Abstract
Birt-Hogg-Dube syndrome (BHDS) is a rare disorder characterized by the triad of cutaneous lesions, renal tumors, lung cysts and inactivation of the gene folliculin (FLCN). Here, we present three female patients diagnosed with BHDS. First case a 55-year-old female had flesh moles histopathology compatible with angiofibroma, multiple cysts in the lung and kidneys, FLCN gene mutations (‘c.1285dupC [p.His429Profs*]’ 11th exon and ‘c.653G>A [p.Arg258His]’ 7th exon). The second case a 76-year-old female had trichodiscoma on her skin, multiple cysts in the lung, spontaneous pneumothorax, FLCN gene mutation ‘c.1285dupC (p.His429Profs*27) 11th exon’ and, her son had renal carcinoma history under 50 years of age. Our third case, also the daughter of case 2, had dermal papules histopathology compatible with trichodiscoma, spontaneous pneumothorax, FLCN gene mutation ‘c.1285dupC (p.His429Profs*27) 11th exon’ and, parotid oncocytoma. Through our cases, we document the first case of two mutations (‘c.1285dupC [p.His429Profs*]’ 11th exon and ‘c.653G>A [p.Arg258His]’ 7th exon) in the same FLCN gene and the 11th known case of parotid oncocytoma associated with BHDS in the light of the literature.
Keywords: Birt-Hogg-Dube syndrome, FLCN gene, Parotid Neoplasms, Pneumothorax, Kidney neoplasms
INTRODUCTION
Birt-Hogg-Dube syndrome (BHDS) is an autosomal dominant inherited genodermatosis characterized by benign tumors of the hair follicle, pulmonary cysts, spontaneous pneumothorax and renal tumors1,2. Germline mutations in the folliculin (FLCN) gene which encode the protein called folliculin and located in the 14th exon of the p11.2 region of chromosome 17 have been found to cause the BHDS3,4. Folliculin seems to take part in the adenosine-monophosphate-activated protein kinase (AMPK) and mechanistic target of rapamycincomplex (mTORC) pathways and is expressed in many tissues including the skin, type 1 pneumocytes and distal nephrons4,5.
Here, we present three female cases diagnosed with BHDS. Through them, we document the first case of two mutations in the same FLCN gene and the11th known case of parotid oncocytoma associated with BHDS in the light of the literature.
Molecular & in silico analysis
Genomic DNA was extracted from peripheral venous blood using the QIAamp® DNA Mini Kit (QIAGEN, Ankara, Turkey) in all cases. FLCN gene mutations detected by next generation sequencing and confirmed by Sanger sequencing.
For in silico analysis protein structure of FLCN-FNIP2-Rag-Ragulator complex was downloaded from the protein data bank (PDB). FLCN in complex with other proteins (PDB ID: 6ULG) was visualized and presented by using PyMol software (http://pymol.sourceforge.net).
The written informed consents from patients for publication of the submitted article, accompanying genetic analyses, photographic materials and the results were obtained after full explanation of the purpose and nature of all procedures used.
