Abstract
Background
Because of the loss of chloroquine (CQ) effectiveness, the Democratic Republic of Congo (DRC)’s malaria treatment policy replaced CQ by sulfadoxine–pyrimethamine (SP) as first-line treatment of uncomplicated malaria in 2003, which in turn was replaced by artemisinin-based combination therapies (ACT) in 2005. The World Health Organization (WHO) recommends monitoring of anti-malarial drug resistance every 2 years. The study aimed to provide baseline data for biennial molecular surveillance of anti-malarial drug resistance by comparing data from a study conducted in 2019 to previously published data from a similar study conducted in 2017 in the DRC.
Methods
From July to November 2019, a cross-sectional study was conducted in ten sites which were previously selected for a similar study conducted in 2017 across the DRC. P. falciparum malaria was diagnosed by a rapid diagnostic test (RDT) or by microscopy and dried blood samples (DBS) were taken from patients who had a positive test. Segments of interest in pfcrt and pfk13 genes were amplified by conventional PCR before sequencing.
Results
Out of 1087 enrolled patients, 906 (83.3%) were PCR-confirmed for P. falciparum. Like in the 2017-study, none of the mutations known to be associated with Artemisinine (ART) resistance in Southeast Asia was detected. However, non-synonymous (NS) mutations with unknown functions were observed among which, A578S was detected in both 2017 and 2019-studies. The overall prevalence of pfcrt-K76T mutation that confers CQ-resistance was 22.7% in 2019-study compared to 28.5% in 2017-study (p-value = 0.069), but there was high variability between sites in the two studies. Like in 2017-study, the pfcrt 72–76 SVMNT haplotype associated with resistance to amodiaquine was not detected.
Conclusion
The study reported, within 2 years, the non-presence of molecular markers currently known to be associated with resistance to ART and to AQ in P. falciparum isolated in the DRC. However, the presence of polymorphisms with as-yet unknown functions was observed, requiring further characterization. Moreover, an overall decrease in the prevalence of CQ-resistance marker was observed in the DRC, but this prevalence remained highly variable from region to region.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07112-z.
Keywords: Biennial, Surveillance, Plasmodium falciparum, Anti-malarial, Resistance, Markers, Democratic Republic of Congo
Background
The spread of resistance to anti-malarial drugs poses a serious public health risk and a real challenge for malaria control in endemic countries. As result of high treatment failure rate (> 10%) with chloroquine (CQ) and sulfadoxine–pyrimethamine (SP) and following the World Health Organization (WHO) recommendation, the Democratic republic of Congo (DRC) malaria national policy implemented artemisinin-based Combination Therapies (ACT) as first-line treatment of uncomplicated Plasmodium falciparum malaria in 2005 [1, 2]. WHO recommends biennial surveillance of anti-malarial drug efficacy to ensure early detection of emergence and spread of resistance. Therapeutic efficacy studies (TESs) are the gold standard for the monitoring of anti-malarial efficacy. However, today, in addition to TETs, the assessment of molecular marker in P. falciparum genes associated with anti-malarial drug resistance provides useful information about the emergence and spread of resistant parasites [3].
Point mutations in the chloroquine resistance transporter gene (pfcrt), notably mutations in amino acids position 72 to 76 have been found to confer resistance to both CQ and amodiaquine (AQ) [4]. The amino acid substitution from lysine (K) to threonine (T) at position 76 of the PFCRT protein (K76T) has been shown to confer CQ resistance [5], while the 72–76 PFCRT haplotype, namely SVMNT (Serine–Valine–Methionine–Asparagine–Threonine) has been linked to AQ resistance [6]. K76T mutation, which is considered as the most reliable molecular marker of CQ resistance, is found in all CQ-resistant isolates of P. falciparum [5]. The decline in this CQ-resistance marker following the official CQ removal from national treatment guidelines varies significantly between countries. In several countries, it was reported that after the discontinuance of CQ use, K76T-mutant parasites have been replaced by wild ones, resulting in the decrease in prevalence of K76T marker [7–10]. In other countries, studies reported the persistence of resistant strains several years after stopping the use of CQ as first-line treatment of uncomplicated malaria [11, 12]. In the DRC, the prevalence of K76T marker was 55.4% in a study conducted with samples obtained from 2007 Demographic and Health Survey (2007-DHS) [13] and 63.9% in a study conducted in 2014 [14]. Moreover, a large variability in this prevalence of K76T was observed across the DRC in 2017, with presence of very high prevalence (89.5%) in one province and very low (1.5%) in another [15].
