcohol-related consequences (Bell et al., 2018, Hamaker and Muthén, 2020). This tests whether the number of alcohol-related consequences is elevated on days when a person reaches a higher versus lower peak TAC (for example), adjusting for their typical peak TAC across the five days. The coefficient for the person-mean feature represents the independent between-person association, sometimes called the “contextual effect” (Bell et al., 2018). This tests whether the mean number of alcohol-related consequences experienced is higher for people who tend to reach higher TAC peaks compared to people who tend to reach lower TAC peaks, assuming they both achieved the same peak that day (Hamaker and Muthén, 2020). All models adjusted for sex, bodyweight in pounds, and social weekend (Thu/Fri/Sat). The first set of models adjusted only for these covariates; the second set added adjustment for day- and person-level morning report drinks; the third set replaced morning report drink controls with day- and person-level episodic EMA daily drink totals. All predictors were grand-mean centered and standardized to allow meaningful comparison of IRR effect sizes. Random slopes were tested throughout using likelihood ratio tests. Scant evidence for random slopes was found so random slopes were omitted from final models. Total consequence models were followed by exploratory tests of single consequences using multilevel logistic regression (glmmTMB, family=binomial). Each TAC feature predicted each consequence in a separate model adjusting for covariates (gender, bodyweight, and social weekend). Odds ratio effect sizes and their 95% CIs were estimated.
RESULTS
Descriptive Statistics
Table 2 shows descriptive statistics for TAC features, morning report drinks, episodic EMA daily drink totals, and alcohol-related consequences. Across all days, participants reported a mean of 2.7 drinks in morning reports (Median=1, IQR:0-5). On morning-report drinking days only, participants reported 5.2 drinks per day on average (Median=5, IQR:2-7), which indicated that drinking days tended to be heavy drinking days. That drinking days tended to be heavy was also supported by episodic EMA. On days with episodic EMA, participants reported a mean of 7 drinks (Median=6, IQR: 3-10.5). A multilevel model testing the difference between episodic EMA and morning report drinks on morning-report drinking days showed that participants reported 1.7 more drinks in episodic EMA than they did in the morning report (p < .001). The mean of alcohol-related consequences reported after drinking days was 0.53, suggesting that alcohol-related consequences occurred following the minority of drinking days, but SDs showed that the number of alcohol-related consequences varied across days and persons.
Table 2.
Descriptive statistics for study variables
| N persons | N days | Mean | Median | Person-level SD | Day-level SD | ICC | ICC: 95% CI* | |
| AUC | 218 | 1272 | 0.43 | 0.01 | 0.35 | 0.75 | 0.18 | 0.12, 0.23 |
| Peak | 218 | 1274 | 0.06 | 0.009 | 0.04 | 0.09 | 0.18 | 0.12, 0.23 |
| Rise Rate (+ TAC / hour) | 218 | 1214 | 0.01 | 0.001 | 0.005 | 0.02 | 0.09 | 0.05, 0.14 |
| Fall Rate (− TAC / hour) | 218 | 1270 | 0.009 | 0.003 | 0.005 | 0.01 | 0.17 | 0.11, 0.22 |
| Duration (hours) | 218 | 1272 | 6.49 | 2.03 | 4.05 | 6.70 | 0.27 | 0.21, 0.33 |
| Morning Report Drinks (all days) | 222 | 1069 | 2.74 | 1.00 | 1.44 | 3.36 | 0.16 | 0.10, 0.21 |
| Morning Report Drinks (on drinking days) | 209 | 554 | 5.17 | 5.00 | 1.89 | 2.94 | 0.29 | 0.19, 0.39 |
| Alcohol-Related Consequences** | 209 | 553 | 0.53 | 0.00 | 0.43 | 0.93 | 0.18 | 0.08, 0.28 |
| Episodic EMA Drinks (daily total) | 204 | 536 | 7.00 | 6.00 | 2.28 | 4.12 | 0.23 | 0.13, 0.33 |
Estimated using bootstrapping with 10,000 sample draws; values represent the 2.5 and 97.5 percentile points of the empirical sampling distribution.
Alcohol-related consequences were only reported on days with one or more drinks reported in the morning report.
