Graphical abstract
The heparin polysaccharide nanoparticles block the interaction between heparan sulfate/S protein and inhibit the infection of both wild-type SARS-CoV-2 pseudovirus and the mutated strains through pulmonary delivery.
KEY WORDS: SARS-CoV-2, Delta variant, Heparan sulfate, Spike protein, Heparin nanoparticle, Pulmonary delivery
To the Editor:
As of January 27, 2022, there were 363,062,293 cases and 5,645,884 deaths from the COVID-19 pandemic1. The prevalent mutated strains have aggravated the global pandemic2. SARS-CoV-2 is highly mutable, and the mutations on the spike (S) protein resulted in a high transmission of COVID-19 and vaccine breakthrough infection3. For example, there is a notable decrease in neutralizing ability of BNT162b2 vaccination-elicited antibodies against the Delta and other variants4,5, and attenuation of peak viral burden and vaccine effectiveness are reduced with Delta variant6.
The development of antiviral drugs against infection of SARS-CoV-2 and its variants is still an urgent need. The blockage of SARS-CoV-2 spike protein against the binding with the receptors on host cells is a promising therapeutic target7, and angiotensin-converting enzyme 2 (ACE2) is the most explored virus receptor on host cells8,9. Recent studies have demonstrated that ACE2-expressing exosomes can inhibit SARS-CoV-2 entry to and infect the host cells10, 11, 12, 13, 14, 15; for instance, we previously reported that the intranasal administration of ACE2+ exosomes can inhibit the colonization and infection of SARS-CoV-2 pseudovirus in the nasal epithelium11. However, SARS-CoV-2 can also infect the cells with low ACE2 expression, suggesting that there are alternative virus receptors in mediating the infection16. Spike protein can bind with some specific glycosaminoglycans (GAGs) on host cell membrane17, 18, 19, 20. In particular, S protein can efficiently bind with heparan sulfate (HS), and the binding allows spike protein to maintain an “open” conformation that facilitates the subsequent binding to ACE2 and TMPRSS2, respectively19,21. It has been revealed that the analog heparin is able to inhibit SARS-CoV-2 entry to host cells by blocking interaction between heparan sulfate and spike protein22,23. Therefore, heparin can be a therapeutic candidate.
However, administration of heparin relies on intravenous (i.v.) injection (only injection formulations clinically available), which has two major disadvantages. First, i.v. injection must be handled by a medical professional in clinical settings. Second, i.v. heparin in systemic circulation has a potential bleeding risk24.
To address these issues and develop a self-administrable and safe heparin-based therapy, we proposed the heparin nanoparticles (NPs) for anti-SARS-CoV-2 infection via inhalation delivery to facilitate the lung-specific drug distribution. Such a system can act as a nano-capturer to neutralize the virus, with a unique benefit of early treatment that is critical for preventing hospitalization and the chronic sequelae of COVID-19, saving lives, and relieving the overburden of medical systems25.
1. Effect of heparin on anti-infection of SARS-CoV-2 pseudovirus
