Abstract
The 14-3-3 family proteins are vital scaffold proteins that ubiquitously expressed in various tissues. They interact with numerous protein targets and mediate many cellular signaling pathways. The 14-3-3 binding motifs are often embedded in intrinsically disordered regions which are closely associated with liquid–liquid phase separation (LLPS). In the past ten years, LLPS has been observed for a variety of proteins and biological processes, indicating that LLPS plays a fundamental role in the formation of membraneless organelles and cellular condensates. While extensive investigations have been performed on 14-3-3 proteins, its involvement in LLPS is overlooked. To date, 14-3-3 proteins have not been reported to undergo LLPS alone or regulate LLPS of their binding partners. To reveal the potential involvement of 14-3-3 proteins in LLPS, in this review, we summarized the LLPS propensity of 14-3-3 binding partners and found that about one half of them may undergo LLPS spontaneously. We further analyzed the phase separation behavior of representative 14-3-3 binders and discussed how 14-3-3 proteins may be involved. By modulating the conformation and valence of interactions and recruiting other molecules, we speculate that 14-3-3 proteins can efficiently regulate the functions of their targets in the context of LLPS. Considering the critical roles of 14-3-3 proteins, there is an urgent need for investigating the involvement of 14-3-3 proteins in the phase separation process of their targets and the underling mechanisms.
Keywords: Protein–protein interaction, Scaffold protein, Intrinsically disordered region, Condensate, Regulation
Introduction
The 14-3-3 proteins are ubiquitously expressed in various tissues and mediate many cellular signaling pathways. The name “14-3-3” was given because these proteins elute in the 14th fraction of bovine brain homogenate on the DEAE cellulose column and the 3.3 fraction in the subsequent starch gel electrophoresis [1]. They interact with numerous protein targets, including kinases, phosphatases, transmembrane receptors, and transcription factors [2, 3]. Through these protein–protein interactions, 14-3-3 proteins participate in cell cycle regulation, gene expression control, apoptosis, signal transduction, and many other vital biological processes [4–6]. Dysregulation of 14-3-3 protein expression has been observed in several cancers [7]. Furthermore, 14-3-3 proteins have chaperone activity which may play a role in neurodegenerative disease progression [8]. Since 14-3-3 proteins are potential therapeutic targets, many 14-3-3 modulators have been discovered/designed to target these specific interactions [9, 10].
The binding targets of 14-3-3 proteins usually carry specific motifs containing phosphorylated serine or threonine. Most of these motifs are embedded in intrinsically disordered regions (IDRs) [11, 12]. In contrast to traditional folded protein domains, IDRs do not fold into stable three dimensional structures in the free state. Their amino acid compositions significantly differ from those of folded proteins. Generally, IDRs lack bulky hydrophobic amino acids and are enriched in charged amino acids [13, 14]. While IDRs are unfolded, they perform critical functions. Structural disorder facilitates post-translational modifications (PTMs), association/dissociation kinetics, conformational changes, and so on [15, 16]. Consequently, numerous IDRs are involved in cellular regulation and signal transduction [17–19].
Recently, IDRs have become a theme of many research efforts because they are closely associated with liquid–liquid phase separation (LLPS), which underlies the formation of membraneless organelles and cellular condensates [20–25]. Increasing evidences suggest that many types of biomolecular condensates, such as stress granules, P granules, and nuclear speckles, are formed through LLPS [24, 26–29]. Enriching interacting components inside droplets has been shown to promote protein aggregation [30–32], enzyme reaction [33, 34], gene expression [35–37], and virus replication [38, 39]. LLPS is driven by multivalent interactions and can be readily regulated by effector molecules or PTMs [22, 29, 40].
To date, 14-3-3 proteins have not been reported to undergo LLPS alone or regulate LLPS of their binding partners. However, the high LLPS propensity of 14-3-3 binding partners and the bivalent nature of 14-3-3 proteins suggest that 14-3-3 proteins could be potentially involved in LLPS regulation. In this review, we first summarized the interacting modes of 14-3-3 and mechanism of LLPS, and then we proposed that 14-3-3 proteins could be recruited into the droplets and regulate the phase separation behavior of their binding partners.
Structure and Function of 14-3-3 Proteins
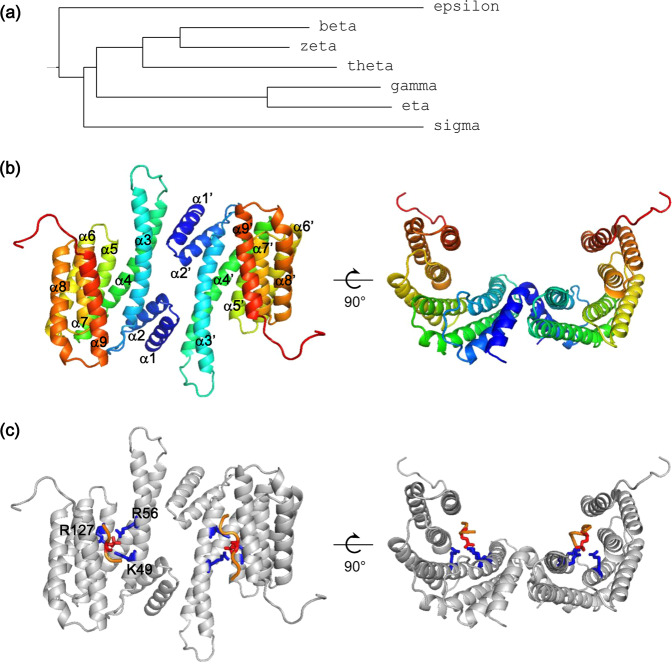
The 14-3-3 proteins are a family of highly conserved scaffold proteins. They are expressed in various types of tissues [41–45]. In mammals, seven 14-3-3 isoforms encoded by different genes have been identified: α/β, γ, ε, δ/ζ, η, θ, and σ, where α and δ are the phosphorylated forms of 14-3-3β and ζ, respectively [46–48]. The length of human 14-3-3 isoform varies from 245 residues to 255 residues. Each 14-3-3 protein sequence contains a divergent N terminus, a divergent C terminus, and a conserved core region. A phylogenetic tree generated from sequence alignment is shown in Fig. 1a. 14-3-3 proteins form stable homodimers or heterodimers with a cup-shaped structure (Fig. 1b). Each protomer consists of nine antiparallel α-helices where α1 and α2 of one protomer interact with α3 and α4 of the other protomer to form the dimer interface [49, 50].
Fig. 1.
Sequence and structure analysis of 14-3-3 proteins. a A phylogenetic tree generated from sequence alignment of seven human 14-3-3 isoforms. b Two perpendicular views of 14-3-3σ dimer. Each protomer is shown as cartoon in rainbow color. The nine antiparallel α-helices in one protomer is labeled from α1 to α9. c Interactions between the pSer214 residue of tau peptide and the conserved basic residues Lys49, Arg56, and Arg127 within the amphipathic groove of 14-3-3σ. The tau peptide is shown as an orange tube, where the pSer214 residue is shown as red sticks. Residues Lys49, Arg56, and Arg127 of 14-3-3σ are shown as blue sticks. Crystal structure of the 14-3-3σ/tau complex (Protein Data Bank ID 4FL5 [60]) is used in b and c for illustration
14-3-3 proteins interact with their binding partners primarily through the amphipathic binding grooves constituted by α-helices α3, α5, α7, and α9 (Fig. 1b). Three distinct 14-3-3 binding motifs are identified: motif I [RXXp(S/T)XP/G], motif II [RX(F/Y)Xp(S/T)X(P/G)], and motif III [p(S/T)X1-2-COOH], where p(S/T) is a phosphorylated serine or threonine, and X is any type of residue [51–55]. The three conserved basic residues Lys49, Arg56, and Arg127 within the amphipathic groove form ionic and hydrogen bonds with the phosphorylated residue (Fig. 1c) [49]. Although the sequences of the seven 14-3-3 isoforms are highly conserved, they bind to the same target with different affinities. Interestingly, their binding affinities obey a hierarchized profile with conserved relative KD ratios [56].
It should be noted that not all interactions with 14-3-3 proteins require the above-mentioned phosphorylated motifs. Some non-phosphorylated peptides also bind to 14-3-3 proteins with high affinity [57, 58]. If a protein contains multiple motifs, they could occupy both amphipathic grooves within a 14-3-3 dimer simultaneously [59–62]. Besides the amphipathic grooves, the last two C-terminal α-helices are also shown to contribute to interactions with some partners [63–65].
So far, hundreds of 14-3-3 binding proteins have been identified [66–68]. Affinity-purification/mass spectrometry combined with domain-based cluster analysis suggested that 14-3-3 proteins can potentially engage around 0.6% of the human proteome [69]. Overall, the 14-3-3 binding proteins show great structural and functional diversity. A recent Gene Ontology analysis of 14-3-3 partners showed that 25% are non-receptor Ser/Thr protein kinases, 20% are DNA binding proteins, 12% are cell cycle proteins, 10% are RNA-binding proteins, and several other categories [12]. Therefore, 14-3-3 binding proteins are extensively involved in cell cycle regulation, apoptosis, autophagy, signal transduction, and gene expression control [4–6].
14-3-3 proteins regulate the function of their targets through several modes [70]: (i) A 14-3-3 dimer can act as a scaffold to bring two interacting proteins in close proximity, thus mediating the formation of protein complexes. For example, 14-3-3β mediates the interaction between Bcr and Raf [71] and 14-3-3ζ stabilizes interaction between α4 integrin and paxillin [72]. (ii) 14-3-3 proteins can occlude interactions between the target proteins and their effectors. Interaction between BAD and Bcl-2 promotes apoptosis. Datta et al. found that phosphorylation of BAD at Ser136 and Ser112 recruits 14-3-3 proteins, which disrupts the binding of BAD to Bcl-2 and thereby promotes cell survival [73]. (iii) 14-3-3 proteins can induce conformational changes on the target proteins, altering their activity. A well-studied example is the serotonin N-acetyltransferase, an enzyme that controls the daily rhythm in melatonin synthesis. 14-3-3 proteins increase the activity of serotonin N-acetyltransferase by modulating the structure of the substrate-binding sites [74, 75]. Sometimes, different modes may work synergetically to modulate the activity of the 14-3-3 binding partners. For example, the binding site of 14-3-3ζ on the kinase domain of ASK1 is in close proximity to the kinase active site. Therefore, 14-3-3ζ may inhibit the activity of ASK1 by blocking its accessibility and/or affecting its conformation [76].
By participating in numerous signaling pathways, dysregulation of 14-3-3 proteins is involved in many diseases [7, 8]. Expression levels of the majority of 14-3-3 isoforms are elevated in many tumors, such as meningioma [77], astrocytoma [78], glioblastoma [79], lung cancer [80, 81], breast cancer [82, 83], and hepatocellular carcinoma [84, 85]. Because 14-3-3σ is a tumor suppressor, its expression in tumors is negatively regulated [77, 86]. By interacting with GSK-3β, LRRK2, or FOXO3a, or co-localizing with α-synuclein in the Lewy bodies or tau in the neurofibrillary tangles, 14-3-3 proteins are associated with Alzheimer’s disease and Parkinson’s disease [87–93]. Furthermore, 14-3-3 proteins are also associated with other neurological disorders, such as lissencephaly, schizophrenia, bipolar disorder, Williams syndrome, and Down syndrome [94, 95].
Liquid–Liquid Phase Separation: Function and Mechanism
Many proteins are able to undergo LLPS when the concentration exceeds a threshold value, in particular under crowded conditions. LLPS usually results in formation of liquid droplets in which proteins are enriched. The protein concentration inside the droplets can be tens of folds higher than that of the surrounding dilute phase [96, 97]. The phase separation behavior of a protein can be readily affected by several environmental conditions, such as salt concentration, pH, and temperature [98, 99].
LLPS can be driven by multivalent interactions [22, 29]. Tandem repeats of binding element are multivalent, which is well examplified by the nephrin–NCK–N-WASP system [100]. Multivalence can also be achieved by oligomerization, as in NPM1 or SynGAP [101, 102]. Furthermore, IDRs form dynamic, multivalent interactions, such as cation–π interactions, electrostatic interactions, and hydrophobic interactions [103–105]. The interaction mode between multivalent molecules can be described by the stickers-and-spacers model [22, 106], in which stickers are groups of residues forming attractive interactions, while spacers connect the stickers and influence their interactions.
