Abstract
Prolactin (PRL) is a peptide hormone secreted from anterior pituitary involved in milk production in the females and regulation of sex drive in both sexes. PRL has pro-inflammatory and anti-inflammatory functions. High PRL serum level or hyperprolactinemia is associated with different viral infections. In coronavirus disease 2019 (Covid-19), which caused by positive-sense single-strand RNA virus known as severe acute respiratory distress syndrome coronavirus type 2 (SARS-CoV-2), PRL serum level is increased. PRL in Covid-19 may exacerbate the underlying inflammatory status by induction release of pro-inflammatory cytokines. However, PRL through its anti-inflammatory effects may reduce the hyperinflammatory status in Covid-19. The underlying mechanism of increasing PRL in Covid-19 is poorly understood. Therefore, in this review we try to find the potential anti-inflammatory or pro-inflammatory role of PRL in Covid-19. As well, this review was aimed to discuss the underlying causes and mechanisms for Covid-19-induced hyperprolactinemia.
Keywords: Prolactin, Covid-19, Anti-inflammatory effects, Pro-inflammatory cytokines
Background
Prolactin (PRL) also known as lactotropin (lactogenic hormone with immune function) is a peptide hormone secreted from anterior pituitary involved in milk production in the females and regulation of sex drive in both sexes [1]. PRL is the only hormone secreted from anterior pituitary not activated by hypothalamic-releasing factor but under control of inhibitory effect of dopamine from the hypothalamus [2]. Dopamine inhibits release of PRL via dopamine receptor type 2 (D2) and deficiency of this receptor contributes into development of hyperprolactinemia [3]. PRL secretion is stimulated by suckling, thyrotropin-releasing hormone (TRH), estrogen, and vasoactive intestinal polypeptide (Fig. 1), [4]. Therefore, D2 agonists like bromocriptine and cabergoline inhibit PRL secretion, while D2 antagonists like sulpiride, risperidone, haloperidol, metoclopramide, and domperidone stimulate PRL secretion [5].
Fig. 1.
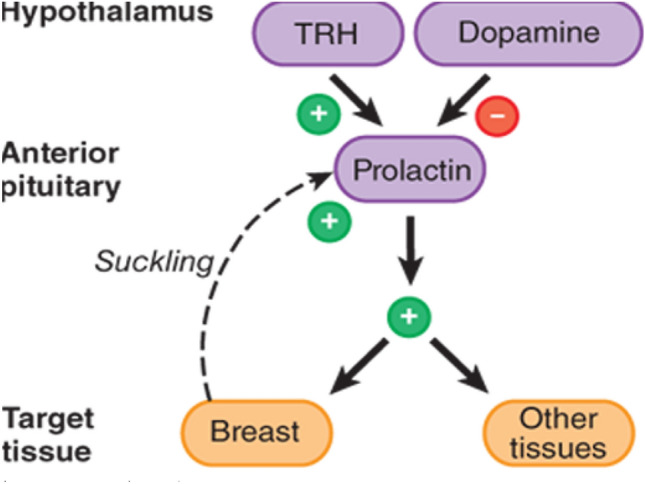
Regulation of prolactin secretion: Thyrotropin-releasing hormone (TRH) stimulates prolactin secretion, while dopamine inhibits prolactin secretion
There are three circulating forms of PRL, which are little-PRL (22 kDa), Big-PRL (48 kDa), and Big-big PRL (150 kDa). Little-PRL or free PRL is the most active one, while Big-big PRL or macro-PRL has very low biological activity and appears to be predominantly circulated in postmenopausal women [6]. PRL acts through specific PRL receptors (PRLRs) belonging to the cytokine receptors super-family, which are also receptors for leptin, erythropoietin, colony-stimulating factor, and interleukin-6 (IL-6) [7].
PRLRs can also act as function receptors for growth hormone and placental lactogen [8]. As well, PRL may act as a cytokine-like action via activation of cytokine receptors in the regulation of immune system [9].
Therefore, the diverse function of PRL is related to activation of PRLRs and cytokine receptors through endocrine, paracrine, and autocrine manners (Fig. 2).
Fig. 2.
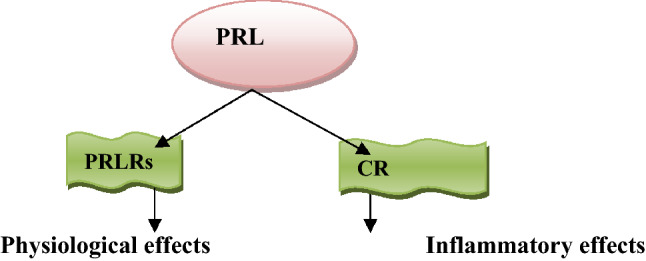
Effects of prolactin (PRL): PRL activates PRL receptors (PRLRs) leading to the physiological effects. PRL activates cytokine receptors (CR) leading to inflammatory reactions
Moreover, PRL may be classified as a gonadotropin because it has luteotropic and weak gonadotropin effects. PRL inhibits secretion of gonadotropin-releasing hormone by unidentified mechanism, although PRLRs have been recognized in the hypothalamus [10].
Similarly, PRL suppresses secretions of testosterone in men and estrogen in women; however, physiological level of PRL improves Leydig cells function and stimulates testosterone secretion [11, 12].
Furthermore, PRL has pleiotropic effects, including improvement formation of myelin sheath of neuronal axons of central nervous system (CNS), fetal neurogenesis, promoting formation of lung surfactant, and immunological tolerance during pregnancy [13].
Extra-pituitary PRL is released from decidua, breast, myometrium, prostate, leukocytes, and lymphocytes that secretion is not affected by dopamine but by superdistal promoters. Extra-pituitary PRL (ePRL) has mainly autocrine and paracrine effects rather than endocrine effects due to different bioactivity and molecular weight from pituitary PRL [14].
PRL serum level is high during sleep and early morning, it can be increased by exercise, emotional stress, high-protein diet, and following epileptic seizure [15]. Therefore, PRL serum level can be used to distinguish epileptic seizure, which associated with high PRL serum level from psychogenic seizure which associated with normal PRL serum level [16].
Pathologically, PRL serum level is elevated in various clinical conditions, such as prolactinoma, primary hypothyroidism, and adrenal failure. Hyperprolactinemia is defined when fasting PRL serum level more than 25 ng/mL in females and 20 ng/mL in males [17].
PRLRs are broadly expressed in different endocrine and target tissues as well as immune cells, including lymphocytes, monocytes, macrophages, granulocytes, microglia, natural killer (NK) cells, and thymic epithelial cells for controlling of immune response and reactions in autoimmune disorders [18].
There are various isoforms of PRLRs, which are large (PRLR-L), intermediate (PRLR-I), and small (PRLR-S) according to the size of intracellular domain, as the extra-cellular domains of PRLRs are identical.
Besides, soluble form of PRLR has been identified, but its precise function is not elucidated [18]. Therefore, PRL may act as an immune–endocrine link between the immune cells and endocrine disorders.
The coronavirus disease 2019 (Covid-19), which is caused by positive-sense single-strand RNA virus known as severe acute respiratory distress syndrome coronavirus type 2 (SARS-CoV-2), leads to acute tissue injury and exaggerated immune response [19]. Covid-19 patients are chiefly asymptomatic or presented with mild respiratory symptoms, although 5–15% of patients may develop pulmonary and extra-pulmonary complications [20]. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the most common pulmonary complications [21].
Besides, extra-pulmonary complications like acute cardiac injury, acute brain injury, testicular injury, and different endocrinopathies could be the presenting symptoms [22, 23]. The diverse effects of SARS-CoV-2 infection is due to wide distribution of angiotensin-converting enzyme 2 (ACE2), which acts as receptor and entry point for this virus [24].
It has been reported that PRL has pro-inflammatory and anti-inflammatory functions [25]; therefore, the aim of the present study is to review the potential role of PRL in Covid-19.