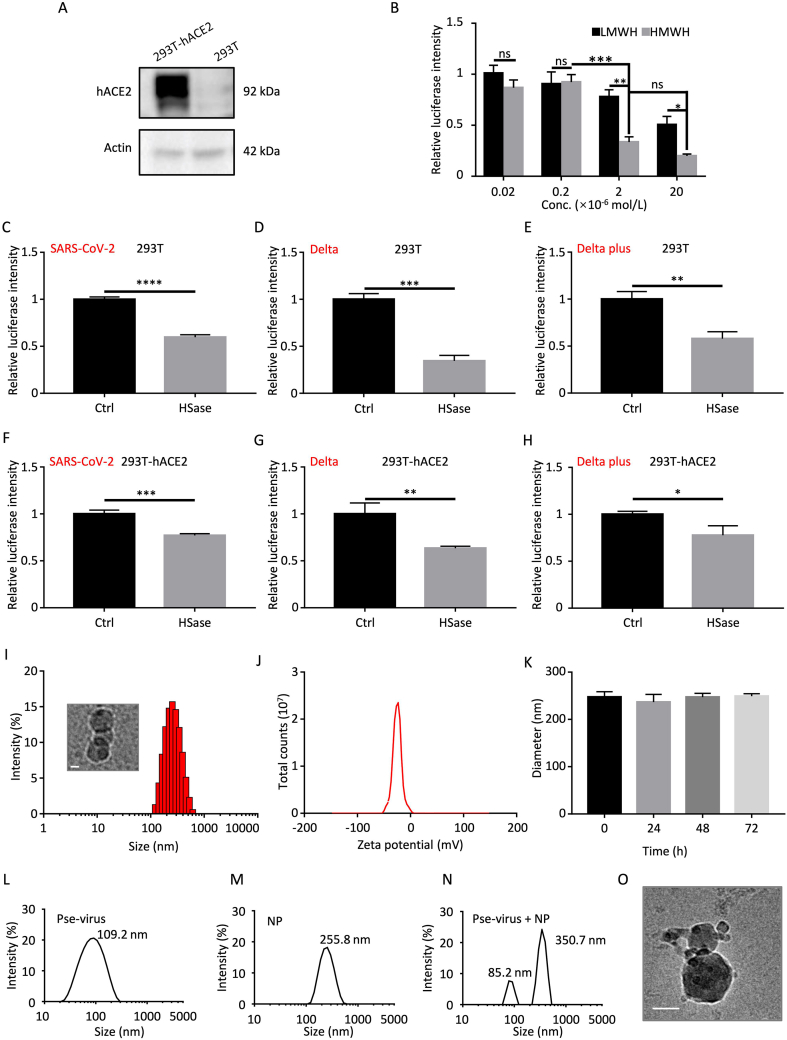
The heparins with different molecular weights (Supporting Information) were used in the study. Both high-molecular-weight heparin (HMWH) and low-molecular-weight heparin (LMWH) exhibited a dose-dependent inhibition against SARS-CoV-2 in 293T-hACE2 cells, and HMWH had a significantly higher efficacy of anti-infection than LMWH (Fig. 1A and B). Therefore, the HMWH was employed for the preparation of heparin nanoparticles. Our results are also consistent with a previous finding that longer heparin chains can bind more efficiently to multiple sites and also potentially create stronger steric hindrance to block the spike protein binding with the cell surface viral receptors22.
Figure 1.
Effect of heparin on anti-infection of SARS-CoV-2 pseudovirus and characterization of heparin NPs. (A) hACE2 expression in 293T-hACE2 and 293T cells. (B) Heparin with different molecular weights blocked SARS-CoV-2 infection in 293T-hACE2 cells. LMWH: MW 5600–6400; HMWH: MW 15,000–19,000). Data were normalized by the control group of the SARS-CoV-2 pseudovirus infected 293T-hACE2 cells without treatment. The 293T cells pretreated with heparinase were infected by SARS-CoV-2 pseudovirus (C), and Delta (D) and Delta plus (E) mutated strains. The 293T-hACE2 cells pretreated with heparinase were infected by SARS-CoV-2 pseudovirus (F), Delta (G), and Delta plus (H) mutated strains. (I) Particle size and the cryo-TEM of the NPs. Scale bar: 100 nm. (J) ζ-potential of the NPs. (K) The colloidal stability of the NPs. (L) Size of the pseudovirus. (M) Particle size of the NPs. (N) Size of the NP/pseudovirus complex. (O) The cryo-TEM image of the complex, scale bar: 100 nm. Data are presented as mean ± SD (n = 3); ns, no significance; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
To verify the role of cell surface heparan in mediating SARS-CoV-2 infection, we pretreated the host cells with heparinase to eliminate the surface heparan. The results showed heparinase pretreatment dramatically reduced the transfection efficiency of SARS-CoV-2 pseudovirus and the mutated strains (e.g., Delta and Delta plus) in both 293T cells and 293T-hACE2 cells (Fig. 1C–H). It indicated that the cell surface heparan was important for SARS-CoV-2 infection. Notably, the removal of heparan affected the infection in the 293T cells more significantly than the 293T-hACE2 cells, suggesting that the virus may heavily rely on heparan to enter the host cells with low ACE2 expression. More importantly, the Delta-mutated strain was very sensitive to the heparan elimination that caused the most significant inhibition against infection in both 293T and 293T-hACE2 among the tested strains.
2. The binding of heparin NPs with the pseudovirus
The poly-anionic heparin can crosslink by the cationic chitosan via charge interaction, thus forming the stable nanoparticles with spherical shape (Fig. 1I and K). Chitosan thus serves as a cross-linker in this nanodecoy system. The net charge of the heparin polysaccharide NPs was negative and thereby reduced the risk of positive charge-related side toxicity (Fig. 1J).