Single-nucleotide polymorphisms (SNPs) occurring within P. falciparum Kelch 13 gene (pfk13), precisely in the propeller domain region, have been described as molecular markers of ART resistance [16, 17]. The list of these SNPs categorized as validated mutations and candidate mutations of ART resistance is updated by the WHO [18]. Originally, these mutations have emerged and spread in Southeast Asia, but nowadays, some of them are increasingly detected in sub-Saharan African countries providing evidence for de novo emergence of resistance to ART in Sub-Saharan African countries [19–22].
To help improve long-term monitoring of anti-malarial drug resistance, the present study aimed to provide baseline data for biennial molecular surveillance of P. falciparum anti-malarial drug resistance by comparing data from the study conducted in 2019 to previously published data from a similar study conducted in 2017 in the DRC [15, 23].
Methods
Study population and blood samples collection
From July to November 2019, a cross-sectional study was conducted in ten sites (sentinel sites of National Malaria Control Programme) located in ten provinces: Kingasani in Kinshasa, Kabondo in Kisangani/Tshopo, Lubumbashi in Haut-Katanga, Bolenge in Equateur, Karawa in Nord-Ubangi, Vanga in Kwilu, Kalima in Maniema, Kamina in Lomami, Fungurume in Lwalaba and Katana in Sud-Kivu (Fig. 1). P. falciparum malaria was diagnosed using a rapid diagnostic test (RDT) or the microscopy in all patients with fever or history of fever in the last 24 h, who presented at one of the selected health structures of each study site. The RDT used was the one available on the site during sample collection (SD Bioline Malaria Ag P.f/Standard Diagnostics/South Korea or CareStart Malaria Pf/Access Bio/South Korea) while the light microscopy of Giemsa-stained thick blood test was used in some referral health structures where it was feasible. To participate in the study, informed consent was obtained from each adult patient (≥ 18 years old) or from a parent or legal guardian for children and adolescents. Blood samples were collected on filter paper as dried blood spots (DBS) and then transported at the University of Kinshasa for storage at – 20 °C until use.
Fig. 1.
Geographical distribution of study sites across the Democratic republic of Congo (outline map: http://johan.lemarchand.free.fr/cartes/cartes-republique-democratique-du-congo.html)
Molecular analysis
Extraction of genomic DNA from DBS was carried out using the QIAamp DNA Mini Kit (QIAGEN GmbH, Germany) according to the manufacturer’s instruction (https://www.qiagen.com). Briefly, the DNA purification was performed following the manufacturer’s manual procedure which included four steps: cell lysis, DNA binding to the QIAamp membrane, washing contaminants and DNA elution. The extracted DNA was kept at − 20 °C until time of use. To detect P. falciparum, a real-time Pf-PCR assay was performed according to a previously described procedure [24], which was previously modified [15]. Samples with PCR-confirmed presence of P. falciparum were used to amplify segments of interest on pfk13 and pfcrt genes. The procedures previously described were used to amplify the interest segment on pfcrt gene [25] and that on the propeller region of pfk13 gene [23]. The analysis of P. falciparum genes polymorphisms was done by DNA bi-directional sequencing using Sanger method as previously described [15, 23]. Nucleotides sequences were analyzed using Vector NTI (Thermo Fisher Scientific, US) and aligned to the reference annotated PF3D7 (PF3D7_0709000 for PFCRT sequences and PF3D7_1343700 for PFK13 sequences) to assess SNPs.
Data analysis
Data were encoded and verified in a Microsoft Excel 2010 database and imported in SPSS Statistics 20.0 for analysis. Only isolates successfully sequenced have been considered to calculate the prevalence of each SNP. Frequencies were statistically compared using Chi-squared tests. Secondary analyses were conducted using logistic regression model to determine if the marginal associations from the univariate model matched those of the multivariate model. The p-values less than 0.05 were considered significant.
Results
Baseline characteristics of enrolled patients from 2019-study compared to 2017-study
In total, 1087 patients were enrolled in 2019-study in comparison to 1070 in 2017-study. Their age ranged from 0 to 77 years old. Table 1 presents the distribution of patients according to different characteristics in comparison to 2017-study patients. The distribution per age range showed a low difference (p-value = 0.01) between 2017-study and 2019-study while no difference was observed when considered per sex. Among enrolled patients, 906 (83.3%) were confirmed positive for P. falciparum by real-time PCR in 2019-study versus 805 (75.3%) in 2017-study (p < 0.001).
Table 1.
