Abstract
Despite tremendous success against hematological malignancies, the performance of chimeric antigen receptor (CAR) T-cells against solid tumors remains poor. In such settings, the lack of success of this groundbreaking immunotherapy is in part mediated by ligand engagement of immune checkpoint molecules on the surface of T-cells in the tumor microenvironment (TME). Though CTLA-4 and PD-1 are well-established checkpoints that inhibit T-cell activity, the engagement of glycans and glycan-binding proteins are a growing area of interest due to their immunomodulatory effects. This review discusses exemplary strategies to neutralize checkpoint molecules through an in-depth overview of genetic engineering approaches aimed at overcoming the inhibitory program death ligand-1 (PD-L1)/program death-1 (PD-1) axis in T-cell therapies and summarizes current knowledge on glyco-immune interactions that mediate T-cell immunosuppression.
Introduction
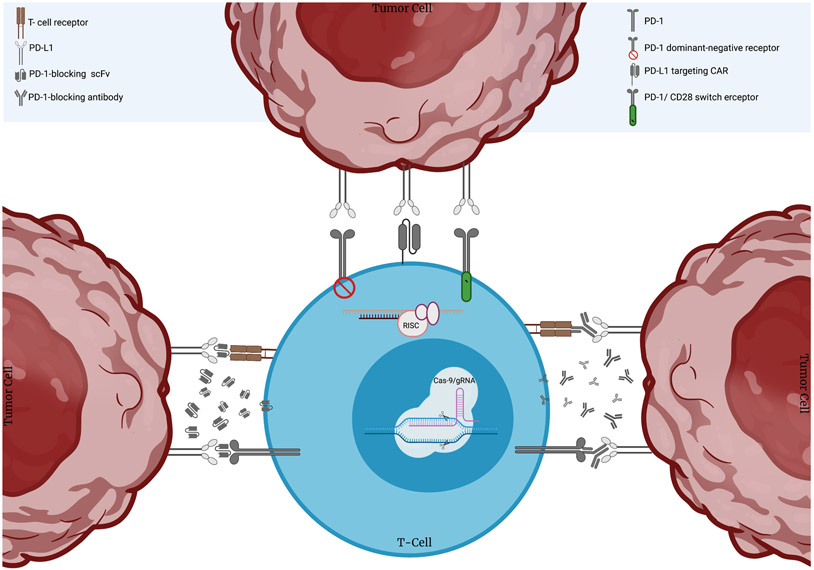
CAR T-cells are often met with an immunosuppressive milieu that contributes to their subpar performance in solid tumors. The establishment of this immunosuppressive microenvironment is partially due to ligands on tumor cells that engage their cognate inhibitory receptors upregulated on the surface of activated T-cells. Such inhibitory receptors, or checkpoint molecules, are the intrinsic brakes of the immune system that protect against autoimmunity under homeostatic conditions. Consequently, treatment with checkpoint inhibitors has revolutionized the treatment of different hematological and solid tumors (1). Indeed, the success of FDA-approved monoclonal antibodies targeting the PD-1/PD-L1 axis in some settings has prompted the investigation of PD-1/PD-L1 blockade combined with the killing prowess of CAR T-cells (2). However, this approach is limited by immune-related adverse effects resulting from the systemic administration of the inhibitors, as well as the high cost of such combination therapies (3-5). To this end, several genetic engineering strategies have been employed to overcome checkpoint-mediated inhibition and render the TME immune-permissive to CAR T-cell performance (Figure 1).
Figure 1. Engineering strategies to target the PD-1/PD-L1 axis and enhance CAR T-cell performance.
Researchers have interfered with PD-1 expression via shRNA or siRNA-mediated knockdown or CRISPR/Cas-9-mediated knockout. CAR T-cells have also been engineered to secrete full length antibodies or single chain variable fragments (scFvs) that block the binding of PD-1 with its cognate ligand PD-L1. Alternatively, groups have generated PD-L1-targeting CAR T-cells as well as CAR T-cells co-expressing PD-1 dominant-negative receptors with no intracellular signaling domains, or PD-1 switch receptors containing the intracellular domain of the costimulatory molecule CD28.
Genetic engineering approaches targeting the PD-L1/PD-1 axis
Many groups have sought to overcome the effects of the PD-1/PD-L1 pathway by PD-1 knockdown using gene silencing technologies such as shRNA and siRNA (6, 7). However, discrepancies on whether this approach enhances effector functions suggests that further research on the effect of PD-1 silencing may be warranted. Furthermore, compensatory mechanisms due to other inhibitory receptors expressed on tumor-infiltrating T-cells render knockdown of more than one checkpoint molecule a promising approach that has been explored by some groups. Accordingly, Simon et al. have shown that dual downregulation of PD-1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) improved the cytotoxicity of CAR T-cells in vitro relative to targeting each checkpoint alone (8), and shRNA-mediated downregulation of PD-1 and T-cell immunoreceptor with Ig and ITIM domains (TIGIT) has been found to yield synergistically beneficial effects on the performance of CD19-targeting CAR T-cells (Y-H. Lee, H. J. Lee, H. C. Kim, Y. Lee, S. K. Nam, C. Hupperetz, J. S. Y. Ma, X. Wang, O. Singer, W. S. Kim, et al., manuscript posted on bioRxiv, DOI: 10.1101/2020.11.07.372334). Another method to overcome PD-1 inhibition is to permanently knockout PD-1 using CRISPR/Cas9, which has enhanced the anti-tumor activity of CAR T-cells in vitro and in vivo across different solid tumor models (9, 10). However, additional studies will be imperative to further elucidate the effect of permanent PD-1 knockout on the long-term survival and toxicity of the engineered CAR T-cells since PD-1-deficiency may hinder inhibition of endogenous auto-reactive T-cell receptors.
An alternative approach to circumvent the potential downsides of PD-1 knockdown or knockout are “armored” CAR T-cells that release factors able to enhance antitumor performance. Though Suarez et al. showed the ability of PD-L1-secreting anti-carbonic anhydrase CAR T-cells to improve anti-tumor activity in vitro and in vivo (11), a greater focus has been placed on engineering of CAR T-cells to instead secrete single-chain variable fragment (scFv) forms of anti-PD-1, as their smaller size and reduced stability compared to full-length antibodies serves to promote localization in the TME and prevent systemic distribution. Several studies have collectively established the ability of scFv secretion by armored CAR T-cells to mediate an immune-permissive environment through blockade and downregulation of PD-1 in the milieu of tumor cells expressing the CAR target antigen (12-16).
