Abstract
Streptococcus pneumoniae is an important cause of community-acquired pneumonia. However, in this setting the diagnostic sensitivity of blood cultures is below 30%. Since during such infections changes in the amounts of S. pneumoniae may also occur in the upper respiratory tract, quantification of these bacteria in nasopharnygeal secretions (NPSs) may offer a suitable diagnostic approach. Real-time PCR offers a sensitive, efficient, and routinely reproducible approach to quantification. Using primers and a fluorescent probe specific for the pneumolysin gene, we were able to detect DNA from serial dilutions of S. pneumoniae cells in which the quantities of DNA ranged from the amounts extracted from 1 to 106 cells. No difference was noted when the same DNA was mixed with DNA extracted from NPSs shown to be deficient of S. pneumoniae following culture, suggesting that this bacterium can be detected and accurately quantitated in clinical samples. DNAs from Haemophilus influenzae, Moraxella catarrhalis, or alpha-hemolytic streptococci other than S. pneumoniae were not amplified or were only weakly amplified when there were ≥106 cells per reaction mixture. When the assay was applied to NPSs from patients with respiratory tract infections, the assay performed with a sensitivity of 100% and a specificity of up to 96% compared to the culture results. The numbers of S. pneumoniae organisms detected by real-time PCR correlated with the numbers detected by semiquantitative cultures. A real-time PCR that targeted the pneumolysin gene provided a sensitive and reliable means for routine rapid detection and quantification of S. pneumoniae present in NPSs. This assay may serve as a tool to study changes in the amounts of S. pneumoniae during lower respiratory tract infections.
Streptococcus pneumoniae is a human pathogen of major importance. It causes both mucosal and invasive diseases including otitis media, pneumonia, arthritis, septicemia, and meningitis (5, 17, 18). In community-acquired pneumonia (CAP), S. pneumoniae is the major bacterial agent (6). In the acute phase, diagnosis of S. pneumoniae-induced CAP is established mainly by detection of the pathogen in blood cultures and also, but rarely, in pleural fluid aspirates. However, bacteremia caused by S. pneumoniae occurs in less than 30% of CAP cases (1, 15). Although S. pneumoniae can be detected by microscopy and following culture of sputum, this diagnostic approach is not applicable in children because they do not expectorate. Thus, more appropriate diagnostic tools need to be developed to improve the etiologic diagnosis of CAP due to S. pneumoniae.
Nasopharyngeal secretions (NPSs) are readily available from children with respiratory tract infections (14). Since quantitative changes in bacterial load may reflect a lower respiratory tract infection with a given pathogen, quantification of bacteria in NPSs may be a candidate diagnostic approach. However, performance of quantitative bacterial cultures is cumbersome, time-consuming, and slow compared to many molecular diagnostic procedures.
One important challenge for the diagnostic detection of S. pneumoniae is the existence of 90 different S. pneumoniae serotypes (11). Thus, diagnostic assays ideally should target genes or their products common to all S. pneumoniae isolates. Pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin are virulence factors expressed by all serotypes of S. pneumoniae (17). For the diagnosis of CAP due to S. pneumoniae, detection of antibodies to pneumolysin in serum (3, 7), detection of pneumolysin antigen in sputum or NPSs (3), and detection of the pneumolysin gene in whole blood by conventional PCR (9) have been used. The aim of the present study was to develop a method for the rapid and quantitative detection of S. pneumoniae that has a high sensitivity and a high specificity and that is amenable to high-throughput sample processing. Here we describe a real-time PCR (TaqMan) assay with primers specific for the pneumolysin gene for the detection and quantification of S. pneumoniae. This diagnostic tool could be useful in future studies to define the correlation of S. pneumoniae numbers in the nose with carriage or infection in the respiratory tract.
MATERIALS AND METHODS
Bacterial strains.
S. pneumoniae strain ATCC 49619, obtained from the American Type Culture Collection (Manassas, Va.), was used as a reference. To test the specificity of the real-time PCR assay, we used alpha-hemolytic streptococci Streptococcus gordonii strain ATCC 12396 and Streptococcus oralis strain ATCC 10557 (both kindly provided by R. Gmür, Zurich, Switzerland) and, from our own collection, Streptococcus anginosus, Streptococcus constellatus, Streptococcus mitis, Streptococcus mutans, Streptococcus salivarius, and Streptococcus sanguis. Haemophilus influenzae type b and Moraxella catarrhalis were used as representatives of gram-negative bacteria associated with the upper respiratory tract.
