Abstract
The purpose of this study was to evaluate the effects of vitamin D supplementation on ovarian reserve markers, including serum anti-Mullerian hormone (AMH) level, follicle-stimulating hormone (FSH) level, and antral follicle count (AFC), in infertile women with diminished ovarian reserve and vitamin D deficiency.
A prospective, nonrandomized, cross-sectional study was conducted. Women aged 18 to 41 years who were unable to become pregnant after 12 months of sexual intercourse and had normal tubal patency, partners with normal semen analysis, diminished ovarian reserve, and 25-hydroxyvitamin D [25(OH)D] deficiency were included. Eligible patients’ AFC and serum levels of AMH, FSH, 25(OH)D, 1,25-dihydroxyvitamin D [1,25(OH)D], calcium, phosphate, alkaline phosphatase, and parathormone were assessed before and after administration of 300,000 IU of vitamin D ampules. Changes in the parameter values after vitamin D supplementation were compared with the initial levels.
The study was conducted in 62 of the 142 participants. The AFC and AMH, 25(OH)D, 1,25(OH)D, phosphate (P < .01), and calcium levels (P < .05) were statistically significantly increased after vitamin D supplementation. Statistically significant decreases in FSH (P < .01) and alkaline phosphatase levels (P < .05) were observed after vitamin D supplementation. No statistically significant correlations were found between 25(OH)D level and AFC, 1,25(OH)D level, AMH level, and FSH level before and after supplementation (P > .05).
As improvements in the ovarian reserve markers were obtained with vitamin D supplementation, vitamin D might be considered as a fertility treatment for patients with diminished ovarian reserve and vitamin D deficiency.
Keywords: anti-mullerian hormone, ovarian reserve, vitamin D
1. Introduction
Vitamin D facilitates the absorption of calcium and promotes bone growth. It is produced in the skin from 7-dehydrocholesterol by ultraviolet-B radiation and yields 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)D] after 2 hydroxylation processes. 25(OH)D level is an easily measured molecule and considered the best indicator of vitamin D status in humans.[1]
Vitamin D plays significant roles in different parts of the body, which are suggested to be due to the vitamin D receptor expression on different organs, including reproductive tissues.[2] An important role of vitamin D in human reproduction has been suggested. Although little is known about the mechanism by which vitamin D may affect reproductive physiology,[3] several studies have addressed the association of vitamin D with different markers of infertility.[4,5] Vitamin D level has been suggested to be associated with the outcome of controlled ovarian stimulation in women undergoing in vitro fertilization (IVF) treatments,[6,7] the features of polycystic ovarian syndrome (PCOS), and endometriosis.[8] Furthermore, vitamin D has been shown to alter anti-Mullerian hormone (AMH) signaling by downregulating the AMH receptor-II gene expression, follicle-stimulating hormone (FSH) sensitivity, and progesterone production. Moreover, it plays important roles in follicular development, differentiation, and luteinization.[9,10] Vitamin D at physiological levels plays an important role in oocyte development, embryo quality and endometrial receptivity.[11] Also a positive correlation has been demonstrated between follicular fluid vitamin D level and percentage of embryo fragmentation[12]
Diminished ovarian reserve (DOR) is one of the most bothersome causes of IVF failures.[13] Ovarian reserve, which indicates the reproductive potential of females, is associated with both oocyte quality and quantity. DOR is demonstrated by changes in markers such as decreased antral follicle count (AFC), increased day 3 FSH level, and decreased AMH level.[14]
At present, an inconsistency in the field has been observed regarding whether vitamin D has the capacity to influence ovarian reserve. Studies in the literature demonstrated conflicting results, with several studies finding a positive correlation between vitamin D level and ovarian reserve markers[10,15,16] while other studies reported largely negative findings.[17–20] The previously mentioned studies that emphasized the association between vitamin D level and ovarian reserve markers have inspired to obtain an improvement in ovarian reserve with appropriate vitamin D supplementation in vitamin D-deficient cases.
Therefore, a prospective, cross-sectional study was conducted to evaluate the effects of vitamin D supplementation on ovarian reserve markers, including serum AMH level, FSH level, and AFC, in infertile women with DOR and vitamin D deficiency.