CASE REPORT
Case 1
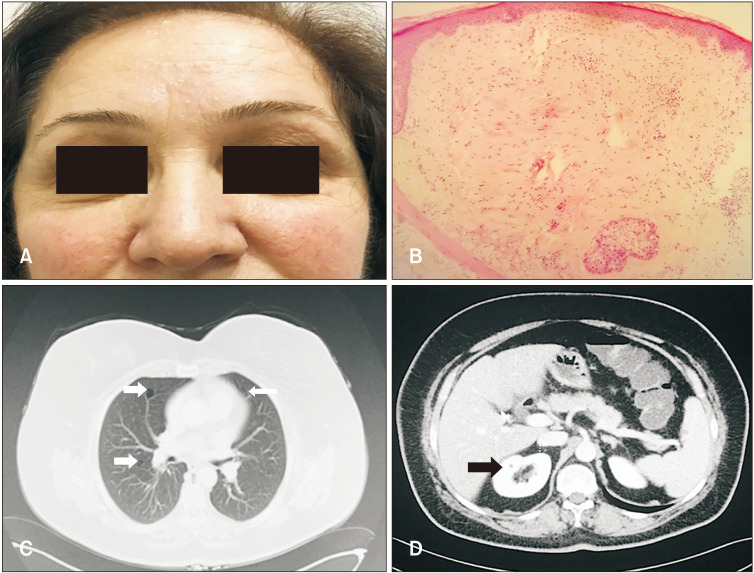
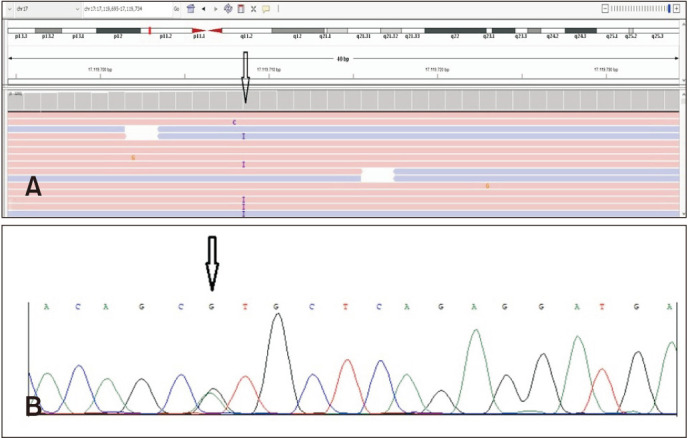
A 55-year-old female was admitted to our outpatient clinic with the complaint of flesh moles on her face for 10 years. She had a family history of colon carcinoma. On dermatological examination, there were numerous 1 to 3 mm diameter skin-colored, asymptomatic, dome-shaped papules on herforehead, malar region, nose and neck (Fig. 1A). Histopathological examination of the skin biopsy was compatible with angiofibroma (Fig. 1B). Thoracic-abdominal-pelvic tomography (CT) of the patient revealed multiple, thin-walled cysts of different sizesin both lobes of the lung parenchyma, and hypodense lesions that were compatible with multiple cysts in kidneys (Fig. 1C, D). As a result of clinical exome sequencing, heterozygous c.1285dupC (p.His429Profs*) (Fig. 2A) mutation in the 11th exon of the FLCN gene and as a second mutation in exon 7, heterozygous c.653G>A (p.Arg258His) variation were detected (Fig. 2B). The diagnosis of BHDS was madedue to the presence of multiple cysts in the lungs and kidneys, facial angiofibromas and FLCN gene mutations.
Fig. 1. (A) Multiple skin-colored papules on the forehead, malar region and nose. (B) Some bizarre-looking fibroblasts, collagen and vascular proliferation in the dermis (H&E, original magnification ×40). (C) Thoracic CT revealed thin-walled cysts of different sizes in the lung parenchyma. (D) Abdominal-pelvic CT revealed hypodense lesions that were compatible with multiple cysts in both kidneys.
Fig. 2. (A) Integrative genomics view of c.1285dupC (p.His429Profs*27) heterozygous change in FLCN gene. (B) Electropherograms of heterozygous genotype of FLCN c.653G>A (p.Arg258His) variation.
Case 2
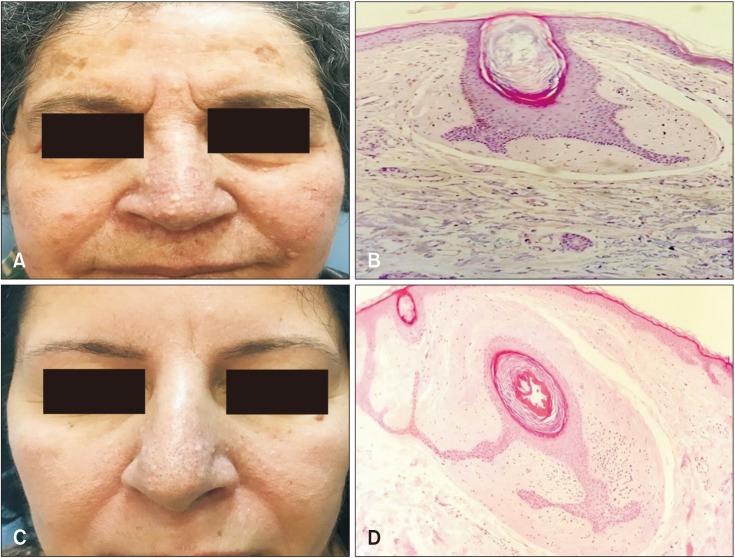
A 76-year-old female was referred to our outpatient clinic with the presence of cystic lung disease for two months and the complaint of facial widespread flesh moles which appeared at her twenties. She had a history of colon cancer treated 10 years ago. Her son had clear cell renal cell carcinoma and her daughter who is also our third patient had a spontaneous pneumothorax history, parotid oncocytoma, and similar flesh moles. On dermatological examination, there were numerous, skin-colored, asymptomatic papules on her face and neck (Fig. 3A). Histopathological examination of the skin biopsy was consistent with trichodiscoma (Fig. 3B). Thoracic CT showed smoothly circumscribed cysts in the bilateral lung parenchyma. Genetic analysis revealed c.1285dupC (p.His429Profs*27) mutation in the 11th exon in the FLCN gene the same as in case 1. The patient was diagnosed as BHDS due to the presence of trichodiscoma of the skin, multiple cysts in the lung, FLCN gene mutation,presence of renal carcinoma under 50 years of age in the family and spontaneous pneumothorax.