Clinical samples.
Surplus samples from 195 NPSs collected from children with respiratory tract infections and sent to the Infectious Diseases Laboratory for a rapid test for respiratory syncytial virus were used for semiquantitative bacterial cultures and PCR amplification of the pneumolysin gene. Immediately after plating for cultures, the patient samples were stored at −20°C until DNA extraction was performed (see below).
Semiquantitative bacterial cultures.
All 195 samples of NPSs were tested by semiquantitative bacterial cultures. The samples were inoculated onto sheep blood agar, chocolate agar, and Columbia colistin-nalidixic acid agar by fractionation with a calibrated wire loop (5 μl), and the plates were incubated at 37°C in 5% CO2 for 48 h. Growth only in the first fraction was defined as low growth (+), growth also in the second fraction was defined as intermediate growth (++), and growth in all three fractions was defined as abundant growth (+++). Species identification was done by standard methods (13).
DNA extraction.
The extraction of DNA was performed as described previously (4). Briefly, 1 ml of a liquid culture or a patient NPS sample was centrifuged at 12,000 × g for 10 min. The pellet was resuspended in 200 μl of digestion buffer (50 mM Tris-HCl [pH 8.5], 1 mM EDTA, 0.5% sodium dodecyl sulfate, 200 mg of proteinase K per ml), and the suspension was incubated with shaking for 1 h at 55°C. The DNA was then purified with a QIAamp DNA Blood Mini kit (article 51106; QIAGEN, Basel, Switzerland) according to the instructions of the supplier, except that the elution step was done with 100 μl (instead of 200 μl) AE elution buffer. Extracts were stored at −70°C until they were required for analysis.
Real-time PCR.
The primers and the fluorogenic probe for the pneumolysin gene (20) of S. pneumoniae (GenBank accession no. M17717) were designed with Primer Express Software (Perkin-Elmer, Applied Biosystems, Foster City, Calif.) and were obtained from Microsynth GmbH (Balgach, Switzerland). The nucleotide sequence of the forward primer was 5′-AGCGATAGCTTTCTCCAAGTGG-3′ (positions 531 to 552), the sequence of the reverse primer was 5′-CTTAGCCAACAAATCGTTTACCG-3′ (positions 605 to 583), and the sequence of the probe was 5′-ACCCCAGCAATTCAAGTGTTCGCG-3′ (positions 556 to 580). The fluorescent reporter dye at the 5′ end of the probe was 6-carboxyfluorescein (FAM); the quencher at the 3′ end was 6-carboxy-N,N,N′,N′-tetramethylrhodamine (TAMRA). The principle of real-time PCR has been described extensively. Briefly, during real-time PCR, the fluorogenic probe and PCR amplimers first hybridize to their DNA targets. With the fluorogenic probe still intact, the emission of the reporter dye is quenched, but during the PCR extension phase the probe is cleaved by the 5′-exonuclease activity of the Taq DNA polymerase. This cleavage interrupts the fluorescence resonance energy transfer and permits the reporter dye to fluoresce, with the level of fluorescence produced being in proportion to the level of PCR product accumulation. Furthermore, the increment in the signal from the degraded fluorogenic probe can be continuously monitored throughout the course of gene amplification. The reporter signal is standardized to an internal passive reference dye, which is typically 5-carboxy-X-rhodamine. The background fluorescence is often determined from cycles 3 to 15, and an automated software feature calculates 10 times the standard deviation to produce a threshold value. The cycle threshold (CT) value is defined as the cycle at which the reporting dye fluorescence first exceeds the calculated background level. A low CT value thus corresponds to a high target concentration. The CT value can also be set manually to be equivalent across experiments. Each run contains both negative (no template) and positive controls.