Immunological role of prolactin
Activation of PRLRs may stimulate various inflammatory signaling pathways including mitogen-activated protein kinase (MAPK), extra-cellular signal regulated kinase 1/2 (ERK1/2), Janus kinase 2 (JAK2), signal transducer and activator of transcription 5 (STAT5), phosphoinositide 3-kinase (PI3K), and protein kinase B (PKB) resulting in pro-inflammatory or anti-inflammatory actions depending on the different isoforms of activated PRLRs [26]. In addition, PRL secretion is stimulated by IL-1, 1L-2, and IL-6 during active phase of chronic inflammatory disorders. Williams et al. observed that activation of macrophages by PRL through STAT3 result in release of anti-inflammatory cytokine IL-10 [27]. However, activation of macrophages by PRL through JAK2/STAT1 pathway results in release of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), IL-1β, IL-12, and interferon gamma (INF-γ) [28].
Similarly, PRL provokes T cells activation via STAT5 signaling pathway leading to expression of T-box transcription factor (TBXF) [29]. Indeed, experimental study demonstrated that psychological stress-induced PRL secretion result in the induction of intestinal inflammation via alteration function of dendritic and T cells [29]. Further molecular studies revealed that PRL can activate expression of inducible nitric oxide synthase (iNOS) in the peripheral mononuclear cells via STAT5 signaling pathway and interferon regulatory factor 1 (IRF-1) [30]. Likewise, experimental study illustrated that expression of ePRL in the macrophages and monocytes are activated by adrenergic pathways with subsequent polarization of macrophages toward the inflammatory one (M1) [31].
Furthermore, PRL has important effects on the adaptive and innate immune responses; it increases expression of IL-2 on the lymphocytes and thymocytes with maturation of CD4+ and CD8+ [32].
A direct association between PRL serum level and CD4+ T lymphocytes has been clarified by induction expression of NF-κB and IRF-1 [33]. Likewise, high PRL serum level and hyperprolactinemia promote auto-reactivity by inhibiting B cells clonal deletion and alteration activation threshold and tolerance for B cells [34]. Indeed, PRL activates dendritic cells (DCs) for antigen presentation and production of INF-α [25].
As well, PRL promotes maturation and development of immune cells expressing major histocompatibility complex II (MHC-II) and co-stimulatory molecules, such as CD86 and CD40 [35].
Therefore, hyperprolactinemia is correlated with high level of auto-antibodies, like anti-pituitary and anti-cardiolipin auto-antibodies [36].
During pregnancy there is an immunological-tolerant state with adaptation due to shifting of immune response from the pro-inflammatory Th1/Th17 toward the anti-inflammatory Th2 immune response with development of regulatory T cells (Treg) [37].
It has been shown that PRL and estrogen have immunostimulatory effects, while progesterone and testosterone have immunoinhibitory effects during pregnancy [38].
Thus, high PRL serum level during pregnancy and lactation may provoke immunological disorders and development of autoimmune diseases, including systemic lupus erythematosus (SLE), peripartum cardiomyopathy, and rheumatoid arthritis [39]. Taken together, PRL has dual inflammatory and anti-inflammatory effects depending on the underlying pathophysiological conditions (Fig. 3).
Fig. 3.
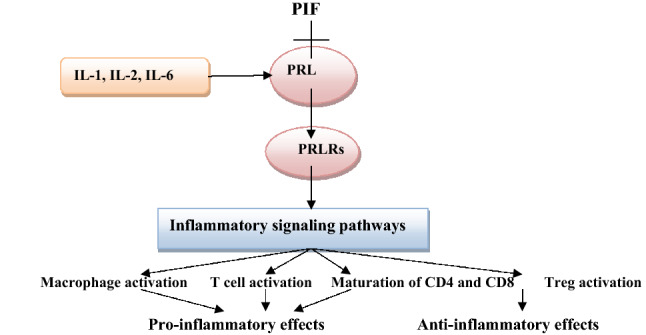
Immunological role of prolactin (PRL): PRL is inhibited by PRL inhibiting factor (PIF) and stimulated by interleukins (ILs). PRL stimulates PRL receptors (PRLRs) causing activation of inflammatory signaling pathways with subsequent inflammatory or anti-inflammatory effects
Inflammatory role of prolactin
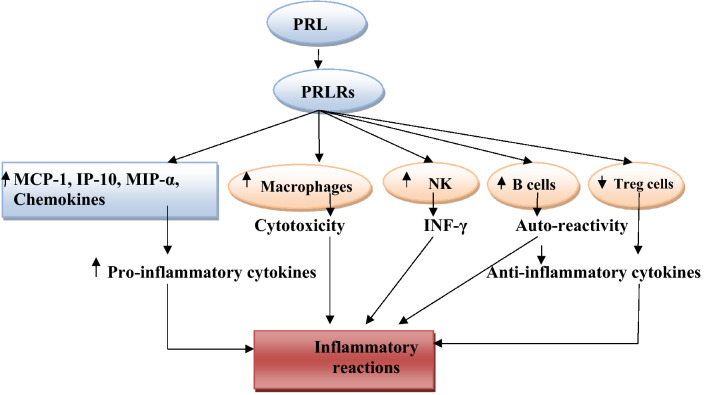
PRL through PRLRs, which distributed in various immune cells, can induce release of pro-inflammatory cytokines. PRL activates release of macrophage inflammatory protein-1α (MIP-1α), interferon protein 10(IP-10), chemokines, and monocyte chemoattractant protein 1 (MCP-1) from activated macrophages [28].
Similarly, PRL triggers macrophage cytotoxicity with generation of ROS, as well it induces expression of IRF-1 and iNOS in the granulocytes [40]. PRL also stimulates natural killer (NK) cells to produce INF-γ with activation survival of T cells and increase percentage of CD4 through inhibition of T cells apoptosis and production of pro-inflammatory cytokines that act as a co-stimulatory molecule promoting T cells survival [41]. In addition, PRL inhibits function of regulatory T cells (Treg) [42].
Alternatively, PRL activates antibody production by stimulating B cells through increment of intracellular calcium, suppression of B cells energy and inhibition expression of pro-apoptotic gene [42]. Thus, persistent elevation of PRL alters B cells function and promoting auto-reactivity.
Therefore, PRL through activation of T and B cells with stimulation of monocyte–macrophage axis promotes the cellular and humoral immune response with induction of inflammatory changes (Fig. 4).
Fig. 4.
Pro-inflammatory role of prolactin (PRL): PRL through PRL receptors (PRLRs) activates release of macrophage inflammatory protein-1α (MIP-1α), interferon protein 10(IP-10), chemokines, and monocyte chemoattractant protein 1(MCP-1). PRL also stimulates natural killer (NK) cells to produce INF-γ, PRL inhibits function of regulatory T cells (Treg), and PRL alters B cells function and promotes auto-reactivity and antibody production with subsequent inflammatory reactions
So, PRL has important inflammatory role that induces and exaggerates different inflammatory disorders [32].
On the other hand, PRL has an anti-inflammatory against different autoimmune diseases, and recent evidences illustrated that PRL does not play a critical role in the development and progression of multiple sclerosis, experimental autoimmune encephalomyelitis, systemic lupus erythematosus, and even in experimental rheumatoid arthritis (RA) [43].
However, there is significant controversy regarding the role the anti-inflammatory and inflammatory role of PRL in RA, since systemic and locally produced PRL have different roles in RA [44]. The anti-inflammatory effects of PRL are documented in different experimental and clinical studies.
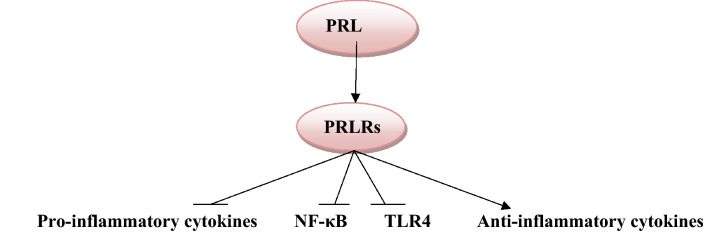
Olmos-Ortiz et al. illustrated that PRL reduces the immune response to the effects of lipopolysaccharide (LPS)-induced inflammation by attenuating expression toll-like receptor 4 (TLR4) and nuclear factor kappa B cells (NF-κB) with subsequent reduction release of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 [45].
