Abstract
Objective
Chronic low back pain (CLBP) is a disabling and costly condition for older adults that is difficult to properly classify and treat. In a cohort study, a subgroup of older adults with CLBP who had elevated hip pain and hip muscle weakness was identified; this subgroup differentiated itself by being at higher risk for future mobility decline. The primary purpose of this clinical trial is to evaluate whether a hip-focused low back pain (LBP) treatment provides better disability and physical performance outcomes for this at-risk group compared with a spine-focused LBP treatment.
Methods
This study is a multisite, single-blinded, randomized controlled, parallel arm, Phase II trial conducted across 3 clinical research sites. A total of 180 people aged between 60 and 85 years with CLBP and hip pain are being recruited. Participants undergo a comprehensive baseline assessment and are randomized into 1 of 2 intervention arms: hip-focused or spine-focused. They are treated twice weekly by a licensed physical therapist for 8 weeks and undergo follow-up assessments at 8 weeks and 6 months after randomization. Primary outcome measures include the Quebec Low Back Disability Scale and the 10-Meter Walk Test, which are measures of self-report and performance-based physical function, respectively.
Impact
This multicenter, randomized clinical trial will determine whether a hip-focused or spine-focused physical therapist intervention results in improved disability and physical performance for a subgroup of older adults with CLBP and hip pain who are at increased risk of mobility decline. This trial will help further the development of effective interventions for this subgroup of older adults with CLBP.
Keywords: Hip Pain, Low Back Pain, Older Adults, Osteoarthritis, Physical Function
Introduction
Chronic low back pain (CLBP) is a prevalent, costly, and disabling problem in the older adult population; the rising prevalence suggests that current treatment approaches are not effective.1–8 Several large, longitudinal studies have demonstrated that CLBP among older adults is independently associated with a steeper rate of decline in performance-based mobility function (ie, gait speed, chair rise performance, balance) compared with older adults without low back pain (LBP).9–11 Because poor and/or declining mobility function in this population is predictive of increased future risk for mortality, institutionalization, and overall disability,12–16 effective interventions are needed to improve function.
Experts in LBP have speculated that older adults with LBP do not belong to 1 homogeneous group but rather belong in subgroups that share similar clinical characteristics.17,18 In younger cohorts, evidence exists that matching treatments to the limitations of an individual patient can improve physical therapy outcomes.19–22 It stands to reason that older adults would similarly benefit from matching treatments to impairments; however, older adults are often excluded from clinical trials.23 The National Institutes of Health (NIH) has recently highlighted the importance of including participants across the lifespan.24 Tailored interventions, which align with recent calls for precision medicine initiatives,25 may be particularly important in older adults with painful conditions, where heterogeneity is particularly prevalent.26,27 Thus, specific classification systems must be developed for this population.
In a previous cohort study involving older adults with CLBP,28 we identified a subgroup with a combination of elevated hip pain and global hip muscle weakness. This subgroup was at risk for markedly worse CLBP, functional outcomes, and self-efficacy over the course of 12 months, putting them at risk for future mobility decline and institutionalization. Compared with older adults without pain, those with CLBP had a greater prevalence of clinical hip symptoms associated with hip osteoarthritis, which were associated with worse health-related quality of life.29 The totality of these results prompted us to develop the current trial, which focuses on evaluating whether a hip-focused LBP treatment will lead to reduced disability and improved physical function compared with a spine-focused LBP treatment. Our primary hypothesis is that members of the at-risk subgroup who receive hip-focused LBP treatment will have decreased pain and better functional outcomes than those who receive spine-focused LBP treatment.
Methods
Design
The Manual Therapy and Strengthening for the Hip in Older Adults With Chronic Low Back Pain Trial (MASH) is a multi-site, single-blind, parallel arms, randomized controlled, Phase II trial and adheres to the SPIRIT guidelines (Figure).30
Figure.
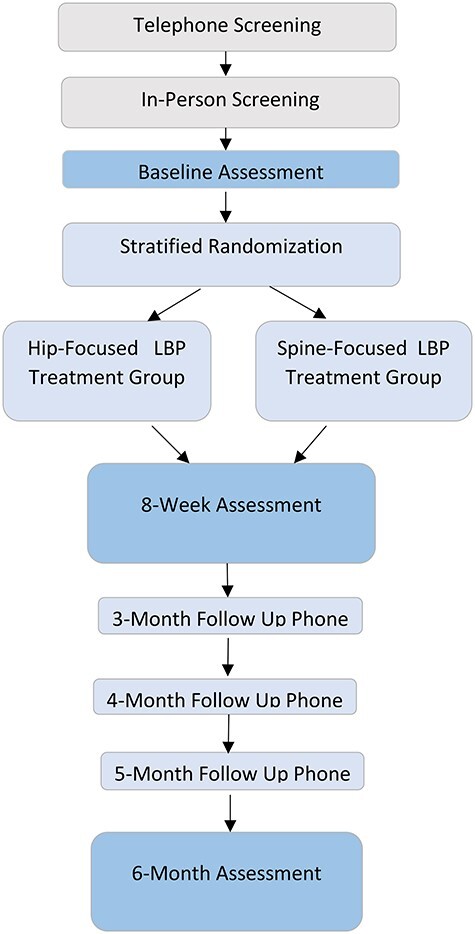
Study overview.