The real-time PCR amplifications were performed in 25-μl reaction volumes containing 2× TaqMan Universal Master Mix (Perkin-Elmer Biosystems), which includes dUTP and uracil-N-glycosylase, each primer at a concentration of 333 nM, fluorescent labeled probe at a concentration of 200 nM, and 1 μl of DNA extract. All reactions were performed in duplicate, and for amplification and detection an ABI PRISM 7700 sequence detection system was used. Standard amplification parameters were used and were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles, each of which comprised 95°C for 15 s and 60°C for 1 min. In later experiments, annealing was produced at 62 or 65°C for 1 min. Real-time data were analyzed with Sequence Detection Systems software, version 1.7.
Sensitivity, detection range, and specificity.
To determine the sensitivity and the detection range of the real-time PCR assay, a standard curve for S. pneumoniae was generated as follows: S. pneumoniae was grown aerobically in 2 ml of Todd-Hewitt broth at 37°C for 4 h to reach the logarithmic phase. This culture was diluted with physiological saline until it reached a McFarland 0.5 standard, representing approximately 108 microorganisms/ml. Starting from this concentration, 10-fold serial dilutions in physiological saline were prepared, and the number of CFU was determined by plating 100 μl of each dilution onto sheep blood agar plates and then aerobic incubation overnight at 37°C. One milliliter of each dilution was used for DNA extraction, followed by in vitro amplification as described above. The calculated CT values were then plotted against the numbers of microorganisms.
To determine the specificity of the real-time PCR assay for S. pneumoniae, serial dilutions of liquid cultures of various strains of alpha-hemolytic streptococci, H. influenzae type b, and M. catarrhalis were tested in the same manner as described above for S. pneumoniae. Finally, the real-time PCR assay was applied to clinical samples, i.e., NPSs, and its sensitivity (including quantitation) and specificity were assessed by comparison of the results with those of the semiquantitative culture procedure.
Statistics.
The results for subpopulations within the NPS group that were culture positive and culture negative for S. pneumoniae were compared with those of the PCR assay by the chi-square test and the two-tailed Fisher's exact test. The Mann-Whitney test was used for comparison of mean ± standard deviation CT values for the groups. A P value of <0.05 was considered statistically significant.
RESULTS
Detection range of the real-time PCR assay for S. pneumoniae.
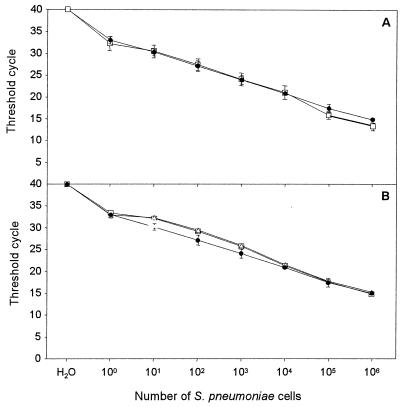
The real-time PCR assay with 10-fold serial dilutions of S. pneumoniae was able to detect bacterial DNA in mixtures in which the quantity of DNA was over a linear range of DNA from between 1 and 106 microorganisms per reaction mixture, with CT values ranging between 15 and 33 (Fig. 1A). The intra- and interassay variabilities with replicates from the same DNA extraction per dilution were 2.0 and 3.3%, respectively. To exclude PCR-inhibiting substances from the clinical samples, two 10-fold serial dilutions of liquid cultures of S. pneumoniae were spiked with DNA extracted from samples of NPSs shown to be negative for S. pneumoniae. The results were almost identical to those obtained with pure bacterial cultures (Fig. 1B).
FIG. 1.
Sensitivity and detection range of the real-time PCR assay for S. pneumoniae. (A) Reproducibility of the assay when testing dilution series of S. pneumoniae followed by DNA extraction in a single assay with replicates from the same DNA extraction (●) and in independent assays with the same DNA extraction (□). The intra- and interassay variabilities were 2.0 and 3.3%, respectively. (B) Absence of inhibition when spiking genomic DNA from S. pneumoniae with DNA from NPS samples shown to be negative for S. pneumoniae. Threshold cycle is the cycle number when the threshold fluorescence is reached. The standard deviations from three measurements are shown in error bars. ●, S. pneumonia DNA alone; ▵, S. pneumonia DNA spiked with DNA from NPS sample 1; □, S. pneumoniae DNA spiked with DNA from NPS sample 2.
Specificity of the real-time PCR assay.