Besides, PRL inhibits expression of TNF-α and IL-1β in human fetal membrane inflammation induced by LPS [46]. Interestingly, PRL increases circulating immunosuppressive progesterone by inhibiting 20-hydroxysteroid dehydrogenase that supports the anti-inflammatory effect of placental PRL (Fig. 5), [46].
Fig. 5.
Anti-inflammatory role of prolactin (PRL): PRL inhibits expression of pro-inflammatory cytokines, nuclear factor kappa B (NF-κB), and toll-like receptor 4 (TLR4) with activation of anti-inflammatory cytokines
Therefore, PRL have both pro-inflammatory and anti-inflammatory roles according to certain conditions, and these dual effects are greatly influenced by molecular compositions of the target cells during inflammatory milieu.
Role of prolactin in viral infection
It has been reported that high PRL serum level or hyperprolactinemia is linked with human immune deficiency (HIV) infection. Hyperprolactinemia in HIV infection is not related to the metabolic disorders, liver disturbance, viral load, and use of antiretroviral treatment [47]. A prospective study involved 192 men patients with HIV infection illustrated that hyperprolactinemia is observed in 21.4% of HIV-infected men patients and associated with higher CD4+ counts [47].
Besides, hyperprolactinemia could be a potential cause of hypogonadism in HIV-infected patients through inhibitory effect of PRL on the release of gonadotropin-releasing factor from the hypothalamus [48]. Although, a cohort study comprising 188 HIV-infected patients showed that hyperprolactinemia-induced hypogonadism in HIV is not related significantly to the inhibition of gonadotropin release [48].
Indeed, hyperprolactinemia is associated with hepatitis C virus infection (HCV), although this association is not linked with the extra-hepatic manifestation of HCV infection, like autoimmunity [49].
Kong et al. in vitro study demonstrated that PRL regulatory element binding (PREB) is regarded as a novel cofactor for HCV infection [50]. PREB is induced by HCV and promotes replication of HCV RNA through formation of HCV replication compartment [50].
These findings give a new insight into the role of PRL in HCV infection.
A prospective study illustrated that high PRL serum level is observed in HCV-infected patients compared to the healthy controls due to induction of PRL mRNA in the peripheral blood mononuclear cells by HCV [51]. Glal et al. observational study showed that high PRL serum level is linked with development of thrombocytopenia in patients with HCV infection as there was a negative correlation between PRL serum level and platelet counts [52].
High PRL serum level in HCV infection may cause immune-mediated thrombocytopenia through induction production of auto-antibodies by interfering with B cells tolerance with induction release of pro-inflammatory cytokines [53].
Furthermore, human cytomegalovirus (HCMV) infection induces expression of PRLRs by stimulating inflammatory signaling pathways including NF-κB and MAPK in ovarian cancer [54]. Both HCMV and PRL may share the same immunological pathway by activating inflammatory pathway and suppression of anti-inflammatory pathway that further improve virus replication and aggravate inflammatory reactions [54].
Accordingly, Wallis in 2021 demonstrated that CMV and other viruses like rubella can use PRLRs as receptor and entry point for viral–host cell interactions [55]. In SARS epidemic 2003, SARS-CoV led to significant increase in PRL serum level due to dysregulation of adenohypophyseal control by direct cytopathic effect or associated pro-inflammatory changes [56].
Moreover, a prospective cohort study involved 32 hospitalized infants with respiratory syncytial virus infection (RSV), which illustrated that severe infection of RSV is linked with high PRL serum level and lymphopenia [57].
Taken together, PRL serum level is augmented in different viral infections due to involvement of PRL in the pathogenesis of viral entry and replication and stimulation of PRL release by the associated activation of inflammatory signaling pathways. However, high PRL serum level in viral infections could be a compensatory mechanism due to the anti-inflammatory of PRL.
Prolactin and Covid-19
In Covid-19, there are noteworthy immunological and inflammatory changes that affect neuroendocrine homeostasis mainly in older patients with underlying co-morbidities. In addition, Covid-19-induced stressful status may affect release of PRL and other stress-mediated hormones [58].
It has been reported that PRL plays an important role in the regulation of immune function during viral infections, and hypoprolactinemia can cause death from opportunistic infections in patients with HIV infection [59]. Therefore, enhancement of PRL serum level toward the physiological values by dopamine antagonists may improve the immunological profile and survival in various critical statuses [60].
Petrulli et al. illustrated that systemic inflammation triggers activation of striatal dopamine with subsequent reduction in the release of PRL [61].
Hence, Sen proposed that dopamine antagonists could improve and boost the immune function in Covid-19 through augmentation release of immune-stimulant PRL [62]. Also, high PRL serum level in pregnant women, children, and cigarette smokers could be a protective factor against Covid-19 [62].
Interestingly, there is a significant controversy regarding PRL serum level in pregnant women, children, and cigarette smokers that might be normal, reduced, or elevated in these conditions [63].
It has been shown that hypothalamic TRH is regarded as a potent stimulator for PRL secretion from anterior pituitary [4]. In this sense, a prospective observational clinical trial in Italy involved 31 male attending care for infertility during Covid-19 lockdown which illustrated that TSH was significantly reduced by unidentified mechanism [64]. Low TSH serum level may trigger release of TRH from the hypothalamus with subsequent activation release of PRL from anterior pituitary in Covid-19. Similarly, a prospective study comprised 41 men patients with Covid-19 to assess the semen quality, which revealed that PRL serum level was elevated in those patients and remains elevated after recovery [65].
These findings suggest that SARS-CoV-2 infection can impair hypothalamic–pituitary–gonadal axis and by this mechanism may increase secretion of PRL from anterior pituitary in Covid-19.
In Covid-19, there are momentous interaction and cross-talk between hypothalamic–pituitary–adrenal (HPA) axis and SARS-CoV-2 infection, since pro-inflammatory cytokines and stress activate HPA, which also regulate response to the inflammatory cytokines [66].
A cohort, prospective study involved 28 Covid-19 patients which illustrated that cortisol and ACTH serum levels were reduced significantly in SARS-CoV-2 infection indicating impairment of adrenocortical response due to central adrenal insufficiency [66].
Of note, PRL serum level is increased in patients with adrenal insufficiency and in experimental studies [17, 67]. Kumar et al. cross-sectional study involved 235 Covid-19 patients, 21(8.5%) of them had hyperprolactinemia without sex difference, and this condition was not correlated with Covid-19 severity and mortality [68]. Besides, 25.1% of Covid-19 patients had primary hypothyroidism [68], which is similar to sick euthyroid syndrome reported in viral infections [69].
In addition, Schwaz et al. retrospective study comprised 54 Covid-19 patients which revealed that low free T3 serum level was correlated with risk of mechanical ventilation [70].
These findings suggest that hyperprolactinemia in Covid-19 may be stress induced as there were no relationship and correlation between PRL serum level and Covid-19 severity in absence of other endocrinopathies. However, secondary hyperprolactinemia in Covid-19 with primary hypothyroidism or adrenal insufficiency is linked with Covid-19 severity.
Previously, a cohort study showed that PRL serum level is increased in patients with septic shock-induced ALI [71] since; PRL has a crucial role in damping and regulation of immune response and inflammatory reactions. However, experimental study demonstrated that repeated administration of domperidone, a dopamine antagonist in experimental mice, leads to induction of ALI by exacerbating inflammatory-induced injury in lung epithelial cells [72].