Characteristics of enrolled patients from 2019-study compared to 2017-study
| Characteristic | 2017-study | 2019-study | p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (year) | 0.010 | ||||
| 0–5 | 465 | 43.5 | 437 | 40.2 | |
| 6–12 | 259 | 24.2 | 225 | 20.7 | |
| 13–19 | 107 | 10.0 | 127 | 11.7 | |
| 20 and + | 239 | 22.3 | 298 | 27.4 | |
| Total | 1070 | 1087 | |||
| Sex | 0.649 | ||||
| Female | 551 | 51.5 | 573 | 52.5 | |
| Male | 519 | 48.5 | 519 | 47.5 | |
| Site | 0.138 | ||||
| Bolenge | 98 | 9.2 | 99 | 8.7 | |
| Fungurume | 92 | 8.6 | 100 | 8.8 | |
| Kalima | 97 | 9.1 | 100 | 8.8 | |
| Kamina | 98 | 9.2 | 99 | 8.7 | |
| Karawa | 97 | 9.1 | 104 | 9.2 | |
| Katana | 105 | 9.8 | 87 | 7.7 | |
| Kingasani | 160 | 15.0 | 150 | 13.2 | |
| Kisangani | 128 | 12.0 | 135 | 11.9 | |
| Lubumbashi | 101 | 9.4 | 157 | 13.8 | |
| Vanga | 94 | 8.8 | 104 | 9.2 | |
| Number episode of malaria last 12 months | < 0.001 | ||||
| 0 | 529 | 49.4 | 458 | 40.4 | |
| 1 | 119 | 11.1 | 295 | 26.0 | |
| 2 | 221 | 20.7 | 170 | 15.0 | |
| 3 | 122 | 11.4 | 123 | 10.8 | |
| 4 and + | 79 | 7.4 | 89 | 7.8 | |
| P. falciparum PCR | < 0.001 | ||||
| Negative | 264 | 24.7 | 181 | 16.7 | |
| Positive | 805 | 75.3 | 906 | 83.3 | |
| Total | 1069 | 100.0 | 1087 | 100.0 | |
N: Number
Prevalence of chloroquine resistance marker according to patients’ characteristics from 2017-study and 2019-study
As shown in Table 2, the prevalence of CQ-resistance marker was not different between age groups and sex categories (p > 0.05). Globally, this prevalence decreased from 28.5% in 2017-study to 22.7% in 2019-study (p = 0.007), but it was highly variable between sites.
Table 2.
Chloroquine resistance marker and patient characteristics from merged 2017/2019 studies
| CQ-resistance | No CQ-resistance | p-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (year) | 0.163 | ||||
| 0–5 | 153 | 23.2 | 506 | 76.8 | |
| 6–12 | 108 | 29.3 | 260 | 70.7 | |
| 13–19 | 48 | 27.9 | 124 | 72.1 | |
| 20 and + | 93 | 26.1 | 264 | 73.9 | |
| Total | 402 | 25.8 | 1154 | 74.2 | |
| Sex | 0.689 | ||||
| Female | 208 | 25.5 | 608 | 74.5 | |
| Male | 196 | 26.4 | 547 | 73.6 | |
| Total | 404 | 25.9 | 1155 | 74.1 | |
| Site | < 0.001 | ||||
| Bolenge | 35 | 26.5 | 97 | 73.5 | |
| Fungurume | 2 | 1.5 | 132 | 98.5 | |
| Kalima | 7 | 4.8 | 140 | 95.2 | |
| Kamina | 14 | 9.2 | 138 | 90.8 | |
| Karawa | 17 | 12.4 | 120 | 87.6 | |
| Katana | 132 | 86.8 | 20 | 13.2 | |
| Kingasani | 112 | 42.4 | 152 | 57.6 | |
| KIsangani | 10 | 5.6 | 170 | 94.4 | |
| Lubumbashi | 6 | 4.4 | 131 | 95.6 | |
| Vanga | 72 | 44.7 | 89 | 55.3 | |
| Number episode of malaria last 12 months | 0.001 | ||||
| 1 | 54 | 18.6 | 236 | 81.4 | |
| 2 | 71 | 26.5 | 197 | 73.5 | |
| 3 | 51 | 28.3 | 129 | 71.7 | |
| 4 and + | 52 | 38.2 | 84 | 61.8 | |
| Total | 407 | 25.5 | 1189 | 74.5 | |
| Study | 0.007 | ||||
| 2017-study | 218 | 28.5 | 545 | 71.4 | |
| 2019-study | 189 | 22.7 | 644 | 77.3 | |