2. Methods
This study was conducted at the In vitro Fertilization Department of the Istanbul Training and Research Hospital, Istanbul, Turkey, between June 2019 and September 2019. It was approved by the ethics committee of the Istanbul Training and Research Hospital (2011-KAEK-50-09/08/2019).
The study was designed as a prospective, nonrandomized, cross-sectional study. All the participating women gave their written informed consent prior to their inclusion in the study. Patients who met the following inclusion criteria were enrolled: Infertile women aged 18 to 41 years who had diminished ovarian reserve and 25(OH) D deficiency, were unable to become pregnant after 12 months of sexual intercourse, had normal tubal patency, had partners with normal semen analysis results and did not use any vitamin supplements were included. Women with any underlying endocrine or metabolic disease, with a history of pelvic surgery, and who had chemotherapy or pelvic radiotherapy, vitamin D supplementation, ovarian masses, endometriosis, hypogonadotropic hypogonadism, hyperprolactinemia, pregnancy, congenital adrenal hyperplasia, androgen-secreting tumors, and Cushingʼs syndrome were excluded. The exclusion criteria also included women with systemic disorders such as thyroid and renal dysfunctions, and hypertension. Patients with endocrine and metabolic diseases were excluded according to anamnesis, physical examination, biochemical tests analyzing blood glucose, thyroid hormone levels, renal and liver function. In addition, any patient who refused to continue the study or did not complete the study was excluded.
All the participants underwent a physical examination. Body mass index (BMI) was calculated by dividing weight (in kilograms) by height (in meters) squared. AFC was evaluated on ultrasonography (Logiq C3 Class 1, GE Medical Systems, China) using a 5- to 9-MHz transvaginal two-dimensional probe by the same clinician for interobserver or interobserver reliability on days 2 to 5 of the cycle. Both ovaries were visualized. The recorded AFC represented the sum of the antral follicles between 2 and 10 mm from the right and left ovaries.
Moreover, all the women underwent blood sampling for the assessment of their serum hormone profiles and vitamin D levels on days 2 to 5 of their menstrual cycle. Once the blood sample for AMH level measurement had been taken, centrifugation was performed within 1 hour, and serum was separated and immediately stored at −80°C until analysis. All the samples were measured with the Gen II Beckman Coulter AMH enzyme-linked immunosorbent assay (ELISA) kit. Once the blood sample for vitamin D concentration measurement was taken, the sample was centrifuged and frozen at −20°C. With all the blood samples obtained for vitamin D concentration measurement, ELISA was used at one time to avoid inter-assay variability by using an electrochemiluminescence binding assay (Roche Diagnostics, Mannheim, Germany) and a Cobas 6000 immunoanalyzer.
Vitamin D deficiency was defined as serum 25(OH)D levels <20 ng/mL in accordance with the Institute of Medicine and Endocrine Society clinical practice guidelines.[21,22] All the included women had vitamin D concentrations <20 ng/mL, which is the accepted cutoff value for vitamin D deficiency.
DOR was defined as the presence of 2 of the following 3 features:
-
1.
maternal age ≥40 years or any other risk factor of poor response,
-
2.
previous poorly responsive cycles (≤3 oocytes retrieved), and
-
3.
an abnormal ovarian reserve test result (AMH level <0.5–1.1 ng/mL or antral follicle count <5–7 follicles).[23]
Istanbul is a city in Turkey that is located in the northern hemisphere, at the intersection of the 41st parallel and 29th meridian. The study was performed between June and September, a period that covers the summer season in Istanbul.
The recorded variables included age, weight, and height (for calculation of BMI). AFC and 25(OH)D, 1,25(OH)D, AMH, FSH, calcium, phosphate, alkaline phosphatase (ALP), and parathormone (PTH) levels were recorded before the treatment. Vitamin D ampules containing 300,000 IU vitamin D3 were administered monthly for 2 months to all eligible infertile women. At the end of 2 months, on days 2 to 5 of their menstrual cycle, AFC and serum levels of 25(OH)D, 1,25(OH)D, AMH, FSH, calcium, phosphate, ALP, and PTH were measured again, and the measurements were compared with the initial laboratory results.