Correspondence of TAC detection, episodic EMA, and morning reports
Of morning report drinking days, TAC episode data were present on 84.4% (95% CI: 79.3%, 88.5%) and episodic EMA data were provided on 89.2% (95% CI: 85.9%, 91.9%). Poisson GEE revealed that morning report drinking days detected by the TAC sensor contained significantly more morning report drinks than days that were not detected (M=5.8 versus 3.4 drinks, IRR=1.69, 95% CI: 1.33, 2.16). Morning report drinking days detected by the TAC sensor were also significantly more likely to be heavy drinking days (64% were heavy drinking days defined by 4+/5+ morning report drinks for females/males) than morning report drinking days that were not detected by the sensor (35%; PR=1.83, 95% CI: 1.17, 2.87). Morning report drinking days with versus without episodic EMA also contained more morning report drinks (5.43 versus 4.41 drinks), but this difference was not significant (IRR=1.23, 95% CI: 0.94, 1.62). Of days with episodic EMA, TAC episode data were present on 83.7% (95% CI: 78.4%, 87.8%). Episodic EMA days detected by the TAC sensor contained significantly more drinks than episodic EMA days not detected (7.67 versus 4.26; IRR=1.80, 95% CI: 1.43, 2.27) and were significantly more likely to be heavy drinking days (70% versus 35%, PR=2.01, 95% CI: 1.39, 2.89).
Intercorrelations of TAC features and drinking self-reports
Day-level results.
Within-person correlations of TAC features and drinking self-reports are presented in the top half of Table 3. Three main findings are present. First, all TAC features were strongly and positively associated within-persons, with the strongest association seen between peak and AUC. AUC and peak showed the strongest associations with other features, followed by rise and fall rates, and lastly by duration. Second, TAC features correlated strongly with morning report drinks. Peak and AUC showed the strongest correlations with morning report drinks, but correlations with morning-report drinks were ≥ 0.60 for all features. Third, episodic EMA drink totals correlated only modestly with TAC features (r=0.30-0.50) and morning report drinks (r=0.57) across days.
Table 3.
Intercorrelations of TAC features and drinking self-reports
| Within persons (N days = 1274) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1. AUC | 1 | ||||||
| 2. Peak | 0.92 | 1 | |||||
| 3. Rise Rate | 0.69 | 0.82 | 1 | ||||
| 4. Fall Rate | 0.64 | 0.78 | 0.82 | 1 | |||
| 5. Duration | 0.64 | 0.69 | 0.57 | 0.55 | 1 | ||
| 6. Morning Report Drinks | 0.73 | 0.74 | 0.65 | 0.60 | 0.60 | 1 | |
| 7. Episodic EMA Drinks | 0.50 | 0.45 | 0.35 | 0.34 | 0.30 | 0.57 | 1 |
| Between persons (N persons = 218) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|
| |||||||
| 1. AUC | 1 | ||||||
| 2. Peak | 0.95 | 1 | |||||
| 3. Rise Rate | 0.76 | 0.85 | 1 | ||||
| 4. Fall Rate | 0.74 | 0.83 | 0.87 | 1 | |||
| 5. Duration | 0.84 | 0.84 | 0.71 | 0.65 | 1 | ||
| 6. Morning Report Drinks | 0.74 | 0.73 | 0.63 | 0.63 | 0.65 | 1 | |
| 7. Episodic EMA Drinks | 0.46 | 0.49 | 0.45 | 0.38 | 0.43 | 0.52 | 1 |
Note: Within-persons correlations were estimated and tested for significance using multilevel models with person-mean centered features. Person-mean centered features were standardized prior to running models to obtain correlation coefficients. At the between-persons level, standard Pearson correlations between person-means were tested among the 218 participants for whom TAC data were collected. All correlations presented were significant at p < 0.001. Episodic EMA Drink reports were only present on 536 days among 204 participants.
Person-level results.
Correlations of person-mean TAC features and drinking self-reports are also presented in Table 3. Results were largely similar to what was seen at the within-person, day-level, with a few exceptions: (1) correlations between person-means of TAC features were larger than TAC feature correlations at the within-person level, (2) person-means of TAC features correlated well with person-mean morning report drinks (r=0.63-0.74), and (3) person-means of episodic EMA drinks correlated only modestly with person-means of TAC features and morning report drinks (r=0.38-0.52).
TAC features as predictors of alcohol-related consequences
Day-level results.