| Total | 407 | 25.5 | 1189 | 74.5 | |
CQ: chloroquine, N: number
Pfk13-propeller polymorphisms and PfCRT-K76T mutation per site in comparison of 2019-study to 2017-study
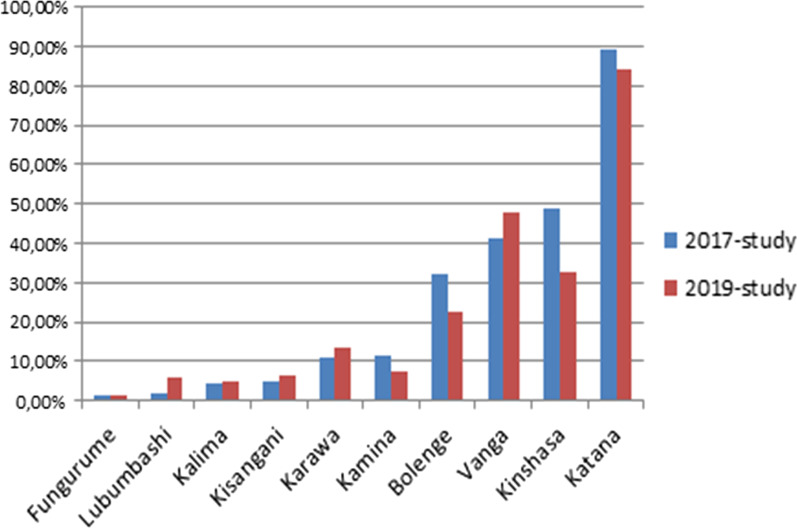
In 2019-study, among 822 isolates successfully sequenced on pfk13-propeller gene, following non-synonymous mutations have been detected: M472L, D516A, V568M, S576T, A578S, D584E and Y588C (Additional file 1: Table S1), A578S and D584E were each found in two isolates. A578S mutation was the only one detected in both 2017 and 2019-studies in Fungurume and in Kinshasa while all other mutations were detected once (p < 0.001). On pfcrt gene, when considered in the same site, the prevalence of K76T mutation did not shown significant difference between 2019-study and 2017-study (p > 0.05), except in Kinshasa (Table 3), while when comparing site by site, this prevalence remained highly variable in the two studies ranging from 1.5 to 89.5% in 2017-study and from 1.4 to 84.2% in 2019-study respectively in Fungurume and in Katana (p < 0.001) (Fig. 2). The 72–76 PfCRT CVIET haplotype was the most prevalent among the K76T-mutant parasites (93.6% in 2019-study versus 95.9% in 2017-study), while the SVMNT haplotype was detected neither by the 2019-study nor the 2017-study.
Table 3.
PfCRT-K76T mutation and pfk13-propeller polymorphisms per site and per study
| Site | pfcrt-K72T | pfk13mutation | |||
|---|---|---|---|---|---|
| 2017-study n/N (%) |
2019-study n/N (%) |
p-value | 2017-study | 2019-study | |
| Bolenge | 18/56 (32.1) | 17/76 (22.4) | 0.21 | A578S | |
| Fungurume | 1/65 (1.5) | 1/69 (1.4) | 0.99 | A578S | A578S |
| Kalima | 3/66 (4.5) | 4/81 (4.9) | 0.99 | – | |
| Kamina | 8/71 (11.3) | 6/81 (7.4) | 0.42 | A569T | D584E, V568M |
| Karawa | 6/54 (11.1) | 11/83 (13.3) | 0.71 | – | D584E |
| Katana | 68/76 (89.5) | 64/76 (84.2) | 0.33 | M472I | S576T |
| Kinshasa | 78/160 (48.8) | 34/104 (32.7) | 0.01 | A578S | A578S, M472L |
| Kisangani | 4/85 (4.7) | 6/95 (6.3) | 0.89 | A578S | D516A |
| Lubumbashi | 1/56 (1.8) | 5/82 (6.1) | 0.43 | – | Y588C |
| Vanga | 31/75 (41.3) | 41/86 (47.7) | 0.42 | V534A | |
| Total | 218/764 (28.5) | 189/833 (22.7) | 0.007 | ||
Pf: Plasmodium falciparum, n: Number of isolates carrying K76T, N: Number of isolates successfully sequenced
Fig. 2.
Distribution of K76T mutation from 2019-study versus 2017-study site per site across Democratic Republic of Congo showing the persistence of high variability of the CQ-resistance marker prevalence between sites
Chloroquine resistance marker and patients’ characteristics by logistic regression model
Unlike univariate model, the multivariate logistic regression model showed no statistical difference between the prevalence of CQ-resistance from 2017-study and that from 2019-study (p = 0.069; OR = 0.76; IC 95%: 0.56–1.02). However, the between sites variability of this prevalence has been confirmed by the logistic regression model, as shown in Table 4.