2.1. Statistical analyses
The sample size based on the AMH level with Δ:0.53 Δ SD:0.36 was 15 participants. This assumes an overall two-sided significance level of b:0.20, a:0.05 and 80% power to detect the stated difference between the treatment arms.[24]
A statistical analysis was performed using the IBM SPSS Statistics 22.0 program. Continuous data were described as mean, standard deviation, and range (i.e., from the minimum to the maximum). Differences between the baseline levels and post-supplementation values were assessed with a paired-sample t test for continuous data, a Wilcoxon signed-rank test for discrete data, and McNemar's test for categorical data. Bivariate correlations were analyzed with Pearson correlation for parametric data. Significance was evaluated at the level of P < .05.
3. Results
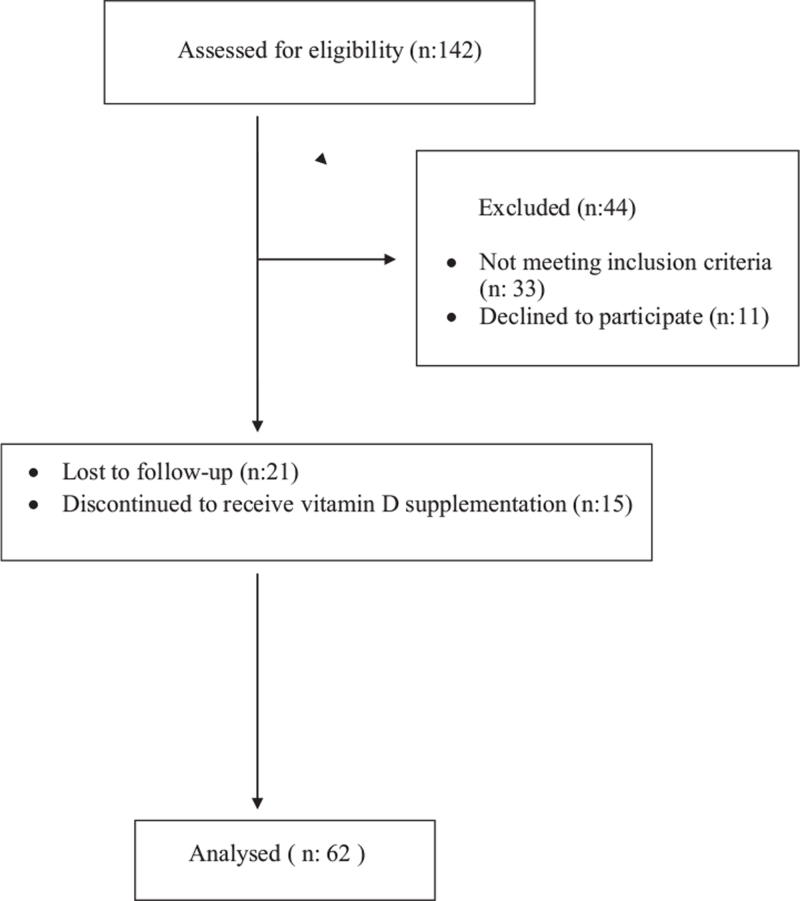
Of the 142 participants, 33 did not meet the inclusion criteria and 11 declined to further participate in the study. Twenty one patients were lost to follow-up, and 15 did not continue the vitamin D supplementation (Fig. 1). Finally, the study was conducted with 62 patients aged between 22 and 41 years (mean, 32.45 ± 4.69 years). The BMI measurements ranged from 19 to 33 kg/m2 (mean, 23.69 ± 3.33 kg/m2).
Figure 1.
Flow-chart of study design.