Yet another promising strategy is the alteration of the PD-1 receptor itself. In one example, engineered T-cells that co-express a mesothelin-targeting CAR and a PD-1 dominant-negative receptor were more efficacious in vitro and mediated greater tumor control in vivo compared to T-cells expressing only the mesothelin-targeting CAR (17). In lieu of solely abrogating PD-1 signaling, PD-1 chimeric switch receptors have also been designed to provide the CD28 costimulatory signal to CAR T-cells upon PD-L1 ligand engagement (18). These receptors have resulted in enhanced antitumor effects of CAR T-cells in vitro and in vivo, which was dependent on the CD28 signaling domain. Alternatively, to render T-cell activation dependent on tumor expression of PD-L1, Qin et al. designed CARs consisting of the extracellular and transmembrane domains of PD-1 (19). This study also assessed the efficacy of T-cells expressing an anti-PD-L1 scFv-based CAR, and although T-cells bearing both constructs showed enhanced anti-tumor activity, anti-PD-L1 scFv-based CAR T-cells provided a greater in vitro and in vivo advantage. These findings hint to the potential benefit of PD-L1-targeting CAR T-cells against solid tumors; however, PD-L1 expression on normal tissues and immune cells – activated T-cells included – suggests a need to assess the safety of these CAR T-cells and ways of enhancing their safety.
New studies are warranted to directly compare the safety and efficacy of the aforementioned genetic engineering strategies and evaluate which approach is the most potent way of abrogating the effects of PD-1/PD-L1 signaling. Furthermore, despite the pre-clinical success shown by these innovative works, tumor progression or relapse observed in some studies reflect the reality that many patients have primary or acquired resistance to PD-1/PD-L1 blockade (20). This suggests that other inhibitory mechanisms are at play, and that the interplay between different checkpoint receptors merits further investigation. Thus, even as the PD-1/PD-L1 axis remains an active area of research, an increased focus on alternative pathways driving T-cell suppression and tumor escape could further help improve the performance of CAR T-cells against solid tumors.
Glyco-Immune Checkpoints
Glycosylation, or the conjugation of carbohydrates to other essential macromolecules, is a key post-translational modification resulting in diverse cell surface glycan structures that regulate many biological processes (21). It is well established that cancer cells are aberrantly glycosylated due to the changes in glycan synthesis pathways-- a hallmark implicated in their proliferation, metastasis, and other tumor-promoting processes. Such tumor-associated glycans commonly mediate their effects by engaging glycan-binding proteins, or lectins, that typically contain one or more carbohydrate-recognition domains. Accordingly, mechanisms by which tumor-specific glycan signatures mediate tumor cell recognition by the immune system have garnered increased attention (22), resulting in growing evidence of lectins modulating anti-tumor innate and adaptive immune responses – including various Sialic-acid binding immunoglobulin-type lectins (Siglecs), galectins, and C-type lectins. The engagement by such lectins of their glycan ligands in immune cells constitute glyco-immune checkpoints and present the potential to develop novel or improved cancer immunotherapeutic modalities, including T-cell therapies.
The immunomodulatory sialoglycan-Siglec axis
Cell surface glycans are often modified with terminal sialic acids that mediate many aspects of cell-cell interaction. In this sense, sialoglycans act as self-associated molecular patterns (23), and tumor cells commonly mask themselves by hypersialylating their surface glycans. Sialoglycans are ligands for Siglecs, which are implicated in tumor cell immunoevasion. These pattern recognition receptors are generally divided into the evolutionary conserved Siglecs and the rapidly evolved CD33-related Siglecs, differing in the sialic acid ligands they recognize and their expression profiles across immune cells whose activities they can inhibit or promote (22). Most Siglec receptors contain intracellular immunotyrosine inhibitory motif (ITIM) domains, which recruit and signal through SHP1 and SHP2, and negatively regulate immune cell activation, proliferation and survival. As such, the sialoglycans overexpressed by tumor cells engage these inhibitory Siglecs, often culminating in the dampening of anti-tumor immune responses.
Various studies have identified sialoglycan ligands for CD33-related Siglecs that mediate tumor cell escape from surveillance and elimination by the immune system (23-29). Though Siglec-9 expression on myeloid and natural killer (NK) cells had previously been implicated in immune modulation (24-26), consistent and prominent expression of this receptor on tumor-infiltrating lymphocytes has also been found in primary samples of non-small cell lung cancer (23). Sialoglycan interaction with Siglec-9 negatively impacts T-cell activation in vitro, while in vivo studies further implicate human Siglec-9 expression in accelerated tumor growth and worse overall survival. A CRISPR inference-based genomic screening approach identified a specific glycoform of CD43 expressed on K562 leukemia cells as the primary ligand for Siglec-7, establishing a novel axis with therapeutic potential (30). As Siglecs continue to emerge as targets to boost the antitumor immune response (31), the identification of ligands that engage these receptors on T-cells and other immune cells will be imperative. Such findings could allow the development of potent engineering strategies targeting the sialoglycan-Siglec axis, particularly within the context of CAR T-cell therapy against solid tumors. Meril et al. designed second generation CAR T-cells based on the extracellular region of the Siglec-7 and Siglec-9 proteins (32). These engineered cells showed anti-tumor activity against hypersialylated tumor targets in vitro and extended the survival of tumor-bearing mice by delaying tumor growth.
Other groups have proposed alternative ways of interfering with Siglec-sialoglycan interactions, including pharmacologically inhibiting sialic acid expression using a sialic acid glycomimetic (33). More recently, Gray et al. demonstrated that an anti-HER-2-sialidase conjugate selectively desialylated breast cancer cells specifically within the TME in vivo (34). This strategy hints at the potential of armored CAR T-cells secreting sialidase or a sialic acid-blocking glycomimetic for the treatment of solid tumors. Such antibody-targeted approaches have the potential to minimize systemic exposure to therapeutic strategies against sialoglycans, as sialoglycan expression on normal tissue may present concerns for off-target activity. Thus, Siglec-based CAR T-cells, as engineered by Meril et al., will require additional assays to assess the risk of toxicity to normal tissues that may express ligands for Siglec-7 and Siglec-9.