Setting
Assessment and treatment sessions take place within clinical laboratories at the University of Delaware, the University of Pittsburgh, and Duke University. The home exercise program (HEP) portion is completed without supervision at each participant’s residence.
Participants
A total of 180 older adults aged 60 to 85 years are being recruited across the aforementioned 3 sites. We are utilizing newspaper advertisements, on-site visits to local senior centers, research registries, referrals from nearby rheumatology and geriatrics clinics, and electronic medical record invitations to recruit participants meeting specific inclusion criteria (Tab. 1). Two of the inclusion criteria are used to classify participants into the at-risk subgroup: hip internal rotation (IR) strength <0.26 (taken isometrically with a handheld dynamometer and normalized to body weight), and Hip Disability Osteoarthritis Outcome Score (HOOS) >5 on items P4–P8. These 5 items from the HOOS pain subscale ascertain the amount of hip pain a person has experienced in the last week during a specified activity; individual items are scored from 0 = no hip pain to 4 = extreme hip pain, with greater summed scores indicating greater severity. Using data from previous work,28 we developed a simple decision tree using regression and receiver operating characteristic curve analyses and found that if individuals met the criteria for hip IR strength and HOOS item scores, they were placed accurately into the group with elevated hip pain and global muscle weakness (ie, our study population of focus) 81% of the time. To maximize safety, we adhere to specific exclusion criteria as well (Tab. 1).
Table 1.
Inclusion/Exclusion Criteria
| Inclusion criteria | Low back pain >3 mo for at least half of the days in last 6 mo50 |
| Moderate low back pain intensity (>3 on 0–10) | |
| Normalized isometric hip internal rotation strength <0.26a | |
| Hip Disability and Osteoarthritis Outcome Score >5 on pain-related items P4–P8a | |
| Exclusion criteria | Previous hip fracture with repair |
| Hip fracture within last 15 y without repair | |
| Total hip replacement | |
| Known spinal pathology other than spinal stenosis and/or osteoarthritis | |
| Severely impaired mobility (ie, requires use of a wheelchair) | |
| Folstein Mini-Mental State Examination Score <2451 | |
| Severe visual or hearing impairment | |
| Red flags such as fever, significant unintentional weight loss >10 pounds, pain that awakens or keeps one awake at night, trauma that preceded the onset of pain, or signs and symptoms of cauda equina | |
| Significant pain in legs greater than the back | |
| Acute illness (eg, COVID-19) | |
| Inability to participate in the study for the full 6 mo (eg, moving residences) | |
| Receipt of manual or exercise therapy for low back or hip within the last 3 mo |
a Criteria defined from decision tree analysis.
Enrollment and Assessments
All potential participants complete an initial phone screen. Individuals deemed preliminarily eligible are invited on-site to participate in an in-person screening, during which they complete the informed consent and answer questionnaires related to demographics. They have their height and weight taken and undergo hip IR strength testing; this information is used to determine final eligibility.
Eligible participants who provide informed consent undergo 3 assessments: (1) baseline (completed at the time of the in-person screening), (2) 8 weeks postrandomization (postintervention), and (3) 6 months postrandomization. Each comprehensive assessment is performed by a licensed physical therapist and includes self-report questionnaires, range of motion and strength measurements, and physical performance tests. Gerontological evidence indicates self-report and performance-based measures of function offer complementary information; therefore, both types should be used in the comprehensive assessment of physical function.13,31–34 For this trial, primary outcome measures are the Quebec Low Back Pain-Related Disability Scale (QBPD)35 and the 10-Meter Walk Test (10MWT).36,37 The QBPD is a disease-specific self-reported measure of physical function that has excellent reliability and good construct validity compared with other pain and disability measures among older adults.35 The 10MWT is a reliable and valid measure of gait speed, which is predictive of disability and mortality in older adults.12–16 Secondary outcome measures were selected to capture constructs such as self-efficacy and pain perception (Tab. 2).
Table 2.