It has been shown that calcitonin gene-related peptide (CGRP) has anti-inflammatory and broncho-protective effects, as well as vasodilator effects and tissue repair [73]. Ochoa-Callejero and colleagues exemplified that CGRP serum level is reduced in Covid-19 due to immune deregulation [74]. Likewise, deficiency of CGRP provokes development of autoimmunity through PRL upregulation and deficiency of anti-inflammatory cytokines [75]. Therefore, high PRL serum level in Covid-19 might be due to reduction of CGRP activity.
Of note, PRL has inflammatory or anti-inflammatory actions depending on the underlying pathological conditions. The anti-inflammatory role of PRL in Covid-19 could be beneficial in Covid-19 by mitigation exaggerated immune response. Paucity and limitation of published study regarding experimental and clinical trial studies concerning role of PRL in Covid-19 made this difficult and hard to prove the anti-inflammatory role of PRL in Covid-19. Of interest PRL has anti-inflammatory effects by attenuating expression TLR4 and NF-κB with succeeding decrease in the release of pro-inflammatory cytokines [45].
Fascinatingly, the anti-inflammatory role of PRL could be by increasing immunosuppressive progesterone [46]. In Covid-19, expression TLR4 and NF-κB are activated and augmented leading induction release of pro-inflammatory cytokines with development of ALI, ARDS, and cytokine with MOFs [76].
Pinna in 2020 suggested that sex steroids including progesterone hormone have anti-inflammatory and immune regulatory effects that protect women from Covid-19 and could have a potential role against Covid-19 in postmenopausal women and men [77].
In addition, Devi et al. experimental study illustrated that PRL inhibits expression of MAPK in the ovary with development of premature ovarian failure [78].
Thus, PRL may inhibit MAPK-mediated endothelial dysfunction and immunothrombosis in Covid-19 [79]. Therefore, high PRL serum level in Covid-19 might be a compensatory mechanism against activated TLR4/NF-κB/MAPK axis and low progesterone in Covid-19.
On the other hand, PRL is regarded as pro-inflammatory agent increased release of pro-inflammatory cytokines and development of immunoinflammatory disorders.
Notably, PRL activates release of macrophage inflammatory protein-1α (MIP-1α), interferon protein 10(IP-10), chemokines, and MCP-1 from activated macrophages [28]. PRL increases macrophage cytotoxicity with generation of ROS [40]. PRL stimulates NK cells to produce INF-γ with activation of T cells and production of pro-inflammatory cytokines [41]. Furthermore, PRL inhibits function of (Treg) cell [42].
Also, PRL activates antibody production by stimulating B by prompting B cell auto-reactivity [42]. Therefore, inflammatory signaling pathways play important role in immune dysregulation during Covid-19.
Zhuo et al. revealed that MIP-1α and IP-10 from activated monocytes and macrophages trigger progression of cytokine storm in patients with severe Covid-19 [80].
As results, high PRL serum level may increase Covid-19 severity by augmentation and release of MIP-1α and IP-10. Besides, a prospective cohort study illustrated that Covid-19 patients are associated with reduction of circulating T, B, and NK cell with macrophage cytotoxicity that reduce the antiviral activity mainly in Covid-19 patients in ICU due to high IL-6 serum level and induction of oxidative stress [81].
Moreover, perturbation of Treg in Covid-19 is correlated with disease severity and complications due to reduction of anti-inflammatory cytokines from Treg [82].
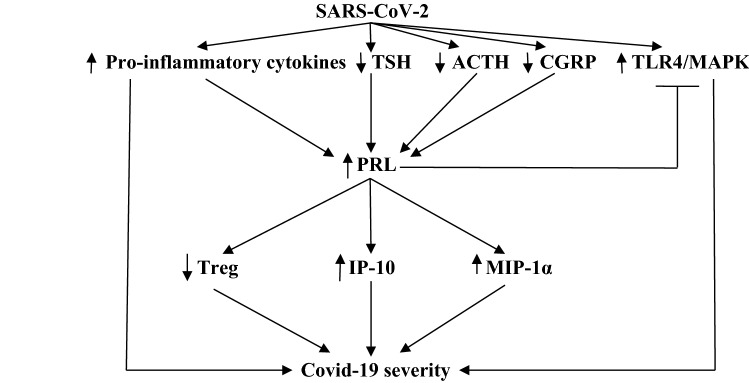
Indeed, Song et al. divulge that auto-reactivity of T and B cells is triggered in Covid-19 with risk of autoimmunity [82]. Therefore, high PRL serum level may increase severity of Covid-19 through activation of immune cells cytotoxicity, release of pro-inflammatory cytokines, inhibition of Treg cells, and induction of B cell auto-reactivity (Fig. 6).
Fig. 6.
Prolactin (PRL) and Covid-19 severity: SARS-CoV-2 induces activation of pro-inflammatory cytokines, toll-like receptor 4 (TLR4), and mitogen-activated protein kinase (MAPK), with inhibition of thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), and calcitonin gene-related peptide (CGRP) leading to activation of PRL release, which in turn stimulate inflammatory protein-1α (MIP-1α) and interferon protein 10 (IP-10) with inhibition of regulatory T cell (Treg), leading to hyperinflammation and Covid-19 severity
The underlying mechanisms of high PRL serum level in Covid-19 are poorly understood, although stress, oxidative, and immune dysregulation could be the potential mechanisms, since stressful conditions in Covid-19 may trigger release of PRL [83]. In a precise manner, dopamine which is an inhibitory releasing factor for PRL release is involved in the pathogenesis of SARS-CoV-2 during CNS invasion [84, 85].
It has been shown that there is co-expression of ACE2 and dopa decarboxylase (DDC), which is an enzyme responsible for synthesis of dopamine and serotonin [86]. As well, ACE2 regulates DDC activity through formation of AngI1-7 [86], therefore downregulation of ACE2 by SARS-CoV-2 may alter dopamine and serotonin biosynthesis [86]. Therefore, reduction of brain dopamine by SARS-CoV-2 can remove the inhibitory effects on PRL leading to hyperprolactinemia as seen in Covid-19 patients.
Mpekoulis et al. observed that expression of DDC with ACE2 is also observed in nasopharyngeal tissue [87]. Indeed, monocyte also expresses DDC which regulates synthesis of dopamine [88].
In Covid-19, lymphopenia and dysregulation of monocyte/macrophages may affect the expression and activity of DDC [89]. Therefore, downregulation of central and peripheral DDC may increase hypothalamic PRL and extra-pituitary PRL leading to hyperprolactinemia in Covid-19.
Furthermore, high inflammatory milieu in Covid-19 could be a proposed mechanism for high PRL serum, since high IL-6 serum in Covid-19 is regarded as a potent stimulator for PRL from anterior pituitary [90, 91]. Previous study revealed that stress induced by immune activation and hyperinflammation may provoke release of PRL from anterior pituitary [92]. Experimental study demonstrated that administration of bacterial endotoxin activates PRL from anterior pituitary through inhibition of hypothalamic dopamine and activation of corticotrophin releasing factor (CRF) at hypothalamus [93].
Besides, pro-inflammatory cytokines mainly IL1β cross BBB and activate release of PRL from anterior pituitary in rat [94]. Moreover, a follicular stellate cell of anterior pituitary is regarded as a source and target for pro-inflammatory cytokines including TNF-α, IL-1, and IL-6 during systemic inflammatory conditions [95]. As well, anterior pituitary cells express TLR4, which involve in PRL release and development of prolactinoma by stimulatory effects of estrogen [96].
Haj-Mirzaian et al. showed that higher activity of HPA axis TLR4 is associated with chronic stress and may lead to inflammation-induced cardiovascular and metabolic disorders [97]. In addition, activation of central TLR4 can inhibit hypothalamic gonadotropin-releasing hormone (GnRH), which stimulate PRL release [98]. Therefore, high pro-inflammatory cytokines and activated TLR4 in Covid-19 may lead to activation release of PRL from anterior pituitary and could explain hyperprolactinemia in Covid-19.