Table 4 shows day-level results of TAC features predicting number of alcohol-related consequences. Morning report drinks (IRR=2.87, 95% CI: 2.30, 3.60) and episodic EMA drinks (IRR=2.17, 95% CI: 1.74, 2.71) each predicted alcohol-related consequences the next morning in unadjusted models. All unadjusted associations between TAC features and alcohol-related consequences were significant. Days with larger AUCs, higher peaks, faster rise rates, faster fall rates, and longer durations in alcohol concentration were significantly associated with more alcohol-related consequences the next morning. After adjusting for morning report drinks, only AUC, peak, and rise rate remained significant predictors of alcohol-related consequences; all features except duration remained significant after adjusting for episodic EMA drinks. Both morning report and episodic EMA drinks were significant predictors of alcohol-related consequences in all models adjusting for TAC features.
Table 4.
Results of multilevel negative binomial models showing prediction of alcohol-related consequences from day-level TAC features in separate models
| Drink Count Unadjusted IRR (95% CI) | Morning Drink Count Adjusted IRR (95% CI) | EMA Drink Count Adjusted IRR (95% CI) | |
|---|---|---|---|
| AUC | 1.83 (1.57, 2.12)*** | 1.42 (1.19, 1.69)*** | 1.54 (1.30, 1.83)*** |
| Morning Report Drinks | -- | 2.13 (1.65, 2.77)*** | -- |
| Episodic EMA Drinks | -- | -- | 1.55 (1.22, 1.98)*** |
| Peak | 1.95 (1.65, 2.31)*** | 1.48 (1.21, 1.81)*** | 1.61 (1.33, 1.95)*** |
| Morning Report Drinks | -- | 2.16 (1.67, 2.80)*** | -- |
| Episodic EMA Drinks | -- | -- | 1.64 (1.29, 2.08)*** |
| Rise Rate | 1.52 (1.28, 1.81)*** | 1.23 (1.02, 1.49)* | 1.27 (1.06, 1.53)** |
| Morning Report Drinks | -- | 2.70 (2.13, 3.43)*** | -- |
| Episodic EMA Drinks | -- | -- | 2.04 (1.62, 2.58)*** |
| Fall Rate | 1.55 (1.31, 1.85)*** | 1.20 (1.00, 1.45)† | 1.24 (1.03, 1.50)* |
| Morning Report Drinks | -- | 2.61 (2.06, 3.31)*** | -- |
| Episodic EMA Drinks | -- | -- | 1.94 (1.54, 2.44)*** |
| Duration | 1.59 (1.24, 2.05)*** | 0.97 (0.74, 1.28) | 1.18 (0.89, 1.56) |
| Morning Report Drinks | -- | 2.88 (2.24, 3.69)*** | -- |
| Episodic EMA Drinks | -- | -- | 2.06 (1.65, 2.59)*** |
Note: All models adjust for person means of TAC features and self-reported drinking (morning and/or EMA drink reports) as well as sex (male = 1, female = 0), bodyweight (in pounds), and social weekend (Thu/Fri/Sat) versus weekday (Sun/Mon/Tue/Wed). Results including coefficients for all independent variables and random effect estimates are included in Supplementary Tables 2a through 2e. All independent variables were z-scored to allow meaningful comparison of IRR effect sizes.
p < .001,
p < .01,
p < .05,
p < .10. IRR = incident rate ratio, interpreted as percent difference using (IRR – 1)*100. IRRs less than 1 indicate a percent decrease; IRRs greater than 1 indicate a percent increase. IRRs with confidence intervals including 1 indicate no significant relationship.
Person-level results.
Supplemental Tables 2a through 2e show the full models – including person-level and covariate associations – behind associations in Table 4. Neither person-means of morning report drinks (IRR=0.82, 95% CI: 0.64, 1.06) nor episodic EMA drinks (IRR=1.06, 95% CI: 0.82, 1.36) significantly predicted alcohol-related consequences. Person-mean TAC features were almost never significantly associated with alcohol-related consequences.
Exploratory single-consequence models
Supplemental Tables 3a through 3e show tests of TAC features predicting single consequences. Only the six most prevalent consequences were tested because estimates for other consequences were unstable in at least one of the models. Day-level results showed that, as with the number of consequences, AUC and peak were the most consistent predictors of single consequences, each predicting hangover, being sick to your stomach, experiencing an argument, throwing up, and blacking out. Fall rate predicted the next most consistently, predicting hangover, sick to your stomach, argument, and intriguingly, blacking out. Rise rate and duration predicted fewer (hangover, sick to your stomach, throwing up). Very little evidence for single-consequence prediction was seen at the person-level for any of the features.