The AFC and AMH, 25(OH)D, 1,25(OH)D, phosphate (P < .01), and Ca levels (P < .05) statistically significantly increased after vitamin D supplementation. No statistically significant change in PTH level was observed (P > .05; Table 1). Statistically significant decreases in FSH (P < .01) and ALP levels (P < .05) were found after vitamin D supplementation (Table 1). No statistically significant correlation was found between the initial 25(OH)D level and the initial AFC and 1,25(OH)D, AMH, and FSH levels (P > .05; Table 2). 25(OH)D level showed no statistically significant correlation with the AFC and levels of AMH and FSH after supplementation (P > .05; Table 2). A moderate statistically significant correlation was found between the 25(OH)D and 1,25(OH)D levels after supplementation (r = 0.508; P < .01; Table 2).
Table 1.
Laboratory and ultrasonographic results before and after vitamin D replacement.
| Before replacement | After replacement | ||
| Mean ± SD | Mean ± SD | P | |
| AFC | 2.85 ± 1.54 | 3.53 ± 1.22 | 2.001∗∗ |
| PTH | 43.85 ± 18.18 | 46.52 ± 16.46 | 1.315 |
| 25(OH)D | 13.71 ± 5.94 | 64.34 ± 37.65 | 1.001∗∗ |
| 1,25(OH)D | 50.90 ± 18.29 | 81.32 ± 32.54 | 1.001∗∗ |
| Ca | 0.35 ± 0.06 | 0.52 ± 0.08 | 1.037∗ |
| P | 3.11 ± 0.38 | 3.38 ± 0.47 | 1.003∗∗ |
| AMH | 0.82 ± 0.87 | 1.05 ± 1.01 | 2.001∗∗ |
| FSH | 16.44 ± 17.47 | 14.16 ± 13.87 | 2.002∗∗ |
| ALP | 72.11 ± 21.92 | 68.17 ± 21.09 | 1.033∗ |
1paired sample t-test.
2Wilcoxon signed-ranks test.
1,25(OH)D = 1,25 dihydroxyvitamin D, 25(OH)D = 25- hydroxyvitamin D, AFC = antral follicle count, ALP = alkalen phosphatase, AMH = anti-Mullerian hormone, Ca = calcium, FSH = follicle stimulating hormone, P = phosphate, PTH = parathormone.
P < .05.
P < .01.
Table 2.
Relation between 25(OH)D level and AFC, 1,25(OH) D, AMH, and FSH.
| 25OH D Vit (Z score) | ||||
| Before replacement | After replacement | |||
| r | P | R | P | |
| AFC (Z score) | −0.087 | .503 | 0.033 | .800 |
| 1,25(OH) D (Z score) | 0.164 | .202 | 0.508 | .001 ∗∗ |
| AMH (Z score) | −0.059 | .649 | 0.037 | .777 |
| FSH (Z score) | 0.184 | .153 | 0.022 | .863 |
r: Pearson's correlation coefficient.
1,25(OH)D = 1,25 dihydroxyvitamin D, 25(OH)D = 25-hydroxyvitamin D, AFC = antral follicle count, AMH = anti-Mullerian hormone, FSH = follicle stimulating hormone.
P < .01.
4. Discussion
In the present study, we found some possible beneficial effects of vitamin D supplementation on ovarian reserve markers. We found statistically significant increases in AFC and AMH level, and decrease in FSH level. Although the ovarian reserve markers showed improvements, no statistically significant association was found between the 25(OH)D levels and ovarian reserve markers before and after supplementation.