On the other hand, downregulation of ACE2 by SARS-CoV-2 leads to reduction of vasodilator Ang1-7 and Ang1-9 with increment in the level of vasoconstrictor AngII [99]. It has been reported that AngII is regarded as a powerful activator of PRL release from anterior pituitary [100]. Similarly, arginine vasopressin (AVP) activates release of PRL from anterior pituitary during stress in peripartum period [101].
Recently, Al-kuraishy et al. [102] illustrated that AVP is augmented in Covid-19 due to high circulating AngII and pro-inflammatory cytokines. Thus, high AVP and AngII serum levels during Covid-19 might be another possible mechanism for Covid-19-induced hyperprolactinemia (Fig. 7).
Fig. 7.
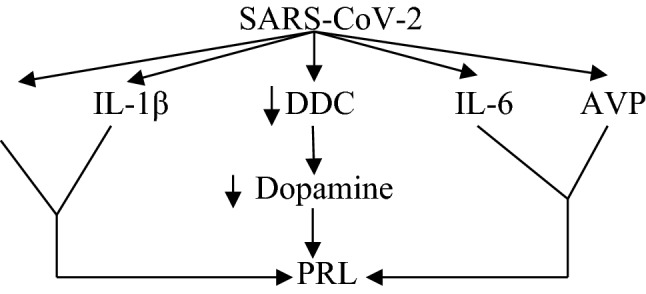
Mechanism of hyperprolactinemia in Covid-19: Prolactin (PRL) increases angiotensin II (AngII), IL-1β, IL-6, and arginine vasopressin (AVP) with reduction of dopa decarboxylase (DDC), with subsequent increase of PRL
Furthermore, these studies revealed that high PRL serum level in Covid-19 could have protective and harmful effects according to the phase of SARS-CoV-2 infection. High PRL serum level in Covid-19 might be a compensatory mechanism to counteracting hyperinflammation and associated endocrinopathies.
The present study has several limitations including paucity of clinical studies regarding PRL serum level in Covid-19 patients. As well, in silico, experimental and clinical trial studies concerning role of PRL were not evaluated. However, this study gives an explanation for the potential role of PRL in Covid-19 and proposed mechanisms for high PRL serum level in Covid-19. Although, experimental, clinical trial and clinical studies are necessary in this regard to confirm the inflammatory and/or anti-inflammatory role of PRL in Covid-19.
Conclusion
In Covid-19, there are noteworthy immunological and inflammatory changes that are affecting neuroendocrine homeostasis mainly in older patients with underlying co-morbidities. Of interest PRL has anti-inflammatory effects by attenuating expression TLR4 and NF-κB with succeeding decrease in the release of pro-inflammatory cytokines. In addition, Covid-19-induced stressful status may affect release of PRL and other stress-mediated hormones. On the other hand, PRL may has a pro-inflammatory agent increase release of pro-inflammatory cytokines and development of immunoinflammatory disorders. Therefore, high PRL serum level may increase severity of Covid-19 through activation of immune cells cytotoxicity, release of pro-inflammatory cytokines, inhibition of Treg cells, and induction of B cell auto-reactivity. The underlying mechanisms of high PRL serum level in Covid-19 are poorly understood, although stress, oxidative, and immune dysregulation could be the potential mechanisms, since stressful conditions in Covid-19 may trigger release of PRL. High PRL serum level in Covid-19 might be a compensatory mechanism to counteracting hyperinflammation and associated endocrinopathies. Besides, high pro-inflammatory cytokines and elevated TRH, AngII and AVP in Covid-19 could be proposed mechanisms for Covid-19-induced hyperprolactinemia. Nevertheless, experimental, clinical trial, and clinical studies are necessary in this regard to confirm the inflammatory and/or anti-inflammatory role of PRL in Covid-19.
Author contributions
Conceptualization: HMA-K and AI A-G; methodology: GE-SB; data curation: HMA-K, AI A-G, and MB; writing—original draft preparation: GE-SB and MB; writing—review and editing, visualization, supervision, and project administration: GE-SB and MB. All authors have contributed to the redaction of this work and have approved the final version.
Funding
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hayder M. Al-Kuraishy, Email: Hayderm36@yahoo.com
Ali I. Al-Gareeb, Email: Dr.alialgareeb78@yahoo.com
Monica Butnariu, Email: monicabutnariu@yahoo.com.
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
References
- 1.Al-Maiahy TJ, Al-Gareeb AI, Al-kuraishy HM. Prolactin and risk of preeclampsia: a single institution, cross-sectional study. Asian Pac J Reprod. 2019;8(3):112. [Google Scholar]
- 2.Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI, Hussien NR, Al-Nami MS. Effects of diabetic pharmacotherapy on prolactin hormone in patients with type 2 diabetes mellitus: bane or boon. J Adv Pharm Technol Res. 2019;10(4):163. doi: 10.4103/japtr.JAPTR_65_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Kuraishy HM, Al-Gareeb AI, Awad MS, Alrifai SB. Assessment of serum prolactin levels in acute myocardial infarction: the role of pharmacotherapy. Indian J Endocrinol Metabol. 2016;20(1):72. doi: 10.4103/2230-8210.172240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Nami MS, Al-Kuraishy HM, Al-Gareeb AI, Al-Mamoori F. Metabolic profile and prolactin serum levels in men with type 2 diabetes mellitus: old-new rubric. Int J Crit Illn Inj Sci. 2019;9(3):120. doi: 10.4103/IJCIIS.IJCIIS_40_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yip SH, Romano N, Gustafson P, Hodson DJ, Williams EJ, Kokay IC, Martin AO, Mollard P, Grattan DR, Bunn SJ. Elevated prolactin during pregnancy drives a phenotypic switch in mouse hypothalamic dopaminergic neurons. Cell Rep. 2019;26(7):1787–1799. doi: 10.1016/j.celrep.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 6.Langevin RH, McPhaul MJ, Hashim IA. Circulating macroprolactin exhibits molecular heterogeneity and is not exclusively an antibody complex. Clin Chim Acta. 2020;514:90–95. doi: 10.1016/j.cca.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Gong N, Ferreira-Martins D, McCormick SD, Sheridan MA. Divergent genes encoding the putative receptors for growth hormone and prolactin in sea lamprey display distinct patterns of expression. Sci Rep. 2020;10(1):1–1. doi: 10.1038/s41598-020-58344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Jiang J, Lepik B, Zhang Y, Zinn KR, Frank SJ. Subdomain 2, not the transmembrane domain, determines the dimerization partner of growth hormone receptor and prolactin receptor. Endocrinology. 2017;158(10):3235–3248. doi: 10.1210/en.2017-00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ihedioha O, Blanchard AA, Balhara J, Okwor I, Jia P, Uzonna J, Myal Y. The human breast cancer-associated protein, the prolactin-inducible protein (PIP), regulates intracellular signaling events and cytokine production by macrophages. Immunol Res. 2018;66(2):245–254. doi: 10.1007/s12026-018-8987-6. [DOI] [PubMed] [Google Scholar]
- 10.Grattan DR. Coordination or coincidence? The relationship between prolactin and gonadotropin secretion. Trends Endocrinol Metab. 2018;29(1):3–5. doi: 10.1016/j.tem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Costello LC, Franklin RB. Testosterone, prolactin, and oncogenic regulation of the prostate gland. A new concept: testosterone-independent malignancy is the development of prolactin-dependent malignancy! Oncol Rev. 2018;12:2. doi: 10.4081/oncol.2018.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman T, Patrick C, Ma C, Nicol GE, Reynolds CF, III, Mulsant BH, Hartz SM, Yingling M, Lenze EJ. Prolactin and estrogen levels in postmenopausal women receiving aripiprazole augmentation treatment for depression. J Clin Psychopharmacol. 2021;41(1):31–35. doi: 10.1097/JCP.0000000000001335. [DOI] [PubMed] [Google Scholar]