Table 4.
Chloroquine resistance marker and patients’ characteristics by logistic regression
| Chloroquine resistance | p-value | ||
|---|---|---|---|
| OR | IC95% | ||
| Age (year) | |||
| 0–5 | 1 | ||
| 6–12 | 1.04 | 0.72–1.48 | 0.825 |
| 13–19 | 0.94 | 0.57–1.55 | 0.812 |
| 20 and + | 0.89 | 0.60–1.33 | 0.601 |
| Sex | |||
| Female | 1 | ||
| Male | 1.08 | 0.81–1.45 | 0.584 |
| Site | |||
| Bolenge | 1 | ||
| Fungurume | 0.04 | 0.01–0.18 | 0.000 |
| Kalima | 0.13 | 0.05–0.31 | 0.000 |
| Kamina | 0.19 | 0.09–0.42 | 0.000 |
| Karawa | 0.33 | 0.17–0.65 | 0.001 |
| Katana | 17.52 | 9.36–32.78 | 0.000 |
| Kingasani | 1.90 | 1.18–3.06 | 0.008 |
| Kisangani | 0.14 | 0.06–0.31 | 0.000 |
| Lubumbashi | 0.12 | 0.05–0.32 | 0.000 |
| Vanga | 2.33 | 1.40–3.89 | 0.001 |
| Number episode of malaria last 12 months | |||
| 0 | 1 | ||
| 1 | 1.03 | 0.65–1.63 | 0.886 |
| 2 | 1.15 | 0.77–1.71 | 0.469 |
| 3 | 0.11 | 0.64–1.59 | 0.961 |
| 4 and + | 1.64 | 0.98–2.75 | 0.057 |
| Study | |||
| Study-2017 | 1 | ||
| Study-2019 | 0.76 | 0.56–1.021.02 | 0.069 |
Discussion
Like in 2017-study [23], the 2019-study did not detect any polymorphisms that are currently known to be associated with resistance to ART. However, other coding substitutions observed, such as A578S, should be considered. A578S mutation, commonly reported in African countries including the DRC [23, 26, 27], was the only one which was detected in both 2019-study and 2017-study. Although, the amino acid substitution in A578S mutation is likely to alter the function of PFK13 protein [28], previous studies have not shown the link with ART resistance [17, 27]. The function of the pfk13 gene is largely unknown and the phenotypic expression of this gene could involve other determinants which could vary from one geographical region to another due to the genetic diversity of P falciparum. Other unusual polymorphisms have been observed by both the 2019-study and 2017-study; some of them (M472I, V534A and A569T) were previously reported in African countries [26]. Since the polymorphisms associated with ART resistance are numerous and occur on a background of genetic diversity, the mutations that are increasingly observed in Africa merit further characterization [29, 30].
Globally, the prevalence of the K76T mutation known to be associated with CQ-resistance continues to decline in the DRC. Indeed, compared to previously reported prevalence which was more than 50% [13, 14, 25], the present study has shown that the prevalence of CQ-resistance marker has decreased to less than 30% (28.5% in 2017-study and 22.7% in 2019-study) in the DRC. However, this prevalence remained very variable from one region to another, with the highest prevalence in Katana (89.5% in 2017-study and 84.2% in 2019-study) and the lowest in Fungurume (1.5% in 2017-study and 1.4% in 2019-study). A possible illicit use of CQ that contributes in maintaining CQ selective pressure on the local P. falciparum population could explain the persistence of high CQ resistance rate in some regions. In addition, the selection for CQ-resistance is largely dependent on the genetic structure of parasite populations, which have been shown to vary greatly between regions of the DRC [30]; this may partly explain the observed regional variability in prevalence of CQ-resistance rate. Other potential explanations for the heterogeneity in prevalence of CQ-resistance marker in different regions of the DRC have been addressed in the previous 2017-study [15].
The return of CQ-susceptible malaria may present a novel opportunity for reintroducing this molecule to prevent malaria, especially in vulnerable populations. Indeed, if the proportion of CQ-resistant parasites declines in African endemic countries to an undetectable level, the reintroduction of CQ to be used alone or in combination with other anti-malarial drugs for prophylaxis or treatment may possibly be considered. The absence of drug pressure is supposed to be the key driver in the reemergence of CQ-sensitivity parasites in the field setting [31]. Unfortunately, with the advent of the COVID-19 pandemic, CQ has been largely used for its supposed antiviral properties in affected countries including the DRC [32]. In this context, CQ has been made widely available in private drugstores with the presence of falsified version containing low amount of CQ, which was discovered in the DRC [33]. This is likely to maintain CQ pressure on P. falciparum populations, thus compromising the hope towards the return of CQ-susceptible malaria in endemic countries particularly in the DRC.