Outcome Measuresa
| Test | Domain | Description | Scoring |
|---|---|---|---|
| QBPD35,b | Low back pain-related disability | Questionnaire: 20 items related to daily activities, which are grouped into 6 categories | Higher scores = greater disability |
| 10MWTb,36,37 | Gait speed | Participants walk along a linear pathway at their “usual pace” and “as quickly as possible” | Higher gait speeds = better outcomes |
| PHQ-952,53 | Depressive symptoms | Questionnaire: 9 items related to depressive symptoms | Scores ≥10/27 indicative of major depression |
| LOBACS54 | Self-efficacy | Questionnaire: 15 items scored according to participant’s level of confidence in performing activities | Higher scores = greater self-efficacy |
| PCS55,56 | Pain perception | Questionnaire: 13 items scored as 3 subscales to evaluate constructs of rumination, magnification, and helplessness | Higher scores = increased catastrophizing |
| Movement-evoked pain57 | Pain provocation with activity | Participants reported pain intensity before, during, and after 6MWT and 30 Second Chair Stand Tests | Smaller increases in pain = lower pain provocation with activity |
| Quantitative sensory testing58–60 | Pain pressure threshold | Algometer used to measure pressure pain sensitivity at 4 sites bilaterally: upper trapezius, posterior superior iliac supine, greater trochanter, and tibialis anterior | Higher values = higher pain pressure threshold |
| Hip strength61,62 | Strength | Hip strength (abduction, extension, internal and external rotation, flexion) taken with a hand-held dynamometer, normalized to body weight | Higher forces = greater strength |
| 6MWT63 | Functional mobility | Participants walk around a predetermined course trying to cover as much ground as possible in 6 min | Longer distances = better outcome |
| 30-Second Chair Stand Test64 | Functional mobility | Participants perform as many sit-to-stands as possible in 30 sec with arms folded across chest |
More sit-to-stands = better functional mobility |
| HOOS65 | Hip-related disability | Questionnaire: 40 items across 5 domains (pain, symptoms, activities of daily living, sport and recreation function, hip-related quality of life) | Higher scores = better hip outcomes |
| PROMIS-2966–68 | Health-related quality of life | Questionnaire: 29 items across 8 domains (physical function, anxiety, depression, fatigue, sleep disturbance, ability to participate in social roles and activities, pain interference, and pain intensity) | Higher scores = greater presence of each outcome |
a 6MWT = 6-Minute Walk Test; 10MWT = 10 Meter Walk Test; HOOS = Hip Disability and Osteoarthritis Outcome Score; LOBACS = Low Back Activity Confidence; PCS = Pain Catastrophizing Scale; PHQ-9 = Patient Health Questionnaire-9 item; PROMIS-29 = Patient-Reported Outcomes Measurement Information System; QBPD = Quebec Back Pain Disability Scale.
b Primary outcome measure.
Modifications Related to COVID-19
Early in study recruitment, the COVID-19 pandemic forced changes to our assessment protocol. Modifying trials to reduce in-person exposure is consistent with current clinical trial recommendations.38,39 We sought to reduce the on-site time and accomplished this by having participants fill out questionnaires remotely.
Randomization and Blinding
The study biostatistician uses statistical software to generate a randomization plan using permuted block with random block sizes to ensure a roughly equal number of participants is assigned to each arm by the end of the study in a 1:1 allocation. Randomization is stratified by site, as well as by sex, due to the reported sex differences in pain conditions among older adults.20 Assignments generated by the statistician are uploaded into the electronic data capture (EDC) system. The site study coordinator retrieves the assignment once a participant is deemed fully eligible and provides this information to the treating therapists (interventionists), thereby concealing allocation. Therapists performing the assessments (assessors) are masked to group assignment. Interventionists are masked to participants’ outcome data. Because treatment assignment cannot be withheld from the participants, both assessors and interventionists ask the participants to avoid discussion of any aspects of treatment with the assessors and other research participants. Interventionists are not assigned to a particular intervention arm and may treat participants in both groups.
Interventions
Eligible participants are randomized into 1 of 2 intervention arms: hip-focused or spine-focused treatment. Participants receive treatment from a licensed physical therapist twice weekly for 8 weeks. All interventionists participate in at least 4 hours of one-on-one training, during which they practice hands-on techniques under the supervision of a therapist trained in the MASH trial treatments; assessments of treatment fidelity occur regularly. Interventionists give participants an HEP that includes a pictorial exercise handout and an exercise log, and ask them to perform it twice weekly on non-therapy days. Although education related to the HEP is primarily focused on correct performance, interventionists also discuss several principles: the promotion and understanding of the anatomical and structural strength of the human spine; the use of active coping strategies, such as exercise; early resumption of daily, vocational, and social activities, even when pain is present; and the importance of improved activity levels, not just pain relief.40 All exercises are progressed based on meeting a quota criterion (eg, 3 sets of 10 repetitions). Interventions are displayed in Table 3. Full manual therapy protocols, exercise protocols, and HEP pictorial handouts for both groups are included in the Supplementary Material. Our intervention development was informed by both our pilot work and current evidence.
Table 3.