DISCUSSION
The current study tested TAC features – peak, rise rate, fall rate, duration, and AUC – as indicators of self-reported alcohol consumption and as predictors of alcohol-related consequences in a sample of regularly heavy drinking young adults. TAC features (a) correlate strongly with number of drinks collected via daily retrospective morning reports and moderately with number of drinks collected via episodic EMA self-reports and (b) predict alcohol-related consequences after adjusting for morning and episodic EMA self-reports. Our results support TAC features as valid measures of alcohol misuse and consequence risk in natural settings.
All TAC features showed significant correlations with self-reported number of drinks. Correlations within and between persons were of comparable magnitude. Correlations between TAC features and daily morning drink reports were suggestive of moderate to strong convergent validity (r=0.60-0.74) but correlations between TAC features and episodic EMA drink reports were suggestive of weaker convergent validity (r=0.30-0.49). Episodic EMA drink totals were higher than morning report drink totals, consistent with prior research showing that more intensive assessment self-report methods are associated with greater reported quantities (Leigh, 2000). Morning report drinks and episodic EMA drinks showed only moderate convergence at within- (r=0.57) and between-person levels (r=0.52). Difficulties in accurately estimating the number of drinks during drinking episodes, which may have arisen from distraction, fatigue, or alcohol intoxication, may have contributed to reductions in convergent validity relative to passively collected TAC features (Piasecki, 2019, Shiffman, 2009). Future research is needed testing whether and how much accuracy of consumption reports might diminish with increasing intoxication (Piasecki, 2019, Shiffman, 2009). However, episodic EMA drinks independently predicted alcohol-related consequences even when adjusted for TAC features. Although episodic drink reports show more modest correspondence with daily drink reports than TAC features, they may nonetheless offer unique prediction of alcohol-related consequences.
Of the five features we tested, peak TAC provided the greatest utility in predicting alcohol-related consequences. Peak performed similarly to AUC in its day-level associations with self-reported drinking, but showed slightly stronger effect sizes in predicting day-level alcohol-related consequences. That peak TAC showed the strongest prediction of alcohol-related consequences may be expected because although not in perfect agreement, higher peak TAC readings correspond with higher BACs, upon which impairment symptoms central to clinical descriptions of acute alcohol intoxication are based (Vonghia et al., 2008). Both AUC and peak showed the most consistent prediction of single consequences, and their odds ratios were of comparable magnitude. Rise and fall rate showed more modest predictive power for alcohol-related consequences. Recent research has begun to explore dynamics in estimated BAC (eBAC) collected from EMA self-reports, showing that faster eBAC rise is associated with more alcohol-related consequences the next day (Carpenter and Merrill, 2020). Our study adds corroborating evidence by demonstrating that faster TAC rise is associated with alcohol-related consequences, and adds a new dimension by showing that faster TAC fall is also associated with consequences. Because the rate of descending alcohol concentration is difficult to obtain via self-reports, this finding demonstrates unique potential of TAC sensors.
Associations of fall rate with self-reported drinking and consequences were in the opposite direction of our hypothesis that faster fall rates would signal more efficient alcohol elimination and fewer alcohol-related consequences as a result. Faster fall rates were instead associated with longer durations, more self-reported drinking, and greater risk for alcohol-related consequences. Intriguingly, fall rate showed a strong association with blacking out in single-consequence models. These results may be explained by evidence suggesting that higher rates of alcohol elimination have been seen at high blood alcohol concentrations in some, but not all, studies (Cederbaum, 2012). Such concentration-dependent elimination may have been indicated in our study, as larger AUCs and higher peaks tended to accompany faster fall rates and predict the same consequences. As such, fall rate may function as a proxy for these features. Faster fall rates at the person-level could suggest more efficient clearing of alcohol from the system, and could present a risk for alcohol dependence if combined with high consumption. Future research should test this.
Self-reported drinking, both from morning reports and episodic EMA, predicted day-level alcohol-related consequences even after adjusting for TAC features. TAC features may therefore be complements but not replacements of drinking self-reports. The inclusion of both sensors and self-reports in assessment protocols may enhance prediction of alcohol-related consequences. The unique associations between self-reported drinking and alcohol-related consequences may be driven by methodological factors such as imperfect TAC-sensor sensitivity and/or shared method variance with alcohol-related consequences (Del Boca and Darkes, 2003). Future research should investigate these issues.