Galectins
A broadly-expressed class of lectins, galectins – previously known as “S-type lectins” due to their dependence on disulfide bonds – specifically bind to β-galactoside carbohydrates and play a valuable role in modulating the TME by regulating the innate and adaptive immune systems (35). Moreover, galectins can positively and negatively regulate T cell death. Of the 11 galectins identified in humans, galectin-1, galectin-3, and galectin-9 have been subject to extensive investigation as it pertains to tumor progression and immune escape (36).
Galectin-1, −3, and −9 can all regulate T-cell death, both intra- and extracellularly. Extracellular galectin-1 and galectin-3 directly induce death of both T-cells and thymocytes. However, galectin-3 possesses both pro- and anti-apoptotic activity as intracellular galectin-3 can also suppress apoptosis (36). Although both galectin-1 and galectin-3 can induce events that lead to cell death, the mechanisms by which each perform its tasks differ in various ways. Galectin-3 binds to a complement of T-cell surface glycoprotein receptors, including CD71, that differ from those recognized by galectin-1. T-cell apoptosis mediated by galectin-1 requires CD7 but not CD45 which contrasts with galectin-3, despite previous work implicating a role for CD7 in galectin-3 induced T-cell death. Lastly, thymocyte subsets vary in susceptibility to galectin-1 and −3 induced cell death. Galectin-1 can kill both double-negative and double-positive human thymocytes, while galectin-3 preferentially induces cell death in double-negative thymocytes.
Galectin-1 is involved in the apoptosis of various activated immune cells – CD8+ T-cells included, the secretion of anti-inflammatory factors such as IL-10 and TGF-β, and maintenance and activity of CD8+CD122+PD-1+ regulatory T-cells (Tregs) (37). In the azoxymethane-dextran sodium sulfate model of colitis-associated colorectal cancer, mice lacking galectin-1 developed fewer tumors and had decreased frequency of such Tregs, suggesting that the regulatory activity of galectin-1 is associated, at least in part, with these cells. Of mouse and human CD4 helper T-cell subsets, galectin-1 selectively induces apoptosis of pro-inflammatory Th1 and Th17 cells, but not naïve, Th2, and regulatory FoxP3+ T-cells (38). This susceptibility to galectin-1-induced apoptosis is associated with decreased N-acetylneuraminic acid α2,6-galactose residues on the surface of Th1 and Th17 cells as well as decreased expression of beta-galactose α2,6-sialyltransferase ST6Gal1. Interestingly, ST6Gal1 expression in CD4+ T-cells is also associated with self-renewing properties and is required for optimal expression of the stemness-associated transcription factor TCF1 (39).
The engagement of galectin-3 and PD-1 leads to tumor-induced immune suppression, and both PD-L1 and galectin-3 have been implicated in M2-macrophage polarization and reduced CD8+ T-cell recruitment to the tumor site (40). Blockade of galectin-3 enhanced the antitumor efficacy of checkpoint inhibitors and T-cell agonists by restoring the function of tumor-reactive T-cells, including restored cytokine production and cytolytic activity (40). This finding was further supported by another study from Vuong et al. showing that treatment with anti-PD-L1 antibody or galectin-3 inhibitor GB1107 alone could not reduce tumor size in mice with NSCLC, whereas combination treatment produced a significant decrease in tumor growth (41). In humans, there is a correlation between patients’ responses to anti PD-1 immunotherapy and galectin-3 expression, which identifies galectin-3 as a potential marker for tumor responsiveness (42). Findings from a pilot study by Capalbo et al. indicate early and dramatic tumor progression for patients with PD-L1+ non-small cell lung carcinoma (NSCLC) and concomitant high expression of galectin-3 treated with pembrolizumab, whereas patients with low-intermediate or negative expression of galectin-3 showed early and durable response.
In addition to galectin-1 and galectin-3, galectin-9 has garnered increased interest due to its involvement in many aspects of tumor cell biology and ability to modulate the immune system. Galectin-9 plays a pivotal role in the regulation of immune suppressive features of gliomas, and patients with high galectin-9 expression were shown to be more susceptible to the development of malignant tumors (43, 44). Galectin-9 recognizes the N-linked glycan chains present within the T-cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) IgV domain, a higher affinity interaction compared to galectin-1 and galectin-3 (45). Tim-3 is a co-inhibitory receptor expressed on IFN-γ-producing T-cells and interaction between Tim-3 and galectin-9 downregulates Th1 immunity (45, 46).
Recent work by Yang et al. has shown that galectin-9 binds to PD-1, an interaction that is highly selective, mediated by glycans, and does not disrupt PD-L1 binding to PD-1 (47). In addition, PD-1 expression desensitizes T-cells to cell death mediated by the interaction of galectin-9 with Tim-3, and galectin-9 expression and secretion is regulated by interferons. Considering that co-expression of PD-1 and Tim-3 indicates the functional exhaustion of CD8+ T-cells, these findings could be leveraged to improve the persistence of CAR T-cell therapy in the TME. In addition, and since galectin-9 is upregulated by IFN signaling similar to IFN-mediated upregulation of PD-L1, targeting IFN-induced galectin-9 expression and secretion may be a promising strategy. Lastly, galectin-9 has an immunosuppressive role in pancreatic ductal adenocarcinoma (PDA). In PDA cell lines HPAFII and CFPAC, which are resistant to tMUC1-CAR T cell therapy treatment, qPCR analysis revealed the overexpression of galectin-9 (48). Targeting galectin-9 with a blocking antibody reduced resistance of these PDA cell lines to tMUC1-CAR T cell therapy, illustrating the immunosuppressive role galectin-9 plays and its potential in the future development of T-cell therapy.
C-type Lectins
Another promising group of immunotherapeutic targets are the C-type lectins, a superfamily of over 1,000 proteins defined by the presence of one or more C-type lectin-like domains (49). Many reports have identified specific axes with immunomodulatory effects, findings that could potentially be exploited to improve CAR T-cell function.