Summary of Intervention Components
| Component | Description | |
|---|---|---|
| Hip-focused | ||
| On-site (45 min) | Hip manual therapy | Four hip mobilization techniques (bilateral) • Long-axis distraction (Grade III sustained) plusmanipulation • Anterior–posterior oscillatory mobilizations (GradeIII/IV) • Lateral femoral glide (Grade III sustained) with hipinternal rotation oscillatory mobilizations (GradeIII/IV) • Posterior–anterior oscillatory mobilizations (GradeIII/IV) Manual stretches (bilateral) • Hamstrings • Hip flexors |
| Functional hip exercises | Two phases • Visits 1–8: partial wall squats, hip abduction withelastic band, • forward step-ups •Visits 9–16: wall squats, side-stepping with band, lateral step-ups |
|
| Home exercise program education (15 min) | Hip strengthening exercises with elastic band | Hip abduction Hip extension Hip internal rotation Hip external rotation |
| Trunk muscle training exercises | Bracing Anterior trunk (eg, curl-ups) Posterior trunk (eg, alternating arm lifts in quadruped) Lateral trunk (eg, side bridges) |
|
| Spine-focused | ||
| On-site (45 min) | Lumbar manual therapy | Central posterior–anterior oscillatory mobilizations (Grade I–II) to L1-L5 levels Effleurage to thoracolumbar area |
| Trunk muscle training exercises | Bracing Anterior trunk (eg, curl-ups) Posterior trunk (eg, alternating arm lifts in quadruped) |
|
| Stationary cycling | Stationary cycle without resistance for submaximal intensity | |
| Home exercise program education (15 min) | Lumbar flexibility exercises | Generalized stretches to enhance lumbar mobility |
| Trunk muscle training exercises | Same as above but lateral trunk exercises omitted | |
Hip-Focused
The hip-focused LBP treatment consists of 2 parts: an on-site session and an HEP. During the on-site session, participants receive manual therapy to both hips and participate in supervised functional hip exercises. Manual therapy to the hip includes joint mobilizations and stretching. Joint mobilizations include long-axis distraction (with a manipulation at the end of the 30 seconds) as well as oscillatory grade III to IV (3 × 30 seconds) joint mobilizations in 3 other directions: anterior–posterior, lateral glide with IR, and posterior–anterior. Hip joint mobilizations were adapted from prior work, where they were shown to be effective in improving various clinical outcomes among individuals with a primary diagnosis of hip osteoarthritis.41,42 Stretches include supine hamstring stretching and either side-lying or supine hip flexor stretching. Functional hip exercises focus on strength, endurance, and control of the hip muscles while completing functional tasks (ie, squatting and stepping). They are performed with therapist supervision for safety. The last 15 minutes of each on-site treatment session are dedicated to moist heat pack application and education regarding the delivery of the HEP: therapists demonstrate all exercises, ensure that participants use proper form, and increase the dosage on HEP logs according to each participant’s progress.
The hip-focused HEP consists of 3 parts: hip stretching, hip strengthening exercises, and trunk muscle training (TMT) exercises. The strengthening exercises, which were adapted from prior work,43 focus on the strengthening of various hip muscles using resistance bands. The TMT exercises, which target muscles in the anterior, posterior, and lateral trunk, are used to help improve neuromuscular control and dynamic spinal stability. TMT exercises were adapted from prior work in which they were shown to improve various clinical outcomes among older adults with CLBP.44
Spine-Focused
The spine-focused LBP treatment consists of 2 parts: an on-site session and an HEP. During the on-site session, participants receive manual therapy to the lumbar spine and participate in TMT exercises and stationary cycling without resistance. Manual therapy includes oscillatory grade I to II central posterior–anterior joint mobilizations to lumbar levels L1 to L5 for pain relief as well as light soft tissue effleurage massage to the thoracolumbar paraspinal muscles. The TMT exercises performed in this group target muscles in the anterior and posterior trunk; lateral trunk exercises are omitted to avoid inadvertently targeting the hip abductor muscles. Participants perform stationary cycling without resistance, because clinical practice guidelines highlight strong evidence supporting the effectiveness of low-intensity submaximal fitness and endurance exercises for adults with CLBP.40 As in the hip-focused group, the last 15 minutes of each session are dedicated to education regarding the delivery of the HEP. Once the HEP education is completed for each session, if there is time remaining, therapists may provide participants with moist heat to the lumbar spine to equalize face-to-face time between intervention groups.
The spine-focused HEP consists of lumbar flexibility exercises and the same TMT performed during the on-site visit. The lumbar flexibility exercises are matched to each participant’s preference and are varied throughout the treatment sessions.
Modifications Related to COVID-19
Due to COVID-19, aspects of the interventions were modified to reduce the overall time on-site. We moved the hip strengthening and TMT exercises (hip-focused group) and lumbar flexibility exercises (spine-focused group) from the on-site sessions to the HEP format.
Adherence
Participants are encouraged to adhere to the intervention arm to which they have been assigned; we define intervention adherence based on the number of in-person and HEP completed sessions (Suppl. Appendix). During the last 15 minutes of each treatment session for all participants, interventionists discuss updates to HEPs while the participants are receiving their moist hot pack application. Participants receive positive feedback and reinforcement if they adhere to their HEP and return their exercise logs. Participants receive treatment free of charge, and receive monetary compensation for follow-up assessments. Participants receive follow-up phone calls at the 3-, 4-, and 5-month post-randomization time points to promote retention and to monitor for adverse events (AEs).