As indicated by the stickers-and-spacers model, LLPS will be altered if the strength of interaction, the valence of molecules, the distribution of stickers, or the length of spacers is changed. PTM is a universal way to regulate LLPS [22, 40, 107, 108]. PTM can induce conformational changes or alter electrostatic interactions and hydrophobic interactions, thereby promoting or suppressing LLPS. Phosphorylation [109–113], methylation [98, 111, 114–116], acetylation [117, 118], polyubiquitination [119, 120], and PARylation [121, 122], have been shown to alter LLPS of many proteins.
Proteins within a droplet can be generally divided into two classes [123]. The first class is scaffolds or drivers, which are multivalent and essential for LLPS. The second class is clients or regulators. Clients and regulators are dispensable for LLPS and are recruited into the droplets via interactions with the scaffolds or drivers. In promyelocytic leukemia (PML) nuclear bodies, the PML protein represents the scaffold, while SUMO-1, Daxx, and Sp100 are the clients and dynamically exchanged at the PML nuclear bodies [124, 125]. It has been shown that partition of clients into droplets varies with the scaffolds concentration or valency [123]. Recently, Orti et al. systematically analyzed the features of drivers, clients, and regulators, and observed some difference among them in disorder content, sequence composition, and prevalence of PTMs [126].
Accumulated evidences suggest that LLPS underlies the formation of various membraneless compartments, such as stress granules, P granules, nuclear speckles, and nucleoli [24, 26–29]. Thus, LLPS plays critical roles in many cellular processes, including assembly of mitotic spindle [127], cellular stress response [128, 129], gene expression [35–37], and RNA splicing [130]. Furthermore, LLPS is also associated with pathological processes, such as protein aggregation and virus replication [30–32, 38, 39]. Consequently, misregulation of LLPS may lead to the occurrence of diseases [31, 97, 131–133].
14-3-3 Proteins are Potential Regulators of Phase Separation
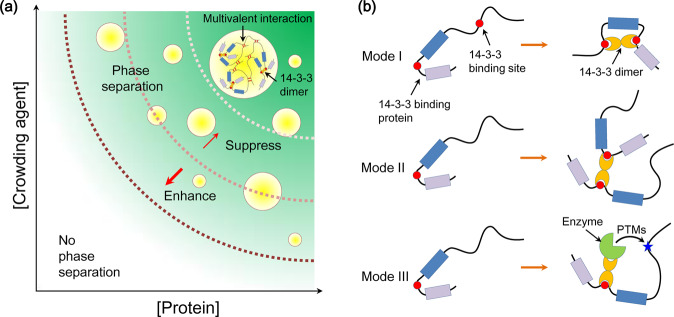
As discussed above, 14-3-3 proteins participate in numerous signaling pathways and regulate various biological processes, where LLPS may occur. However, the involvement of 14-3-3 proteins in LLPS has not been investigated. Since LLPS of 14-3-3 proteins has not been reported, we first predicted their LLPS propensities using the FuzDrop server [134]. FuzDrop prediction gives the probability of LLPS (pLLPS) for human 14-3-3β, γ, ε, ζ, η, θ, and σ are 0.2884, 0.1581, 0.1586, 0.2169, 0.1364, 0.1318, and 0.2124, respectively, suggesting that all seven 14-3-3 isoforms will not phase separate spontaneously. We further analyzed the LLPS propensities for the binding partners of human 14-3-3 proteins. 1370 14-3-3 binding partners were collected from the IntAct database [135] and then subjected to FuzDrop prediction (Table S1). Surprisingly, more than one half of the 14-3-3 binders show pLLPS values larger than 0.6 (Fig. 2a), suggesting that a large number of the 14-3-3 binders could undergo phase separation. A search of literature showed that LLPS of some 14-3-3 binding partners has been characterized experimentally (Table 1). The FuzDrop prediction profiles of five representative 14-3-3 binders were shown in Fig. 2b. Given the high LLPS propensities of 14-3-3 binding partners and the above discussion on the modes through which 14-3-3 proteins regulate their targets, we speculate that 14-3-3 proteins could enhanced or suppressed LLPS of their binding partners (Fig. 3a). Some 14-3-3 binding partners contain multiple 14-3-3 binding sites which may be occupied by a 14-3-3 dimer. Upon 14-3-3 proteins binding, significant conformational changes may occur within the 14-3-3 binding partners. Therefore, LLPS of the 14-3-3 binding partners may be modulated (Mode I in Fig. 3b). 14-3-3 proteins could also act as cross-linkers by binding to multiple partners simultaneously, thus increasing the valence of interaction for LLPS and promoting phase separation (Mode II in Fig. 3b). PTMs are key factors regulating LLPS. 14-3-3 proteins could modulate LLPS of their binding partners by facilitating the occurrence of PTMs by recruiting enzymes (Mode III in Fig. 3b). In the following text, we summarized the phase separation properties of five representative 14-3-3 binders and discussed how 14-3-3 proteins could play a role.
Fig. 2.
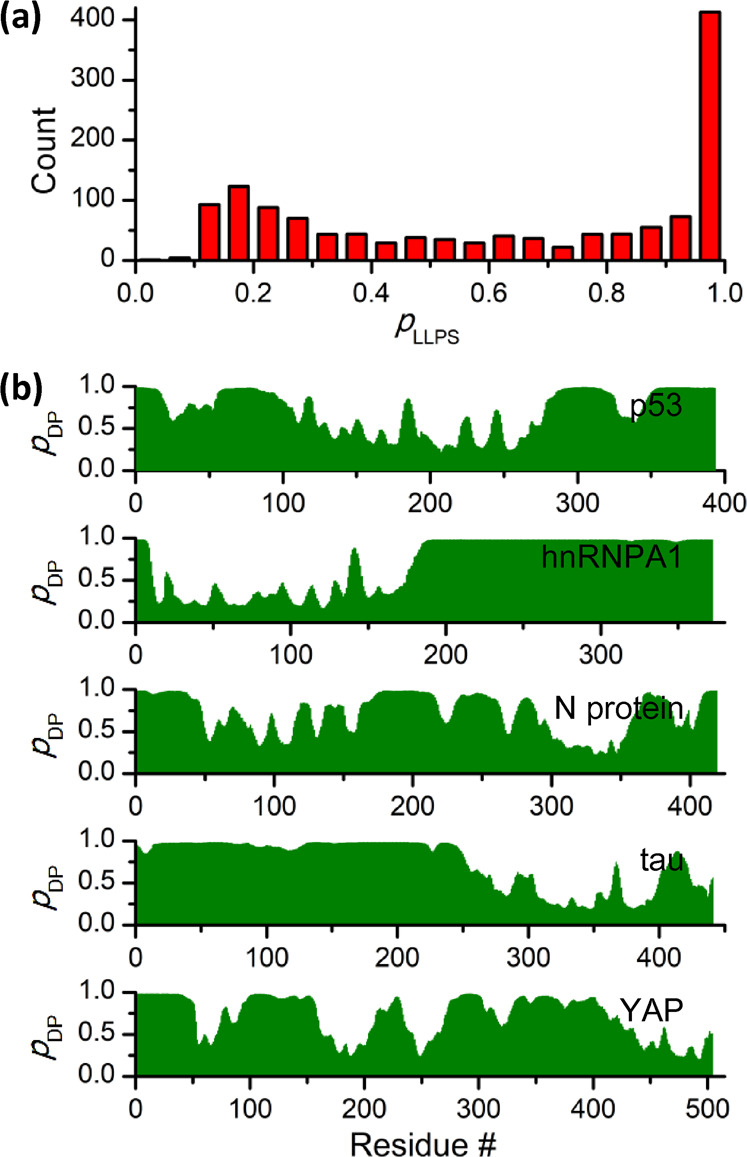
LLPS propensity prediction of 14-3-3 binding partners using FuzDrop [134]. a Statistical analysis of LLPS probability (pLLPS) for 14-3-3 binding partners. According to FuzDrop, proteins with pLLPS greater than 0.61 likely form droplets spontaneously. pLLPS values for all proteins are provided in Supplementary Table S1. b FuzDrop predicted droplet-promoting propensity profiles (pDP) of p53, hnRNPA1, SARS-CoV-2 nucleocapsid protein, tau, and YAP. Residues with pDP > 0.60 are likely to mediate LLPS
Table 1.
Experimental characterization of phase separation of 14-3-3 binding partners
| Protein | UniProt ID | Function | Phase separation properties | References |
|---|---|---|---|---|
| APC | P25054 | Cellular signaling | LLPS in vitro; LLPS with Axin in cells. |
[206] [207] |
| CEP152 | O94986 | Scaffold protein | LLPS with Cep63 in vitro and in cells. | [208] |
| CHAF1A | Q13111 | DNA binding protein | LLPS in cells | [209] |
| DACT1 | Q9NYF0 | Cellular signaling | LLPS in cells | [210] |
| DCP1A | Q9NPI6 | mRNA decapping factor | LLPS in cells. | [211] |
| DDX3X | O00571 | RNA helicase | LLPS in vitro and in cells. | [118] |
| EDC3 | Q96F86 | Scaffold protein | LLPS in vitro. | [212–215] |
| ERC1 | Q8IUD2 | Scaffold protein | LLPS in cells. | [216] |
| GIT1 | Q9Y2X7 | GTPase activator | LLPS in vitro and in cells. | [217] |
| H1.2 | P16403 | DNA binding protein | LLPS in vitro | [218] |
| hnRNPA1 | P09651 | RNA-binding protein | LLPS in vitro and in cells. | [32, 176, 177, 179] |
| hnRNPA2 | P22626 | RNA-binding protein | LLPS in vitro, in cells, and in C. elegans. | [116, 219–222] |
| hnRNPD | Q14103 | RNA-binding protein | LLPS in cells. | [222] |
| HSF1 | Q00613 | Transcription factor | LLPS in cells. | [223, 224] |
| HTT | P42858 | microtubule-mediated transport | LLPS in cells. | [225] |
| LATS1 | O95835 | Cellular signaling | LLPS in cells | [226] |
| MPRIP | Q6WCQ1 | actin filament binding protein | LLPS in cells. | [227] |
| NELFE | P18615 | Transcriptional regulation | LLPS with NELFA in vitro and in cells. | [228] |
| NPM1 | P06748 | Nucleolar chaperone | LLPS in vitro and in cells. | [101, 229–231] |
| p53 | P04637 | Transcription factor | LLPS in vitro and in cells. | [35, 164–168] |
| PCNT | O95613 | Scaffold protein | LLPS in cells | [232] |
| PLK4 | O00444 | Serine/threonine-protein kinase | LLPS in vitro and in cells. | [233, 234] |
| RAD52 | P43351 | DNA repair protein | LLPS in vitro and in cells. | [235, 236] |
| SARS-CoV-2 nucleocapsid protein | P0DTC9 | RNA-binding protein | LLPS in vitro and in cells. | [38, 39, 189–199, 237, 238] |
| SOX-2 | P48431 | Transcription factor | LLPS in vitro. | [239] |
| SRRM2 | Q9UQ35 | Splicing factor | LLPS with SON in cells. | [240] |
| SynGAP | F6SEU4 | Cellular signaling | LLPS with PSD-95 in vitro and in cells | [102, 241, 242] |
| tau | P10636 | Microtubule-associated protein | LLPS in vitro and in cells. | [30, 96, 97, 99, 104, 105, 154–157, 159, 160, 179, 243–251] |
| TFE3 | P19532 | Transcription factor | LLPS in cells. | [252] |
| TFEB | P19484 | Transcription factor | LLPS in vitro and in cells. | [253] |
| UBQLN4 | Q9NRR5 | Cellular signaling | LLPS in vitro. | [254] |
| USP42 | Q9H9J4 | Deubiquitinating enzyme | LLPS in vitro and in cells. | [255] |
| YAP | P46937 | Transcription factor | LLPS in vitro and in cells. | [203, 204] |
Fig. 3.