The efficient binding of heparin NPs and pseudovirus was demonstrated by the morphological change (cryo-TEM image, Fig. 1O). It also showed the increased particle size of the NP/pseudovirus complex (Fig. 1L–N). It should be mentioned that there is a minor peak of 82 nm, which could be the exosomes from the packing cells. Due to the close size of the exosomes and virus, both were collected by ultracentrifugation without further separation.
3. Neutralization of wide-type SARS-CoV-2 pseudovirus and Delta variants
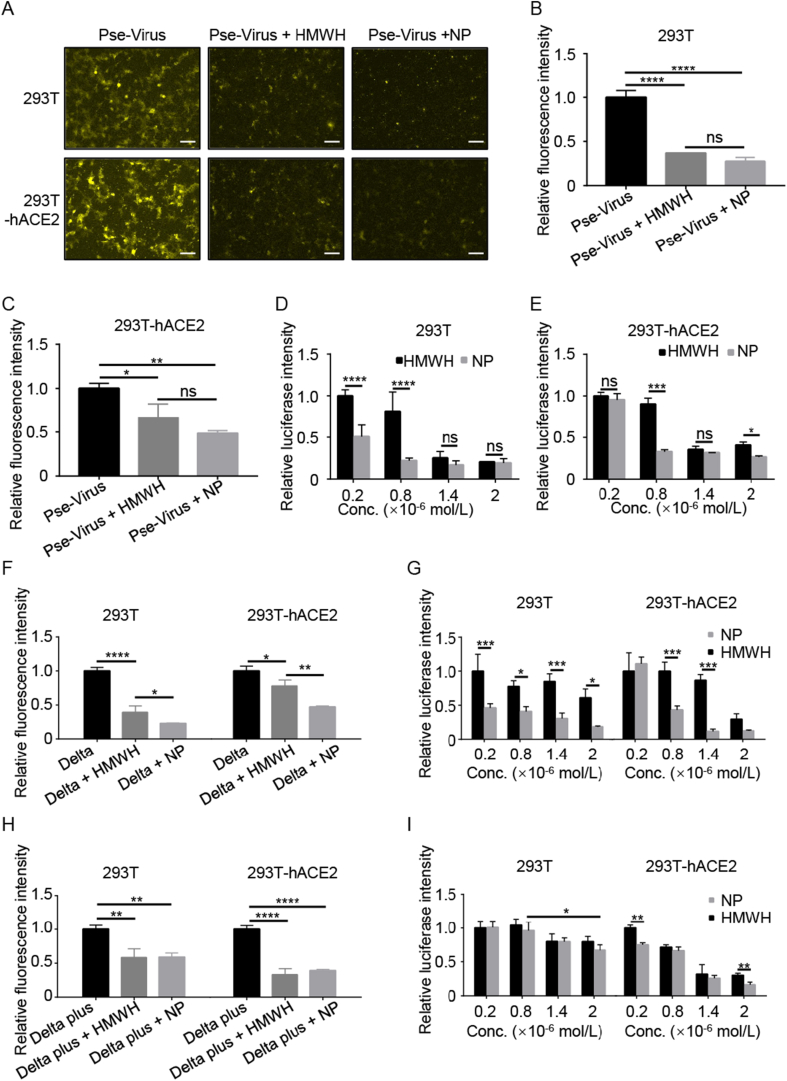
The 293T and 293T-hACE2 cells were used in this study because 293T cells is a common cell model for SARS-CoV-2 infection via ACE2 or HS receptors26. Also, 293T cells are convenient for genetic engineering for stable expression of a target protein (e.g., hACE2). Both HMWH and the heparin NPs efficiently inhibited the cell entry of SARS-CoV-2 pseudovirus in 293T cells and 293T-hACE2 cells as shown in the fluorescence images (Fig. 2A). The inhibition results were further confirmed by flow cytometry detection (Fig. 2B and C), which showed that the positive cells containing the Dil dye-labeled pseudovirus were dramatically decreased by treatment with HMWH or the heparin NPs. Accordingly, the transfection of pseudovirus in 293T and 293T-hACE2 cells was significantly suppressed by the treatment, as reflected by the decreasing level of luciferase (a reporter gene of pseudovirus) (Fig. 2D and E). Notably, the heparin NPs had a better inhibition effect than HMWH. This may due to the higher surface area in the nanoparticles27. In addition, the NPs can provide multiple ligands to bind with viruses and thus efficiently block the cell entry of viruses.