Fig. 3. (A) Multiple skin-colored papules on the face. (B, D) Follicle structures showing epithelial proliferation surrounded by fibrocollagenous tissue (H&E, original magnification ×40). (C) Diffuse whitish papules on the face.
Case 3
A 54-year-old female, the daughter of case 2, presented with a complaint of flesh moles on her face, which has been around for 30 years. She had a history of spontaneous pneumothorax 15 years ago and right parotid glandoncocytoma 10 years ago. Dermatological examination revealed multiple, skin-colored, asymptomatic papules on her face and neck (Fig. 3C). Histopathological examination of the skin biopsy was consistent with trichodiscoma (Fig. 3D). The c.1285dupC (p.His429Profs*) mutation in the FLCN geneis inherited from the mother (case 2) and same as the mutation detected in case 1.
In silico finding and functional predictions
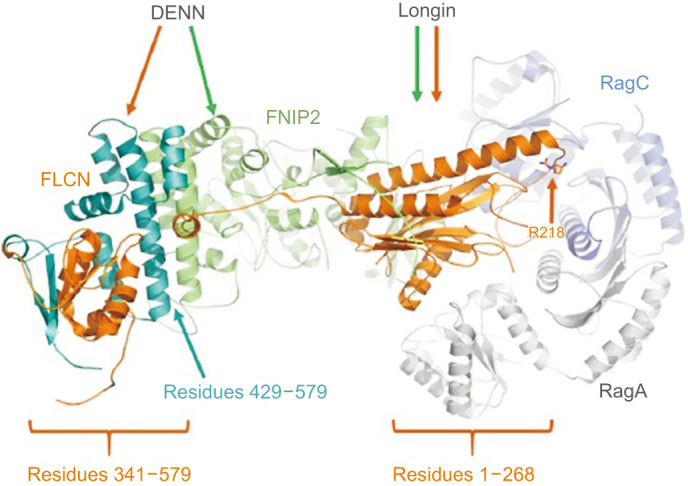
FLCN with its interacting proteins FNIP1 or FNIP2 (folliculin interacting protein 1 or 2) bind to AMPK and TFEB5,6, hence they regulate TFEB dependent transcription7. In addition, FLCN-FNIP complex binds to Rag GTPases to initiate the GTP hydrolysis by RagC/D8,9. Rag complex interacts with mTORC19. FLCN and FNIP are mainly composed of Longin and DENN domains (Fig. 4) where they heterodimerize to interact with the nucleotide binding domain of Rag heterodimer10. Structural information shows that mutant p.His429Profs*27 protein is mainly lack of FNIP2 interacting residues in the DENN domain (Fig. 4) which can attenuate their dimerization. Since FLCN-FNIP dimer regulates the GTP hydrolysis in Rag complex and the mTORC1 activity, lack of dimerization may adversely affect the appropriate mTORC1 activity.
Fig. 4. Structure of FLCN in complex with FNIP2, RagA, and RagC (PDB ID: 6ULG) where proteins are shown in orange, green, white, and navy ribbons, respectively. Residue numbers of FLCN forming the Longin and DENN domains are provided.
DISCUSSION
In 1977, Birt, Hogg, and Dubé described a pedigree in which members had skin lesions consisting of “trichodiscomas, acrochordons, and fibrofolliculomas1”. BHDS may be phenotypically heterogeneous. Therefore, diagnostic criteria for BHDS have been reported2.
Fibrofoliculomas are the most common skin manifestations of BHDS. It occurs in the third decade in 84% of the patients and presents with painless, dome-shaped whitish papules on the face, neck, chest, and back11. Recent studies show that Asian patients with BHDS present a lower incidence of skin lesions(25.0%~48.7%) than Caucasian patients12,13. Other skin lesions associated with BHDS are trichodiscomas and acrocordons. Less frequently, angiofibroma, angiolipoma, epidermal cysthave also been reported2. The skin lesions of our cases were compatible with angiofibromas andtrichodiscomas.