Potential influence of 14-3-3 proteins on the LLPS propensity of 14-3-3 binding partners. a A phase diagram illustrating that LLPS of 14-3-3 binding partners may be regulated by 14-3-3 proteins. Yellow circles indicate the droplets formed. Multivalent interactions driving LLPS and recruitment of 14-3-3 into the droplets are indicated. When 14-3-3 proteins promote LLPS of their partners, the phase boundary is shifted towards lower protein concentration and lower crowder concentration. On the contrary, if 14-3-3 proteins suppress LLPS of their partners, the phase boundary is shifted towards higher protein concentration and higher crowder concentration. b Schematic illustration of modes through which 14-3-3 proteins may regulate the LLPS propensities of their targets. In mode I, a 14-3-3 dimer binds to its target and induces conformational change. In mode II, a 14-3-3 dimer binds to multiple targets, thus increasing the valence of interaction for LLPS. In mode III, a 14-3-3 dimer bridges its target with the modifying enzyme, thus facilitating the occurrence of PTMs
Microtubule-Associated Protein Tau
Tau is a microtubule-associated protein mainly distributed in axons. Its primary function is to regulate the assembly and spatial organization of microtubule [136, 137]. Tau also plays a role in cellular signaling and chromosome stability [138, 139]. Because hyperphosphorylation and abnormal aggregation of tau have been observed in a number of neurodegenerative diseases, tau is considered as an important target for neurodegenerative disease treatment [140–143].
The interactions between 14-3-3 and tau have been recognized for a long time and may contribute to tau aggregation [144–146]. Crystal structures and binding affinity characterization show that tau segments embracing phosphorylated residues pSer214 and pSer324 bind to the amphipathic grooves of 14-3-3 [60]. However, amphipathic groove-specific binding ligands are not able to completely inhibit binding of tau to 14-3-3 [147]. Moreover, Stefanoska et al. showed that an N-terminal 11-amino acid-long motif (residues 18–28) modulates interactions between tau and 14-3-3 [148].
Recently, it was found that LLPS is an intrinsic property of tau and may be involved in its function and aggregation [149–153]. For example, tau droplets can concentrate tubulin and nucleate microtubule bundles as well as regulate the activity of microtubule-severing enzymes and the movement of molecular motors [154, 155]. LLPS also promotes tau aggregation or formation of toxic oligomers [30, 96, 97, 99, 156, 157].
LLPS of tau is mainly driven by electrostatic interactions between the negatively charged N-terminal and the C-terminal domains and the positively charged proline-rich domain and the microtubule-binding domain [104], whereas hydrophobic interactions are also involved [97, 105]. Many factors promoting tau aggregation have been found to promote tau phase separation [105, 153], and several proteins, including EFhd2 [158], PDI [159], Hsp22 [160], and TIA1 [157], have been found to regulate the LLPS of tau. Considering the extensive interactions between tau and 14-3-3 proteins, we speculate that the conformational changes on tau induced by 14-3-3 may influence the phase separation of tau (mode I in Fig. 3a). Furthermore, 14-3-3 proteins promote tau phosphorylation [161], which may also regulate tau LLPS (mode III in Fig. 3a).
Tumor Suppressor p53
The tumor suppressor p53 protein is a transcription factor. Its inactivation is found in many cancer types. 14-3-3 proteins regulate the transcriptional activity, stability, and cellular localization of p53 [6]. So far, three 14-3-3 binding sites located in the disordered C-terminal domain of p53 have been characterized experimentally [6].
Early studies have demonstrated that p53 is uptaken into membrane-less cellular bodies, such as Cajal bodies and promyelocytic leukemia protein bodies [162, 163]. Droplets of p53 were first reported by Boija et at. when they mixed p53 with transcriptional coactivator MED1 [35]. Later, anomalous liquid condensates of p53 were observed by Safari et al. when they explored the aggregation process of p53 at near-physiological conditions and in crowded environments [164]. p53 also forms droplets when overexpressed in yeast [165]. While experiments using recombinant p53 suggest that electrostatic interactions between the negatively charged N-terminal domain and the positively charged C-terminal domain play a key role in mediating p53 droplet formation [166], some mutations are found to markedly promote p53 condensation in cancer cells [167, 168]. Though the function of p53 condensates remains elusive [169], formation of p53 droplets may be related to p53 aggregation [164, 165, 168, 170].
Oligomerization is a key factor in LLPS as it generates multi valences and enhances intermolecular interactions [109, 132, 171]. Interactions with 14-3-3 proteins are found to enhance the tetramerization of p53 [172]. In this context, 14-3-3 proteins are expected to enhance the LLPS propensity of p53 and have an effect on the subsequent functioning process (mode II in Fig. 3a).
Heterogeneous Nuclear Ribonucleoprotein hnRNPA1
Heterogeneous nuclear ribonucleoproteins (hnRNPs) are a family of RNA-binding proteins that regulate diverse biological processes. hnRNPA1 is concentrated in the nucleus and involved in mRNA processing. During stress, hnRNPA1 is sequestered in the cytoplasm and forms stress granules with other RNA-binding proteins and mRNA [173]. A 14-3-3 binding motif is located on the N-terminus, where phosphorylation on Ser4/6 is required for hnRNPA1/14-3-3 interactions [174]. 14-3-3 can mediate re-entry of hnRNPA1 into the nucleus or cooperate with hnRNPA1 to control splicing of genes under stress conditions [174, 175].
hnRNPA1 consists of two folded RNA recognition motifs and a low complexity domain. While LLPS of hnRNPA1 is mainly driven by the low complexity domain via aromatic-aromatic and aromatic-arginine interactions, the folded RNA recognition motifs modify the phase behavior through intramolecular electrostatic interactions between the low complexity domain and the RNA recognition motifs [32, 176]. LLPS of hnRNPA1 has been suggested to contribute to the assembly of stress granules and the formation of amyloid-like fibers [32, 177, 178]. Since conditions that favor LLPS also enhance hnRNPA1 aggregation, it is suggested that LLPS is on pathway to its aggregation [179].
14-3-3 proteins have been shown to regulate the component of stress granules. MK2-induced phosphorylation of tristetraprolin at Ser52 and Ser178 promotes formation of tristetraprolin/14-3-3 complexes, resulting in exclusion of tristetraprolin from arsenite-induced stress granules [180]. Moreover, 14-3-3 proteins were able to regulate the distribution of hMex-3B in P-bodies and stress granules [181]. Importantly, both tristetraprolin and hMex-3B are predicted to undergo phase separation spontaneous with pLLPS values of 0.9986 and 0.9988, respectively. Modulating the component of stress granules by 14-3-3 or building up physical cross-linking between hnRNPA1 may influence the material properties or functions of stress granules (mode II in Fig. 3a).
SARS-CoV-2 Nucleocapsid Protein
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the worldwide pandemic of the coronavirus disease 2019 (COVID-19) [182]. The SARS-CoV-2 genome encodes about 30 proteins, among which the nucleocapsid (N) protein is the most abundant viral protein in infected cells [183–185]. N protein is a multifunctional protein. It packages viral RNA into virions and plays a critical role in virus transcription and assembly [186].
14-3-3 proteins have been reported to regulate the shuttling of SARS-CoV N protein between the nucleus and the cytoplasm [187]. Recently, Tugaeva et al. characterized the interactions between N protein and 14-3-3 proteins [188]. They found that N protein interacts with all seven human 14-3-3 isoforms with Kd values in low micromolar range. The main binding site for N protein to bind 14-3-3 is phosphorylated Ser197, which is located in the central IDR.
The phase separation propensity of N protein has been subjected to extensive studies. N protein undergoes LLPS with RNA where the N-terminal RNA-binding domain, the central IDR, and C-terminal dimerization domain play an essential role [39, 189–194]. LLPS of N protein is modulated by phosphorylation at the serine/arginine-rich region by CDK1, GSK-3β, or SRPK1 [38, 193, 195]. The N protein/RNA condensates recruit RNA polymerase complex of SARS-CoV-2, which may provide a mechanism for efficient transcription of viral RNA [38]. N protein also phase separates with G3BP or hnRNPA2 into stress granules [190, 193, 196, 197]. Furthermore, LLPS of N protein may be associated with the dysfunctional inflammatory responses and antiviral immunity [194, 198]. Consequently, modulating the phase separation propensity of N protein could be a potential treatment for COVID-19 [39, 199].
Though the influence of 14-3-3 on LLPS of N protein has not been investigated so far, binding of 14-3-3 to N protein is expected to modify the conformation of the central IDR or introduce intermolecular crossing-linking, which could play a role in modulating the phase separation propensity of N protein (mode I and mode II in Fig. 3a). Recruitment of 14-3-3 into N protein condensates may also regulate the signaling pathways involved in immune response.
Transcriptional Coactivator YAP
The Yes-associated protein (YAP) is a key transcriptional coactivator of the Hippo pathway [200]. Unphosphorylated YAP is located in the nucleus and interacts with the TEAD transcription factors [201]. Upon association with TEAD, YAP induces expression of genes involved in anti-apoptotic processes [202]. On the contrary, when YAP is phosphorylated by LATS1-2, it binds to 14-3-3 proteins and remains in the cytosol [202]. Therefore, 14-3-3 proteins play a role in regulating the Hippo pathway.
To date, there are two studies showing that YAP undergoes phase separation. In one study, Yu et al. found that interferon-γ promotes phase separation of YAP after anti-PD-1 therapy in tumor cells [203]. YAP droplets induced by interferon-γ are localized in the nucleus and recruit TAZ, TEAD4, EP300, and MED1, forming a transcriptional hub for gene expression. Therefore, disrupting LLPS of YAP suppresses tumor cell growth [203]. In the other study, Cai et al. investigated the phase separation behavior of YAP under hyperosmotic stress [204]. In hyperosmotically stressed cells, YAP condensates are observed in both cytoplasm and nucleus. Importantly, the components of cytoplasmic and nuclear YAP condensates are different. Cytoplasmic YAP condensates concentrate kinase, including LATS1 and NLK, whereas nuclear YAP condensates contain transcription factor and transcription regulator, such as TEAD1 and TAZ. While both studies confirm the essential role of C-terminal IDR of YAP in LLPS, formation of YAP condensates is regulated by Ser127 and Ser128 phosphorylation [204]. The Ser127 phosphorylation creates a binding site for 14-3-3 proteins and promotes formation of cytoplasmic YAP condensates, whereas phosphorylation on Ser128 abolishes YAP/14-3-3 binding and suppresses LLPS of YAP in the cytoplasm [204, 205]. Therefore, 14-3-3 proteins may play a critical role in regulating the formation of cytoplasmic YAP condensates which sequester YAP from the nucleus (mode I and II in Fig. 3a).
Conclusions and Perspectives
In the past ten years, our understanding on LLPS has achieved rapid development. Increasing studies show that LLPS plays a fundamental role in formation of membraneless organelles and cellular condensates, and dysregulation of LLPS is closely related to human diseases. 14-3-3 proteins interact with various targets and form an interacting hub for many cellular signaling pathways. While numerous investigations have been performed on 14-3-3 proteins, its involvement in LLPS is overlooked. To reveal the potential correlation between 14-3-3 proteins and LLPS, in this review, we summarized the LLPS propensity of 14-3-3 binding partners and found that about one half of them may undergo LLPS spontaneously. We further analyzed the phase separation behavior of representative 14-3-3 binders and discussed how 14-3-3 protein may be involved. By modulating the conformation and valence of interactions and recruiting other molecules, we speculate that 14-3-3 proteins can efficiently regulate the functions of their targets in the context of LLPS. Considering the critical roles of 14-3-3 proteins, there is an urgent need for investigating the involvement of 14-3-3 proteins in the phase separation process of their targets and the underlying mechanisms.
Supplementary Information
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant 32000883 to M.G.], and funding from Hubei University of Technology [Y.H.]
Abbreviations
- IDRs
intrinsically disordered regions
- PTMs
post-translational modifications
- LLPS
liquid–liquid phase separation
- PML
promyelocytic leukemia
- hnRNPs
heterogeneous nuclear ribonucleoproteins
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- COVID-19
coronavirus disease 2019
- YAP
Yes-associated protein
Author Contributions
Y.H. and Z.S. had the idea for the article; X.H., Z.Z., and Y.W. performed the literature search and data analysis; X.H., Z.Z., Y.W., Y.H., and M.G. drafted the work; Y.H., M.G., and Z.S. critically revised the work.
Data Availability
All data are included in the article and its supplementary information files.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xianlong Huang, Zhiwen Zheng, Yixin Wu
Supplementary information
The online version contains supplementary material available at 10.1007/s12013-022-01067-3.
References
- 1.Moore, B. W., Perez, V. J. (1967). Specific acid proteins in the nervous system. In F. D. Carlson (Ed.), Physiological and biochemical aspects of nervous integration. Prentice-Hall, Englewood Cliffs, NJ.