Vitamin D deficiency is a worldwide problem.[25] As much as 20% to 52% of women of reproductive age have been shown to be vitamin D deficient.[26] The main reason is inadequate exposure to sunshine, which is the main source of vitamin D.[25,26]
Vitamin D is thought to act via vitamin D receptors. The expressions of vitamin D receptors in granulosa cells,[27] having a vitamin D response element on the promoter region for the AMH gene,[28] seasonal variations in ovulation, conception rate, and birth rate have increased the interest regarding vitamin D and characterized vitamin D as a “fertility vitamin.”[29]
The most commonly used markers of ovarian reserve are AFC and levels of AMH and FSH. AMH is produced by the granulosa cells of the preantral and antral follicles and reflects the primordial follicle pool. AMH was suggested to be relatively stable throughout the menstrual cycle.[30] AFC can be measured in the context of a primary ultrasonographic evaluation. A quick result can be obtained with good inter-cycle and interobserver reliabilities.[30] Several studies have shown that AFC and AMH level are both good predictors of ovarian response in the context of hormonal stimulation for IVF.[30] FSH is a marker with poor sensitivity toward the detection of DOR. It has several major limitations, including significant inter-cycle and intra-cycle variabilities.[31]
Several studies reported that serum 25(OH)D concentration is positively associated with ovarian reserve markers.[9,16,32,33] Vitamin D was shown to have a direct effect on AMH production, and women with higher vitamin D concentrations maintained their ovarian reserve for longer.[34] In another study, lower serum 25(OH)D levels have been accompanied by higher serum FSH levels.[10] On the other hand, other studies reported that vitamin D levels were unrelated to ovarian reserve.[15,35,36] A recent cross-sectional study showed no significant association between serum vitamin D and AMH levels or AFC in women with infertility.[37]
Currently, whether vitamin D has the capacity to influence ovarian reserve has been a controversial issue in the field of fertility. Numerous observational studies and few interventional studies were aimed at evaluating the relationship between serum vitamin D level and ovarian reserve markers. Several studies have shown a positive correlation between vitamin D levels and ovarian reserve markers,[10,15,16] while other studies reported negative findings.[17–20] A recent systematic review and meta-analysis of interventional studies revealed that vitamin D supplementation affects serum AMH levels but have opposite effects depending on the ovulatory statuses of women.[38] Vitamin D supplementation appeared to decrease the AMH levels of anovulatory women with PCOS, while vitamin D supplementation appeared to increase the AMH levels in ovulatory non-PCOS women.[38]
A study similar to our work evaluated the effects of 25(OH)D administration on the serum AMH levels of women with infertility and revealed that the serum levels of both 25(OH)D and AMH increased significantly after treatment.[24] A significant correlation was found, and higher 25(OH)D levels were accompanied by higher AMH levels. The study was nonrandomized and performed in 30 infertile women who had low serum levels of both 25(OH)D and the AMH. Vitamin D was prescribed 50,000 IU weekly for up to 3 months.[24] The results of the study by Naderi et al were primitive because of the small sample size and evaluation of only the AMH serum levels.[24] In our study, the effects of 25(OH)D supplementation on the ovarian reserve markers of the infertile women with DOR were evaluated. We prospectively recruited a larger number of infertile women with DOR. Women with 25(OH)D deficiency were included. After 2 months of vitamin D supplementation, our results showed statistically significant increases in AFC and AMH levels and a statistically significant decrease in FSH level after supplementation. Although improvements in the ovarian reserve markers were observed, 25(OH)D level showed no statistically significant association with AFC and the levels of AMH and FSH before and after supplementation.
Significant seasonal variations in serum vitamin D levels were observed between summer and winter, with higher levels in summer and autumn and lower levels in winter and spring; however, serum AMH levels remained unaffected by season.[30] Thus, our study was completed in the summer.
Our study has several limitations. The nonrandomized cross-sectional nature and small sample size of the study limited the generalizability of the results. The lack of standardized lifestyle conditions in terms of dietary habits and clothing styles of the participants and absence of a control group could also be considered limitations of this study. The strengths of our study are its prospective design and the stringent inclusion criteria used to eliminate confounders that might have affected the increase in ovarian reserve. Furthermore, we not only evaluated AMH serum levels in association with 25-OH vitamin D but also assessed other important markers, AFC and FSH level. In addition, blood samples for the assessments of hormone profile, 25-OHD level, and AMH level were sampled on the same day.
5. Conclusion
In the present study, we found some possible beneficial effects of vitamin D supplementation on ovarian reserve markers. Although it is premature to draw final conclusions, vitamin D supplementation might be considered in the fertility treatment of patients with DOR, as it causes an increase in AFC and AMH level and a decrease in FSH level. Further studies that include fertilization, clinical pregnancy, and live birth rates are needed.
Acknowledgments
The authors would like to thank Enago (www.enago.com) for the English language review and Mrs Nihal Ozdemir for statistical analysis (The person gave permission to be named).