Adverse Events
All study staff are responsible for monitoring for the occurrence of AEs, which are operationalized as any unfavorable or untoward occurrence to participants during their involvement in the clinical trial, including any abnormal sign, symptom, or disease that may or may not be related to research participation. Study staff catalogue new signs, symptoms, or diseases within Possible Adverse Event Report forms. Site investigators adjudicate Possible Adverse Event Reports and report any serious AEs or unanticipated problems to site and sponsor regulatory officials. We utilize the Common Terminology Criteria for Adverse Events classification and NIH common data elements (eg, severity) in recording AE classifications.45
Process Evaluation
Employing a multisite trial design allows for exploration of the generalizability of the approach and findings but requires a focused approach to ensure that internal validity is maximized. We employ a rigorous assessment and treatment fidelity approach across sites to promote uniform and high-quality data acquisition: we have implemented a quality control program that includes the use of detailed operations manuals, therapist training, and periodic data review by the principal investigator. Protocols describe each testing or treatment procedure and contain an exemplar script. We have regular training workshops for all study staff and monthly meetings for both assessors and interventionists. The study investigators meet once monthly to discuss AEs and address any issues that arose over the prior month.
We have implemented an internal process review system (Tab. 4) designed to promote adherence to the study design and provide excellent participant care. Specifically related to treatment fidelity, we utilize processes that increase the likelihood of consistent treatment delivery across sites: automated chart review, manual chart review, and on-site observation of treatment sessions. Compliance issues are mitigated by on-site targeted retraining.
Table 4.
Internal Review Processes
| Process | Type | Reviewer | Description |
|---|---|---|---|
| Automated | Chart | Web-based application | Significant deviations from standardized protocols (eg, omission of treatment components) automatically flagged in real-time by electronic data capture system |
| Manual | Chart | Study staff member | ≥25% of charted treatment sessions reviewed for fidelity to treatment prescription and progression in mo 1–6; ≥10% of treatment sessions every year thereafter |
| On-site | Live | Study staff member | ≥5 Audits per interventionist in first year observed for fidelity to treatment prescription and progression; ≥2 evaluations per year per interventionist thereafter |
Data Analysis Plan
All analyses will be performed using intention-to-treat methodology. We will use the QBPD score and gait speed result to test our primary hypotheses regarding changes in disability and physical function both in the short term (immediately posttreatment) and longer term (6 months postrandomization). We will use constrained baseline linear mixed models with time (baseline, 8 weeks, and 6 months) and the group by time interaction as fixed effects while controlling for repeated measures using an unstructured correlation matrix between time points. We will treat the effect of time as nominal. We will also control for site and sex, because these are stratification factors in the randomization. If the interaction effect is significant, the simple main effect of group will be tested at each follow-up time point. If model normality and homoscedasticity assumptions are violated, we will either consider transformations that can be applied but maintain interpretability or use robust standard errors. We will test the secondary outcome measures using the same statistical models to identify which outcomes should be considered in future trials with a more conservative alpha.
The sample size for the trial was determined using the GLIMMPSE framework assuming the group by time interactions were significant for disability and physical function.46 With alpha = .05 and 80% power, we will be able to detect a moderate effect, d = .45, for a total sample of 150 or 75 per treatment group. This assumes a correlation of repeated measures of 0.5. This effect size translates to a between-group difference of 6.8 points in QBPD scores and 0.095 m/s in gait speed over time between groups. For our final sample size of 150, a total of 180 participants will be enrolled, accounting for a dropout rate of 17% based on our previous trial of older adults with CLBP.44 Sensitivity analyses using logistic regression models will be performed to identify predictors of missed assessment visits using baseline characteristics; if significant predictors are found, they will be included in a secondary analysis of the primary outcomes. If their inclusion results in estimates that differ from the initial analysis, these results will be reported because they would be unbiased if data are missing at random with respect to baseline characteristics.47 The mixed models yield accurate parameter estimates if data are missing at random and have been shown to perform as well as multiple imputation given the same assumption of the missing data mechanism.48 We will conduct several sensitivity analyses assuming non-ignorable missingness with differential imputation of poor change scores and pattern mixture models.49 We will compare the results from these analyses to our primary analyses with all observed data to assess the robustness of our findings.
Ethics
This study was approved by the institutional review boards at each of the participating study sites; each site provides continuing reviews per their site requirements. Oversight of the entire trial is provided by an independent data and safety monitoring board. At each site, participants complete the informed consent process and are assigned an identification code. Data are then entered into the EDC, which is password protected and securely stored. Enrollment for the trial began in November 2019 and will continue through 2022; trial recruitment and enrollment were suspended between March 2020 and August 2020 due to site shutdowns related to COVID-19 but were reinitiated as each site reopened.