- 2.Obsil T, Obsilova V. Structural basis of 14-3-3 protein functions. Seminars in Cell and Developmental Biology. 2011;22:663–672. doi: 10.1016/j.semcdb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Obsilova V, Obsil T. The 14-3-3 proteins as important allosteric regulators of protein kinases. International Journal of Molecular Sciences. 2020;21:8824. doi: 10.3390/ijms21228824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aitken A. 14-3-3 proteins: a historic overview. Seminars in Cancer Biology. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochemical Journal. 2004;381:329–342. doi: 10.1042/BJ20031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falcicchio M, Ward JA, Macip S, Doveston RG. Regulation of p53 by the 14-3-3 protein interaction network: new opportunities for drug discovery in cancer. Cell Death Discovery. 2020;6:126. doi: 10.1038/s41420-020-00362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermeking H. The 14-3-3 cancer connection. Nature Reviews Cancer. 2003;3:931–943. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- 8.Fan X, Cui L, Zeng Y, Song W, Gaur U, Yang M. 14-3-3 proteins are on the crossroads of cancer, aging, and age-related neurodegenerative disease. International Journal of Molecular Sciences. 2019;20:3518. doi: 10.3390/ijms20143518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevers LM, Sijbesma E, Botta M, MacKintosh C, Obsil T, Landrieu I, Cau Y, Wilson AJ, et al. Modulators of 14-3-3 protein-protein interactions. Journal of Medicinal Chemistry. 2018;61:3755–3778. doi: 10.1021/acs.jmedchem.7b00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottmann C. Small-molecule modulators of 14-3-3 protein-protein interactions. Bioorganic and Medicinal Chemistr. 2013;21:4058–4062. doi: 10.1016/j.bmc.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Bustos DM. The role of protein disorder in the 14-3-3 interaction network. Molecular Biosystems. 2012;8:178–184. doi: 10.1039/c1mb05216k. [DOI] [PubMed] [Google Scholar]
- 12.Sluchanko NN, Bustos DM. Intrinsic disorder associated with 14-3-3 proteins and their partners. Progress in Molecular Biology and Translational Science. 2019;166:19–61. doi: 10.1016/bs.pmbts.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Tompa P. Intrinsically unstructured proteins. Trends in Biochemical Sciences. 2002;27:527–533. doi: 10.1016/S0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 14.Uversky VN. Natively unfolded proteins: a point where biology waits for physics. Protein Science. 2002;11:739–756. doi: 10.1110/ps.4210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlow RB, Dyson HJ, Wright PE. Functional advantages of dynamic protein disorder. FEBS Letters. 2015;589:2433–2440. doi: 10.1016/j.febslet.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu ZR, Huang YQ. Advantages of proteins being disordered. Protein Science. 2014;23:539–550. doi: 10.1002/pro.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nature Reviews Molecular Cell Biology. 2015;16:18–29. doi: 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallmann A, Kesten C. Common functions of disordered proteins across evolutionary distant organisms. International Journal of Molecular Sciences. 2020;21:2105. doi: 10.3390/ijms21062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uversky VN. Protein intrinsic disorder and structure-function continuum. Progress in Molecular Biology and Translational Science. 2019;166:1–17. doi: 10.1016/bs.pmbts.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Uversky VN. Recent developments in the field of intrinsically disordered proteins: intrinsic disorder-based emergence in cellular biology in light of the physiological and pathological liquid-liquid phase transitions. Annual Review of Biophysics. 2021;50:135–156. doi: 10.1146/annurev-biophys-062920-063704. [DOI] [PubMed] [Google Scholar]
- 21.Uversky VN, Kuznetsova IM, Turoverov KK, Zaslavsky B. Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates. FEBS Letters. 2015;589:15–22. doi: 10.1016/j.febslet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Borcherds W, Bremer A, Borgia MB, Mittag T. How do intrinsically disordered protein regions encode a driving force for liquid-liquid phase separation? Current Opinion in Structural Biology. 2021;67:41–50. doi: 10.1016/j.sbi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong S, Mir M. Towards decoding the sequence-based grammar governing the functions of intrinsically disordered protein regions. Journal of Molecular Biology. 2021;433:166724. doi: 10.1016/j.jmb.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Lafontaine DLJ, Riback JA, Bascetin R, Brangwynne CP. The nucleolus as a multiphase liquid condensate. Nature Reviews Molecular Cell Biology. 2021;22:165–182. doi: 10.1038/s41580-020-0272-6. [DOI] [PubMed] [Google Scholar]
- 25.Youn JY, Dyakov BJA, Zhang J, Knight JDR, Vernon RM, Forman-Kay JD, Gingras AC. Properties of stress granule and P-body proteomes. Molecular Cell. 2019;76:286–294. doi: 10.1016/j.molcel.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Wolozin B, Ivanov P. Stress granules and neurodegeneration. Nature Reviews Neuroscience. 2019;20:649–666. doi: 10.1038/s41583-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darling AL, Liu Y, Oldfield CJ, Uversky VN. Intrinsically disordered proteome of human membrane-less organelles. Proteomics. 2018;18:e1700193.. doi: 10.1002/pmic.201700193. [DOI] [PubMed] [Google Scholar]
- 28.Seydoux G. The P granules of C. elegans: a genetic model for the study of RNA-protein condensates. Journal of Molecular Biology. 2018;430:4702–4710. doi: 10.1016/j.jmb.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nature Reviews Molecular Cell Biology. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyko S, Surewicz K, Surewicz WK. Regulatory mechanisms of tau protein fibrillation under the conditions of liquid-liquid phase separation. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:31882–31890. doi: 10.1073/pnas.2012460117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 32.Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peeples W, Rosen MK. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nucleic Acids Research. 2021;17:693–702. doi: 10.1038/s41589-021-00801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji S, Luo Y, Cai Q, Cao Z, Zhao Y, Mei J, Li C, Xia P, et al. LC domain-mediated coalescence is essential for Otu enzymatic activity to extend Drosophila lifespan. Molecular Cell. 2019;74:363–377. doi: 10.1016/j.molcel.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 2018;175:1842–1855. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A phase separation model for transcriptional control. Cell. 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terlecki-Zaniewicz S, Humer T, Eder T, Schmoellerl J, Heyes E, Manhart G, Kuchynka N, Parapatics K, et al. Biomolecular condensation of NUP98 fusion proteins drives leukemogenic gene expression. Nature Structural and Molecular Biology. 2021;28:190–201. doi: 10.1038/s41594-020-00550-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savastano A, Ibanez de Opakua A, Rankovic M, Zweckstetter M. Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates. Nature Communications. 2020;11:6041. doi: 10.1038/s41467-020-19843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao M, Yu Y, Sun LM, Xing JQ, Li T, Zhu Y, Wang M, Yu Y, et al. GCG inhibits SARS-CoV-2 replication by disrupting the liquid phase condensation of its nucleocapsid protein. Nature Communications. 2021;12:2114. doi: 10.1038/s41467-021-22297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofweber M, Dormann D. Friend or foe-Post-translational modifications as regulators of phase separation and RNP granule dynamics. Journal of Biological Chemistry. 2019;294:7137–7150. doi: 10.1074/jbc.TM118.001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baxter HC, Fraser JR, Liu WG, Forster JL, Clokie S, Steinacker P, Otto M, Bahn E, et al. Specific 14-3-3 isoform detection and immunolocalization in prion diseases. Biochemical Society Transactions. 2002;30:387–391. doi: 10.1042/bst0300387. [DOI] [PubMed] [Google Scholar]
- 42.Boston PF, Jackson P, Thompson RJ. Human 14-3-3 protein: radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. Journal of Neurochemistry. 1982;38:1475–1482. doi: 10.1111/j.1471-4159.1982.tb07928.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Herrmann CJ, Simonovic M, Szklarczyk D, von Mering C. Version 4.0 of PaxDb: protein abundance data, integrated across model organisms, tissues, and cell-lines. Proteomics. 2015;15:3163–3168. doi: 10.1002/pmic.201400441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, et al. A subcellular map of the human proteome. Science. 2017;356:eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 45.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 46.Ichimura T, Isobe T, Okuyama T, Takahashi N, Araki K, Kuwano R, Takahashi Y. Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7084–7088. doi: 10.1073/pnas.85.19.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toker A, Sellers LA, Amess B, Patel Y, Harris A, Aitken A. Multiple isoforms of a protein kinase C inhibitor (KCIP-1/14-3-3) from sheep brain. Amino acid sequence of phosphorylated forms. European Journal of Biochemistry. 1992;206:453–461. doi: 10.1111/j.1432-1033.1992.tb16946.x. [DOI] [PubMed] [Google Scholar]
- 48.Aitken A, Howell S, Jones D, Madrazo J, Patel Y. 14-3-3 alpha and delta are the phosphorylated forms of raf-activating 14-3-3 beta and zeta. In vivo stoichiometric phosphorylation in brain at a Ser-Pro-Glu-Lys MOTIF. Journal of Biological Chemistry. 1995;270:5706–5709. doi: 10.1074/jbc.270.11.5706. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, Lee WH, Sobott F, Papagrigoriou E, Robinson CV, Grossmann JG, Sundstrom M, Doyle DA, et al. Structural basis for protein-protein interactions in the 14-3-3 protein family. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17237–17242. doi: 10.1073/pnas.0605779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu D, Bienkowska J, Petosa C, Collier RJ, Fu H, Liddington R. Crystal structure of the zeta isoform of the 14-3-3 protein. Nature. 1995;376:191–194. doi: 10.1038/376191a0. [DOI] [PubMed] [Google Scholar]
- 51.Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, et al. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 52.Rittinger K, Budman J, Xu J, Volinia S, Cantley LC, Smerdon SJ, Gamblin SJ, Yaffe MB. Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Molecular Cell. 1999;4:153–166. doi: 10.1016/s1097-2765(00)80363-9. [DOI] [PubMed] [Google Scholar]
- 53.Ganguly S, Weller JL, Ho A, Chemineau P, Malpaux B, Klein DC. Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1222–1227. doi: 10.1073/pnas.0406871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paiardini A, Aducci P, Cervoni L, Cutruzzola F, Di Lucente C, Janson G, Pascarella S, Rinaldo S, et al. The phytotoxin fusicoccin differently regulates 14-3-3 proteins association to mode III targets. IUBMB Life. 2014;66:52–62. doi: 10.1002/iub.1239. [DOI] [PubMed] [Google Scholar]
- 55.Coblitz B, Wu M, Shikano S, Li M. C-terminal binding: an expanded repertoire and function of 14-3-3 proteins. FEBS Letters. 2006;580:1531–1535. doi: 10.1016/j.febslet.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Gogl G, Tugaeva KV, Eberling P, Kostmann C, Trave G, Sluchanko NN. Hierarchized phosphotarget binding by the seven human 14-3-3 isoforms. Nature Communications. 2021;12:1677. doi: 10.1038/s41467-021-21908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ottmann C, Yasmin L, Weyand M, Veesenmeyer JL, Diaz MH, Palmer RH, Francis MS, Hauser AR, et al. Phosphorylation-independent interaction between 14-3-3 and exoenzyme S: from structure to pathogenesis. The EMBO Journal. 2007;26:902–913. doi: 10.1038/sj.emboj.7601530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang B, Yang H, Liu YC, Jelinek T, Zhang L, Ruoslahti E, Fu H. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry. 1999;38:12499–12504. doi: 10.1021/bi991353h. [DOI] [PubMed] [Google Scholar]
- 59.Masters SC, Fu H. 14-3-3 proteins mediate an essential anti-apoptotic signal. Journal of Biological Chemistry. 2001;276:45193–45200. doi: 10.1074/jbc.M105971200. [DOI] [PubMed] [Google Scholar]