Figure 2.
Heparin NPs inhibited cell entry and transfection of SARS-CoV-2 pseudovirus, Delta and Delta plus mutated strains in 293T and 293T-hACE2 cells. (A) The cell entry of the Dil-labeled SARS-CoV-2 pseudovirus inhibited by HMWH and heparin NPs, scale bar = 100 μm. Flow cytometry assay of the cell entry of SARS-CoV-2 pseudovirus in 293T (B) and 293T-hACE2 cells (C). The transfection of SARS-CoV-2 pseudovirus inhibited by HMWH and heparin NPs in 293T cells (D) and 293T-hACE2 cells (E). (F) The cell entry inhibition of Delta pseudovirus by the NPs. (G) The inhibition of Delta pseudovirus transfection by the NPs in 293T cells and 293T-hACE2 cells. (H) The cell entry inhibition of Delta plus pseudovirus. (I) The inhibition of Delta plus pseudovirus transfection by the NPs. Data are presented as mean ± SD (n = 3); ns, no significance; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
The Delta variant SARS-CoV-2 has been a main threat in the current global pandemic28. Therefore, to develop a broad-spectrum antiviral agent is a pressing need under the current circumstance. It was discovered that the cell entry of Delta pseudovirus was significantly blocked by either HMWH or the heparin NPs, as reflected by the decreased amount of the positive cells containing the Dil-labeled Delta pseudovirus (Fig. 2F). Furthermore, both HMWH and the heparin NPs were able to inhibit Delta pseudovirus infection (Fig. 2G). Similarly, the heparin NPs had better efficacy against Delta pseudovirus infection. The result also implys that the heparin NPs could block the infection of Delta pseudovirus.
Another SARS-CoV-2 variant, Delta plus, has also raised public concerns for its increased transmissibility and reduction in monoclonal antibody response29. We also tested the inhibition efficacy against Delta plus variant. The results were promising that the heparin NPs were also highly efficient for suppressing the cell entry and infection of the Delta plus variant (Fig. 2H and I).
The findings demonstrate that the heparin NPs can serve as a potent antiviral agent effectively in various SARS-CoV-2 variants.
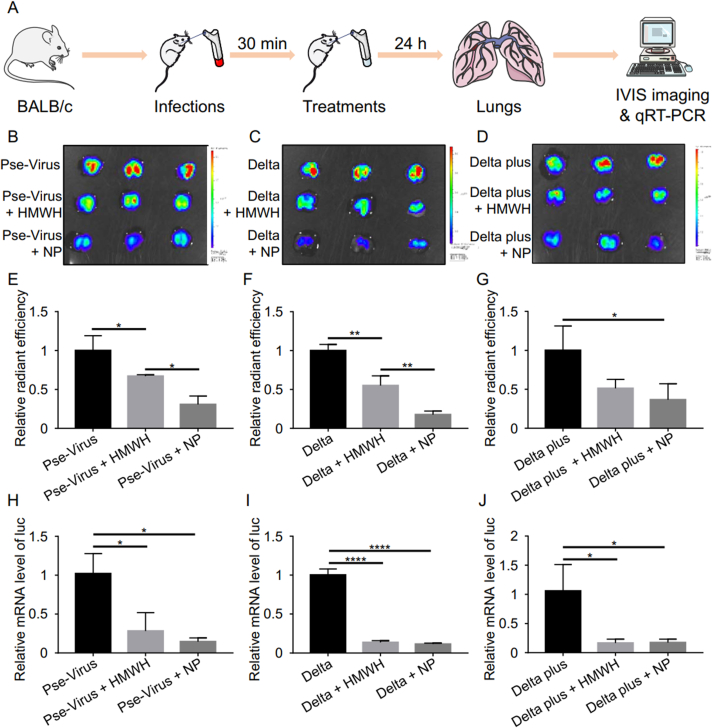
4. In vivo blockage of SARS-CoV-2 and Delta variants
Lung is the primary target organ in SARS-CoV-2 infection. Pulmonary drug delivery is generally considered to be an ideal administration route for anti-COVID-19 therapy due to direct access of drug to the lung30,31. Moreover, inhaled formulations also possess the benefits of self-administration, rapid onset of action, good patient compliance, and reduced systemic side effects31. The mouse model was established by introducing the pseudovirus to infect the lung tissue through the bronchus. The inhibition efficacy of the heparin NPs against infection was evaluated by inhaled administration. After treatment with either HMWH or the heparin NPs, the accumulation of the pseudovirus (including the wide-type, Delta, and Delta plus) in the lung was significantly reduced (Fig. 3B–G). The heparin NPs showed a higher anti-viral efficacy than HMWH. Moreover, the inhibition efficacy of the heparin NPs against transfection by different pseudovirus was evaluated by monitoring the level of the reporter gene luciferase in the lung. The data further demonstrate that the NPs could significantly reduce infection of all the test variants of pseudovirus (Fig. 3H–J), suggesting that the heparin NPs can serve as a broad-spectrum anti-SARS-CoV-2 drug candidate against various variants.