The PFCRT 72–76 haplotype, SVMNT has been correlated with high-level resistance to the AQ metabolite desethylamodiaquine (DEAQ) in in vitro tests [34]. Like in 2017-study, the SVMNT haplotype associated with AQ-resistance was not detected in 2019-study. AQ is one of the partner drugs of ACTs used in many endemic countries like the DRC for the treatment of uncomplicated P. falciparum malaria. Generally, AQ was found to be effective in Africa, even in the face of increasing CQ-resistance [35] that would constitute a risk factor of emergence of AQ-resistance in Africa. However, high prevalence of SVMNT has been reported in P. falciparum from South America and Asia [36] and the presence of this haplotype was also reported in Tanzania [37] and in Angola [38]. AQ is structurally related to CQ with both belonging to the group of 4-aminoquinolines. There is a possibility that AQ could continue to contribute to selection for the mutant pfcrt-K76T parasites even after discontinuance of CQ usage [39].
The present study contributed to ongoing malaria surveillance providing baseline data for the biennial surveillance of P. falciparum molecular markers associated with resistance to anti-malarial drugs in the DRC. Regular studies with size of samples taking into account the regional variability and a broader exploration of the genome of P. falciparum could help to provide more information about the landscape of P. falciparum drug resistance in the DRC.
Conclusions
The study did not report, within 2 years, the presence of molecular markers currently known to be associated with resistance to ART and to AQ in P. falciparum isolated in the DRC. Moreover, the overall prevalence of CQ-resistance marker continues to decrease in the DRC, but there was observed a high variability from region to region with persistence of high prevalence of parasites carrying CQ-resistant haplotype. Further characterization of unusual detected polymorphisms is required and regular surveillance should continue in order to ensure early detection and containment of the emerging anti-malarial drug resistance in the DRC.
Supplementary Information
Additional file 1: Table S1. Plasmodium falciparum K13 nucleotide sequences from 2019-study.
Acknowledgements
The study was conducted in collaboration with the National Malaria Control Programme (NMCP). We would like to thank all patients and their guardians, as well as the staff of all collecting sites for their participation in this study.
Abbreviations
- ACT
Artemisinin-based combination therapy
- AL
Artemether–lumefantrine
- AQ
Amodiaquine
- ASAQ
Artesunate–amodiaquine
- CQ
Chloroquine
- DBS
Dried blood spot
- DNA
Deoxyribonucleic acid
- DRC
Democratic Republic of Congo
- NMCP
National Malaria Control Programme
- PCR
Polymerase chain reaction
- pfcrt
Plasmodium falciparum Chloroquine resistance transporter
- pfk13
Plasmodium falciparum Kelch 13
- US
United States of America
- RDT
Rapid diagnostic test
- SNP
Single nucleotide polymorphisms
- SP
Sulfadoxine–pyrimethamine
- WHO
World Health Organization
Authors’ contributions
DMY, DMM, PDM, GLM and MPH initiated the study. DMY and NKK carried out the data and sample collection. DMY and RB performed the molecular analysis. PZA carried out the statistical analysis. DMY designed the study and drafted the manuscript. NKK, DMM, RB, PZK, PZA, HNTS, PDM, NS, GLM and MPH contributed to the interpretation of data and they substantively revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Académie de Recherche et d'Enseignement Supérieur (ARES), Belgium. The funding body had no role in the study design, interpretation of data and in writing and publication of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The protocol and the informed consent form were approved by the Ethics Committee of the Faculty of Medicine, University of Kinshasa. All participants involved in the study signed an informed consent form. For participants aged less than 18 years old, the consent form was approved and signed by their parent or guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kazadi WM, Vong S, Makina BN, Mantshumba JC, Kabuya W, Kebela BI, et al. Assessing the efficacy of chloroquine and sulfadoxine-pyrimethamine for treatment of uncomplicated Plasmodium falciparum malaria in the Democratic Republic of Congo. Trop Med Int Health. 2003;8:868–875. doi: 10.1046/j.1365-3156.2003.01098.x. [DOI] [PubMed] [Google Scholar]
- 2.Swarthout TD, Van den Broek IV, Kayembe G, Montgomery J, Pota H, Roper C. Artesunate+amodiaquine and artesunate+sulphadoxine–pyrimethamine for treatment of uncomplicated malaria in Democratic Republic of Congo: a clinical trial with determination of sulphadoxine and pyrimethamine-resistant haplotypes. Trop Med Int Health. 2006;11(10):1503–1511. doi: 10.1111/j.1365-3156.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 3.OMS. Lutte contre le paludisme: Surveillance, suivi et évaluation. Un manuel de référence. Organisation Mondiale de la Santé, Genève; 2019. https://apps.who.int › handle › 9789242565577-fre.pdf. Accessed 01 Jan 2021.