Role of the Funding Source
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Discussion
The development of safe, alternative treatments that lessen the burden of CLBP and promote the independence of community-dwelling older adults is essential. Although experts agree that tailored approaches to treatment are necessary, there is a scarcity of evidence relating to older adults. Our study is unique in that by identifying a subgroup with hip impairments, we can determine the effectiveness of a tailored versus standard approach and address key initiatives set forth by the NIH.
This trial has limitations to consider when results are reported. We specifically exclude people with total hip replacements as well as certain individuals with a history of hip fracture. This decision was made with participant safety in mind, because hip mobilization is contraindicated in many of those individuals; however, we recognize that this may exclude participants who would fall into our subgroup of interest. Further, this is a single-blind trial, and there is no way to mask treatment allocation from the interventionists or participants. This leaves open the possibility of a participant inadvertently revealing allocation; we have attempted to mitigate this by consistently reminding participants to discuss treatments with only interventionists and not assessors or other participants. Another potential area of bias may arise if interventionists believe that 1 treatment arm is more effective than the other; we have attempted to mitigate this by incorporating the concept of equipoise (ie, the understanding that we do not yet know which treatment arm is indeed more effective) into the therapist training modules, because both interventions target impairments commonly experienced in this subgroup. The concept of equipoise is important in this trial due to the potential misperception that 1 randomly assigned treatment group was favored by the investigator team. Accordingly, we have created a protocol that ensures both treatment groups are receiving similar proportions of active exercise/intervention and both groups received the same type of evidence-informed information regarding exercise and CLBP.40
Our trial has several strengths. It is a multisite study involving 3 sites with access to a broad demographic of older adults. We used specific inclusion criteria to identify members of a subgroup with increased risk of mobility decline. We incorporated tablet technology and an EDC to streamline data collection and reduce operator error. We incorporated internal review processes to promote delivery of standardized interventions and provide treatments with excellence. Importantly, the treatments used in this trial require no specialized equipment or advanced training and should translate well into clinical practice.
Supplementary Material
Appendix 1.
Contributor Information
Jenifer M Pugliese, Department of Physical Therapy, University of Delaware, Newark, Delaware, USA.
Peter C Coyle, Department of Physical Therapy, University of Delaware, Newark, Delaware, USA.
Patrick J Knox, Department of Physical Therapy, University of Delaware, Newark, Delaware, USA.
J Megan Sions, Department of Physical Therapy, University of Delaware, Newark, Delaware, USA.
Charity G Patterson, Clinical and Translational Sciences Institute, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania USA.
Ryan T Pohlig, Department of Epidemiology, University of Delaware, Newark, Delaware, USA.
Corey B Simon, Duke Clinical Research Institute, Duke University, Durham, North Carolina, USA.
Debra K Weiner, Geriatric Research Education and Clinical Center, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, Pennsylvania, USA; Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Department of Anesthesiology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Department of Medicine, Division of Geriatric Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Steven Z George, Department of Orthopedic Surgery, Duke School of Medicine, Durham, North Carolina, USA.
Sara Piva, Department of Physical Therapy, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Gregory E Hicks, Department of Physical Therapy, University of Delaware, Newark, Delaware, USA.
Author Contributions
Concept/idea/research design: P.C. Coyle, J.M. Sions, C.G. Patterson, R.T. Pohlig, C.B. Simon, S.Z. George, G.E. Hicks
Writing: J.M. Pugliese, P.C. Coyle, P.J. Knox, J.M. Sions, C.G. Patterson, R.T. Pohlig, C.B. Simon, D.K. Weiner, S.Z. George, S. Piva, G.E. Hicks
Data collection: J.M. Pugliese, P.C. Coyle, P.J. Knox, J.M. Sions, C.B. Simon, G.E. Hicks
Data analysis: C.G. Patterson, R.T. Pohlig, G.E. Hicks
Project management: J.M. Pugliese, P.C. Coyle, P.J. Knox, J.M. Sions, S.Z. George, G.E. Hicks
Fund procurement: G.E. Hicks
Providing participants: C.B. Simon, S.Z. George, G.E. Hicks
Providing facilities/equipment: S. Piva, G.E. Hicks
Providing institutional liaisons: S. Piva, G.E. Hicks
Clerical/secretarial support: J.M. Pugliese, P.C. Coyle, J.M. Sions
Consultation (including review of manuscript before submitting): J.M. Pugliese, P.C. Coyle, P.J. Knox, J.M. Sions, C.B. Simon, D.K. Weiner, S.Z. George, S. Piva, G.E. Hicks
Funding
This trial is funded by the National Institutes of Health’s National Institute on Aging (grant no: 2 R01 AG041202).
Ethics Approval
This study was approved by the institutional review boards at each of the participating study sites; each site provides continuing reviews per their site requirements. Oversight of the entire trial is provided by an independent data and safety monitoring board.