Figure 3.
Heparin NPs inhibited infection in lung tissues. (A) Schematic illustrating the inhibition of pseudovirus infection to the lung in vivo. Ex vivo imaging of the lung tissues exposed to SARS-CoV-2 pseudovirus (B), Delta pseudovirus (C), and Delta plus pseudovirus (D); the fluorescence intensity indicated the amount of DiR-labeled pseudovirus in the lung. Ex vivo radiant efficiency of the lung tissues exposed to SARS-CoV-2 pseudovirus (E), Delta pseudovirus (F), and Delta plus pseudovirus (G). The luciferase (luc) mRNA level in the lung exposed to SARS-CoV-2 pseudovirus (H), Delta (I), and Delta plus pseudovirus (J). Data are presented as mean ± SD (n = 3); ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001.
5. Clearance of the NP/virus via phagocytosis by macrophages
Macrophages are the major scavenger for clearing away pathogens via phagocytosis32. For instance, the immune complexes formed by the binding of antibody/virus to the NPs, can be identified and phagocytized by macrophages33. Our results show that the viruses neutralized by the heparin NPs were increasingly captured by the macrophages, compared to the control groups (Supporting Information Fig. S1), suggesting that the thus-formed NP/virus complexes were efficiently identified by the macrophages as an exogenous component and removed away.
A previous study revealed that a large number of monocyte-derived macrophages were involved in COVID-19 patients’ lungs34. Macrophages play an important role in mitigating respiratory virus infection35. Phagocytosis by macrophages is highly dependent on the component sizes; e.g., the macrophages exhibited an enhanced ability to phagocytose the nanoparticles less than 500 nm36. Therefore, the NP/virus complex with a relatively large size about 350 nm could facilitate the capture by the macrophages.
6. The clinical application prospects
Both heparin and chitosan have been widely used in pharmaceutical products. Specifically, inhaled delivery of heparin and chitosan has been widely explored in either clinical or preclinical studies, and the biosafety has been demonstrated37, 38, 39. Heparin is an anticoagulation drug and has been used for the patients in the advanced stage of COVID-1940. A retrospective analysis suggests the treatment benefits of heparin in the patients with severe COVID-19 meeting sepsis-induced coagulopathy (SIC) criteria or with markedly elevated D-dimer41. In-hospital heparin treatment in severely ill COVID-19 patients and in those with strong coagulation activation was associated with a lowering mortality42. Heparin can also be used as an immunomodulatory drug to treat the hyperinflammatory response43. But most clinical trials just emphasized the coagulation-related symptoms of advanced COVID-19. The early prevention of SARS-CoV-2 using heparin is still under-explored.
Nanotechnology has been actively explored for the application against SARS-CoV-244, 45, 46. In this work, it was demonstrated that the efficacy of the heparin NPs served as nanodecoy for neutralizing SARS-CoV-2 and the Delta-mutated strains. Importantly, heparin can inhibit the enzymatic activity of Furin that cleaves many viral glycoproteins with polybasic residues (e.g., SARS-CoV-2 spike protein), thus activating the S protein47. Notably, a simulation study revealed that the omicron variant has the increased furin-binding ability compared to the wide-type SARS-CoV-2, suggesting that the stronger furin binding could lead to the more efficient fusion at molecular level and higher viral load on the host48. Therefore, it is expected that this heparin nanodecoy strategy is potentially workable in anti-Omicron variant infection.