- 4.Ecker A, Lehane AM, Clain J, Fidock DA. PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 2012;28:504–514. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourté Y, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 6.Beshir K, Sutherland CJ, Merinopoulos I, Durrani N, Leslie T, Rowland M, et al. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT Allele at Codons 72 to 76. Antimicrob Agents Chemother. 2010;54:3714–3716. doi: 10.1128/AAC.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed A, Ndaro A, Kalinga A, Manjurano A, Mosha JF, Mosha DF, et al. Trends in chloroquine resistance marker, Pfcrt-K76T mutation ten years after chloroquine withdrawal in Tanzania. Malar J. 2013;12:415. doi: 10.1186/1475-2875-12-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemming-Schroeder E, Umukoro E, Lo E, Fung B, Tomás-Domingo P, Zhou G, et al. Impacts of antimalarial drugs on Plasmodium falciparum drug resistance markers, Western Kenya, 2003–2015. Am J Trop Med Hyg. 2018;98:692–699. doi: 10.4269/ajtmh.17-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwanza S, Joshi S, Nambozi M, Chileshe J, Malunga P, Kabuya J-BB, et al. The return of chloroquine-susceptible Plasmodiumfalciparum malaria in Zambia. Malar J. 2016;15:584. doi: 10.1186/s12936-016-1637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afoakwah R, Boampong JN, Egyir-Yawson A, Nwaefuna EK, Verner ON, Asare KK. High prevalence of PfCRT K76T mutation in Plasmodium falciparum isolates in Ghana. Acta Trop. 2014;136:32–36. doi: 10.1016/j.actatropica.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G. High prevalence of pfcrt-CVIET haplotype in isolates from asymptomatic and symptomatic patients in south-central Oromia Ethiopia. Malar J. 2014;13:120. doi: 10.1186/1475-2875-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meshnick SR, Janko M, Tshefu AK, Taylor SM, Emch M, Antonia AL. A cross-sectional survey of Plasmodiumfalciparum pfcrt mutant haplotypes in the Democratic Republic of Congo. Am J Trop Med Hyg. 2014;90:1094–1097. doi: 10.4269/ajtmh.13-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mvumbi DM, Bobanga TL, Kayembe J-N, Mvumbi GL, Situakibanza H-T, Benoit-Vical F, et al. Molecular surveillance of Plasmodium falciparum resistance to artemisinin-based combination therapies in the Democratic Republic of Congo. PLoS ONE. 2017;12:e0179142. doi: 10.1371/journal.pone.0179142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yobi DM, Kayiba NK, Mvumbi DM, Boreux R, Kabututu PZ, Situakibanza HNT, et al. Molecular surveillance of anti-malarial drug resistance in Democratic Republic of Congo: high variability of chloroquinoresistance and lack of amodiaquinoresistance. Malar J. 2020;19:121. doi: 10.1186/s12936-020-03192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Status report on artemisinin resistance and ACT efficacy. World Health Organization/Global Malaria programme; August 2018. https://www.who.int/malaria/publications/atoz/artemisinin-resistance/august2018/en/. Accessed 20 Apr 2019.