Clinical Trial Registration
This trial is registered at ClinicalTrials.gov NCT04009837.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Bressler HB, Keyes WJ, Rochon PA, Badley E. The prevalence of low back pain in the elderly. A systematic review of the literature. Spine (Phila Pa 1976). 1999;24:1813–1819. [DOI] [PubMed] [Google Scholar]
- 2. Koch HK, Smith MC. Office-based Ambulatory Care for Patients 75 Years Old and Over: National Ambulatory Medical Care Survey, 1980 and 1981. Hyattsville, MD, USA: U.S. Dept. of Health and Human Services, Public Health Service, National Center for Health Statistics; 1985. [Google Scholar]
- 3. Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154:2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cypress BK. Characteristics of physician visits for back symptoms: a national perspective. Am J Public Health. 1983;73:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a US national survey. Spine. 1995;20:11–19. [DOI] [PubMed] [Google Scholar]
- 6. Docking RE, Fleming J, Brayne C, Zhao J, Macfarlane GJ, Jones GT. Epidemiology of back pain in older adults: prevalence and risk factors for back pain onset. Rheumatology (Oxford). 2011;50:1645–1653. [DOI] [PubMed] [Google Scholar]
- 7. Rundell SD, Sherman KJ, Heagerty PJ, Mock CN, Jarvik JG. The clinical course of pain and function in older adults with a new primary care visit for back pain. J Am Geriatr Soc. 2015;63:524–530. [DOI] [PubMed] [Google Scholar]
- 8. Jarvik JG, Gold LS, Tan K, et al. Long-term outcomes of a large, prospective observational cohort of older adults with back pain. Spine J. 2018;18:1540–1551. [DOI] [PubMed] [Google Scholar]
- 9. Reid MC, Williams CS, Gill TM. Back pain and decline in lower extremity physical function among community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60:793–797. [DOI] [PubMed] [Google Scholar]
- 10. Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–1424. [DOI] [PubMed] [Google Scholar]
- 11. Leveille SG, Guralnik JM, Hochberg M, et al. Low back pain and disability in older women: independent association with difficulty but not inability to perform daily activities. J Gerontol A Biol Sci Med. 1999;54:M487–M493. [DOI] [PubMed] [Google Scholar]
- 12. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 14. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PubMed] [Google Scholar]
- 15. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. [DOI] [PubMed] [Google Scholar]
- 16. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–887. [DOI] [PubMed] [Google Scholar]
- 18. Bigos S, Bowyer O, Braen G, Brown K, Deyo R, Haldeman S. Acute Low Back Problems in Adults. Rockville, MD, USA: Agency for Health Care Policy and Research. Public Health Service, US Department of Health and Human Services; 1994. [Google Scholar]
- 19. Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: results of a randomized clinical trial. Spine. 2006;31:623–631. [DOI] [PubMed] [Google Scholar]
- 20. Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141:920–928. [DOI] [PubMed] [Google Scholar]
- 21. Fritz JM, Delitto A, Erhard RE. Comparison of classification-based physical therapy with therapy based on clinical practice guidelines for patients with acute low back pain: a randomized clinical trial. Spine. 2003;28:1363–1371. [DOI] [PubMed] [Google Scholar]
- 22. Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine. 2004;29:2593–2602. [DOI] [PubMed] [Google Scholar]
- 23. Paeck T, Ferreira ML, Sun C, Lin CW, Tiedemann A, Maher CG. Are older adults missing from low back pain clinical trials? A systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2014;66:1220–1226. [DOI] [PubMed] [Google Scholar]
- 24. Bernard MA, Clayton JA, Lauer MS. Inclusion across the lifespan: NIH policy for clinical research. JAMA. 2018;320:1535–1536. [DOI] [PubMed] [Google Scholar]
- 25. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. James RJE, Walsh DA, Ferguson E. Trajectories of pain predict disabilities affecting daily living in arthritis. Br J Health Psychol. 2019;24:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hicks GE, Pohlig RT, Coyle PC, et al. Classification of geriatric low back pain based on hip characteristics with a 12-month longitudinal exploration of clinical outcomes: findings from Delaware spine studies. Phys Ther. 2020;pzab227. doi: 10.1093/ptj/pzab227. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hicks GE, Sions JM, Velasco TO. Hip symptoms, physical performance, and health status in older adults with chronic low back pain: a preliminary investigation. Arch Phys Med Rehabil. 2018;99:1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoeymans N, Feskens EJ, van den Bos GA, Kromhout D. Measuring functional status: cross-sectional and longitudinal associations between performance and self-report (Zutphen Elderly Study 1990-1993). J Clin Epidemiol. 1996;49:1103–1110. [DOI] [PubMed] [Google Scholar]
- 32. Reuben DB, Seeman TE, Keeler E, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59:1056–1061. [DOI] [PubMed] [Google Scholar]
- 33. Stretton CM, Latham NK, Carter KN, Lee AC, Anderson CS. Determinants of physical health in frail older people: the importance of self-efficacy. Clin Rehabil. 2006;20:357–366. [DOI] [PubMed] [Google Scholar]