Ligation of galectin-9 by dectin-1, mostly expressed on the surface of macrophages and other myeloid cells, has been implicated in producing tolerogenic macrophages that lead to adaptive immune suppression and disease progression (50, 51). Blockade of this axis or dectin-1 deletion increased the anti-tumor activity of T-cells in vivo (51). In addition, CD8+ T-cells from dectin-1-deficient mice show significantly decreased PD-1 induction (52). Towards therapeutics, an exosome-based dual delivery system containing surface oxaliplatin prodrug and loaded with siRNA targeting the galectin-9/Dectin-1 axis reversed tumor-associated macrophage (TAM)-mediated immunosuppression, downregulated regulatory T-cells, and promoted the recruitment of cytotoxic immune cells (53).
NKG2 proteins are another group of C-type lectins expressed on NK cells that most notably dimerize with CD94 on the cell surface (54, 55). With regards to cancer, two of the more well-studied members of this family are the inhibitory NKG2A and activating NKG2D receptors, the latter of which is also expressed on CD8+ T-cells among other cells. Consequently, blockade of the NKG2A-CD94 axis has been found to stimulate CD8+ T-cells and NK cells (55). Some groups have alternatively sought to interfere with NKG2A expression via shRNA and lentiviral transduction of protein expression blockers (56, 57). NKG2D ligand binding is normally an activating signal for NK and CD8+ T-cells, when ligands are expressed on the cell surface. However, continuous stimulation of NKG2D has been shown to be detrimental to NK and T-cell immune function (58, 59), further exacerbated by ligation from soluble ligands secreted by tumors, which bind NKG2D, but do not activate receptor-mediated signaling. This is a strategy employed by many different tumor types (58, 60-64) contributing to the immunosuppressive TME. One approach to enhance NKG2D-mediated elimination of tumors is a fusion protein comprised of the NKG2D ligand MICA and an anti-CD20 single chain variable fragment; this MICA-scFv recombinant protein ligated MICA on the surface of CD20+ leukemia cells, which activated NKG2D+ NK cells to induce apoptosis (65). Targeting these soluble ligands via antibody blockade can abrogate their immunosuppressive effects and enhance CD8+ T-cell effector functions, especially when used in concert with other therapies such as PD-1/PD-L1 blockade (66), suggesting this approach could be used to improve CAR T-cell cytotoxicity.
The macrophage galactose-type lectin (MGL) is most commonly expressed on dendritic cells and macrophages and binds GalNAc residues, galactose, O-linked Tn-antigen and TF-antigen (67, 68). Tn antigen engagement by MGL results in the polarization of tolerogenic dendritic cells and immunosuppressive macrophages (69, 70). In addition, MGL binding of CD45 on effector T-cells suppresses activation and leads to apoptosis (71). In a model of lung cancer, Tn antigen expression on tumor cells engaging MGL2 (mouse homolog for human MGL) on antigen presenting cells mediated recruitment of IL-10 secreting T-cells and an immunosuppressive milieu (72). Similarly, in a mouse model of glioma, Tn+ glioma tumors influenced local recruitment of PD-L1+CCR2+ tumor-associated macrophages as well as an expansion of these cells in the bone marrow (70), suggesting that the existence of a MGL/Tn antigen immunosuppressive checkpoint axis.
Dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) is another C-type lectin also expressed on APCs (73, 74), and its interaction with Lewis X antigens on tumor cells leads to immune suppression through various mechanisms driven by TAMs, including increased PD-L1 expression (75, 76). Importantly, blockade of DC-SIGN can abrogate immunosuppressive activity from TAMs and increase anti-tumor activity of CD8+ T-cells, while working synergistically with PD-1 immunotherapies in vitro (76).
The P-selectin glycoprotein ligand-1 (PSGL-1) is most widely known for its interactions with selectins and role in cellular migration (77); additional evidence points to its ability to also hinder T-cell activity by interfering with IL-2 and IL-7 signaling while increasing IL-10 production (78-80). In melanoma models marked by T-cell dysfunction, a deficiency in PSGL-1 improved T-cell response and tumor control (80, 81). Additionally, PSGL-1 can bind to V-domain immunoglobulin suppressor of T cell activation (VISTA) to mediate T-cell suppression in acidic environments, characteristic of many TMEs (82). Consequently, blockade with antibodies specific to this PSGL-1/VISTA axis reversed immunosuppression in vivo, and ongoing clinical trials are assessing the blockade of VISTA. In light of these findings, PSGL-1 could also be a promising therapeutic target to overcome T-cell suppression (83, 84).
E-selectin receptor is expressed on vascular endothelial cells and is known to be one of the key players in the processes of cell adherence and homing of cells circulating throughout the body. (85, 86). Ligation of E-selectin by its associated tetra-saccharide ligand sialyl-Lewis X, expressed on many circulating cells, leads to their adherence and infiltration (86). Mondal et al. utilize ex vivo fucosylation of CAR T-cells as a glycoengineering strategy to increase sialyl-Lewis X expression and improve their homing to the bone marrow (87). The ability of immunotherapies, such as CAR T-cells, to induce tumor regression is highly contingent on whether or not the cells are able to sufficiently penetrate and accumulate in the tumor site itself (88, 89). Improved homing of CAR T-cells to targeted tissues may mean that a lower dose could achieve the same amount of infiltration, potentially combatting treatment-related toxicities that arise from large immunotherapy doses (87, 90).
Conclusion
Although CAR T-cell therapy has revolutionized the treatment of cancer, great focus remains on overcoming the immunosuppressive microenvironment that lessens their efficacy against solid tumors. This has given rise to innovative approaches aimed at reducing inhibitory effects stemming from the interaction of checkpoint receptors on T-cells with their cognate ligands, with many groups targeting the PD-L1/PD-1 axis. However, primary and secondary resistance to PD-L1/PD-1 blockade in the clinic signals the presence of alternative immunosuppressive mechanisms, such as the inhibition of immune cells driven by lectins engaged by tumor cell-surface glycans. Such inhibitory glycan-lectin interactions present new and exciting avenues to improve immunotherapeutic modalities, and existing engineering strategies aiming to disrupt the PD-L1/PD-1 axis could serve as a blueprint to target such glyco-immune checkpoints.