Author contributions
Conceptualization: Besim Haluk Bacanakgil, Gülşah İlhan.
Data curation: Karolin Ohanoğlu.
Formal analysis: Besim Haluk Bacanakgil.
Investigation: Besim Haluk Bacanakgil, Gülşah İlhan, Karolin Ohanoğlu.
Methodology: Gülşah İlhan.
Resources: Gülşah İlhan.
Supervision: Besim Haluk Bacanakgil.
Visualization: Gülşah İlhan.
Writing – original draft: Gülşah İlhan.
Writing – review & editing: Besim Haluk Bacanakgil, Gülşah İlhan.
Footnotes
Abbreviations: [1,25(OH)D] = 1,25-dihydroxyvitamin D, [25(OH)D] = 25-hydroxyvitamin D, AFC = antral follicle count, ALP = alkaline phosphatase, AMH = anti-Mullerian hormone, BMI = body mass index, COS = controlled ovarian stimulation, DOR = diminished ovarian reserve, FSH = follicle-stimulating hormone, IVF = in vitro fertilization, PCOS = polycystic ovary syndrome.
How to cite this article: Bacanakgil BH, İlhan G, Ohanoğlu K. Effects of vitamin D supplementation on ovarian reserve markers in infertile women with diminished ovarian reserve. Medicine. 2022;101:6(e28796).
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999;69:842–56. [DOI] [PubMed] [Google Scholar]
- [2].Ranjana H. Role of vitamin D in infertility. J Public Health Policy Plan 2017;1:08–10. [Google Scholar]
- [3].Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol 2012;166:765–78. [DOI] [PubMed] [Google Scholar]
- [4].Aleyasin A, Hosseini MA, Mahdavi A, et al. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol 2011;159:132–7. [DOI] [PubMed] [Google Scholar]
- [5].Pacis MM, Fortin CN, Zarek SM, Mumford SL, Segars JH. Vitamin D and assisted reproduction: should vitamin D be routinely screened and repleted prior to ART? A systematic review. J Assist Reprod Genet 2015;32:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Butts SF, Seifer DB, Koelper N, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J Clin Endocrinol Metab 2019;104:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhao J, Liu S, Wang Y, et al. Vitamin D improves in-vitro fertilization outcomes in infertile women with polycystic ovary syndrome and insulin resistance. Minerva Med 2019;110:199–208. [DOI] [PubMed] [Google Scholar]
- [8].Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract 2013;67:225–35. [DOI] [PubMed] [Google Scholar]
- [9].Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril 2014;102:460–8. [DOI] [PubMed] [Google Scholar]
- [10].Jukic AM, Steiner AZ, Baird DD. Association between serum 25-hydroxyvitamin D and ovarian reserve in premenopausal women. Menopause 2015;22:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Laganà AS, Vitale SG, Ban Frangež H, Vrtačnik-Bokal E, D’Anna R. Vitamin D in human reproduction: the more, the better? An evidence-based critical appraisal. Eur Rev Med Pharmacol Sci 2017;21:4243–51. [PubMed] [Google Scholar]
- [12].Jeremic A, Mikovic Z, Soldatovic I, Sudar-Milovanovic E, Isenovic ER, Perovic M. Follicular and serum levels of vitamin D in women with unexplained infertility and their relationship with in vitro fertilization outcome: an observational pilot study. Arch Med Sci 2021;17:1418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Soleimani Rad S, Abbasalizadeh S, Ghorbani Haghjo A, Sadagheyani M, Montaseri A, Soleimani Rad J. Evaluation of the melatonin and oxidative stress markers level in serum of fertile and infertile women. Iran J Reprod Med 2015;13:439–44. [PMC free article] [PubMed] [Google Scholar]
- [14].Tal R, Seifer DB. Ovarian reserve testing: a user's guide. Am J Obstet Gynecol 2017;217:129–40. [DOI] [PubMed] [Google Scholar]
- [15].Wong HYQ, Li HWR, Lam KSL, et al. Independent association of serum vitamin D with anti-Mullerian hormone levels in women with polycystic ovary syndrome. Clin Endocrinol 2018;89:634–41. [DOI] [PubMed] [Google Scholar]
- [16].Dennis NA, Houghton LA, Jones GT, Van Rij AM, Morgan K, McLennan IS. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab 2012;97:2450–5. [DOI] [PubMed] [Google Scholar]