- 13.Bernard V, Young J, Binart N. Prolactin—a pleiotropic factor in health and disease. Nat Rev Endocrinol. 2019;15(6):356–365. doi: 10.1038/s41574-019-0194-6. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar-Rojas A, Huerta-Reyes M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells. Oncol Rep. 2009;22(5):981–990. doi: 10.3892/or_00000525. [DOI] [PubMed] [Google Scholar]
- 15.Saleem M, Martin H, Coates P. Prolactin biology and laboratory measurement: an update on physiology and current analytical issues. Clin Biochem Rev. 2018;39(1):3. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YQ, Wen Y, Wang MM, Zhang YW, Fang ZX. Prolactin levels as a criterion to differentiate between psychogenic non-epileptic seizures and epileptic seizures: a systematic review. Epilepsy Res. 2020;24:106508. doi: 10.1016/j.eplepsyres.2020.106508. [DOI] [PubMed] [Google Scholar]
- 17.Vilar L, Vilar CF, Lyra R, da Conceicao FM. Pitfalls in the diagnostic evaluation of hyperprolactinemia. Neuroendocrinology. 2019;109(1):7–19. doi: 10.1159/000499694. [DOI] [PubMed] [Google Scholar]
- 18.Aoki M, Wartenberg P, Grünewald R, Phillipps HR, Wyatt A, Grattan DR, Boehm U. Widespread cell-specific prolactin receptor expression in multiple murine organs. Endocrinology. 2019;160(11):2587–2599. doi: 10.1210/en.2019-00234. [DOI] [PubMed] [Google Scholar]
- 19.Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Alexiou A, Batiha GE. Levamisole therapy in COVID-19. Viral Immunol. 2021;1318:449. doi: 10.1089/vim.2021.0042. [DOI] [PubMed] [Google Scholar]
- 20.Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N, Batiha GE. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type ii diabetes mellitus: the anti-inflammatory role of metformin. Front Med. 2021;8:110. doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Cruz-Martins N, Batiha GE. Sequential doxycycline and colchicine combination therapy in Covid-19: the salutary effects. Pulmonary Pharmacol Therapeut. 2021;67:102008. doi: 10.1016/j.pupt.2021.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GE. The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr. 2021;8:1. doi: 10.3389/fnut.2021.649128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Kuraishy HM, Al-Gareeb AI, Almulaiky YQ, Cruz-Martins N, Batiha GE. Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of Covid-19: the enigmatic entity. Eur J Pharmacol. 2021;15:174196. doi: 10.1016/j.ejphar.2021.174196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-kuraishy HM, Al-Gareeb AI. Acute kidney injury and COVID-19. Egypt J Internal Med. 2021;33(1):1–5. doi: 10.1186/s43162-021-00064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and autoimmunity: the hormone as an inflammatory cytokine. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101324. doi: 10.1016/j.beem.2019.101324. [DOI] [PubMed] [Google Scholar]
- 26.Abramicheva PA, Smirnova OV. Prolactin receptor isoforms as the basis of tissue-specific action of prolactin in the norm and pathology. Biochem Mosc. 2019;84(4):329–345. doi: 10.1134/S0006297919040011. [DOI] [PubMed] [Google Scholar]
- 27.Williams LM, Sarma U, Willets K, Smallie T, Brennan F, Foxwell BM. Expression of constitutively active STAT3 can replicate the cytokine-suppressive activity of interleukin-10 in human primary macrophages. J Biol Chem. 2007;282(10):6965–6975. doi: 10.1074/jbc.M609101200. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi A, Sodhi A. Prolactin-induced production of cytokines in macrophages in vitro involves JAK/STAT and JNK MAPK pathways. Int Immunol. 2008;20(3):327–336. doi: 10.1093/intimm/dxm145. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Sun M, Zhang HP, Chen T, Wu R, Liu C, Yang G, Geng XR, Feng BS, Liu Z, Liu Z. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut. 2014;63(12):1883–1892. doi: 10.1136/gutjnl-2013-306083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retnoningrum D, Hendrianingtyas M, Istiadi H, Jaludamascena A. Correlation between Prolactin Serum with Neutrophil Lymphocyte Ratio (NLR) in Systemic Inflammatory Response Syndrome. Diponegoro Int Med J. 2021;2(1):10–13. [Google Scholar]
- 31.Barrett R, Narasimhulu CA, Parthasarathy S. Adrenergic hormones induce extrapituitary prolactin gene expression in leukocytes-potential implications in obesity. Sci Rep. 2018;8(1):1–4. doi: 10.1038/s41598-018-20378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez AL, López-Rincón G, Neri PA, Estrada-Chávez C. Prolactin in inflammatory response. Recent Adv Prolact Res. 2015;2015:243–264. doi: 10.1007/978-3-319-12114-7_11. [DOI] [PubMed] [Google Scholar]
- 33.Brand JM, Frohn C, Cziupka K, Brockmann C, Kirchner H, Luhm J. Prolactin triggers pro-inflammatory immune responses in peripheral immune cells. Eur Cytokine Netw. 2004;15(2):99–104. [PubMed] [Google Scholar]
- 34.Saha S, Gonzalez J, Rosenfeld G, Keiser H, Peeva E. Prolactin alters the mechanisms of B cell tolerance induction. Arthritis Rheumat. 2009;60(6):1743–1752. doi: 10.1002/art.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavez-Rueda K, Hérnández J, Zenteno E, Leaños-Miranda A, Legorreta-Haquet MV, Blanco-Favela F. Identification of prolactin as a novel immunomodulator on the expression of co-stimulatory molecules and cytokine secretions on T and B human lymphocytes. Clin Immunol. 2005;116(2):182–191. doi: 10.1016/j.clim.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Pivonello R, Bellastella A, Bizzarrol A. Annamaria De Bellis," Annamaria Colao. Autoimmunity Part C. 2007;1107(1107):129–135. doi: 10.1196/annals.1381.014. [DOI] [PubMed] [Google Scholar]
- 37.Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. J Lab Phys. 2018;10(03):276–282. doi: 10.4103/JLP.JLP_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Carolis S, Moresi S, Rizzo F, Monteleone G, Tabacco S, Salvi S, Garufi C, Lanzone A. Autoimmunity in obstetrics and autoimmune diseases in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2019;60:66–76. doi: 10.1016/j.bpobgyn.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and autoimmunity. Front Immunol. 2018;9:73. doi: 10.3389/fimmu.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malaguarnera L, Musumeci M, Licata F, Di Rosa M, Messina A, Musumeci S. Prolactin induces chitotriosidase gene expression in human monocyte-derived macrophages. Immunol Lett. 2004;94(1–2):57–63. doi: 10.1016/j.imlet.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Filipin MD, Brazao V, Santello FH, da Costa CM, Toldo MP, de Morais FR, et al. Does Prolactin treatment trigger imunoendocrine alterations during experimental T. cruzi infection? Cytokine. 2019;121:154736. doi: 10.1016/j.cyto.2019.154736. [DOI] [PubMed] [Google Scholar]