Besides, heparin can inhibit another protease, Factor Xa, in host cells, which is necessary for processing the spike protein49. Therefore, heparin-based neutralization therapy may involve multiple mechanisms against the infection of SARS-CoV-2 and its variants. Yet, further experiments will be needed to confirm the prediction.
It should be noted that a recent clinical trial revealed that LMWH (i.e., Enoxaparin) had a better treatment outcome in hospitalized COVID-19 patients than unfractionated heparin, but the analysis just focused on the impact of the COVID-associated coagulation cascades50. Heparin is expected to have multiple synergistic functions when applied to the treatment of COVID-1951, apart from the neutralization effect. In terms of neutralization effect, our results suggest that heparin with high molecular weight yields better efficacy of anti-SARS-CoV-2 infection.
For early treatment purposes, a self-administrable dosage form is the priority in drug development. We developed a nanodecoy strategy for anti-COVID-19 by using the heparin NPs to neutralize SARS-CoV-2. The results reveal the superiority of the heparin NPs over heparin. The NPs serve as an effective inhibitor against infection of both wide type virus and Delta mutations in the pseudovirus test. Pulmonary delivery of the heparin NPs can direct access the lung and reduce the unwanted drug exposure to other organs. The preparation of the NPs is easy and rapid. Therefore, the heparin NPs have a potential value for clinical translation.
Acknowledgments
We are thankful for the support of the National Key Research and Development Program of China (2021YFE0103100, China), National Natural Science Foundation of China of China (81925035 and 81521005), Shanghai Sci-Tech Innovation Initiative (19431903100, 18430740800, China), and the Sanofi-SIBS Yong Faculty Award (China), and the Youth Innovation Promotion Association (China). We thank the Molecular Imaging Center and TEM Facility at Shanghai Institute of Materia Medica for the technical support.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.01.019.
Contributor Information
Huiyuan Wang, Email: wanghuiyuan@simm.ac.cn.
Yongzhuo Huang, Email: yzhuang@simm.ac.cn.
Author contributions
Yongzhuo Huang and Huiyuan Wang designed the research and performed data analysis. Bin Tu and Huiyuan Wang carried out the experiments. Xinran An, Jingkun Qu, Qianqian Li, Yanrong Gao, and Mingjie Shi participated part of the experiments. Bin Tu and Huiyuan Wang wrote the manuscript. Hong Qiu assisted the concept development and result analysis. Yongzhuo Huang finalized the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Covid-19 coronavirus pandemic. Available at: https://www.worldometers.info/coronavirus/. (Accessed Jan 27, 2022).
- 2.Vilar S., Isom D.G. One year of SARS-CoV-2: how much has the virus changed? Biology (Basel) 2021;10:91. doi: 10.3390/biology10020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B., Goh Y.S., Fong S.W., Young B.E., Ngoh E.Z.X., Chavatte J.M., et al. Resistance of SARS-CoV-2 Delta variant to neutralization by BNT162b2-elicited antibodies in Asians. Lancet Reg Health West Pac. 2021;15:100276. doi: 10.1016/j.lanwpc.2021.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., Liu Y., Xia H., Zou J., Weaver S.C., Swanson K.A., et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596:273–275. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 6.Pouwels K.B., Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Vihta K.D., et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021 doi: 10.1038/s41591-021-01548-7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., Wu Y., Yao S., Ge H., Zhu Y., Chen K., et al. Discovery of potential small molecular SARS-CoV-2 entry blockers targeting the spike protein. Acta Pharmacol Sin. 2021 doi: 10.1038/s41401-021-00735-z. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian J., Li Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm Sin B. 2021;11:1–12. doi: 10.1016/j.apsb.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C., Xu Q., Wang H., Tu B., Zeng J., Zhao P., et al. Neutralization of SARS-CoV-2 pseudovirus using ACE2-engineered extracellular vesicles. Acta Pharm Sin B. 2022;3:1523–1533. doi: 10.1016/j.apsb.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocozza F., Nevo N., Piovesana E., Lahaye X., Buchrieser J., Schwartz O., et al. Extracellular vesicles containing ACE2 efficiently prevent infection by SARS-CoV-2 Spike protein-containing virus. J Extracell Vesicles. 