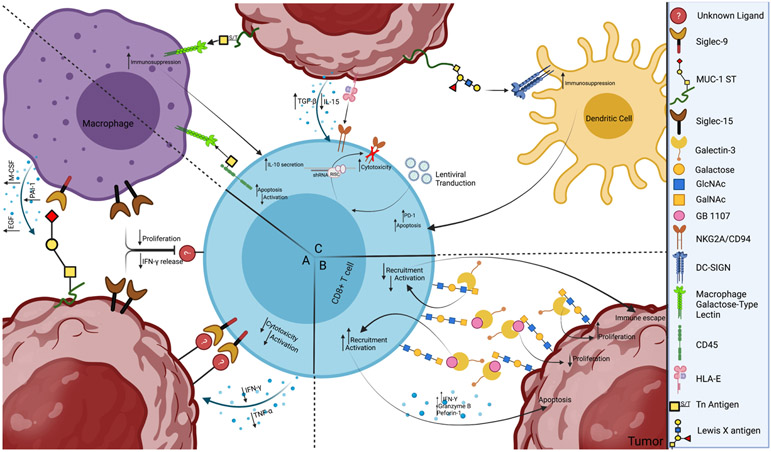
Figure 2. Aberrantly glycosylated tumor cells can engage lectins to inhibit the activity of CD8+ T-cells.
a) Examples of tumor-associated sialoglycan engagement of sialic acid-binding immunoglobulin-like lectins (Siglecs) to modulate the immune response. Specific axes that modulate myeloid and natural killer cells have been identified, such as the binding of MUC1 aberrantly glycosylated with short, sialylated O-glycans (MUC1-ST) to Siglec-9 expressed on macrophages to promote a tumor-associated macrophage-like phenotype. Although Siglec-9 upregulation on T-cells and Siglec-15 expression on macrophages and tumor cells have been implicated in the inhibition of CD8+ T-cells, the specific ligands that mediate their effects have not been identified. b.) Galectin-3 regulates proliferation and cytokine production by CD8+ T-cells. Galectin-3 binding to β-galactoside glycan structures, N-acetyllactosamine (LacNAc), causes an increase in tumor cell proliferation and immune escape. The galectin-3 inhibitor GB1107 reduced mouse and human lung adenocarcinoma growth and caused an increased expression of cytotoxic (IFNγ, granzyme B, perforin-1) and apoptotic effector molecules, recruitment and activation in CD8+ T-cells, and decreased tumor cell proliferation. c.) Examples of C-type Lectin modulation of adaptive immune function. Macrophage galactose-type lectin (MGL) interacts with terminal α-GalNAc residues (Tn antigen) on tumor cells to induce an immunosuppressive phenotype as well as on CD45 on effector T-cells to directly inhibit activity. NKG2A/CD94 recognizes HLA-E on tumor cells, leading to immunosuppression through increased TGF-β and decreased IL-15 secretion. This effect has been prevented through lentiviral transduction to produce shRNA against NKG2A transcripts, leading to increased NK and T-cell cytotoxicity. The interaction of DC-SIGN expressed on dendritic cells with Lewis X antigens on the tumor surface causes adaptive immunosuppression through many pathways, including increased PD-1 expression on T-cells, leading to apoptosis.
Acknowledgements
A.D.P. is supported by grant funding from the U.S. Department of Veterans Affairs (VA) (IK2 BX004183), V Foundation for Cancer Research, American Association for Cancer Research and Lustgarten Foundation, and Gabrielle’s Angel Foundation for Cancer Research.
Footnotes
Disclosures
A.D.P. is an inventor on patent applications relevant to the manuscript’s content. Dr. Posey also serves on the board of directors for GO Therapeutics and Stromatis Pharma and receives research support from Tmunity Therapeutics.
References
- 1.Wei SC, Anang NAS, Sharma R, Andrews MC, Reuben A, Levine JH, Cogdill AP, Mancuso JJ, Wargo JA, Pe'er D, and Allison JP. 2019. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A 116: 22699–22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosser R, Cherkassky L, Chintala N, and Adusumilli PS. 2019. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 36: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, and Schuster SJ. 2017. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129: 1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID, Brenner MK, and Brown MP. 2016. GD2-specific CAR T Cells Undergo Potent Activation and Deletion Following Antigen Encounter but can be Protected From Activation-induced Cell Death by PD-1 Blockade. Mol Ther 24: 1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Y, Boesteanu AC, Binder ZA, Xu C, Reid RA, Rodriguez JL, Cook DR, Thokala R, Blouch K, McGettigan-Croce B, Zhang L, Konradt C, Cogdill AP, Panjwani MK, Jiang S, Migliorini D, Dahmane N, Posey AD Jr., June CH, Mason NJ, Lin Z, O'Rourke DM, and Johnson LA. 2018. Checkpoint Blockade Reverses Anergy in IL-13Ralpha2 Humanized scFv-Based CAR T Cells to Treat Murine and Canine Gliomas. Mol Ther Oncolytics 11: 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J, Luo C, Wang Y, Guo Y, Dai H, Tong C, Ti D, Wu Z, and Han W. 2019. PD-1 silencing impairs the anti-tumor function of chimeric antigen receptor modified T cells by inhibiting proliferation activity. J Immunother Cancer 7: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou JE, Yu J, Wang Y, Wang H, Wang J, Wang Y, Yu L, and Yan Z. 2021. ShRNA-mediated silencing of PD-1 augments the efficacy of chimeric antigen receptor T cells on subcutaneous prostate and leukemia xenograft. Biomed Pharmacother 137: 111339. [DOI] [PubMed] [Google Scholar]
- 8.Simon B, Harrer DC, Schuler-Thurner B, Schaft N, Schuler G, Dorrie J, and Uslu U. 2018. The siRNA-mediated downregulation of PD-1 alone or simultaneously with CTLA-4 shows enhanced in vitro CAR-T-cell functionality for further clinical development towards the potential use in immunotherapy of melanoma. Exp Dermatol 27: 769–778. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Jiang H, Shi B, Zhou M, Zhang H, Shi Z, Du G, Luo H, Wu X, Wang Y, Sun R, and Li Z. 2018. Disruption of PD-1 Enhanced the Anti-tumor Activity of Chimeric Antigen Receptor T Cells Against Hepatocellular Carcinoma. Front Pharmacol 9: 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, and Wei F. 2019. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother 68: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez ER, Chang de K, Sun J, Sui J, Freeman GJ, Signoretti S, Zhu Q, and Marasco WA. 2016. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget 7: 34341–34355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ping Y, Li F, Nan S, Zhang D, Shi X, Shan J, and Zhang Y. 2020. Augmenting the Effectiveness of CAR-T Cells by Enhanced Self-Delivery of PD-1-Neutralizing scFv. Front Cell Dev Biol 8: 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou JT, Liu JH, Song TT, Ma B, Amidula N, and Bai C. 2020. EGLIF-CAR-T Cells Secreting PD-1 Blocking Antibodies Significantly Mediate the Elimination of Gastric Cancer. Cancer Manag Res 12: 8893–8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, Kim YJ, Mac J, Lu Z, Wang S, Han X, and Wang P. 2017. Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor-Modified T Cells Engineered to Secrete Checkpoint Inhibitors. Clin Cancer Res 23: 6982–6992. [DOI] [PubMed] [Google Scholar]