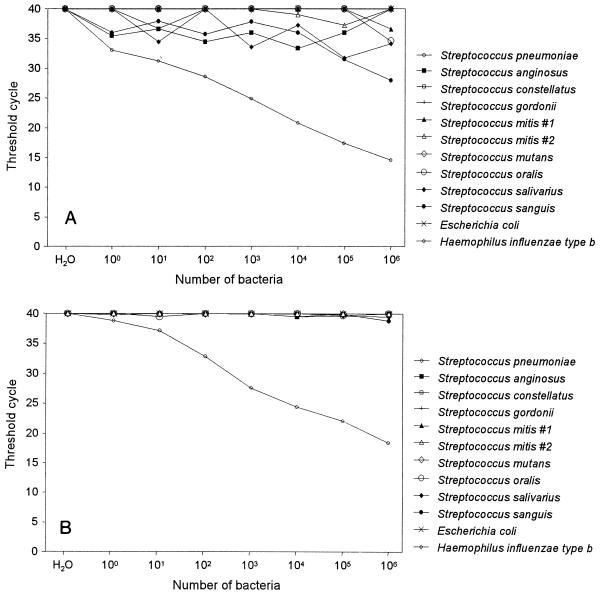
The specificity of the real-time PCR assay for S. pneumoniae with primers specific for the pneumolysin gene was investigated by testing 10-fold serial dilutions of H. influenzae type b, M. catarrhalis, and alpha-hemolytic streptococci including S. anginosus, S. constellatus, S. gordonii, S. mitis, S. mutans, S. oralis, S. salivarius, and S. sanguis (Fig. 2). Amplifications of H. influenzae type b and M. catarrhalis remained negative at all dilutions, whereas the CT values for all alpha-hemolytic streptococci except S. sanguis were between 40 and 31; for S. sanguis only at a concentration of 106 microorganisms the CT value was 28. By comparison, a CT value of <15 was achieved for S. pneumoniae at the same concentration of 106 microorganisms (Fig. 2A). Since the specificity of PCR amplification is temperature dependent, we performed the assay at an annealing temperature of 65°C. This resulted in CT values above 38, even with the largest number of microorganisms tested, except for S. pneumoniae, which gained approximately 4 to 5 CT units compared to the values obtained by the same test performed at 60°C (Fig. 2B).
FIG. 2.
Specificity of the real-time PCR assay for S. pneumoniae. Serial dilutions of liquid cultures from different bacterial species were subjected to DNA extraction and quantitation by the real-time PCR assay targeting the pneumolysin gene. The values are the means of duplicate measurements. (A) Assay executed at an annealing temperature of 60°C. (B) Assay executed at an annealing temperature of 65°C.
Application of the real-time PCR assay for S. pneumoniae to NPSs.
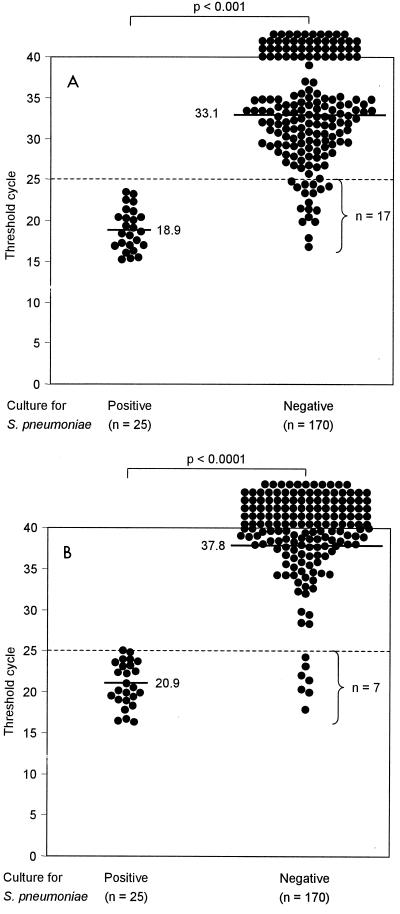
Culture of S. pneumoniae was possible for 25 of the 195 NPS samples. As shown in Fig. 3A, the real-time PCR assay applied to these 25 culture-positive NPS samples at an annealing temperature of 60°C resulted in CT values between 15.2 and 23.4 (mean, 18.9; median, 18.6; Fig. 3A) and thus performed with a calculated sensitivity of 100%. By contrast, when the real-time PCR assay was applied to the 170 culture-negative NPS samples, CT values were between 16.8 and 40 (mean, 33.1; median 32.2) (P < 0.001). While 153 (90%) of these 170 NPS samples had CT values >25 (49 NPS samples had a CT value of 40), 17 (10%) NPS samples had CT values in a range similar to those for the 25 NPS samples culture positive for S. pneumoniae (Fig. 3A). The proportion of NPS samples that grew S. pneumoniae versus the proportion of NPS samples that did not grow this bacterium and that had CT values ≤25 was statistically significantly different (P < 0.001). By assessing an NPS sample with a CT value >25 as being negative for S. pneumoniae, the calculated specificity of the real-time PCR assay in relation to the results of culture was 90%.