- 42.Wu W, Liu C, Yang PC, Liu Z. P030 Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. J Crohns Colitis. 2014;8:S76. doi: 10.1136/gutjnl-2013-306083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costanza M, Binart N, Steinman L, Pedotti R. Prolactin: a versatile regulator of inflammation and autoimmune pathology. Autoimmun Rev. 2015;14(3):223–230. doi: 10.1016/j.autrev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Tang MW, Garcia S, Gerlag DM, Tak PP, Reedquist KA. Insight into the endocrine system and the immune system: a review of the inflammatory role of prolactin in rheumatoid arthritis and psoriatic arthritis. Front Immunol. 2017;8:720. doi: 10.3389/fimmu.2017.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olmos-Ortiz A, Déciga-García M, Preciado-Martínez E, Bermejo-Martínez L, Flores-Espinosa P, Mancilla-Herrera I, Irles C, Helguera-Repetto AC, Quesada-Reyna B, Goffin V, Díaz L. Prolactin decreases LPS-induced inflammatory cytokines by inhibiting TLR-4/NFκB signaling in the human placenta. Mol Hum Reprod. 2019;25(10):660–667. doi: 10.1093/molehr/gaz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flores-Espinosa P, Preciado-Martínez E, Mejía-Salvador A, Sedano-González G, Bermejo-Martínez L, Parra-Covarruvias A, Estrada-Gutiérrez G, Vega-Sánchez R, Méndez I, Quesada-Reyna B, Olmos-Ortiz A. Selective immuno-modulatory effect of prolactin upon pro-inflammatory response in human fetal membranes. J Reprod Immunol. 2017;123:58–64. doi: 10.1016/j.jri.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Collazos J, Ibarra S, Martínez E, Mayo J. Serum prolactin concentrations in patients infected with human immunodeficiency virus. HIV Clin Trials. 2002;3(2):133–138. doi: 10.1310/QAQQ-XTCJ-8AL4-6F5P. [DOI] [PubMed] [Google Scholar]
- 48.Collazos J, Esteban M. Has prolactin a role in the hypogonadal status of HIV-infected patients? J Int Assoc Phys AIDS Care. 2009;8(1):43–46. doi: 10.1177/1545109708330908. [DOI] [PubMed] [Google Scholar]
- 49.Sousa GM, Oliveira RC, Pereira MM, Paraná R, Sousa-Atta ML, Atta AM. Autoimmunity in hepatitis C virus carriers: involvement of ferritin and prolactin. Autoimmun Rev. 2011;10(4):210–213. doi: 10.1016/j.autrev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Kong L, Fujimoto A, Nakamura M, Aoyagi H, Matsuda M, Watashi K, Suzuki R, Arita M, Yamagoe S, Dohmae N, Suzuki T. Prolactin regulatory element binding protein is involved in hepatitis C virus replication by interaction with NS4B. J Virol. 2016;90(6):3093–3111. doi: 10.1128/JVI.01540-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishii R, Saito T, Shao L, Okumoto K, Nishise Y, Watanabe H, Makino N, Fukao A, Kitanaka C, Kayama T, Ueno Y. Serum prolactin levels and prolactin m RNA expression in peripheral blood mononuclear cells in hepatitis C virus infection. J Med Virol. 2013;85(7):1199–1205. doi: 10.1002/jmv.23599. [DOI] [PubMed] [Google Scholar]
- 52.Glal AZ, Shoeib SA, Abdelhafez MA, Osman NF, Eldin WS, Abdelsala AE, Elgheriany WM. Prolactin contributes to the pathogenesis of thrombocytopenia in patients with hepatitis C virus. Menoufia Med J. 2017;30(1):162. [Google Scholar]
- 53.Shahin D. Thrombocytopenia and leukocytosis are independent predictors of hyperprolactinemia in systemic lupus erythematosus patients. Egypt Rheumatol. 2011;33(2):77–83. [Google Scholar]
- 54.Rahbar A, AlKharusi A, Costa H, Pantalone MR, Kostopoulou ON, Cui HL, Carlsson J, Rådestad AF, Söderberg-Naucler C, Norstedt G. Human cytomegalovirus infection induces high expression of prolactin and prolactin receptors in ovarian cancer. Biology. 2020;9(3):44. doi: 10.3390/biology9030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallis M. Do some viruses use growth hormone, prolactin and their receptors to facilitate entry into cells? Episodic evolution of hormones and receptors suggests host-virus arms races; related placental lactogens may provide protective viral decoys. BioEssays. 2021;43(4):2000268. doi: 10.1002/bies.202000268. [DOI] [PubMed] [Google Scholar]
- 56.Wei L, Sun S, Zhang J, Zhu H, Xu Y, Ma Q, McNutt MA, Korteweg C, Gu J. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS) Biochem Cell Biol. 2010;88(4):723–730. doi: 10.1139/O10-022. [DOI] [PubMed] [Google Scholar]
- 57.Tasker RC, Roe MF, Bloxham DM, White DK, Ross-Russell RI, O’Donnell DR. The neuroendocrine stress response and severity of acute respiratory syncytial virus bronchiolitis in infancy. Intensive Care Med. 2004;30(12):2257–2262. doi: 10.1007/s00134-004-2470-7. [DOI] [PubMed] [Google Scholar]
- 58.Jara LJ, López-Zamora B, Ordoñez-González I, Galaviz-Sánchez MF, Gutiérrez-Melgarejo CI, Saavedra MA, Vera-Lastra OL, Cruz-Domínguez MP, Medina G. The immune-neuroendocrine system in COVID-19, advanced age and rheumatic diseases. Autoimmun Rev. 2021;10:102946. doi: 10.1016/j.autrev.2021.102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaid D, Greenman Y. Human immunodeficiency virus infection and the endocrine system. Endocrinol Metab. 2019;34(2):95–105. doi: 10.3803/EnM.2019.34.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinoli M, Marino F, Cosentino M. Dopaminergic regulation of innate immunity: a review. J Neuroimmune Pharmacol. 2017;12(4):602–623. doi: 10.1007/s11481-017-9749-2. [DOI] [PubMed] [Google Scholar]
- 61.Petrulli JR, Kalish B, Nabulsi NB, Huang Y, Hannestad J. Morris E (2017) Systemic inflammation enhances stimulant-induced striatal dopamine elevation. Transl Psychiatry. 2017;7(3):e1076. doi: 10.1038/tp.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sen A. Repurposing prolactin as a promising immunomodulator for the treatment of COVID-19: are common antiemetics the wonder drug to fight coronavirus? Med Hypoth. 2020;144:110208. doi: 10.1016/j.mehy.2020.110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirzaei F, Tavilani A, Asefy Z, Abbasi E (2021) Prolactin and susceptibility to COVID-19 infection. Medical hypotheses [DOI] [PMC free article] [PubMed]
- 64.Brigante G, Spaggiari G, Rossi B, Granata A, Simoni M, Santi D. A prospective, observational clinical trial on the impact of COVID-19-related national lockdown on thyroid hormone in young males. Sci Rep. 2021;11(1):1–7. doi: 10.1038/s41598-021-86670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, Zhang YW, Xu B, Chen H, Zheng XY, Luo SH. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23(5):479. doi: 10.4103/aja.aja_31_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alzahrani AS, Mukhtar N, Aljomaiah A, Aljamei H, Bakhsh A, Alsudani N, Elsayed T, Alrashidi N, Fadel R, Alqahtani E, Raef H. The impact of COVID-19 viral infection on the hypothalamic-pituitary-adrenal axis. Endocr Pract. 2021;27(2):83–89. doi: 10.1016/j.eprac.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waddankeri S (2020) The hypothyroid adrenals, transient thyro-adrenal failure-functional or real? In: 22nd European Congress of Endocrinology 21 Aug 2020, vol 70. BioScientifica
- 68.Kumar B, Gopalakrishnan M, Garg MK, Purohit P, Banerjee M, Sharma P, Khichar S, Kothari N, Bhatia P, Nag VL, Misra S. Endocrine dysfunction among patients with COVID-19: a single-center experience from a tertiary hospital in India. Indian J Endocrinol Metab. 2021;25(1):14. doi: 10.4103/ijem.IJEM_577_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patki V, Kumbhojkar A, Khilnani P. Sick euthyroid syndrome: a myth or reality. J Pediatr Critical Care. 2017;4(4):44. [Google Scholar]
- 70.Schwarz Y, Percik R, Oberman B, Yaffe D, Zimlichman E, Tirosh A. Sick euthyroid syndrome on presentation of patients With COVID-19: a potential marker for disease severity. Endocr Pract. 2021;27(2):101–109. doi: 10.1016/j.eprac.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borkowski J, Siemiatkowski A, Jedynak M, Czaban SL, Wołczyński S. Serum levels of luteinizing hormone, testosterone and prolactin in patients with septic shock. Przegl Lek. 2003;60(11):706–709. [PubMed] [Google Scholar]