Although TAC sensors were a strength of the current study, they may have led self-reports to be more accurate than they would have otherwise been – the “bogus pipeline” effect (Roese and Jamieson, 1993). TAC sensors may have changed the number of drinks reported in morning and episodic EMA data streams and inflated the associations we observed. We are confident that the number of drinks and their associations with alcohol-related consequences are commensurate with studies that do not include sensors. Classic experimental studies on memory have shown that participants’ quantity estimates are not improved by knowledge that they will be subject to validation (e.g., Greene, 1984) and recent studies show that self-reports do not differ when TAC sensors are versus are not worn (Neville et al., 2013, Simons et al., 2015). However, the possibility that TAC sensors influenced drink report accuracy cannot be removed. Future research might test this through random assignment of sensor wear.
The following limitations should be considered. First, the generalizability of our findings is limited to surrounding areas of other large universities throughout the rural United States. Additionally, results may not generalize to populations with more severe AUD risk who may differ in the amount, pacing, and duration of alcohol consumption. Future research using TAC sensors and EMA could elucidate differences in these consumption dynamics between populations and contribute to a more nuanced understanding of consequence risk among younger versus older as well as more versus less alcohol-dependent drinkers. Second, our alcohol use quantity and frequency measures at screening and baseline may have lumped drinkers of differing severity in the same category (e.g., 3-5 days a week) preventing more fine-grained parsing. Third, data were collected across a single week in participants’ daily lives. Data across multiple weeks will be needed to determine when drinking behavior and alcohol-related consequences are congruent with a person’s “true” average. Fourth, our study did not include young adults under legal drinking age. These young adults were excluded to avoid collecting objective drinking data among participants for whom alcohol consumption was illegal. Although we do not believe results will differ between underage and legal-aged drinkers, we believe it will be important to document these associations among both. Fifth, the SCRAM-CAM device showed imperfect sensitivity, missing ~15% of self-reported drinking days. These missed days tended to be lower-intensity drinking days, consistent with prior research (e.g., Barnett et al., 2014), and this missed detection occurred even though we used a research algorithm shown to improve sensitivity of detection for low—to-moderate drinking events (Roache et al., 2019). Sixth, the high interrelatedness of TAC features prevented us from being able to separate their unique associations with consequences in multiple-predictor models. On average, each feature shared 75% of its day-level variance with other features, creating multicollinearity and preventing efficient tests of independent feature associations (M tolerance=0.25, M variance inflation factor=5.97). We believe our results comparing effect sizes across separate models for each feature nonetheless provide useful evidence to the field. Seventh, Winsorizing daily episodic EMA totals at 15 may have underestimated consumption among the heaviest drinkers. As stated previously, this was done to facilitate comparison with retrospective morning reports (which had “15+” as the highest response option) and to remove the influence of rare and extreme values. To reduce the possibility of underestimation, future research might investigate the performance of higher maximum values in retrospective morning reports (e.g., “25+”) in correctly detecting heavy drinking events while continuing to minimize outlier influence. Research incorporating wearable sensors as a heavy drinking criterion may be particularly useful in this regard.
For research, our findings demonstrate that TAC features are indicative of drinking intensity and predictive of alcohol-related consequences independent of self-reported drinking. Our findings may also have implications for research protocols without TAC sensors by facilitating the development of novel self-report items tapping these features. Once developed, these could be used in large-scale studies for which cost considerations may make use of TAC sensors impractical or infeasible. For intervention, our findings suggest that TAC features could be useful in prevention and treatment protocols focused on alcohol-related harm reduction. For example, peak TAC could be emphasized as part of personalized feedback to provide insight to clients in motivational enhancement therapies (Barnett, 2015), and protective behavioral strategies could be dynamically implemented as strategies to prevent high peak levels and their attendant risks (Kilmer and Logan, 2012, Martens et al., 2007).
CONCLUSION
In a sample of 222 young adults, we derived and tested features from TAC sensor trajectories as indicators of self-reported drinking and predictors of alcohol-related consequences in natural settings. TAC features appear strongly associated with daily morning self-reported number of drinks, and more modestly associated with daily drink totals from EMA. TAC features are also independently predictive of alcohol-related consequences, even after adjusting for self-reported number of drinks (morning report and episodic EMA). Higher peaks and larger AUCs were most strongly associated with alcohol-related consequences, followed by faster rise and fall rates. The current study offers an initial blueprint for feature extraction in research with TAC sensors and supports the utility of TAC sensors in future studies of young-adult drinking.