- 60.Joo Y, Schumacher B, Landrieu I, Bartel M, Smet-Nocca C, Jang A, Choi HS, Jeon NL, et al. Involvement of 14-3-3 in tubulin instability and impaired axon development is mediated by Tau. The FASEB Journal. 2015;29:4133–4144. doi: 10.1096/fj.14-265009. [DOI] [PubMed] [Google Scholar]
- 61.Stevers LM, Lam CV, Leysen SF, Meijer FA, van Scheppingen DS, de Vries RM, Carlile GW, Milroy LG, et al. Characterization and small-molecule stabilization of the multisite tandem binding between 14-3-3 and the R domain of CFTR. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1152–E1161. doi: 10.1073/pnas.1516631113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu J, Sun XM, Su JY, Zhao YF, Chen YX. Different phosphorylation and farnesylation patterns tune Rnd3-14-3-3 interaction in distinct mechanisms. Chemical Science. 2021;12:4432–4442. doi: 10.1039/d0sc05838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sluchanko NN, Beelen S, Kulikova AA, Weeks SD, Antson AA, Gusev NB, Strelkov SV. Structural basis for the interaction of a human small heat shock protein with the 14-3-3 universal signaling regulator. Structure. 2017;25:305–316. doi: 10.1016/j.str.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alblova M, Smidova A, Docekal V, Vesely J, Herman P, Obsilova V, Obsil T. Molecular basis of the 14-3-3 protein-dependent activation of yeast neutral trehalase Nth1. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E9811–E9820. doi: 10.1073/pnas.1714491114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karlberg T, Hornyak P, Pinto AF, Milanova S, Ebrahimi M, Lindberg M, Pullen N, Nordstrom A, et al. 14-3-3 proteins activate Pseudomonas exotoxins-S and -T by chaperoning a hydrophobic surface. Nature Communications. 2018;9:3785. doi: 10.1038/s41467-018-06194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson C, Tinti M, Wood NT, Campbell DG, Toth R, Dubois F, Geraghty KM, Wong BH, et al. Visualization and biochemical analyses of the emerging mammalian 14-3-3-phosphoproteome. Molecular Cell Proteomics. 2011;10(M110):005751. doi: 10.1074/mcp.M110.005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tinti M, Johnson C, Toth R, Ferrier DE, Mackintosh C. Evolution of signal multiplexing by 14-3-3-binding 2R-ohnologue protein families in the vertebrates. Open Biology. 2012;2:120103. doi: 10.1098/rsob.120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uhart M, Bustos DM. Human 14-3-3 paralogs differences uncovered by cross-talk of phosphorylation and lysine acetylation. PLoS One. 2013;8:e55703. doi: 10.1371/journal.pone.0055703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, Metalnikov P, O’Donnell P, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Current Biology. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 70.Obsilova V, Kopecka M, Kosek D, Kacirova M, Kylarova S, Rezabkova L, Obsil T. Mechanisms of the 14-3-3 protein function: regulation of protein function through conformational modulation. Physiological Research. 2014;63:S155–S164. doi: 10.33549/physiolres.932659. [DOI] [PubMed] [Google Scholar]
- 71.Braselmann S, McCormick F. Bcr and Raf form a complex in vivo via 14-3-3 proteins. The EMBO Journal. 1995;14:4839–4848. doi: 10.1002/j.1460-2075.1995.tb00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deakin NO, Bass MD, Warwood S, Schoelermann J, Mostafavi-Pour Z, Knight D, Ballestrem C, Humphries MJ. An integrin-alpha4-14-3-3zeta-paxillin ternary complex mediates localised Cdc42 activity and accelerates cell migration. Journal of Cell Science. 2009;122:1654–1664. doi: 10.1242/jcs.049130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Molecular Cell. 2000;6:41–51. doi: 10.1016/S1097-2765(05)00012-2. [DOI] [PubMed] [Google Scholar]
- 74.Obsil T, Ghirlando R, Klein DC, Ganguly S, Dyda F. Crystal structure of the 14-3-3zeta:serotonin N-acetyltransferase complex. a role for scaffolding in enzyme regulation. Cell. 2001;105:257–267. doi: 10.1016/s0092-8674(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 75.Ganguly S, Gastel JA, Weller JL, Schwartz C, Jaffe H, Namboodiri MA, Coon SL, Hickman AB, et al. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8083–8088. doi: 10.1073/pnas.141118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petrvalska O, Kosek D, Kukacka Z, Tosner Z, Man P, Vecer J, Herman P, Obsilova V, et al. Structural insight into the 14-3-3 protein-dependent inhibition of protein kinase ASK1 (Apoptosis Signal-regulating kinase 1) Journal of Biological Chemistry. 2016;291:20753–20765. doi: 10.1074/jbc.M116.724310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Tian RF, Li YM, Liu WP, Cao L, Yang XL, Cao WD, Zhang X. The expression of seven 14-3-3 isoforms in human meningioma. Brain Research. 2010;1336:98–102. doi: 10.1016/j.brainres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Yang X, Cao W, Lin H, Zhang W, Lin W, Cao L, Zhen H, Huo J, et al. Isoform-specific expression of 14-3-3 proteins in human astrocytoma. Journal of the Neurological Sciences. 2009;276:54–59. doi: 10.1016/j.jns.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 79.Petri MK, Koch P, Stenzinger A, Kuchelmeister K, Nestler U, Paradowska A, Steger K, Brobeil A, et al. PTPIP51, a positive modulator of the MAPK/Erk pathway, is upregulated in glioblastoma and interacts with 14-3-3beta and PTP1B in situ. Histology and Histopathology. 2011;26:1531–1543. doi: 10.14670/HH-26.1531. [DOI] [PubMed] [Google Scholar]
- 80.Raungrut P, Wongkotsila A, Lirdprapamongkol K, Svasti J, Geater SL, Phukaoloun M, Suwiwat S, Thongsuksai P. Prognostic significance of 14-3-3gamma overexpression in advanced non-small cell lung cancer. Asian Pacific Journal of Cancer Prevention. 2014;15:3513–3518. doi: 10.7314/apjcp.2014.15.8.3513. [DOI] [PubMed] [Google Scholar]
- 81.Qi W, Liu X, Qiao D, Martinez JD. Isoform-specific expression of 14-3-3 proteins in human lung cancer tissues. International Journal of Cancer. 2005;113:359–363. doi: 10.1002/ijc.20492. [DOI] [PubMed] [Google Scholar]
- 82.Hiraoka E, Mimae T, Ito M, Kadoya T, Miyata Y, Ito A, Okada M. Breast cancer cell motility is promoted by 14-3-3gamma. Breast Cancer. 2019;26:581–593. doi: 10.1007/s12282-019-00957-4. [DOI] [PubMed] [Google Scholar]
- 83.Mei J, Liu Y, Xu R, Hao L, Qin A, Chu C, Zhu Y, Liu X. Characterization of the expression and prognostic value of 14-3-3 isoforms in breast cancer. Aging (Albany NY) 2020;12:19597–19617. doi: 10.18632/aging.103919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ko BS, Chang TC, Hsu C, Chen YC, Shen TL, Chen SC, Wang J, Wu KK, et al. Overexpression of 14-3-3epsilon predicts tumour metastasis and poor survival in hepatocellular carcinoma. Histopathology. 2011;58:705–711. doi: 10.1111/j.1365-2559.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 85.Liu TA, Jan YJ, Ko BS, Liang SM, Chen SC, Wang J, Hsu C, Wu YM, et al. 14-3-3epsilon overexpression contributes to epithelial-mesenchymal transition of hepatocellular carcinoma. PLoS One. 2013;8:e57968. doi: 10.1371/journal.pone.0057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou R, Shao Z, Liu J, Zhan W, Gao Q, Pan Z, Wu L, Xu L, et al. COPS5 and LASP1 synergistically interact to downregulate 14-3-3sigma expression and promote colorectal cancer progression via activating PI3K/AKT pathway. International Journal of Cancer. 2018;142:1853–1864. doi: 10.1002/ijc.31206. [DOI] [PubMed] [Google Scholar]
- 87.Yuan Z, Agarwal-Mawal A, Paudel HK. 14-3-3 binds to and mediates phosphorylation of microtubule-associated tau protein by Ser9-phosphorylated glycogen synthase kinase 3beta in the brain. Journal of Biological Chemistry. 2004;279:26105–26114. doi: 10.1074/jbc.M308298200. [DOI] [PubMed] [Google Scholar]
- 88.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 89.Berg D, Riess O, Bornemann A. Specification of 14-3-3 proteins in Lewy bodies. Annals of Neurology. 2003;54:135. doi: 10.1002/ana.10621. [DOI] [PubMed] [Google Scholar]
- 90.Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H. 14-3-3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. Journal of Neuropathology and Experimental Neurology. 2002;61:245–253. doi: 10.1093/jnen/61.3.245. [DOI] [PubMed] [Google Scholar]
- 91.Umahara T, Uchihara T, Tsuchiya K, Nakamura A, Iwamoto T, Ikeda K, Takasaki M. 14-3-3 proteins and zeta isoform containing neurofibrillary tangles in patients with Alzheimer’s disease. Acta Neuropathologica. 2004;108:279–286. doi: 10.1007/s00401-004-0885-4. [DOI] [PubMed] [Google Scholar]
- 92.Soulie C, Nicole A, Delacourte A, Ceballos-Picot I. Examination of stress-related genes in human temporal versus occipital cortex in the course of neurodegeneration: involvement of 14-3-3 zeta in this dynamic process. Neuroscience Letters. 2004;365:1–5. doi: 10.1016/j.neulet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 93.Pair FS, Yacoubian TA. 14-3-3 proteins: novel pharmacological targets in neurodegenerative diseases. Trends in Pharmacological Sciences. 2021;42:226–238. doi: 10.1016/j.tips.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cho E, Park JY. Emerging roles of 14-3-3gamma in the brain disorder. BMB Reports. 2020;53:500–511. doi: 10.5483/BMBRep.2020.53.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foote M, Zhou Y. 14-3-3 proteins in neurological disorders. International Journal of Biochemistry and Molecular Biology. 2012;3:152–164. [PMC free article] [PubMed] [Google Scholar]
- 96.Kanaan NM, Hamel C, Grabinski T, Combs B. Liquid-liquid phase separation induces pathogenic tau conformations in vitro. Nature Communications. 2020;11:2809. doi: 10.1038/s41467-020-16580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. The EMBO Journal. 2018;37:e98049. doi: 10.15252/embj.201798049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Molecular Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ambadipudi S, Biernat J, Riedel D, Mandelkow E, Zweckstetter M. Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein tau. Nature Communications. 2017;8:275.. doi: 10.1038/s41467-017-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Nourse A, Deniz AA, et al. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. Elife. 2016;5:e13571.. doi: 10.7554/eLife.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell. 2016;166:1163–1175. doi: 10.1016/j.cell.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J, Choi JM, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 2018;174:688–699. doi: 10.1016/j.cell.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyko S, Qi X, Chen TH, Surewicz K, Surewicz WK. Liquid-liquid phase separation of tau protein: the crucial role of electrostatic interactions. Journal of Biological Chemistry. 2019;294:11054–11059. doi: 10.1074/jbc.AC119.009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin Y, Fichou Y, Zeng Z, Hu NY, Han S. Electrostatically driven complex coacervation and amyloid aggregation of tau are independent processes with overlapping conditions. ACS Chemical Neuroscience. 2020;11:615–627. doi: 10.1021/acschemneuro.9b00627. [DOI] [PubMed] [Google Scholar]
- 106.Choi JM, Holehouse AS, Pappu RV. Physical principles underlying the complex biology of intracellular phase transitions. Annual Review of Biophysics. 2020;49:107–133. doi: 10.1146/annurev-biophys-121219-081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Owen I, Shewmaker F. The role of post-translational modifications in the phase transitions of intrinsically disordered proteins. International Journal of Molecular Sciences. 2019;20:5501.. doi: 10.3390/ijms20215501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bratek-Skicki A, Pancsa R, Meszaros B, Van Lindt J, Tompa P. A guide to regulation of the formation of biomolecular condensates. The FEBS Journal. 2020;287:1924–1935. doi: 10.1111/febs.15254. [DOI] [PubMed] [Google Scholar]