- 15.Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, Yan S, Xiang J, Ma X, Seshan VE, Hendrickson RC, Liu C, and Brentjens RJ. 2018. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 36: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima M, Sakoda Y, Adachi K, Nagano H, and Tamada K. 2019. Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR-T cells. Cancer Sci 110: 3079–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, and Adusumilli PS. 2016. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 126: 3130–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Lo A, June CH, Zhao Y, and Moon EK. 2016. A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second-Generation CAR T Cells in Advanced Solid Tumors. Cancer Res 76: 1578–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin L, Zhao R, Chen D, Wei X, Wu Q, Long Y, Jiang Z, Li Y, Wu H, Zhang X, Wu Y, Cui S, Wei W, Yao H, Liu Z, Cao S, Yao Y, Zhang Z, and Li P. 2020. Chimeric antigen receptor T cells targeting PD-L1 suppress tumor growth. Biomark Res 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, and Lo RS. 2016. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reily C, Stewart TJ, Renfrow MB, and Novak J. 2019. Glycosylation in health and disease. Nat Rev Nephrol 15: 346–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Kooyk Y, and Rabinovich GA. 2008. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol 9: 593–601. [DOI] [PubMed] [Google Scholar]
- 23.Stanczak MA, Siddiqui SS, Trefny MP, Thommen DS, Boligan KF, von Gunten S, Tzankov A, Tietze L, Lardinois D, Heinzelmann-Schwarz V, von Bergwelt-Baildon M, Zhang W, Lenz HJ, Han Y, Amos CI, Syedbasha M, Egli A, Stenner F, Speiser DE, Varki A, Zippelius A, and Laubli H. 2018. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Invest 128: 4912–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Demoulins T, Schneider C, Wehrli M, Hunger RE, Baerlocher GM, Simon HU, Romero P, Munz C, and von Gunten S. 2014. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest 124: 1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laubli H, Alisson-Silva F, Stanczak MA, Siddiqui SS, Deng L, Verhagen A, Varki N, and Varki A. 2014. Lectin galactoside-binding soluble 3 binding protein (LGALS3BP) is a tumor-associated immunomodulatory ligand for CD33-related Siglecs. J Biol Chem 289: 33481–33491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laubli H, Pearce OM, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, Deng L, Verhagen A, Secrest P, Lusk C, Schwartz AG, Varki NM, Bui JD, and Varki A. 2014. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci U S A 111: 14211–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ, and Weissman IL. 2019. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572: 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, Maher J, Noll T, Crocker PR, Taylor-Papadimitriou J, and Burchell JM. 2016. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol 17: 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida A, Ohta M, Toda M, Murata T, Usui T, Akita K, Inoue M, and Nakada H. 2008. Mucin-induced apoptosis of monocyte-derived dendritic cells during maturation. Proteomics 8: 3342–3349. [DOI] [PubMed] [Google Scholar]
- 30.Wisnovsky S, Mockl L, Malaker SA, Pedram K, Hess GT, Riley NM, Gray MA, Smith BAH, Bassik MC, Moerner WE, and Bertozzi CR. 2021. Genome-wide CRISPR screens reveal a specific ligand for the glycan-binding immune checkpoint receptor Siglec-7. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, Zhang J, Song C, Zarr M, Zhou X, Han X, Archer KA, O'Neill T, Herbst RS, Boto AN, Sanmamed MF, Langermann S, Rimm DL, and Chen L. 2019. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 25: 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meril S, Harush O, Reboh Y, Matikhina T, Barliya T, and Cohen CJ. 2020. Targeting glycosylated antigens on cancer cells using siglec-7/9-based CAR T-cells. Mol Carcinog 59: 713–723. [DOI] [PubMed] [Google Scholar]
- 33.Bull C, Boltje TJ, van Dinther EA, Peters T, de Graaf AM, Leusen JH, Kreutz M, Figdor CG, den Brok MH, and Adema GJ. 2015. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 9: 733–745. [DOI] [PubMed] [Google Scholar]
- 34.Gray MA, Stanczak MA, Mantuano NR, Xiao H, Pijnenborg JFA, Malaker SA, Miller CL, Weidenbacher PA, Tanzo JT, Ahn G, Woods EC, Laubli H, and Bertozzi CR. 2020. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat Chem Biol 16: 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modenutti CP, Capurro JIB, Di Lella S, and Marti MA. 2019. The Structural Biology of Galectin-Ligand Recognition: Current Advances in Modeling Tools, Protein Engineering, and Inhibitor Design. Front Chem 7: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, and Baum LG. 2006. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176: 778–789. [DOI] [PubMed] [Google Scholar]
- 37.Cagnoni AJ, Giribaldi ML, Blidner AG, Cutine AM, Gatto SG, Morales RM, Salatino M, Abba MC, Croci DO, Marino KV, and Rabinovich GA. 2021. Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8(+) regulatory T cells. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, and Rabinovich GA. 2007. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol 8: 825–834. [DOI] [PubMed] [Google Scholar]