Supplementary Material
ACKNOWLEDGEMENTS
This research was funded by a pilot mentoring and professional development award through P50DA039838 (National Institute on Drug Abuse, PI: Collins) and departmental funds awarded to Michael Russell. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Sarah Ackerman for her editorial assistance.
Sources of support:
This research was funded by a pilot mentoring and professional development award through P50DA039838 (National Institute on Drug Abuse, PI: Collins) and departmental funds awarded to Michael Russell. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest for this work.
REFERENCES
- Abma IL, Rovers M & Van Der Wees PJ 2016. Appraising convergent validity of patient-reported outcome measures in systematic reviews: Constructing hypotheses and interpreting outcomes. BMC Research Notes, 9, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson S 2016. Better options than self-report of consumption. Addiction, 111, 1727. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB & Monteiro MG 2001. The alcohol use disorders identification test: Guidelines for use in primary care, Department of Mental Health and Substance Dependence, World Health Organization. [Google Scholar]
- Barnett NP 2015. Alcohol sensors and their potential for improving clinical care. Addiction, 110, 1–3. [DOI] [PubMed] [Google Scholar]
- Barnett NP, Meade EB & Glynn TR 2014. Predictors of detection of alcohol use episodes using a transdermal alcohol sensor. Experimental and Clinical Psychopharmacology, 22, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R & Colby SM 2011. Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug and Alcohol Dependence, 118, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A, Jones K & Fairbrother M 2018. Understanding and misunderstanding group mean centering: A commentary on Kelley et al.’s dangerous practice. Quality & Quantity, 52, 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JC, Greenfield TK, Patterson D & Kerr WC 2014. Adjustments for drink size and ethanol content: New results from a self-report diary and transdermal sensor validation study. Alcoholism-Clinical and Experimental Research, 38, 3060–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo AJ, Prince MA & Pearson MR 2017. College-related alcohol beliefs and problematic alcohol consumption: Alcohol protective behavioral strategies as a mediator. Substance Use & Misuse, 52, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AS, Kim J & Wang J 2018. Wearable electrochemical alcohol biosensors. Current Opinion in Electrochemistry, 10, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RW & Merrill JE 2020. How much and how fast: Alcohol consumption patterns, drinking-episode affect, and next-day consequences in the daily life of underage heavy drinkers. Drug and Alcohol Dependence, 108407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI 2012. Alcohol metabolism. Clinics in Liver Disease, 16, 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp JD, Madden DR, Gonzalez Villasanti H, Giraldo LF, Passino KM, Reed MB & Fernandez Puentes I 2018. A system dynamic model of drinking events: Multi-level ecological approach. Systems Research and Behavioral Science, 35, 265–281. [Google Scholar]
- Clapp JD, Madden DR, Mooney DD & Dahlquist KE 2017. Examining the social ecology of a bar-crawl: An exploratory pilot study. PLoS one, 12, e0185238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronce JM, Bittinger JN, Liu J & Kilmer JR 2015. Electronic feedback in college student drinking prevention and intervention. Alcohol Research: Current Reviews, 36, 47–62. [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK & Darkes J 2003. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction, 98, 1–12. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang YY, Karns TE, Lake SL, Cates SE & Roache JD 2015. The potential clinical utility of transdermal alcohol monitoring data to estimate the number of alcoholic drinks consumed. Addictive Disorders & Their Treatment, 14, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers PHC & Marx BD 1996. Flexible smoothing with b-splines and penalties. Statistical Science, 11, 89–121. [Google Scholar]
- Esser MB, Kanny D, Brewer RD & Naimi TS 2012. Binge drinking intensity: A comparison of two measures. American Journal of Preventive Medicine, 42, 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE, Bresin K, Kang D, Rosen IG, Ariss T, Luczak SE, Barnett NP & Eckland NS 2018. A multimodal investigation of contextual effects on alcohol’s emotional rewards. Journal of Abnormal Psychology, 127, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE & Kang D 2019. Temporal dynamics of transdermal alcohol concentration measured via new-generation wrist-worn biosensor. Alcoholism-Clinical and Experimental Research, 43, 2060–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE, Rosen IG, Luczak SE & Venerable WJ 2019. Estimating the quantity and time course of alcohol consumption from transdermal alcohol sensor data: A combined laboratory-ambulatory study. Alcohol, 81, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RL 1984. Incidental learning of event frequency. Memory & Cognition, 12, 90–95. [DOI] [PubMed] [Google Scholar]