- 109.Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S, et al. Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature. 2017;547:236–240. doi: 10.1038/nature22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, O’Meally R, Dignon GL, et al. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. The EMBO Journal. 2017;36:2951–2967. doi: 10.15252/embj.201696394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsang B, Arsenault J, Vernon RM, Lin H, Sonenberg N, Wang LY, Bah A, Forman-Kay JD. Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:4218–4227. doi: 10.1073/pnas.1814385116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C, Honigmann A. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell. 2019;179:923–936. doi: 10.1016/j.cell.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Zhang C, Yang W, Shao S, Xu X, Sun Y, Li P, Liang L, et al. LIMD1 phase separation contributes to cellular mechanics and durotaxis by regulating focal adhesion dynamics in response to force. Developmental Cell. 2021;56:1313–1325. doi: 10.1016/j.devcel.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, Farber P, Lin H, Forman-Kay JD. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife. 2018;7:e31486. doi: 10.7554/eLife.31486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qamar S, Wang G, Randle SJ, Ruggeri FS, Varela JA, Lin JQ, Phillips EC, Miyashita A, et al. FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation-pi interactions. Cell. 2018;173:720–734. doi: 10.1016/j.cell.2018.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryan VH, Dignon GL, Zerze GH, Chabata CV, Silva R, Conicella AE, Amaya J, Burke KA, et al. Mechanistic view of hnRNPA2 low-complexity domain structure, interactions, and phase separation altered by mutation and arginine methylation. Molecular Cell. 2018;69:465–479. doi: 10.1016/j.molcel.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bock AS, Murthy AC, Tang WS, Jovic N, Shewmaker F, Mittal J, Fawzi NL. N-terminal acetylation modestly enhances phase separation and reduces aggregation of the low-complexity domain of RNA-binding protein fused in sarcoma. Protein Science. 2021;30:1337–1349. doi: 10.1002/pro.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saito M, Hess D, Eglinger J, Fritsch AW, Kreysing M, Weinert BT, Choudhary C, Matthias P. Acetylation of intrinsically disordered regions regulates phase separation. Nucleic Acids Research. 2019;15:51–61. doi: 10.1038/s41589-018-0180-7. [DOI] [PubMed] [Google Scholar]
- 119.Sun D, Wu R, Zheng J, Li P, Yu L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Research. 2018;28:405–415. doi: 10.1038/s41422-018-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gwon Y, Maxwell BA, Kolaitis RM, Zhang PP, Kim HJ, Taylor JP. Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science. 2021;372:eabf6548. doi: 10.1126/science.abf6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grofte M, Rask MD, Streicher W, et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose) Nature Communications. 2015;6:8088. doi: 10.1038/ncomms9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leung AKL. Poly(ADP-ribose): a dynamic trigger for biomolecular condensate formation. Trends in Cell Biology. 2020;30:370–383. doi: 10.1016/j.tcb.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, Rosen MK. Compositional control of phase-separated cellular bodies. Cell. 2016;166:651–663. doi: 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dellaire G, Eskiw CH, Dehghani H, Ching RW, Bazett-Jones DP. Mitotic accumulations of PML protein contribute to the re-establishment of PML nuclear bodies in G1. Journal of Cell Science. 2006;119:1034–1042. doi: 10.1242/jcs.02817. [DOI] [PubMed] [Google Scholar]
- 125.Weidtkamp-Peters S, Lenser T, Negorev D, Gerstner N, Hofmann TG, Schwanitz G, Hoischen C, Maul G, et al. Dynamics of component exchange at PML nuclear bodies. Journal of Cell Science. 2008;121:2731–2743. doi: 10.1242/jcs.031922. [DOI] [PubMed] [Google Scholar]
- 126.Orti F, Navarro AM, Rabinovich A, Wodak SJ, Marino-Buslje C. Insight into membraneless organelles and their associated proteins: drivers, clients and regulators. Computational and Structural Biotechnology Journal. 2021;19:3964–3977. doi: 10.1016/j.csbj.2021.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woodruff JB. Assembly of mitotic structures through phase separation. Journal of Molecular Biology. 2018;430:4762–4772. doi: 10.1016/j.jmb.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 128.Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nuske E, Richter D, Baumeister W, et al. Phase separation of a yeast prion protein promotes cellular fitness. Science. 2018;359:eaao5654. doi: 10.1126/science.aao5654. [DOI] [PubMed] [Google Scholar]
- 129.Riback JA, Katanski CD, Kear-Scott JL, Pilipenko EV, Rojek AE, Sosnick TR, Drummond DA. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell. 2017;168:1028–1040. doi: 10.1016/j.cell.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Ramirez Montero D, et al. A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. The EMBO Journal. 2018;37:e97452.. doi: 10.15252/embj.201797452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang S, Fagman JB, Chen C, Alberti S, Liu B. Protein phase separation and its role in tumorigenesis. Elife. 2020;9:e60264.. doi: 10.7554/eLife.60264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Conicella AE, Zerze GH, Mittal J, Fawzi NL. ALS mutations disrupt phase separation mediated by alpha-helical structure in the TDP-43 low-complexity C-terminal domain. Structure. 2016;24:1537–1549. doi: 10.1016/j.str.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, Annu K, Baker M, et al. TIA1 Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Promote Phase Separation and Alter Stress Granule Dynamics. Neuron. 2017;95:808.. doi: 10.1016/j.neuron.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hardenberg M, Horvath A, Ambrus V, Fuxreiter M, Vendruscolo M. Widespread occurrence of the droplet state of proteins in the human proteome. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:33254–33262. doi: 10.1073/pnas.2007670117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Orchard S, Ammari M, Aranda B, Breuza L, Briganti L, Broackes-Carter F, Campbell NH, Chavali G, et al. The MIntAct project–IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Research. 2014;42:D358–D363. doi: 10.1093/nar/gkt1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathologica. 2017;133:665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sayas CL, Tortosa E, Bollati F, Ramirez-Rios S, Arnal I, Avila J. Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells. Journal of Neurochemistry. 2015;133:653–667. doi: 10.1111/jnc.13091. [DOI] [PubMed] [Google Scholar]
- 138.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sotiropoulos I, Galas MC, Silva JM, Skoulakis E, Wegmann S, Maina MB, Blum D, Sayas CL, et al. Atypical, non-standard functions of the microtubule associated tau protein. Acta neuropathologica communications. 2017;5:91. doi: 10.1186/s40478-017-0489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li C, Gotz J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nature Reviews Drug Discovery. 2017;16:863–883. doi: 10.1038/nrd.2017.155. [DOI] [PubMed] [Google Scholar]
- 141.Medina M. An overview on the clinical development of tau-based therapeutics. International Journal of Molecular Sciences. 2018;19:1160.. doi: 10.3390/ijms19041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chong FP, Ng KY, Koh RY, Chye SM. Tau proteins and tauopathies in Alzheimer’s disease. Cellular and Molecular Neurobiology. 2018;38:965–980. doi: 10.1007/s10571-017-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Y, Chen X, Yao Z, Shi Y, Xiong J, Zhou J, Su Z, Huang Y. 14-3-3/Tau Interaction and Tau Amyloidogenesis. Journal of Molecular Neuroscience. 2019;68:620–630. doi: 10.1007/s12031-019-01325-9. [DOI] [PubMed] [Google Scholar]
- 145.Sadik G, Tanaka T, Kato K, Yamamori H, Nessa BN, Morihara T, Takeda M. Phosphorylation of tau at Ser214 mediates its interaction with 14-3-3 protein: implications for the mechanism of tau aggregation. Journal of Neurochemistry. 2009;108:33–43. doi: 10.1111/j.1471-4159.2008.05716.x. [DOI] [PubMed] [Google Scholar]
- 146.Qureshi HY, Li T, MacDonald R, Cho CM, Leclerc N, Paudel HK. Interaction of 14-3-3 zeta with microtubule-associated protein tau within Alzheimer’s disease neurofibrillary tangles. Biochemistry. 2013;52:6445–6455. doi: 10.1021/bi400442d. [DOI] [PubMed] [Google Scholar]
- 147.Andrei SA, Meijer FA, Neves JF, Brunsveld L, Landrieu I, Ottmann C, Milroy LG. Inhibition of 14-3-3/tau by hybrid small-molecule peptides operating via two different binding modes. ACS Chemical Neuroscience. 2018;9:2639–2654. doi: 10.1021/acschemneuro.8b00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stefanoska K, Volkerling A, Bertz J, Poljak A, Ke YD, Ittner LM, Ittner A. An N-terminal motif unique to primate tau enables differential protein-protein interactions. Journal of Biological Chemistry. 2018;293:3710–3719. doi: 10.1074/jbc.RA118.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Najafi S, Lin Y, Longhini AP, Zhang X, Delaney KT, Kosik KS, Fredrickson GH, Shea JE, et al. Liquid-liquid phase separation of Tau by self and complex coacervation. Protein Science. 2021;30:1393–1407. doi: 10.1002/pro.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rai SK, Savastano A, Singh P, Mukhopadhyay S, Zweckstetter M. Liquid-liquid phase separation of tau: From molecular biophysics to physiology and disease. Protein Science. 2021;30:1294–1314. doi: 10.1002/pro.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wegmann S. Liquid-liquid phase separation of tau protein in neurobiology and pathology. Advances in Experimental Medicine and Biology. 2019;1184:341–357. doi: 10.1007/978-981-32-9358-8_25. [DOI] [PubMed] [Google Scholar]
- 152.Kosik KS, Han S. Tau condensates. Advances in Experimental Medicine and Biology. 2019;1184:327–339. doi: 10.1007/978-981-32-9358-8_24. [DOI] [PubMed] [Google Scholar]
- 153.Zeng Y, Yang J, Zhang B, Gao M, Su Z, Huang Y. The structure and phase of tau: from monomer to amyloid filament. Cellular and Molecular Life Sciences. 2021;78:1873–1886. doi: 10.1007/s00018-020-03681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hernandez-Vega A, Braun M, Scharrel L, Jahnel M, Wegmann S, Hyman BT, Alberti S, Diez S, et al. Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Reports. 2017;20:2304–2312. doi: 10.1016/j.celrep.2017.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tan R, Lam AJ, Tan T, Han J, Nowakowski DW, Vershinin M, Simo S, Ori-McKenney KM, et al. Microtubules gate tau condensation to spatially regulate microtubule functions. Nature Cell Biology. 2019;21:1078–1085. doi: 10.1038/s41556-019-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lin Y, Fichou Y, Longhini AP, Llanes LC, Yin P, Bazan GC, Kosik KS, Han S. Liquid-liquid phase separation of tau driven by hydrophobic interaction facilitates fibrillization of tau. Journal of Molecular Biology. 2021;433:166731. doi: 10.1016/j.jmb.2020.166731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ash PEA, Lei S, Shattuck J, Boudeau S, Carlomagno Y, Medalla M, Mashimo BL, Socorro G, et al. TIA1 potentiates tau phase separation and promotes generation of toxic oligomeric tau. Proceedings of the National Academy of Sciences of the United States of America. 2021;118:e2014188118.. doi: 10.1073/pnas.2014188118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vega IE, Umstead A, Kanaan NM. EFhd2 affects tau liquid-liquid phase separation. Frontiers in Neuroscience. 2019;13:845.. doi: 10.3389/fnins.2019.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang K, Liu JQ, Zhong T, Liu XL, Zeng Y, Qiao X, Xie T, Chen Y, et al. Phase separation and cytotoxicity of tau are modulated by protein disulfide isomerase and s-nitrosylation of this molecular chaperone. Journal of Molecular Biology. 2020;432:2141–2163. doi: 10.1016/j.jmb.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 160.Darling AL, Dahrendorff J, Creodore SG, Dickey CA, Blair LJ, Uversky VN. Small heat shock protein 22 kDa can modulate the aggregation and liquid-liquid phase separation behavior of tau. Protein Science. 2021;30:1350–1359. doi: 10.1002/pro.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Agarwal-Mawal A, Qureshi HY, Cafferty PW, Yuan Z, Han D, Lin R, Paudel HK. 14-3-3 connects glycogen synthase kinase-3 beta to tau within a brain microtubule-associated tau phosphorylation complex. Journal of Biological Chemistry. 2003;278:12722–12728. doi: 10.1074/jbc.M211491200. [DOI] [PubMed] [Google Scholar]
- 162.Cioce M, Lamond AI. Cajal bodies: a long history of discovery. Annual Review of Cell and Developmental Biology. 2005;21:105–131. doi: 10.1146/annurev.cellbio.20.010403.103738. [DOI] [PubMed] [Google Scholar]
- 163.Guo A, Salomoni P, Luo J, Shih A, Zhong S, Gu W, Pandolfi PP. The function of PML in p53-dependent apoptosis. Nature Cell Biology. 2000;2:730–736. doi: 10.1038/35036365. [DOI] [PubMed] [Google Scholar]