- 39.Shin B, Kress RL, Kramer PA, Darley-Usmar VM, Bellis SL, and Harrington LE. 2018. Effector CD4 T cells with progenitor potential mediate chronic intestinal inflammation. J Exp Med 215: 1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curti BD, Koguchi Y, Leidner RS, Rolig AS, Sturgill ER, Sun Z, Wu Y, Rajamanickam V, Bernard B, Hilgart-Martiszus I, Fountain CB, Morris G, Iwamoto N, Shimada T, Chang S, Traber PG, Zomer E, Horton JR, Shlevin H, and Redmond WL. 2021. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J Immunother Cancer 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vuong L, Kouverianou E, Rooney CM, McHugh BJ, Howie SEM, Gregory CD, Forbes SJ, Henderson NC, Zetterberg FR, Nilsson UJ, Leffler H, Ford P, Pedersen A, Gravelle L, Tantawi S, Schambye H, Sethi T, and MacKinnon AC. 2019. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res 79: 1480–1492. [DOI] [PubMed] [Google Scholar]
- 42.Capalbo C, Scafetta G, Filetti M, Marchetti P, and Bartolazzi A. 2019. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy: The Galectin-3 Signature in NSCLCs. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan F, Ming H, Wang Y, Yang Y, Yi L, Li T, Ma H, Tong L, Zhang L, Liu P, Li J, Lin Y, Yu S, Ren B, and Yang X. 2020. Molecular and clinical characterization of Galectin-9 in glioma through 1,027 samples. J Cell Physiol 235: 4326–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang T, Wang X, Wang F, Feng E, and You G. 2019. Galectin-9: A Predictive Biomarker Negatively Regulating Immune Response in Glioma Patients. World Neurosurg 132: e455–e462. [DOI] [PubMed] [Google Scholar]
- 45.Das M, Zhu C, and Kuchroo VK. 2017. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev 276: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, and Kuchroo VK. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 47.Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, Yan M, Chang WC, Hsu JM, Cha JH, Hsu JL, Chou CW, Sun X, Deng Y, Chou CK, Yu D, and Hung MC. 2021. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun 12: 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yazdanifar M, Zhou R, Grover P, Williams C, Bose M, Moore LJ, Wu ST, Maher J, Dreau D, and Mukherjee AP. 2019. Overcoming Immunological Resistance Enhances the Efficacy of A Novel Anti-tMUC1-CAR T Cell Treatment against Pancreatic Ductal Adenocarcinoma. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown GD, Willment JA, and Whitehead L. 2018. C-type lectins in immunity and homeostasis. Nat Rev Immunol 18: 374–389. [DOI] [PubMed] [Google Scholar]
- 50.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, and Underhill DM. 2011. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature 472: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSB, Savadkar S, Torres-Hernandez A, Nayak S, Wang D, Hundeyin M, Diskin B, Aykut B, Werba G, Barilla RM, Rodriguez R, Chang S, Gardner L, Mahal LK, Ueberheide B, and Miller G. 2017. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med 23: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhaskaran N, Jayaraman S, Quigley C, Mamileti P, Ghannoum M, Weinberg A, Thuener J, Pan Q, and Pandiyan P. 2021. The Role of Dectin-1 Signaling in Altering Tumor Immune Microenvironment in the Context of Aging. Front Oncol 11: 669066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Zhang Y, Li C, Chu Y, Sun T, and Jiang C. 2021. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 268: 120546. [DOI] [PubMed] [Google Scholar]
- 54.Vazquez-Gonzalez WG, Martinez-Alvarez JC, Arrazola-Garcia A, and Perez-Rodriguez M. 2019. Haplotype block 1 variant (HB-1v) of the NKG2 family of receptors. Hum Immunol 80: 842–847. [DOI] [PubMed] [Google Scholar]
- 55.Creelan BC, and Antonia SJ. 2019. The NKG2A immune checkpoint - a new direction in cancer immunotherapy. Nat Rev Clin Oncol 16: 277–278. [DOI] [PubMed] [Google Scholar]
- 56.Kamiya T, Seow SV, Wong D, Robinson M, and Campana D. 2019. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest 129: 2094–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Figueiredo C, Seltsam A, and Blasczyk R. 2009. Permanent silencing of NKG2A expression for cell-based therapeutics. J Mol Med (Berl) 87: 199–210. [DOI] [PubMed] [Google Scholar]
- 58.Wang LP, Niu H, Xia YF, Han YL, Niu P, Wang HY, and Zhou QL. 2015. Prognostic significance of serum sMICA levels in non-small cell lung cancer. Eur Rev Med Pharmacol Sci 19: 2226–2230. [PubMed] [Google Scholar]
- 59.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, and Spies T. 2001. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol 2: 255–260. [DOI] [PubMed] [Google Scholar]
- 60.Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, Steinle A, Schadendorf D, and Ugurel S. 2009. Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res 15: 5208–5215. [DOI] [PubMed] [Google Scholar]
- 61.Luo Q, Luo W, Zhu Q, Huang H, Peng H, Liu R, Xie M, Li S, Li M, Hu X, and Zou Y. 2020. Tumor-Derived Soluble MICA Obstructs the NKG2D Pathway to Restrain NK Cytotoxicity. Aging Dis 11: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu G, Lu S, Wang X, Page ST, Higano CS, Plymate SR, Greenberg NM, Sun S, Li Z, and Wu JD. 2013. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Invest 123: 4410–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, and Dranoff G. 2008. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A 105: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, and Salih HR. 2012. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol 189: 1360–1371. [DOI] [PubMed] [Google Scholar]
- 65.Zou Y, Luo W, Guo J, Luo Q, Deng M, Lu Z, Fang Y, and Zhang CC. 2018. NK cell-mediated anti-leukemia cytotoxicity is enhanced using a NKG2D ligand MICA and anti-CD20 scfv chimeric protein. Eur J Immunol 48: 1750–1763. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Larrocha PS, Zhang B, Wainwright D, Dhar P, and Wu JD. 2019. Antibody targeting tumor-derived soluble NKG2D ligand sMIC provides dual co-stimulation of CD8 T cells and enables sMIC(+) tumors respond to PD1/PD-L1 blockade therapy. J Immunother Cancer 7: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh SK, Streng-Ouwehand I, Litjens M, Weelij DR, Garcia-Vallejo JJ, van Vliet SJ, Saeland E, and van Kooyk Y. 2009. Characterization of murine MGL1 and MGL2 C-type lectins: distinct glycan specificities and tumor binding properties. Mol Immunol 46: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 68.Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, Kindt A, Van den Bergh R, Leenen PJ, De Baetselier P, and Ghassabeh GH. 2005. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol 77: 321–327. [DOI] [PubMed] [Google Scholar]