- Greenfield TK, Bond J & Kerr WC 2014. Biomonitoring for improving alcohol consumption surveys the new gold standard? Alcohol Research-Current Reviews, 36, 39–45. [PMC free article] [PubMed] [Google Scholar]
- Greenfield TK & Kerr WC 2008. Alcohol measurement methodology in epidemiology: Recent advances and opportunities. Addiction, 103, 1082–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker EL & Muthén B 2020. The fixed versus random effects debate and how it relates to centering in multilevel modeling. Psychological Methods, 25, 365–379. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Roache JD, Liang YY, Karns TE, Cates SE & Dougherty DM 2015. Accounting for sex-related differences in the estimation of breath alcohol concentrations using transdermal alcohol monitoring. Psychopharmacology, 232, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M & Wechsler H 2005. Magnitude of alcohol-related mortality and morbidity among US college students ages 18–24: Changes from 1998 to 2001. Annual Review of Public Health, 26, 259–279. [DOI] [PubMed] [Google Scholar]
- Hingson R, Zha W & Smyth D 2017. Magnitude and trends in heavy episodic drinking, alcohol-impaired driving, and alcohol-related mortality and overdose hospitalizations among emerging adults of college ages 18–24 in the United States, 1998–2014. Journal of Studies on Alcohol and Drugs, 78, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Zha W & Weitzman ER 2009. Magnitude of and trends in alcohol-related mortality and morbidity among US college students ages 18-24, 1998-2005. Journal of Studies on Alcohol and Drugs, Supplement, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder H 2006. Alcohol and the community: A systems approach to prevention, Cambridge, UK, Cambridge University Press. [Google Scholar]
- Kahler CW, Strong DR & Read JP 2005. Toward efficient and comprehensive measurement of the alcohol problems continuum in college students: The brief young adult alcohol consequences questionnaire. Alcoholism: Clinical and Experimental Research, 29, 1180–1189. [DOI] [PubMed] [Google Scholar]
- Karns-Wright TE, Dougherty DM, Hill-Kapturczak N, Mathias CW & Roache JD 2018. The correspondence between transdermal alcohol monitoring and daily self reported alcohol consumption. Addictive Behaviors, 85, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns-Wright TE, Roache JD, Hill-Kapturczak N, Liang YY, Mullen J & Dougherty DM 2017. Time delays in transdermal alcohol concentrations relative to breath alcohol concentrations. Alcohol and Alcoholism, 52, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmer JR & Logan DE 2012. Applying harm-reduction strategies on college campuses. In: Correia CJ, Murphy JG & Barnett NP (eds.) College student alcohol abuse: A guide to assessment, intervention, and prevention. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, Luczak S, Rosen G, Dougherty DM & Barnett NP 2013. Continuous objective monitoring of alcohol use: Twenty-first century measurement using transdermal sensors. Alcoholism-Clinical and Experimental Research, 37, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh BC 2000. Using daily reports to measure drinking and drinking patterns. Journal of Substance Abuse, 12, 51–65. [DOI] [PubMed] [Google Scholar]
- Martens MP, Cimini MD, Barr AR, Rivero EM, Vellis PA, Desemone GA & Horner KJ 2007. Implementing a screening and brief intervention for high-risk drinking in university-based health and mental health care settings: Reductions in alcohol use and correlates of success. Addictive Behaviors, 32, 2563–2572. [DOI] [PubMed] [Google Scholar]
- Mun EY, Li X, Businelle MS, Hébert ET, Tan Z, Barnett NP & Walters ST 2021. Ecological momentary assessment of alcohol consumption and its concordance with transdermal alcohol detection and timeline follow-back self-report among adults experiencing homelessness. Alcoholism: Clinical and Experimental Research, 45, 864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Nelson DE & Brewer RD 2010. The intensity of binge alcohol consumption among US adults. American Journal of Preventive Medicine, 38, 201–207. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health 2018. Accessed: November 15, 2019 from https://www.Datafiles.Samhsa.Gov/study-dataset/national-survey-drug-use-and-health-2018-nsduh-2018-ds0001-nid18758.