- 164.Safari MS, Wang Z, Tailor K, Kolomeisky AB, Conrad JC, Vekilov PG. Anomalous dense liquid condensates host the nucleation of tumor suppressor p53 fibrils. iScience. 2019;12:342–355. doi: 10.1016/j.isci.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park SK, Park S, Pentek C, Liebman SW. Tumor suppressor protein p53 expressed in yeast can remain diffuse, form a prion, or form unstable liquid-like droplets. iScience. 2021;24:102000.. doi: 10.1016/j.isci.2020.102000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kamagata K, Kanbayashi S, Honda M, Itoh Y, Takahashi H, Kameda T, Nagatsugi F, Takahashi S. Liquid-like droplet formation by tumor suppressor p53 induced by multivalent electrostatic interactions between two disordered domains. Scientific Reports. 2020;10:580.. doi: 10.1038/s41598-020-57521-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lemos C, Schulze L, Weiske J, Meyer H, Braeuer N, Barak N, Eberspacher U, Werbeck N, et al. Identification of small molecules that modulate mutant p53 condensation. iScience. 2020;23:101517.. doi: 10.1016/j.isci.2020.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Petronilho EC, Pedrote MM, Marques MA, Passos YM, Mota MF, Jakobus B, de Sousa GDS, Pereira da Costa F, et al. Phase separation of p53 precedes aggregation and is affected by oncogenic mutations and ligands. Chemical Science. 2021;12:7334–7349. doi: 10.1039/d1sc01739j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Alexander KA, Cote A, Nguyen SC, Zhang L, Gholamalamdari O, Agudelo-Garcia P, Lin-Shiao E, Tanim KMA, et al. p53 mediates target gene association with nuclear speckles for amplified RNA expression. Molecular Cell. 2021;81:1666–1681. doi: 10.1016/j.molcel.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang DS, Saeedi A, Davtyan A, Fathi M, Sherman MB, Safari MS, Klindziuk A, Barton MC, et al. Mesoscopic protein-rich clusters host the nucleation of mutant p53 amyloid fibrils. Proceedings of the National Academy of Sciences of the United States of America. 2021;118:e2015618118.. doi: 10.1073/pnas.2015618118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou M, Li W, Li J, Xie L, Wu R, Wang L, Fu S, Su W, et al. Phase-separated condensate-aided enrichment of biomolecular interactions for high-throughput drug screening in test tubes. Journal of Biological Chemistry. 2020;295:11420–11434. doi: 10.1074/jbc.RA120.012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rajagopalan S, Jaulent AM, Wells M, Veprintsev DB, Fersht AR. 14-3-3 activation of DNA binding of p53 by enhancing its association into tetramers. Nucleic Acids Research. 2008;36:5983–5991. doi: 10.1093/nar/gkn598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Low YH, Asi Y, Foti SC, Lashley T. Heterogeneous nuclear ribonucleoproteins: implications in neurological diseases. Molecular Neurobiology. 2021;58:631–646. doi: 10.1007/s12035-020-02137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roy R, Durie D, Li H, Liu BQ, Skehel JM, Mauri F, Cuorvo LV, Barbareschi M, et al. hnRNPA1 couples nuclear export and translation of specific mRNAs downstream of FGF-2/S6K2 signalling. Nucleic Acids Research. 2014;42:12483–12497. doi: 10.1093/nar/gku953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cloutier A, Shkreta L, Toutant J, Durand M, Thibault P, Chabot B. hnRNP A1/A2 and Sam68 collaborate with SRSF10 to control the alternative splicing response to oxaliplatin-mediated DNA damage. Scientific Reports. 2018;8:2206.. doi: 10.1038/s41598-018-20360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martin EW, Thomasen FE, Milkovic NM, Cuneo MJ, Grace CR, Nourse A, Lindorff-Larsen K, Mittag T. Interplay of folded domains and the disordered low-complexity domain in mediating hnRNPA1 phase separation. Nucleic Acids Research. 2021;49:2931–2945. doi: 10.1093/nar/gkab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lin Y, Protter DS, Rosen MK, Parker R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Molecular Cell. 2015;60:208–219. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE, Brangwynne CP. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell. 2017;168:159–171. doi: 10.1016/j.cell.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tsoi PS, Quan MD, Choi KJ, Dao KM, Ferreon JC, Ferreon ACM. Electrostatic modulation of hnRNPA1 low-complexity domain liquid-liquid phase separation and aggregation. Protein Science. 2021;30:1408–1417. doi: 10.1002/pro.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. The EMBO Journal. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Courchet J, Buchet-Poyau K, Potemski A, Bres A, Jariel-Encontre I, Billaud M. Interaction with 14-3-3 adaptors regulates the sorting of hMex-3B RNA-binding protein to distinct classes of RNA granules. Journal of Biological Chemistry. 2008;283:32131–32142. doi: 10.1074/jbc.M802927200. [DOI] [PubMed] [Google Scholar]
- 182.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bojkova D, Klann K, Koch B, Widera M, Krause D, Ciesek S, Cinatl J, Munch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Correa Marrero M, Polacco BJ, Melnyk JE, et al. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020;182:685–712. doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sender R, Bar-On YM, Gleizer S, Bernshtein B, Flamholz A, Phillips R, Milo R. The total number and mass of SARS-CoV-2 virions. Proceedings of the National Academy of Sciences of the United States of America. 2021;118:e2024815118.. doi: 10.1073/pnas.2024815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McBride R, van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Surjit M, Kumar R, Mishra RN, Reddy MK, Chow VT, Lal SK. The severe acute respiratory syndrome coronavirus nucleocapsid protein is phosphorylated and localizes in the cytoplasm by 14-3-3-mediated translocation. Journal of Virology. 2005;79:11476–11486. doi: 10.1128/JVI.79.17.11476-11486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tugaeva KV, Hawkins D, Smith JLR, Bayfield OW, Ker DS, Sysoev AA, Klychnikov OI, Antson AA, et al. The mechanism of SARS-CoV-2 nucleocapsid protein recognition by the human 14-3-3 proteins. Journal of Molecular Biology. 2021;433:166875.. doi: 10.1016/j.jmb.2021.166875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen H, Cui Y, Han X, Hu W, Sun M, Zhang Y, Wang PH, Song G, et al. Liquid-liquid phase separation by SARS-CoV-2 nucleocapsid protein and RNA. Cell Research. 2020;30:1143–1145. doi: 10.1038/s41422-020-00408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Perdikari TM, Murthy AC, Ryan VH, Watters S, Naik MT, Fawzi NL. SARS-CoV-2 nucleocapsid protein phase-separates with RNA and with human hnRNPs. The EMBO Journal. 2020;39:e106478.. doi: 10.15252/embj.2020106478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Iserman C, Roden CA, Boerneke MA, Sealfon RSG, McLaughlin GA, Jungreis I, Fritch EJ, Hou YJ, et al. Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Molecular Cell. 2020;80:1078–1091. doi: 10.1016/j.molcel.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu C, Qavi AJ, Hachim A, Kavian N, Cole AR, Moyle AB, Wagner ND, Sweeney-Gibbons J, et al. Characterization of SARS-CoV-2 nucleocapsid protein reveals multiple functional consequences of the C-terminal domain. iScience. 2021;24:102681.. doi: 10.1016/j.isci.2021.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu S, Ye Q, Singh D, Cao Y, Diedrich JK, Yates JR, Villa E, Cleveland DW, et al. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nature Communications. 2021;12:502.. doi: 10.1038/s41467-020-20768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang S, Dai T, Qin Z, Pan T, Chu F, Lou L, Zhang L, Yang B, et al. Targeting liquid-liquid phase separation of SARS-CoV-2 nucleocapsid protein promotes innate antiviral immunity by elevating MAVS activity. Nature Cell Biology. 2021;23:718–732. doi: 10.1038/s41556-021-00710-0. [DOI] [PubMed] [Google Scholar]
- 195.Carlson CR, Asfaha JB, Ghent CM, Howard CJ, Hartooni N, Safari M, Frankel AD, Morgan DO. Phosphoregulation of phase separation by the SARS-CoV-2 N protein suggests a biophysical basis for its dual functions. Molecular Cell. 2020;80:1092–1103. doi: 10.1016/j.molcel.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang J, Shi C, Xu Q, Yin H. SARS-CoV-2 nucleocapsid protein undergoes liquid-liquid phase separation into stress granules through its N-terminal intrinsically disordered region. Cell Discovery. 2021;7:5.. doi: 10.1038/s41421-020-00240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luo L, Li Z, Zhao T, Ju X, Ma P, Jin B, Zhou Y, He S, et al. SARS-CoV-2 nucleocapsid protein phase separates with G3BPs to disassemble stress granules and facilitate viral production. Science Bulletin. 2021;66:1194–1204. doi: 10.1016/j.scib.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu Y, Ma L, Cai S, Zhuang Z, Zhao Z, Jin S, Xie W, Zhou L, et al. RNA-induced liquid phase separation of SARS-CoV-2 nucleocapsid protein facilitates NF-kappaB hyper-activation and inflammation. Signal Transduction Targeted Therapy. 2021;6:167.. doi: 10.1038/s41392-021-00575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao D, Xu W, Zhang X, Wang X, Ge Y, Yuan E, Xiong Y, Wu S, et al. Understanding the phase separation characteristics of nucleocapsid protein provides a new therapeutic opportunity against SARS-CoV-2. Protein Cell. 2021 doi: 10.1007/s13238-021-00832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Morciano G, Vezzani B, Missiroli S, Boncompagni C, Pinton P, Giorgi C. An updated understanding of the role of YAP in driving oncogenic responses. Cancers. 2021;13:3100.. doi: 10.3390/cancers13123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiological Reviews. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 202.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes and Development. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang C, Lin J, Dong T, et al. Interferon-gamma induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Molecular Cell. 2021;81:1216–1230. doi: 10.1016/j.molcel.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 204.Cai D, Feliciano D, Dong P, Flores E, Gruebele M, Porat-Shliom N, Sukenik S, Liu Z, et al. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nature Cell Biology. 2019;21:1578–1589. doi: 10.1038/s41556-019-0433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hong AW, Meng Z, Yuan HX, Plouffe SW, Moon S, Kim W, Jho EH, Guan KL. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Reports. 2017;18:72–86. doi: 10.15252/embr.201642681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nong J, Kang K, Shi Q, Zhu X, Tao Q, Chen YG. Phase separation of Axin organizes the beta-catenin destruction complex. Journal of Cell Biology. 2021;220:e202012112.. doi: 10.1083/jcb.202012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li TM, Ren J, Husmann D, Coan JP, Gozani O, Chua KF. Multivalent tumor suppressor adenomatous polyposis coli promotes Axin biomolecular condensate formation and efficient beta-catenin degradation. Scientific Reports. 2020;10:17425. doi: 10.1038/s41598-020-74080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ahn JI, Park JE, Meng L, Zhang L, Kim TS, Kruhlak MJ, Kim BY, Lee KS. Phase separation of the Cep63-Cep152 complex underlies the formation of dynamic supramolecular self-assemblies at human centrosomes. Cell Cycle. 2020;19:3437–3457. doi: 10.1080/15384101.2020.1843777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ma X, Chen T, Peng Z, Wang Z, Liu J, Yang T, Wu L, Liu G, et al. Histone chaperone CAF-1 promotes HIV-1 latency by leading the formation of phase-separated suppressive nuclear bodies. The EMBO Journal. 2021;40:e106632.. doi: 10.15252/embj.2020106632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Esposito M, Fang C, Cook KC, Park N, Wei Y, Spadazzi C, Bracha D, Gunaratna RT, et al. TGF-beta-induced DACT1 biomolecular condensates repress Wnt signalling to promote bone metastasis. Nature Cell Biology. 2021;23:257–267. doi: 10.1038/s41556-021-00641-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jalihal AP, Pitchiaya S, Xiao L, Bawa P, Jiang X, Bedi K, Parolia A, Cieslik M, et al. Multivalent proteins rapidly and reversibly phase-separate upon osmotic cell volume change. Molecular Cell. 2020;79:978–990. doi: 10.1016/j.molcel.