2020;10 doi: 10.1002/jev2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q., Jeppesen D.K., Higginbotham J.N., Franklin J.L., Crowe J.E., Jr., Coffey R.J. Angiotensin-converting enzyme 2-containing small extracellular vesicles and exomeres bind the severe acute respiratory syndrome coronavirus 2 spike protein. Gastroenterology. 2021;160:958–961 e3. doi: 10.1053/j.gastro.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie F., Su P., Pan T., Zhou X., Li H., Huang H., et al. Engineering extracellular vesicles enriched with palmitoylated ACE2 as COVID-19 therapy. Adv Mater. 2021 doi: 10.1002/adma.202103471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Huang F., Xia B., Yuan Y., Yu F., Wang G., et al. The interferon-stimulated exosomal hACE2 potently inhibits SARS-CoV-2 replication through competitively blocking the virus entry. Signal Transduct Target Ther. 2021;6:189. doi: 10.1038/s41392-021-00604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troyer Z., Alhusaini N., Tabler C.O., Sweet T., de Carvalho K.I.L., Schlatzer D.M., et al. Extracellular vesicles carry SARS-CoV-2 spike protein and serve as decoys for neutralizing antibodies. J Extracell Vesicles. 2021;10 doi: 10.1002/jev2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Xu Q., Ma L., Wu D., Gao J., Chen G., et al. Systematic profiling of ACE2 expression in diverse physiological and pathological conditions for COVID-19/SARS-CoV-2. J Cell Mol Med. 2020;24:9478–9482. doi: 10.1111/jcmm.15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Donnell C.D., Shukla D. The importance of heparan sulfate in herpesvirus infection. Virol Sin. 2008;23:383–393. doi: 10.1007/s12250-008-2992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao W., Ma B., Li Z., Wang X., Gao X., Li Y., et al. Binding of the SARS-CoV-2 spike protein to glycans. Sci Bull (Beijing) 2021;66:1205–1214. doi: 10.1016/j.scib.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mycroft-West C.J., Su D., Pagani I., Rudd T.R., Elli S., Gandhi N.S., et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb Haemost. 2020;120:1700–1715. doi: 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L., Chopra P., Li X., Bouwman K.M., Tompkins S.M., Wolfert M.A., et al. Heparan sulfate proteoglycans as attachment factor for SARS-CoV-2. ACS Cent Sci. 2021;7:1009–1018. doi: 10.1021/acscentsci.1c00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057 e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tree J.A., Turnbull J.E., Buttigieg K.R., Elmore M.J., Coombes N., Hogwood J., et al. Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations. Br J Pharmacol. 2021;178:626–635. doi: 10.1111/bph.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S.Y., Jin W., Sood A., Montgomery D.W., Grant O.C., Fuster M.M., et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 2020;181:104873. doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsh J. Heparin induced bleeding. Nouv Rev Fr Hematol. 1984;26:261–266. [PubMed] [Google Scholar]
- 25.Kim P.S., Read S.W., Fauci A.S. Therapy for early COVID-19: a critical need. JAMA. 2020;324:2149–2150. doi: 10.1001/jama.2020.22813. [DOI] [PubMed] [Google Scholar]
- 26.Yue J., Jin W., Yang H., Faulkner J., Song X., Qiu H., et al. Heparan sulfate facilitates spike protein-mediated SARS-CoV-2 host cell invasion and contributes to increased infection of SARS-CoV-2 G614 mutant and in lung cancer. Front Mol Biosci. 2021;8:649575. doi: 10.3389/fmolb.2021.649575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tammam S.N., El Safy S., Ramadan S., Arjune S., Krakor E., Mathur S. Repurpose but also (nano)-reformulate! the potential role of nanomedicine in the battle against SARS-CoV2. J Control Release. 2021;337:258–284. doi: 10.1016/j.jconrel.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin E.J., Baden L.R., Udwadia Z.F., Morrissey S. Audio interview: India's covid-19 crisis. N Engl J Med. 2021;384:e84. doi: 10.1056/NEJMe2107728. [DOI] [PubMed] [Google Scholar]