- 19.Uwimana A, Legrand E, Stokes BH, Ndikumana J-LM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020 doi: 10.1038/s41591-020-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacoli C, Gai P, Bayingana C, Sifft K, Geus D, Ndoli J, et al. Artemisinin resistance-associated K13 polymorphisms of Plasmodium falciparum in Southern Rwanda, 2010–2015. Am J Trop Med Hyg. 2016;95:1090–1093. doi: 10.4269/ajtmh.16-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda M, Kaneko M, Tachibana SI, Balikagala B, Sakurai-Yatsushiro M, Yatsushiro S, et al. Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis. 2018;24:718–726. doi: 10.3201/eid2404.170141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayiba NK, Yobi DM, Tshibangu-Kabamba E, Tuan VP, Yamaoka Y, Devleesschauwer B, et al. Spatial and molecular mapping of Pfkelch13 gene polymorphism in Africa in the era of emerging Plasmodium falciparum resistance to artemisinin: a systematic review. Lancet Infect Dis. 2020;21:e82–e92. doi: 10.1016/S1473-3099(20)30493-X. [DOI] [PubMed] [Google Scholar]
- 23.Yobi DM, Kayiba NK, Mvumbi DM, Boreux R, Bontems S, Kabututu PZ, et al. The lack of K13-propeller mutations associated with artemisinin resistance in Plasmodium falciparum in Democratic Republic of Congo (DRC) PLoS ONE. 2020;15:e0237791. doi: 10.1371/journal.pone.0237791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cnops L, Jacobs J, Esbroeck MV. Validation of a four-primer real-time PCR as a diagnostic tool for single and mixed Plasmodium infections. Clin Microbiol Infect. 2011;17:1101–1107. doi: 10.1111/j.1469-0691.2010.03344.x. [DOI] [PubMed] [Google Scholar]
- 25.Mvumbi DM, Boreux R, Sacheli R, Lelo M, Lengu B, Nani-Tuma S, et al. Assessment of pfcrt 72–76 haplotypes eight years after chloroquine withdrawal in Kinshasa, Democratic Republic of Congo. Malar J. 2013;12:459. doi: 10.1186/1475-2875-12-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménard D, Khim N, Beghain J, Adegnika AA, ShafiulAlam M, Amodu O, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374(25):2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WWARN K13 Genotype-Phenotype Study Group Association of mutations in the Plasmodium falciparum (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments—a WWARN individual patient data meta-analysis. BMC Med. 2019;17:1. doi: 10.1186/s12916-018-1207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohon A, Alam M, Bayih A, Folefoc A, Shahinas D, Haque R, et al. Mutations in Plasmodium falciparum K13 propeller gene from Bangladesh (2009–2013) Malar J. 2014;13(1):431. doi: 10.1186/1475-2875-13-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straimer J, Gnadig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2014;347(6220):428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verity R, Aydemir O, Brazeau NF, Watson OJ, Hathaway NJ, Mwandagalirwa MK, et al. The impact of antimalarial resistance on the genetic structure of Plasmodiumfalciparum in the DRC. Nat Commun. 2020;11:2107. doi: 10.1038/s41467-020-15779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kublin JG, Cortese JF, Njunju EM, Mukadam RAG, Wirima JJ, Kazembe PN, et al. Re-emergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 32.Belayneh A. Off-label use of chloroquine and hydroxychloroquine for COVID-19 treatment in Africa against WHO recommendation. Res Rep Trop Med. 2020;11:61–72. doi: 10.2147/RRTM.S269936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gnegel G, Hauk C, Neci R, Mutombo G, Nyaah F, Wistuba D, et al. Identification of falsified chloroquine tablets in Africa at the time of the COVID-19 pandemic. Am J Trop Med Hyg. 2020;103(1):73–76. doi: 10.4269/ajtmh.20-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sa JM, Twu O, Hayton K, Reyes S, Fay MP, Ringwald P, et al. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc Natl Acad Sci USA. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olliaro P, Mussano P. Amodiaquine for treating malaria. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000016. [DOI] [PubMed] [Google Scholar]
- 36.Mehlotra RK, Mattera G, Bockarie MJ, Maguire JD, Baird JK, Sharma YD, et al. Discordant patterns of genetic variation at two chloroquine resistance loci in worldwide populations of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52(6):2212–2222. doi: 10.1128/AAC.00089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alifrangis M, Dalgaard MB, Lusingu JP, Vestergaard LS, Staalsoe T, Jensen ATR, et al. Occurrence of the Southeast Asian/South American SVMNT haplotype of the chloroquine resistance transporter gene in Plasmodium falciparum in Tanzania. J Infect Dis. 2006;193:1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- 38.Gama BE, Pereira-Carvalho GA, Lutucuta Kosi FJ, AlmeidadeOliveira NK, Fortes F, Rosenthal PJ, et al. Plasmodiumfalciparum isolates from Angola show the SVMNT haplotype in the pfcrt gene. Malar J. 2010;9:174. doi: 10.1186/1475-2875-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank M, Lehners N, Mayengue PI, Gabor J, Dal-Bianco M, Kombila DU, et al. A thirteen-year analysis of Plasmodium falciparum populations reveals high conservation of the mutant pfcrt haplotype despite the withdrawal of chloroquine from national treatment guidelines in Gabon. Malar J. 2011;10:304. doi: 10.1186/1475-2875-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Plasmodium falciparum K13 nucleotide sequences from 2019-study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.