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Damman R, Schutz S, Luo Y, Weingarth M, Sprangers R, Baldus M. Atomic-level insight into mRNA processing bodies by combining solid and solution-state NMR spectroscopy. Nature Communications. 2019;10:4536.. doi: 10.1038/s41467-019-12402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schutz S, Noldeke ER, Sprangers R. A synergistic network of interactions promotes the formation of in vitro processing bodies and protects mRNA against decapping. Nucleic Acids Research. 2017;45:6911–6922. doi: 10.1093/nar/gkx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tibble RW, Depaix A, Kowalska J, Jemielity J, Gross JD. Biomolecular condensates amplify mRNA decapping by biasing enzyme conformation. Nucleic Acids Research. 2021;17:615–623. doi: 10.1038/s41589-021-00774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fromm SA, Kamenz J, Noldeke ER, Neu A, Zocher G, Sprangers R. In vitro reconstitution of a cellular phase-transition process that involves the mRNA decapping machinery. Angew. Angewandte Chemie. 2014;53:7354–7359. doi: 10.1002/anie.201402885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sala K, Corbetta A, Minici C, Tonoli D, Murray DH, Cammarota E, Ribolla L, Ramella M, et al. The ERC1 scaffold protein implicated in cell motility drives the assembly of a liquid phase. Scientific Reports. 2019;9:13530.. doi: 10.1038/s41598-019-49630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhu J, Zhou Q, Xia Y, Lin L, Li J, Peng M, Zhang R, Zhang M. GIT/PIX condensates are modular and ideal for distinct compartmentalized cell signaling. Molecular Cell. 2020;79:782–796. doi: 10.1016/j.molcel.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 218.Hollmuller E, Geigges S, Niedermeier ML, Kammer KM, Kienle SM, Rosner D, Scheffner M, Marx A, et al. Site-specific ubiquitylation acts as a regulator of linker histone H1. Nature Communications. 2021;12:3497.. doi: 10.1038/s41467-021-23636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ryan VH, Perdikari TM, Naik MT, Saueressig CF, Lins J, Dignon GL, Mittal J, Hart AC, et al. Tyrosine phosphorylation regulates hnRNPA2 granule protein partitioning and reduces neurodegeneration. The EMBO Journal. 2021;40:e105001.. doi: 10.15252/embj.2020105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Amaya J, Ryan VH, Fawzi NL. The SH3 domain of Fyn kinase interacts with and induces liquid-liquid phase separation of the low-complexity domain of hnRNPA2. Journal of Biological Chemistry. 2018;293:19522–19531. doi: 10.1074/jbc.RA118.005120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ryan VH, Watters S, Amaya J, Khatiwada B, Venditti V, Naik MT, Fawzi NL. Weak binding to the A2RE RNA rigidifies hnRNPA2 RRMs and reduces liquid-liquid phase separation and aggregation. Nucleic Acids Research. 2020;48:10542–10554. doi: 10.1093/nar/gkaa710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gueroussov S, Weatheritt RJ, O’Hanlon D, Lin ZY, Narula A, Gingras AC, Blencowe BJ. Regulatory expansion in mammals of multivalent hnRNP assemblies that globally control alternative splicing. Cell. 2017;170:324–339. doi: 10.1016/j.cell.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 223.Watanabe K, Ohtsuki T. Inhibition of HSF1 and SAFB granule formation enhances apoptosis induced by heat stress. International Journal of Molecular Sciences. 2021;22:4982.. doi: 10.3390/ijms22094982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gaglia G, Rashid R, Yapp C, Joshi GN, Li CG, Lindquist SL, Sarosiek KA, Whitesell L, et al. HSF1 phase transition mediates stress adaptation and cell fate decisions. Nature Cell Biology. 2020;22:151–158. doi: 10.1038/s41556-019-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Aktar F, Burudpakdee C, Polanco M, Pei S, Swayne TC, Lipke PN, Emtage L. The huntingtin inclusion is a dynamic phase-separated compartment. Life Sci Alliance. 2019;2:e201900489.. doi: 10.26508/lsa.201900489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li, R. H., Tian, T., Ge, Q. W., He, X. Y., Shi, C. Y., Li, J. H., Zhang, Z., Liu, F. Z., et al. (2021) A phosphatidic acid-binding lncRNA SNHG9 facilitates LATS1 liquid-liquid phase separation to promote oncogenic YAP signaling. Cell Res, In press. 10.1038/s41422-021-00530-9. [DOI] [PMC free article] [PubMed]
- 227.Balaban C, Sztacho M, Blazikova M, Hozak P. The F-actin-binding MPRIP forms phase-separated condensates and associates with PI(4,5)P2 and active RNA polymerase II in the cell nucleus. Cells. 2021;10:848.. doi: 10.3390/cells10040848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rawat P, Boehning M, Hummel B, Aprile-Garcia F, Pandit AS, Eisenhardt N, Khavaran A, Niskanen E, et al. Stress-induced nuclear condensation of NELF drives transcriptional downregulation. Molecular Cell. 2021;81:1013–1026. doi: 10.1016/j.molcel.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.White MR, Mitrea DM, Zhang P, Stanley CB, Cassidy DE, Nourse A, Phillips AH, Tolbert M, et al. C9orf72 poly(PR) dipeptide repeats disturb biomolecular phase separation and disrupt nucleolar function. Molecular Cell. 2019;74:713–728. doi: 10.1016/j.molcel.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mitrea DM, Cika JA, Stanley CB, Nourse A, Onuchic PL, Banerjee PR, Phillips AH, Park CG, et al. Self-interaction of NPM1 modulates multiple mechanisms of liquid-liquid phase separation. Nature Communications. 2018;9:842.. doi: 10.1038/s41467-018-03255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ferrolino MC, Mitrea DM, Michael JR, Kriwacki RW. Compositional adaptability in NPM1-SURF6 scaffolding networks enabled by dynamic switching of phase separation mechanisms. Nature Communications. 2018;9:5064.. doi: 10.1038/s41467-018-07530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jiang X, Ho DBT, Mahe K, Mia J, Sepulveda G, Antkowiak M, Jiang L, Yamada S, et al. Condensation of pericentrin proteins in human cells illuminates phase separation in centrosome assembly. Journal of Cell Science. 2021;134:jcs258897.. doi: 10.1242/jcs.258897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Park JE, Zhang L, Bang JK, Andresson T, DiMaio F, Lee KS. Phase separation of Polo-like kinase 4 by autoactivation and clustering drives centriole biogenesis. Nature Communications. 2019;10:4959.. doi: 10.1038/s41467-019-12619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Vitiello E, Moreau P, Nunes V, Mettouchi A, Maiato H, Ferreira JG, Wang I, Balland M. Acto-myosin force organization modulates centriole separation and PLK4 recruitment to ensure centriole fidelity. Nature Communications. 2019;10:52.. doi: 10.1038/s41467-018-07965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Oshidari R, Huang R, Medghalchi M, Tse EYW, Ashgriz N, Lee HO, Wyatt H, Mekhail K. DNA repair by Rad52 liquid droplets. Nature Communications. 2020;11:695.. doi: 10.1038/s41467-020-14546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Min J, Wright WE, Shay JW. Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Genes and Development. 2019;33:814–827. doi: 10.1101/gad.324905.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dang M, Li Y, Song J. ATP biphasically modulates LLPS of SARS-CoV-2 nucleocapsid protein and specifically binds its RNA-binding domain. Biochemical and Biophysical Research Communications. 2021;541:50–55. doi: 10.1016/j.bbrc.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cubuk J, Alston JJ, Incicco JJ, Singh S, Stuchell-Brereton MD, Ward MD, Zimmerman MI, Vithani N, et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nature Communications. 2021;12:1936.. doi: 10.1038/s41467-021-21953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Krainer G, Welsh TJ, Joseph JA, Espinosa JR, Wittmann S, de Csillery E, Sridhar A, Toprakcioglu Z, et al. Reentrant liquid condensate phase of proteins is stabilized by hydrophobic and non-ionic interactions. Nature Communications. 2021;12:1085.. doi: 10.1038/s41467-021-21181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ilik IA, Malszycki M, Lubke AK, Schade C, Meierhofer D, Aktas T. SON and SRRM2 are essential for nuclear speckle formation. Elife. 2020;9:e60579.. doi: 10.7554/eLife.60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cinar H, Oliva R, Lin YH, Chen X, Zhang M, Chan HS, Winter R. Pressure sensitivity of SynGAP/PSD-95 condensates as a model for postsynaptic densities and its biophysical and neurological ramifications. Chemistry. 2020;26:11024–11031. doi: 10.1002/chem.201905269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Araki Y, Hong I, Gamache TR, Ju S, Collado-Torres L, Shin JH, Huganir RL. SynGAP isoforms differentially regulate synaptic plasticity and dendritic development. Elife. 2020;9:e56273.. doi: 10.7554/eLife.56273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang X, Lin Y, Eschmann NA, Zhou H, Rauch JN, Hernandez I, Guzman E, Kosik KS, et al. RNA stores tau reversibly in complex coacervates. PLOS Biology. 2017;15:e2002183.. doi: 10.1371/journal.pbio.2002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ferreon JC, Jain A, Choi KJ, Tsoi PS, MacKenzie KR, Jung SY, Ferreon AC. Acetylation disfavors tau phase separation. International Journal of Molecular Sciences. 2018;19:1360.. doi: 10.3390/ijms19051360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lin Y, McCarty J, Rauch JN, Delaney KT, Kosik KS, Fredrickson GH, Shea JE, Han S. Narrow equilibrium window for complex coacervation of tau and RNA under cellular conditions. Elife. 2019;8:e42571.. doi: 10.7554/eLife.42571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ukmar-Godec T, Hutten S, Grieshop MP, Rezaei-Ghaleh N, Cima-Omori MS, Biernat J, Mandelkow E, Soding J, et al. Lysine/RNA-interactions drive and regulate biomolecular condensation. Nature Communications. 2019;10:2909.. doi: 10.1038/s41467-019-10792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rane JS, Kumari A, Panda D. The acetyl mimicking mutation, K274Q in tau, enhances the metal binding affinity of tau and reduces the ability of tau to protect DNA. ACS Chemical Neuroscience. 2020;11:291–303. doi: 10.1021/acschemneuro.9b00455. [DOI] [PubMed] [Google Scholar]
- 248.Singh V, Xu L, Boyko S, Surewicz K, Surewicz WK. Zinc promotes liquid-liquid phase separation of tau protein. Journal of Biological Chemistry. 2020;295:5850–5856. doi: 10.1074/jbc.AC120.013166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang X, Vigers M, McCarty J, Rauch JN, Fredrickson GH, Wilson MZ, Shea JE, Han S, et al. The proline-rich domain promotes Tau liquid-liquid phase separation in cells. Journal of Cell Biology. 2020;219:e202006054.. doi: 10.1083/jcb.202006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Savastano A, Flores D, Kadavath H, Biernat J, Mandelkow E, Zweckstetter M. Disease-associated tau phosphorylation hinders tubulin assembly within tau condensates. Angewandte Chemie. 2021;60:726–730. doi: 10.1002/anie.202011157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Siegert A, Rankovic M, Favretto F, Ukmar-Godec T, Strohaker T, Becker S, Zweckstetter M. Interplay between tau and alpha-synuclein liquid-liquid phase separation. Protein Science. 2021;30:1326–1336. doi: 10.1002/pro.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang B, Gan W, Han X, Liu N, Ma T, Li D. The positive regulation loop between NRF1 and NONO-TFE3 fusion promotes phase separation and aggregation of NONO-TFE3 in NONO-TFE3 tRCC. International Journal of Biological Macromolecules. 2021;176:437–447. doi: 10.1016/j.ijbiomac.2021.02.061. [DOI] [PubMed] [Google Scholar]
- 253.Chen D, Wang Z, Zhao YG, Zheng H, Zhao H, Liu N, Zhang H. Inositol polyphosphate multikinase inhibits liquid-liquid phase separation of TFEB to negatively regulate autophagy activity. Developmental Cell. 2020;55:588–602. doi: 10.1016/j.devcel.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 254.Gerson JE, Linton H, Xing J, Sutter AB, Kakos FS, Ryou J, Liggans N, Sharkey LM, et al. Shared and divergent phase separation and aggregation properties of brain-expressed ubiquilins. Scientific Reports. 2021;11:287.. doi: 10.1038/s41598-020-78775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu S, Wang T, Shi Y, Bai L, Wang S, Guo D, Zhang Y, Qi Y, et al. USP42 drives nuclear speckle mRNA splicing via directing dynamic phase separation to promote tumorigenesis. Cell Death and Differentitation. 2021;28:2482–2498. doi: 10.1038/s41418-021-00763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the article and its supplementary information files.