FIG. 3.
Sensitivity and specificity of the real-time PCR assay for S. pneumoniae in clinical samples (NPSs) compared with culture results. The dashed line indicates the arbitrary cutoff CT value. CT values below 25 were regarded as positive for S. pneumoniae. Horizontal bars indicate medians, which are also given as absolute values. (A) Assay executed at an annealing-extension temperature of 60°C. (B) Assay executed at an annealing-extension temperature of 65°C.
Two of the 17 patients whose NPS samples did not grow S. pneumoniae in culture but whose NPS samples had CT values <25 by the real-time PCR assay had been treated with β-lactam antimicrobials before the samples were collected. Attempts to culture the NPSs from these two patients showed no other bacterial growth. The NPS samples from the other 15 patients not treated with antimicrobials grew normal mouth and nasal flora.
Since the specificity of our real-time PCR assay had been shown to increase in a temperature-dependent manner, we also tested all clinical samples at an annealing temperature of 65°C (Fig. 3B). The CT values for the 25 culture-positive NPS samples ranged between 16.3 and 25.0 (mean, 20.9; median, 20.5; Fig. 3B). The real-time PCR assay applied to the 170 NPS culture-negative samples resulted in CT values between 17.7 and 40 (mean, 37.8; median 40) (P < 0.001); 163 (96%) of these 170 NPS samples had CT values >25 (91 NPS samples had a CT value of 40), and 7 (4%) NPS samples had CT values that covered ranged similar to those for the 25 NPS samples culture positive for S. pneumoniae (Fig. 3B).
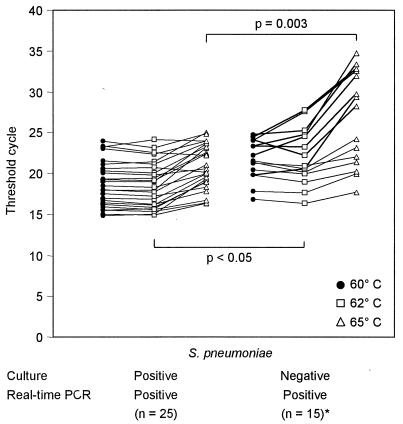
To further document and analyze the effect of raising the annealing temperature on specificity, we tested the 25 S. pneumoniae culture-positive and real-time PCR-positive (concordant) samples and 15 of the 17 S. pneumoniae culture-negative but real-time PCR-positive NPS (discordant) samples (excluding the 2 samples from the 2 patients treated with β-lactam antimicrobials) at an annealing extension temperature of 60°C and also at annealing extension temperatures of 62 and 65°C. As shown in Fig. 4, all 25 (concordant) NPS samples that grew S. pneumoniae continued to exhibit CT values of ≤5, despite the increased annealing extension temperature, whereas the CT values increased to >25 in 3 (20%; P = 0.048) and 8 (53%; P < 0.001) of the 15 discordant NPS samples at 60 and 65°C, respectively. Thus, raising of the annealing extension temperature to 65°C increased the calculated specificity of the real-time PCR assay to 96% compared to the results of culture but did not change the sensitivity. Furthermore, the statistical differences (P values) in CT values between concordant NPS and discordant NPS samples at 62 and 65°C were 0.049 and <0.003, respectively (Fig. 4).
FIG. 4.