- 29.Kannan S.R., Spratt A.N., Cohen A.R., Naqvi S.H., Chand H.S., Quinn T.P., et al. Evolutionary analysis of the Delta and Delta plus variants of the SARS-CoV-2 viruses. J Autoimmun. 2021;124:102715. doi: 10.1016/j.jaut.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebrahimi S., Shamloo A., Alishiri M., Mofrad Y.M., Akherati F. Targeted pulmonary drug delivery in coronavirus disease (COVID-19) therapy: a patient-specific in silico study based on magnetic nanoparticles-coated microcarriers adhesion. Int J Pharm. 2021;609:121133. doi: 10.1016/j.ijpharm.2021.121133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashid M.A., Muneer S., Mendhi J., Sabuj M.Z.R., Alhamhoom Y., Xiao Y., et al. Inhaled Edoxaban dry powder inhaler formulations: development, characterization and their effects on the coagulopathy associated with COVID-19 infection. Int J Pharm. 2021;608:121122. doi: 10.1016/j.ijpharm.2021.121122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016;44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Tay M.Z., Wiehe K., Pollara J. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front Immunol. 2019;10:332. doi: 10.3389/fimmu.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 35.Newton A.H., Cardani A., Braciale T.J. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38:471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M., Rosenberg J., Cai X., Hsuan Lee A.C., Shi J., Nguyen M., et al. Nanotraps for the containment and clearance of SARS-CoV-2. Matter. 2021;4:2059–2082. doi: 10.1016/j.matt.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bendstrup K.E., Gram J., Jensen J.I. Effect of inhaled heparin on lung function and coagulation in healthy volunteers. Eur Respir J. 2002;19:606–610. doi: 10.1183/09031936.02.00105202. [DOI] [PubMed] [Google Scholar]
- 38.Yildiz-Pekoz A., Ozsoy Y. Inhaled heparin: therapeutic efficacy and recent formulations. J Aerosol Med Pulm Drug Deliv. 2017;30:143–156. doi: 10.1089/jamp.2015.1273. [DOI] [PubMed] [Google Scholar]
- 39.Rasul R.M., Tamilarasi Muniandy M., Zakaria Z., Shah K., Chee C.F., Dabbagh A., et al. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr Polym. 2020;250:116800. doi: 10.1016/j.carbpol.2020.116800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi C., Tingting W., Li J.P., Sullivan M.A., Wang C., Wang H., et al. Comprehensive landscape of heparin therapy for COVID-19. Carbohydr Polym. 2021;254:117232. doi: 10.1016/j.carbpol.2020.117232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Castelnuovo A., Costanzo S., Antinori A., Berselli N., Blandi L., Bonaccio M., et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: the multicenter Italian CORIST study. Thromb Haemost. 2021;121:1054–1065. doi: 10.1055/a-1347-6070. [DOI] [PubMed] [Google Scholar]
- 43.Mulloy B., Hogwood J., Gray E., Lever R., Page C.P. Pharmacology of heparin and related drugs. Pharmacol Rev. 2016;68:76–141. doi: 10.1124/pr.115.011247. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., et al. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H., Zhu W., Jin Q., Pan F., Zhu J., Liu Y., et al. Inhalable nanocatchers for SARS-CoV-2 inhibition. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2102957118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao L., Xia S., Xu W., Tian R., Yu G., Gu C., et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci U S A. 2020;117:27141–27147. doi: 10.1073/pnas.2014352117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng J., Meng Y., Chen S.Y., Zhao G., Wang L., Zhang E.X., et al. Structural characteristics of Heparan sulfate required for the binding with the virus processing Enzyme Furin. Glycoconj J. 2021 doi: 10.1007/s10719-021-10018-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jawaid M.Z., Baidya A., Mahboubi-Ardakani R., Davis L.R., Cox L.D. Simulation of the omicron variant of SARS-CoV-2 shows broad antibody escape, weakened ACE2 binding, and modest increase in furin binding. bioRxiv. 2021 doi: 10.1128/spectrum.01213-22. 2021.12.14.472704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kastenhuber E.R., Jaimes J.A., Johnson J.L., Mercadante M., Muecksch F., Weisblum Y., et al. Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry. bioRxiv. 2021 doi: 10.7554/eLife.77444. 2021.03.31.437960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pawlowski C., Venkatakrishnan A.J., Kirkup C., Berner G., Puranik A., O'Horo J.C., et al. Enoxaparin is associated with lower rates of mortality than unfractionated Heparin in hospitalized COVID-19 patients. EClinicalMedicine. 2021;33:100774. doi: 10.1016/j.eclinm.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gozzo L., Viale P., Longo L., Vitale D.C., Drago F. The potential role of heparin in patients with COVID-19: beyond the anticoagulant effect. A review. Front Pharmacol. 2020;11:1307. doi: 10.3389/fphar.2020.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.