- 34. Latham NK, Mehta V, Nguyen AM, et al. Performance-based or self-report measures of physical function: which should be used in clinical trials of hip fracture patients? Arch Phys Med Rehabil. 2008;89:2146–2155. [DOI] [PubMed] [Google Scholar]
- 35. Beurskens AJ, De Vet H, Köke AJ, van der Heijden GJ, Knipschild PG. Measuring the functional status of patients with low back pain. Assessment of the quality of four disease-specific questionnaires. Spine. 1995;20:1017–1028. [DOI] [PubMed] [Google Scholar]
- 36. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36:24–30. [DOI] [PubMed] [Google Scholar]
- 37. Takacs J, Garland SJ, Carpenter MG, Hunt MA. Validity and reliability of the community balance and mobility scale in individuals with knee osteoarthritis. Phys Ther. 2014;94:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Dorn A. COVID-19 and readjusting clinical trials. Lancet. 2020;396:523–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323:2135–2136. [DOI] [PubMed] [Google Scholar]
- 40. Delitto A, George SZ, Van Dillen L, et al. Low back pain. J Orthop Sports Phys Ther. 2012;42:A1–A57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoeksma HL, Dekker J, Ronday HK, et al. Comparison of manual therapy and exercise therapy in osteoarthritis of the hip: a randomized clinical trial. Arthritis Rheum. 2004;51:722–729. [DOI] [PubMed] [Google Scholar]
- 42. Mac Donald CW, Whitman JM, Cleland JA, Smith M, Hoeksma HL. Clinical outcomes following manual physical therapy and exercise for hip osteoarthritis: a case series. J Orthop Sports Phys Ther. 2006;36:588–599. [DOI] [PubMed] [Google Scholar]
- 43. Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and hip strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42:22–29. [DOI] [PubMed] [Google Scholar]
- 44. Hicks GE, Sions JM, Velasco TO, Manal TJ. Trunk muscle training augmented with neuromuscular electrical stimulation appears to improve function in older adults with chronic low back pain: a randomized preliminary trial. Clin J Pain. 2016;32:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cancer Therapy Evaluation Program (CTEP) . Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. U.S. Department of Health and Human Services, National Cancer Institute. 2017. Accessed December 28, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5.0.xlsx.
- 46. Kreidler SM, Muller KE, Grunwald GK, et al. GLIMMPSE: online power computation for linear models with and without a baseline covariate. J Stat Softw. 2013;54:i10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 48. Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol. 2013;66:1022–1028. [DOI] [PubMed] [Google Scholar]
- 49. Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- 50. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on Research Standards for Chronic Low Back Pain. J Pain 2014;15:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Creavin ST, Wisniewski S, Noel-Storr AH, et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev. 2016;1:CD011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42:1194–1201. [DOI] [PubMed] [Google Scholar]
- 54. Davenport TE, Cleland JA, Yamada KA, Kulig K. Measurement properties of the Low Back Activity Confidence Scale (LoBACS). Eval Health Prof. 2016;39:204–214. [DOI] [PubMed] [Google Scholar]
- 55. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524. [Google Scholar]
- 56. George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40:197–205. [DOI] [PubMed] [Google Scholar]
- 57. Corbett DB, Simon CB, Manini TM, George SZ, Riley JL 3rd, Fillingim RB. Movement-evoked pain: transforming the way we understand and measure pain. Pain. 2019;160:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30:115–126. [DOI] [PubMed] [Google Scholar]
- 59. Jones DH, Kilgour RD, Comtois AS. Test-retest reliability of pressure pain threshold measurements of the upper limb and torso in young healthy women. J Pain. 2007;8:650–656. [DOI] [PubMed] [Google Scholar]
- 60. Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23:760–766. [DOI] [PubMed] [Google Scholar]
- 61. Pua YH, Wrigley TV, Cowan SM, Bennell KL. Intrarater test-retest reliability of hip range of motion and hip muscle strength measurements in persons with hip osteoarthritis. Arch Phys Med Rehabil. 2008;89:1146–1154. [DOI] [PubMed] [Google Scholar]
- 62. Thorborg K, Petersen J, Magnusson SP, Hölmich P. Clinical assessment of hip strength using a hand-held dynamometer is reliable. Scand J Med Sci Sports. 2010;20:493–501. [DOI] [PubMed] [Google Scholar]
- 63. Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837–841. [DOI] [PubMed] [Google Scholar]
- 64. Unver B, Kahraman T, Kalkan S, Yuksel E, Karatosun V, Gunal I. Test-retest reliability of the 50-foot timed walk and 30-second chair stand test in patients with total hip arthroplasty. Acta Orthop Belg. 2015;81:435–441. [PubMed] [Google Scholar]
- 65. Nilsdotter AK, Lohmander LS, Klässbo M, Roos EM. Hip Disability and Osteoarthritis Outcome Score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hahn EA, DeVellis RF, Bode RK, et al. Measuring social health in the Patient-Reported Outcomes Measurement Information System (PROMIS): item bank development and testing. Qual Life Res. 2010;19:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