Influence of temperature on CT values and on specificity. The standard amplification parameters used were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles with each cycle comprising 95°C for 15 s and either 60, 62, or 65°C for 1 min.
Comparison of the real-time PCR assay with semiquantitative cultures.
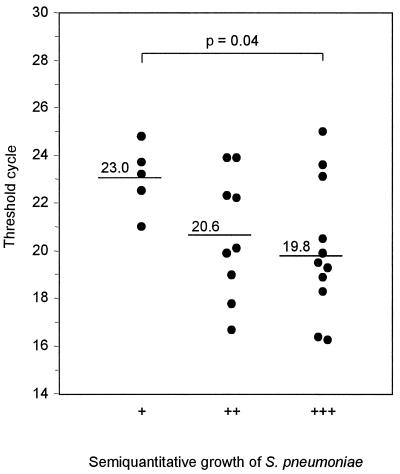
Five of the 25 cultures that grew S. pneumoniae showed low growth (+), 9 intermediate showed growth (++), and 11 showed abundant growth (+++). The corresponding mean CT values obtained by the real-time PCR assay conducted at an annealing temperature of 60°C were 20.6 (range, 18.1 to 23.2), 19.4 (range, 16.0 to 23.4), and 17.6 (range, 15.2 to 20.3), respectively. The difference in CT values between cultures with low levels of growth and those with high levels of growth was statistically significant (P = 0.02). Similar statistically significant differences were noted when an annealing temperature of 65°C was used. The mean CT values for cultures with low levels of growth, intermediate levels of growth, and abundant growth were 23.0 (range, 21.0 to 24.8), 20.6 (range, 16.7 to 23.9), and 20.1 (range, 16.3 to 25.0), respectively (P = 0.04) (Fig. 5).
FIG. 5.
Comparison of quantification of S. pneumoniae by real-time PCR with semiquantitative cultures. The samples were inoculated onto sheep blood agar by fractionation with a calibrated wire loop (5 μl). Growth only in the first fraction was defined a low growth (+), growth also in the second fraction was defined as intermediate growth (++), and growth in all three fractions was defined as abundant growth (+++). The real-time PCR assay was executed at an annealing temperature of 65°C.
DISCUSSION
Herein is described a real-time PCR assay with primers specific for the pneumolysin gene which proved to be highly sensitive (100%) and specific (up to 96%) for the detection of S. pneumoniae in NPSs. The numbers of S. pneumoniae isolates quantified by the real-time PCR assay corresponded to the numbers detected by semiquantitative cultures. Since the assay can be completed within a working day, it is considerably faster than conventional culturing and subsequent identification.
The linear detection range of the real-time PCR assay was 6 orders of magnitude above the background, ranging from 1 to 106 organisms per reaction mixture. Previously reported real-time PCR assays for the detection of bacteria including Porphyromonas gingivalis (10), Borrelia burgdorferi (16), and Mycobacterium tuberculosis (2) also showed detection ranges of 5 to 6 orders of magnitude and encompassed similar sensitivities. In our assay, there were also high intra- and interexperimental reproducibilities (within 2 to 3%) (Fig. 1A). The numbers of S. pneumoniae organisms detected in semiquantitative cultures from the clinical samples were within the detection range of the assay.
The real-time PCR assay with primers specific for the pneumolysin gene was positive for all NPS samples that grew S. pneumoniae. The CT values, which are inversely related to the quantity of organisms, ranged between 15.2 and 23.4 at an annealing extension temperature of 60°C (Fig. 3A) and between 16.3 and 25 at an annealing extension temperature of 65°C (Fig. 3B). These CT values corresponded to 105 to 103 organisms when the values were fitted onto the standard curve generated by using the results for serial dilutions of liquid cultures of S. pneumoniae (Fig. 1). For comparison, the more bacteria that grew in the semiquantitative cultures, the lower the observed CT values in the real-time PCR assay were, and in addition, the differences between CT values for NPS samples with low levels of growth and those for NPS samples with abundant growth were statistically significant (P = 0.04) (Fig. 5).