- 69.van Vliet SJ, van Liempt E, Geijtenbeek TB, and van Kooyk Y. 2006. Differential regulation of C-type lectin expression on tolerogenic dendritic cell subsets. Immunobiology 211: 577–585. [DOI] [PubMed] [Google Scholar]
- 70.Dusoswa SA, Verhoeff J, Abels E, Mendez-Huergo SP, Croci DO, Kuijper LH, de Miguel E, Wouters V, Best MG, Rodriguez E, Cornelissen LAM, van Vliet SJ, Wesseling P, Breakefield XO, Noske DP, Wurdinger T, Broekman MLD, Rabinovich GA, van Kooyk Y, and Garcia-Vallejo JJ. 2020. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc Natl Acad Sci U S A 117: 3693–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Vliet SJ, Gringhuis SI, Geijtenbeek TB, and van Kooyk Y. 2006. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol 7: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 72.da Costa V, van Vliet SJ, Carasi P, Frigerio S, Garcia PA, Croci DO, Festari MF, Costa M, Landeira M, Rodriguez-Zraquia SA, Cagnoni AJ, Cutine AM, Rabinovich GA, Osinaga E, Marino KV, and Freire T. 2021. The Tn antigen promotes lung tumor growth by fostering immunosuppression and angiogenesis via interaction with Macrophage Galactose-type lectin 2 (MGL2). Cancer Lett 518: 72–81. [DOI] [PubMed] [Google Scholar]
- 73.Soilleux EJ, Barten R, and Trowsdale J. 2000. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol 165: 2937–2942. [DOI] [PubMed] [Google Scholar]
- 74.Granelli-Piperno A, Pritsker A, Pack M, Shimeliovich I, Arrighi JF, Park CG, Trumpfheller C, Piguet V, Moran TM, and Steinman RM. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol 175: 4265–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dominguez-Soto A, Sierra-Filardi E, Puig-Kroger A, Perez-Maceda B, Gomez-Aguado F, Corcuera MT, Sanchez-Mateos P, and Corbi AL. 2011. Dendritic cell-specific ICAM-3-grabbing nonintegrin expression on M2-polarized and tumor-associated macrophages is macrophage-CSF dependent and enhanced by tumor-derived IL-6 and IL-10. J Immunol 186: 2192–2200. [DOI] [PubMed] [Google Scholar]
- 76.Hu B, Wang Z, Zeng H, Qi Y, Chen Y, Wang T, Wang J, Chang Y, Bai Q, Xia Y, Wang Y, Liu L, Zhu Y, Dai B, Guo J, Xu L, Zhang W, and Xu J. 2020. Blockade of DC-SIGN(+) Tumor-Associated Macrophages Reactivates Antitumor Immunity and Improves Immunotherapy in Muscle-Invasive Bladder Cancer. Cancer Res 80: 1707–1719. [DOI] [PubMed] [Google Scholar]
- 77.Ley K, and Kansas GS. 2004. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol 4: 325–335. [DOI] [PubMed] [Google Scholar]
- 78.Nunez-Andrade N, Lamana A, Sancho D, Gisbert JP, Gonzalez-Amaro R, Sanchez-Madrid F, and Urzainqui A. 2011. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J Pathol 224: 212–221. [DOI] [PubMed] [Google Scholar]
- 79.Urzainqui A, Martinez del Hoyo G, Lamana A, de la Fuente H, Barreiro O, Olazabal IM, Martin P, Wild MK, Vestweber D, Gonzalez-Amaro R, and Sanchez-Madrid F. 2007. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol 179: 7457–7465. [DOI] [PubMed] [Google Scholar]
- 80.Tinoco R, Carrette F, Barraza ML, Otero DC, Magana J, Bosenberg MW, Swain SL, and Bradley LM. 2016. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity 44: 1470. [DOI] [PubMed] [Google Scholar]
- 81.Meeth K, Wang JX, Micevic G, Damsky W, and Bosenberg MW. 2016. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res 29: 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnston RJ, Su LJ, Pinckney J, Critton D, Boyer E, Krishnakumar A, Corbett M, Rankin AL, Dibella R, Campbell L, Martin GH, Lemar H, Cayton T, Huang RY, Deng X, Nayeem A, Chen H, Ergel B, Rizzo JM, Yamniuk AP, Dutta S, Ngo J, Shorts AO, Ramakrishnan R, Kozhich A, Holloway J, Fang H, Wang YK, Yang Z, Thiam K, Rakestraw G, Rajpal A, Sheppard P, Quigley M, Bahjat KS, and Korman AJ. 2019. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 574: 565–570. [DOI] [PubMed] [Google Scholar]
- 83.Tagliamento M, Bironzo P, and Novello S. 2020. New emerging targets in cancer immunotherapy: the role of VISTA. ESMO Open 4: e000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeRogatis JM, Viramontes KM, Neubert EN, and Tinoco R. 2021. PSGL-1 Immune Checkpoint Inhibition for CD4(+) T Cell Cancer Immunotherapy. Front Immunol 12: 636238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schweitzer KM, Drager AM, van der Valk P, Thijsen SF, Zevenbergen A, Theijsmeijer AP, van der Schoot CE, and Langenhuijsen MM. 1996. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol 148: 165–175. [PMC free article] [PubMed] [Google Scholar]
- 86.Tabe Y, and Konopleva M. 2014. Advances in understanding the leukaemia microenvironment. Br J Haematol 164: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mondal N, Silva M, Castano AP, Maus MV, and Sackstein R. 2019. Glycoengineering of chimeric antigen receptor (CAR) T-cells to enforce E-selectin binding. J Biol Chem 294: 18465–18474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, and Allen PM. 2000. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity 13: 265–276. [DOI] [PubMed] [Google Scholar]
- 89.Shrikant P, and Mescher MF. 1999. Control of syngeneic tumor growth by activation of CD8+ T cells: efficacy is limited by migration away from the site and induction of nonresponsiveness. J Immunol 162: 2858–2866. [PubMed] [Google Scholar]
- 90.Sackstein R 2018. The First Step in Adoptive Cell Immunotherapeutics: Assuring Cell Delivery via Glycoengineering. Front Immunol 9: 3084. [DOI] [PMC free article] [PubMed] [Google Scholar]