- 72.Barreto TR, Costola-de-Souza C, Margatho RO, Queiroz-Hazarbassanov N, Rodrigues SC, Felício LF, Palermo-Neto J, Zager A. Repeated domperidone treatment modulates pulmonary cytokines in LPS-induced acute lung injury in mice. Int Immunopharmacol. 2018;56:43–50. doi: 10.1016/j.intimp.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Lo CC, Moosavi SM, Bubb KJ. The regulation of pulmonary vascular tone by neuropeptides and the implications for pulmonary hypertension. Front Physiol. 2018;9:1167. doi: 10.3389/fphys.2018.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ochoa-Callejero L, García-Sanmartín J, Villoslada-Blanco P, Íñiguez M, Pérez-Matute P, Pujadas E, Fowkes ME, Brody R, Oteo JA, Martínez A. Circulating levels of calcitonin gene-related peptide are lower in COVID-19 patients. J Endocr Soc. 2021;5(3):199. doi: 10.1210/jendso/bvaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Q, Lin Y, Zhang S, Chen M, Chen Q, Rui H, Wang F, Lv X, Gao F. CGRP-mediated prolactin upregulation: a possible pathomechanism in IgG4-related disease. Inflammation. 2021;44(2):536–548. doi: 10.1007/s10753-020-01350-6. [DOI] [PubMed] [Google Scholar]
- 76.Chen B, Han J, Chen S, Xie R, Yang J, Zhou T, Zhang Q, Xia R. MicroLet-7b regulates neutrophil function and dampens neutrophilic inflammation by suppressing the canonical TLR4/NF-κB pathway. Front Immunol. 2021;12:856. doi: 10.3389/fimmu.2021.653344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinna G (2020) Progesterone and estradiol may be beneficial for the treatment of COVID-19 (HYPOTHESIS PAPER). Authorea Preprints. 2020 May 11
- 78.Devi YS, Seibold AM, Shehu A, Maizels E, Halperin J, Le J, Binart N, Bao L, Gibori G. Inhibition of MAPK by prolactin signaling through the short form of its receptor in the ovary and decidua: involvement of a novel phosphatase. J Biol Chem. 2011;286(9):7609–7618. doi: 10.1074/jbc.M110.166603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grimes JM, Grimes KV. p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M, Mencarini J, Caporale R, Peruzzi B, Antonelli A, Trotta M. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Investig. 2020;130:9. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galván-Peña S, Leon J, Chowdhary K, Michelson DA, Vijaykumar B, Yang L, Magnuson AM, Chen F, Manickas-Hill Z, Piechocka-Trocha A, Worrall DP. Profound Treg perturbations correlate with COVID-19 severity. Proc Natl Acad Sci USA. 2021;118:37. doi: 10.1073/pnas.2111315118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song E, Bartley C, Chow R, Ngo T, Jiang R, Zamecnik C, Dandekar R, McAlpine L, Spudich SS, DeRisi J, Iwasaki A. Divergent and self-reactive immune responses in the CNS of COVID-19 patients. Top Antiviral Med. 2021;2021:50–51. doi: 10.1016/j.xcrm.2021.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saricali M, Satici SA, Satici B, Gocet-Tekin E, Griffiths MD. Fear of COVID-19, mindfulness, humor, and hopelessness: a multiple mediation analysis. Int J Ment Heal Addict. 2020;19:1–4. doi: 10.1007/s11469-020-00419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. 2020;27:1. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fasano A, Elia AE, Dallocchio C, Canesi M, Alimonti D, Sorbera C, Alonso-Canovas A, Pezzoli G. Predictors of COVID-19 outcome in Parkinson's disease. Parkinsonism Relat Disord. 2020;78:134–137. doi: 10.1016/j.parkreldis.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mpekoulis G, Frakolaki E, Taka S, Ioannidis A, Vassiliou AG, Kalliampakou KI, Patas K, Karakasiliotis I, Aidinis V, Chatzipanagiotou S, Angelakis E. Alteration of L-dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS ONE. 2021;16(6):e0253458. doi: 10.1371/journal.pone.0253458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrett R (2021) Regulation of extra-pituitary prolactin in monocytes and macrophages. Electronic theses and dissertations. http://purl.fcla.edu/fcla/etd/CFE0007309
- 89.Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID-19. J Med Virol. 2020;1:4. doi: 10.1002/jmv.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomaszewska-Zaremba D, Haziak K, Tomczyk M, Herman AP. Inflammation and LPS-binding protein enable the stimulatory effect of endotoxin on prolactin secretion in the ovine anterior pituitary: Ex vivo study. Mediators Inflamm. 2018;14:2018. doi: 10.1155/2018/5427089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanli DE, Altundag A, Kandemirli SG, Yildirim D, Sanli AN, Saatci O, Kirisoglu CE, Dikensoy O, Murrja E, Yesil A, Bastan S. Relationship between disease severity and serum IL-6 levels in COVID-19 anosmia. Am J Otolaryngol. 2021;42(1):102796. doi: 10.1016/j.amjoto.2020.102796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lennartsson AK, Jonsdottir IH. Prolactin in response to acute psychosocial stress in healthy men and women. Psychoneuroendocrinology. 2011;36(10):1530–1539. doi: 10.1016/j.psyneuen.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Traslaviña GA, Franci CR. The CRH-R1 receptor mediates luteinizing hormone, prolactin, corticosterone and progesterone secretion induced by restraint stress in estrogen-primed rats. Brain Res. 2011;1421:11–19. doi: 10.1016/j.brainres.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Rettori V, Jurcovicova J, McCann SM. Central action of interleukin-1 in altering the release of TSH, growth hormone, and prolactin in the male rat. J Neurosci Res. 1987;18(1):179–183. doi: 10.1002/jnr.490180125. [DOI] [PubMed] [Google Scholar]
- 95.Meilleur MA, Akpovi CD, Pelletier RM, Vitale ML. Tumor necrosis factor-α-induced anterior pituitary folliculostellate TtT/GF cell uncoupling is mediated by connexin 43 dephosphorylation. Endocrinology. 2007;148(12):5913–5924. doi: 10.1210/en.2007-0767. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Ding Q, Wang S, Wu Q, Ni P, Zhang H, Wang X, Chen Y, Wu J. Estrogen promotes pituitary prolactinoma by upregulating TLR4/NF-κB/p38MAPK pathway. Res Square. 2021 doi: 10.21203/rs.3.rs-957520/v1. [DOI] [Google Scholar]
- 97.Haj-Mirzaian A, Ramezanzadeh K, Shariatzadeh S, Tajik M, Khalafi F, Tafazolimoghadam A, Radmard M, Rahbar A, Pirri F, Kazemi K, Khosravi A. Role of hypothalamic-pituitary adrenal-axis, toll-like receptors, and macrophage polarization in pre-atherosclerotic changes induced by social isolation stress in mice. Sci Rep. 2021;11(1):1–5. doi: 10.1038/s41598-021-98276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haziak K, Herman AP, Wojtulewicz K, Pawlina B, Paczesna K, Bochenek J, Tomaszewska-Zaremba D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intracerebroventricular administration of LPS. J Anim Sci Biotechnol. 2018;9(1):1. doi: 10.1186/s40104-018-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, Lungnier C. Renin-Angiotensin system and fibrinolytic pathway in COVID-19: one-way skepticism. Biomed Biotechnol Res J. 2020;4(5):33. [Google Scholar]
- 100.Aguilera G, Hyde CL, Catt KJ. Angiotensin II receptors and prolactin release in pituitary lactotrophs. Endocrinology. 1982;111(4):1045–1050. doi: 10.1210/endo-111-4-1045. [DOI] [PubMed] [Google Scholar]
- 101.Erickson EN, Carter CS, Emeis CL. Oxytocin, vasopressin and prolactin in new breastfeeding mothers: relationship to clinical characteristics and infant weight loss. J Hum Lact. 2020;36(1):136–145. doi: 10.1177/0890334419838225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Atanu FO, Batiha GE. Arginine vasopressin and pathophysiology of COVID-19: an innovative perspective. Biomed Pharmacother. 2021;15:112193. doi: 10.1016/j.biopha.2021.112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.