The pneumolysin gene was shown to be highly specific for S. pneumoniae compared with its specificity for other alpha-hemolytic streptococci, H. influenzae, and M. catarrhalis. For S. pneumoniae a linear titration curve was demonstrated when 10-fold serial dilutions were investigated. When other alpha-hemolytic streptococcal strains were similarly tested, only S. sanguis showed minimal amplification at the highest concentrations of the microorganism tested (105 to 106), with the CT values always remaining above 25 at an annealing extension temperature of 60°C (Fig. 2A). Increasing the annealing extension temperature to 65°C further increased the specificity (Fig. 2B).
All 170 NPS samples shown to be devoid of S. pneumoniae following culture had CT values above 25, but 17 (10%) displayed CT values ranging from 16.8 to 24.7. Two of these 17 NPS samples were collected from patients who had been treated with a β-lactam antimicrobial. Thus, it seems likely that the antibiotic pretreatment may have impeded the growth of S. pneumoniae in culture, and therefore, the real-time PCR assay may have detected DNA from dead bacteria present in the NPS. The remaining 15 of the 17 NPS samples with discordant results grew normal mouth and nasal flora, as determined by culture. Two of the remnant 15 NPS samples grew optochin-resistant alpha-hemolytic streptococci. It is known that there are S. pneumoniae strains which are resistant to optochin (8, 12). Such atypical isolates of S. pneumoniae can be detected by the detection of pneumolysin (A. M. Kearns, J. Wheeler, R. Freeman, P. R. Seiders, J. Perry, A. M. Whatmore, and C. G. Dowson, Letter, J. Clin. Microbiol. 38:1309–1310, 2000). This could explain the discordant results of cultures and the real-time PCR for these two patient samples. A possible explanation for the remaining discordant results could be that this real-time PCR assay with primers specific for the pneumolysin gene targeted pneumolysin homologues from other microorganisms, particularly from alpha-hemolytic streptococci other than S. pneumoniae. The closest relative to pneumolysin, a member of the family of thiol-activated cytolysins, is the enzyme suilysin, which is expressed by S. suis (19). Nevertheless, the alignment of known gene sequences between pneumolysin and suilysin shows that it is very unlikely that this assay amplifies suilysin. Furthermore, the real-time PCR assay (TaqMan) has enhanced specificity compared to that of conventional PCR, gained through the use of a hydrolysis probe. Finally, microorganisms closely related to S. mitis and harboring genes encoding the virulence determinants pneumolysin and autolysin classically associated with S. pneumoniae have been reported recently (21). Thus, our real-time PCR assay may have detected such organisms, which may account for the discordant results for the remaining 13 NPS samples. Nevertheless, we were able to increase the specificity for clinical samples from 90 to 96% by performing the assay at an annealing temperature of 65°C (Fig. 3 and 4). This in turn suggests that the optimal reaction temperature for the primers and the probe, as calculated by the Primer Express software, is a good starting point, but for the detection of pathogens it may need further optimization, as demonstrated by our study.
In summary, we developed a new diagnostic assay on the basis of real-time PCR that allowed the fast (analysis of numerous patient samples, from biological material to analyzed results, within 24 h), sensitive, specific, reproducible, and simple high-throughput detection and quantification of the pneumolysin gene from both typical and atypical S. pneumoniae. This assay is faster and more precise in terms of quantification than conventional culture and identification procedures and may therefore provide a reliable tool for clinical studies aimed at assessing the quantities of S. pneumoniae in the upper respiratory tract during infections of the lower respiratory tract with this organism.
ACKNOWLEDGMENTS
We thank Pia Beck for superb organization of logistics, support, and technical expertise and R. Gmür for providing alpha-hemolytic streptococcal strains.
The study was partly supported by the Swiss National Foundation (grant 31-55553.98).