- Neville FG, Williams DJ, Goodall CA, Murer JS & Donnelly PD 2013. An experimental trial exploring the impact of continuous transdermal alcohol monitoring upon alcohol consumption in a cohort of male students. Plos One, 8, e67386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman T, Peacock A, Ferguson SG, Kuntsche E & Bruno R 2020. Combining transdermal and breath alcohol assessments, real-time drink logs and retrospective self-reports to measure alcohol consumption and intoxication across a multi-day music festival. Drug and Alcohol Review, Early View. [DOI] [PubMed] [Google Scholar]
- Northcote J & Livingston M 2011. Accuracy of self-reported drinking: Observational verification of ‘last occasion’ drink estimates of young adults. Alcohol and Alcoholism, 46, 709–713. [DOI] [PubMed] [Google Scholar]
- Nyman E & Palmlöv A 1936. The elimination of ethyl alcohol in sweat. Skandinavisches Archiv Für Physiologie, 74, 155–159. [Google Scholar]
- Patrick ME & Maggs JL 2011. College students’ evaluations of alcohol consequences as positive and negative. Addictive Behaviors, 36, 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Terry-Mcelrath YM, Evans-Polce RJ & Schulenberg JE 2020. Negative alcohol-related consequences experienced by young adults in the past 12 months: Differences by college attendance, living situation, binge drinking, and sex. Addictive Behaviors, 105, 106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MR 2013. Use of alcohol protective behavioral strategies among college students: A critical review. Clinical Psychology Review, 33, 1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MR, Kirouac M & Witkiewitz K 2016. Questioning the validity of the 4+/5+ binge or heavy drinking criterion in college and clinical populations. Addiction, 111, 1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM 2019. Assessment of alcohol use in the natural environment. Alcoholism-Clinical and Experimental Research, 43, 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team 2020. R: A language and environment for statistical computing, Vienna, Austria, R Foundation for Statistical Computing. [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y & Patra J 2009. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet, 373, 2223–2233. [DOI] [PubMed] [Google Scholar]
- Roache JD, Karns-Wright TE, Goros M, Hill-Kapturczak N, Mathias CW & Dougherty DM 2019. Processing transdermal alcohol concentration (TAC) data to detect low-level drinking. Alcohol, 81, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roese NJ & Jamieson DW 1993. Twenty years of bogus pipeline research: A critical review and meta-analysis. Psychological Bulletin, 114, 363–375. [Google Scholar]
- Russell MA, Linden-Carmichael AN, Lanza ST, Fair EV, Sher K & Piasecki TM 2020. Affect relative to day-level drinking initiation: Analyzing ecological momentary assessment data with multilevel spline modeling. Psychology of Addictive Behaviors, 34, 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Long RJ & Crowley TJ 2006. Validity of transdermal alcohol monitoring: Fixed and self-regulated dosing. Alcoholism-Clinical and Experimental Research, 30, 26–33. [DOI] [PubMed] [Google Scholar]
- Schulenberg J, Johnston LD, O’malley PM, Bachman JG, Miech RA & Patrick ME 2019. Monitoring the future national survey results on drug use, 1975–2018, volume II: College students and adults ages 19-60, Ann Arbor, MI, Institute for Social Research, The University of Michigan. Available at http://monitoringthefuture.org/pubs.html#monographs. [Google Scholar]
- Shiffman S 2009. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment, 21, 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Wills TA, Emery NN & Marks RM 2015. Quantifying alcohol consumption: Self-report, transdermal assessment, and prediction of dependence symptoms. Addictive Behaviors, 50, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift R 2000. Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcoholism-Clinical and Experimental Research, 24, 422–423. [PubMed] [Google Scholar]
- Swift R 2003. Direct measurement of alcohol and its metabolites. Addiction, 98, 73–80. [DOI] [PubMed] [Google Scholar]
- Van Egmond K, Jc Wright C, Livingston M & Kuntsche E 2020. Wearable transdermal alcohol monitors: A systematic review of detection validity, relationship between transdermal and breath alcohol concentration and influencing factors. Alcoholism: Clinical and Experimental Research, 44, 1918–1932. [DOI] [PubMed] [Google Scholar]
- Vonghia L, Leggio L, Ferrulli A, Bertini M, Gasbarrini G, Addolorato G & Group ATS 2008. Acute alcohol intoxication. European Journal of Internal Medicine, 19, 561–567. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fridberg DJ, Shortell DD, Leeman RF, Barnett NP, Cook RL & Porges EC 2021. Wrist-worn alcohol biosensors: Applications and usability in behavioral research. Alcohol, 92, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G 2004. A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology, 159, 702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