- [17].Bakeer E, Radwan R, El Mandoury A, El Rahman AA, Gad M, El Maksoud SA. Anti-müllerian hormone as a diagnostic marker in Egyptian infertile polycystic ovary syndrome females: Correlations with vitamin D, total testosterone, dyslipidemia and anthropometric parameters. J Med Biochem 2018;37:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arslan E, Gorkem U, Togrul C. Is There a relationship between vitamin D deficiency status and PCOS in infertile women? Geburtshilfe Frauenheilkd 2019;79:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu X, Zhang W, Xu Y, et al. Effect of vitamin D status on normal fertilization rate following in vitro fertilization. Reprod Biol Endocrinol 2019;17:59.doi: 10.1186/s12958-019-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shapiro AJ, Darmon SK, Barad DH, Gleicher N, Kushnir VA. Vitamin D levels are not associated with ovarian reserve in a group of infertile women with a high prevalance of diminished ovarian reserve. Fertil Steril 2018;110:761–6. [DOI] [PubMed] [Google Scholar]
- [21].Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol 2010;121:297–300. [DOI] [PubMed] [Google Scholar]
- [22].Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- [23].Ferraretti AP, Marca AL, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L. ESHRE working group on poor ovarian response definition. ESHRE consensus on the definition of ’poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011;26:1616–24. [DOI] [PubMed] [Google Scholar]
- [24].Naderi Z, Kashanian M, Chenari L, Sheikhansari N. Evaluating the effects of administration of 25-hydroxyvitamin D supplement on serum anti-mullerian hormone (AMH) levels in infertile women. Gynecol Endocrinol 2018;34:409–12. [DOI] [PubMed] [Google Scholar]
- [25].Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080–6. [DOI] [PubMed] [Google Scholar]
- [26].Pagliardini L, Vigano P, Molgora M, et al. High prevalence of vitamin D deficiency in infertile women referring for assisted reproduction. Nutrients 2015;7:9972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004;158:531–7. [DOI] [PubMed] [Google Scholar]
- [28].Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000;141:1317–24. [DOI] [PubMed] [Google Scholar]
- [29].Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology 2009;150:1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rojansky N, Brzezinski A, Schenker JG. Seasonality in human reproduction: an update. Human Reproduction 1992;7:735–45. [DOI] [PubMed] [Google Scholar]
- [31].La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 2010;16:113–30. [DOI] [PubMed] [Google Scholar]
- [32].Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006;12:685–718. [DOI] [PubMed] [Google Scholar]
- [33].Moslehi N, Mirmiran P, Tehrani FR, Azizi F. Current evidence on associations of nutritional factors with ovarian reserve and timing of menopause: A systematic review. Adv Nutr 2017;8:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Merhi ZO, Seifer DB, Weedon J, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: women's interagency HIV study. Fertil Steril 2012;98:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Grzechocinska B, Dabrowski FA, Cyganek A, Wielgos M. The role of vitamin D in impaired fertility treatment. Neuro Endocrinol Lett 2013;34:756–62. [PubMed] [Google Scholar]
- [36].Drakopoulos P, van de Vijver A, Schutyser V, et al. The effect of serum vitamin D levels on ovarian reserve markers: a prospective cross-sectional study. Hum Reprod 2017;32:208–14. [DOI] [PubMed] [Google Scholar]
- [37].Pearce K, Gleeson K, Tremellen K. Serum anti-Mullerian hormone production is not correlated with seasonal fluctuations of vitamin D status in ovulatory or PCOS women. Hum Reprod 2015;30:2171–7. [DOI] [PubMed] [Google Scholar]
- [38].Moridi I, Chen A, Tal O, Tal R. The association between vitamin D and anti-Müllerian hormone: a systematic review and meta-analysis. Nutrients 2020;12:1567.doi: 10.3390/nu12061567. [DOI] [PMC free article] [PubMed] [Google Scholar]