REFERENCES
- 1.Chalasani N P, Valdecanas M A, Gopal A K, McGowan J E, Jurado R L. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932–936. doi: 10.1378/chest.108.4.932. [DOI] [PubMed] [Google Scholar]
- 2.Desjardin L E, Chen Y, Perkins M D, Teixeira L, Cave M D, Eisenach K D. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J Clin Microbiol. 1998;36:1964–1968. doi: 10.1128/jcm.36.7.1964-1968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummond P, Clark J, Wheeler J, Galloway A, Freeman R, Cant A. Community acquired pneumonia—a prospective UK study. Arch Dis Child. 2000;83:408–412. doi: 10.1136/adc.83.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer-Romero C, Luthy-Hottenstein J, Altwegg M. Development and evaluation of a broad-range PCR-ELISA assay with Borrelia burgdorferi and Streptococcus pneumoniae as model organisms for reactive arthritis and bacterial meningitis. J Microbiol Methods. 2000;40:79–88. doi: 10.1016/s0167-7012(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg D L. Septic arthritis. Lancet. 1998;351:197–202. doi: 10.1016/S0140-6736(97)09522-6. [DOI] [PubMed] [Google Scholar]
- 6.Heiskanen-Kosma T, Korppi M, Jokinen C, Kurki S, Heiskanen L, Juvonen H, Kallinen S, Stén M, Tarkainen A, Pirjo-Riita R, Kleemola M, Mekälä P H, Leinonen M. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J. 1998;17:986–991. doi: 10.1097/00006454-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Juven T, Mertsola J, Waris M, Leinonen M, Meurman O, Roivainen M, Eskola J, Saikku P, Ruuskanen O. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Kontiainen S, Sivonen A. Optochin resistance in Streptococcus pneumoniae strains isolated from blood and middle ear fluid. Eur J Clin Microbiol. 1987;6:422–424. doi: 10.1007/BF02013101. [DOI] [PubMed] [Google Scholar]
- 9.Lorente M L, Falguera M, Nogues A, Gonzalez A R, Merino M T, Caballero M R. Diagnosis of pneumococcal pneumonia by polymerase chain reaction (PCR) in whole blood: a prospective clinical study. Thorax. 2000;55:133–137. doi: 10.1136/thorax.55.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons S R, Griffen A L, Leys E J. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J Clin Microbiol. 2000;38:2362–2365. doi: 10.1128/jcm.38.6.2362-2365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison K E, Lake D, Crook J, Carlone G M, Ades E, Facklam R, Sampson J S. Confirmation of psaA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J Clin Microbiol. 2000;38:434–437. doi: 10.1128/jcm.38.1.434-437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundy L S, Janoff E N, Schwebke K E, Shanholtzer C J, Willard K E. Ambiguity in the identification of Streptococcus pneumoniae. Optochin, bile solubility, quellung, and the AccuProbe DNA probe tests. Am J Clin Pathol. 1998;109:55–61. doi: 10.1093/ajcp/109.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. [Google Scholar]
- 14.Nadal D, Bossart W, Zucol F, Steiner F, Berger C, Lips U, Altwegg M. Community-acquired pneumonia in children due to Mycoplasma pneumoniae: diagnostic performance of a seminested 16S rDNA-PCR. Diagn Microbiol Infect Dis. 2001;39:15–19. doi: 10.1016/s0732-8893(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 15.Nohynek H, Eskola J, Laine E, Halonen P, Ruutu P, Saikku P, Kleemola M, Leinonen M. The causes of hospital-treated acute lower respiratory tract infection in children. Am J Dis Child. 1991;145:618–622. doi: 10.1001/archpedi.1991.02160060036016. [DOI] [PubMed] [Google Scholar]
- 16.Pahl A, Kuhlbrandt U, Brune K, Rollinghoff M, Gessner A. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol. 1999;37:1958–1963. doi: 10.1128/jcm.37.6.1958-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapola S, Jantti V, Haikala R, Syrjanen R, Carlone G M, Sampson J S, Briles D E, Paton J C, Takala A K, Kilpi T M, Kayhty H. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J Infect Dis. 2000;182:1146–1152. doi: 10.1086/315822. [DOI] [PubMed] [Google Scholar]
- 18.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 19.Segers R P, Kenter T, de Haan L A, Jacobs A A. Characterisation of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol Lett. 1998;167:255–261. doi: 10.1111/j.1574-6968.1998.tb13236.x. [DOI] [PubMed] [Google Scholar]
- 20.Walker J A, Allen R L, Falmagne P, Johnson M K, Boulnois G J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987;55:1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whatmore A M, Efstratiou A, Pickerill A P, Broughton K, Woodard G, Sturgeon D, George R, Dowson C G. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect Immun. 2000;68:1374–1382. doi: 10.1128/iai.68.3.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]