Multiple lung cysts are seen on CT in more than 80% of BHDS cases14,15. The probability of first pneumothorax is 75% by the sixth decade15. About 75% of the patients will experience recurrent pneumothorax14. All our three cases had a history of lung cysts and case 3 had a history of pneumothorax at age 39 years.
The most serious complication of BHDS is renal cancers. It is seen in the 50s in 15%~25% of the patients, mostly multiple and bilateral16. It is reported that the patients diagnosed with BHDShad a 7-fold increased risk of developing renal neoplasia17. Chromophobe renal cancer, mixed chromophobe and oncocytic renal tumors are typical for BHDS16. Therefore, all newly diagnosed patients should undergo abdominal imagingto exclude renal tumors2. The son of case 2 had a history of clear cell renal cell carcinoma with no skin symptoms.
Other tumors reported to be associated with BHDS include parathyroid adenoma/oncocytoma, colorectal carcinoma, and melanoma2,18. Our case 2 had a treated colon carcinoma and case 3 had a history of treated parotid oncocytoma. To the best of our knowledge, case 3 is the 11th reported case of BHDS with parotid oncocytoma11,14,19.
To date, 285 unique mutations have been identifiedin the FLCN gene and documented in the FLCN Leiden Open Variation Database (https://databases.lovd.nl/shared/genes/FLCN).
As a result of the genetic analysis performed in case 1, it was found that c.1285dupC (p.His429Profs*) mutation in the 11th exon of FLCN gene was heterozygous. The identified mutation is described in the Human genetic mutation Database (HGMD) and has been associated with BHDS (HGMD ID: C1023308). This mutation has been reported pathogenic in the ClinVar database. In case 1, change of c.653G>A in exon 7 (p.Arg258His) was also determined as a second mutation. The clinical significance in the ClinVar database has been reported as uncertain.It has also been reported as a variant of uncertain clinical significance in Varsome. The population frequency of the mutation was 0.00000795 in gnomAD Exomes ver. 2.11 (Broad Institute, Cambridge, MA, USA). It was stated that it could be disease-causing in the predictive databases (such as Mutation Taster, FATHMM, PROVEAN). Segregation of this variant could not be demonstrated because the patient’s parents were not alive. Because of this reason this variant could not be demonstrated whether it is in cis or trans position. Hitherto, a case of two mutations in the same FLCN gene has not been reported in the literature.
According to the results of a study conducted in Japan, the mutation of c.1285dupC (p.His429Profs*) in the FLCN gene is among the mutations detected in the majority of cases, and this mutation is thought to be a hotspot mutation in the FLCN gene13. The same mutation was observed in our cases, although they were independent of each other. Therefore, to determine whether this mutation is a hotspot mutation for the Turkish population needs to be screening in larger series of patients.
Analysis of genotype-phenotype correlations for FLCN mutations demonstrated a significantly higher risk of colorectal neoplasia in c.1285dupC mutation carriers than in c.610delGCinsTA mutation carriers20. However, in terms of genotype-phenotype correlations, our case 1, who had compound heterozygous FLCN mutations, did not show more severe symptoms in the skin, lungs and kidneys than the BHDS patients reported previously.
There are still many aspects of BHDS to be explored. Therefore, BHDS should be kept in mind in the differential diagnosis of patients presenting with papular lesions. The prompt and accurate diagnosis is necessary for appropriate management of patients and genetic counselling. Additionally, the detection of two mutations in the same FLCN gene in our cases expands the knowledge of FLCN mutations and will provide insight into the genetic diagnosis of BHDS.
ACKNOWLEDGMENT
The authors wish to express their thanks to the patients for participating in this study.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: None.
References
- 1.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- 2.Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, et al. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 3.Khoo SK, Bradley M, Wong FK, Hedblad MA, Nordenskjöld M, Teh BT. Birt-Hogg-Dubé syndrome: mapping of a novel hereditary neoplasia gene to chromosome 17p12-q11.2. Oncogene. 2001;20:5239–5242. doi: 10.1038/sj.onc.1204703. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 5.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi Y, Kobayashi T, Shiono M, Wang L, Piao X, Sun G, et al. Interaction of folliculin (Birt-Hogg-Dubé gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene. 2008;27:5339–5347. doi: 10.1038/onc.2008.261. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu J, Detraux D, Kuppers D, Wang Y, Cavanaugh C, Sidhu S, et al. Folliculin regulates mTORC1/2 and WNT pathways in early human pluripotency. Nat Commun. 2019;10:632. doi: 10.1038/s41467-018-08020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panchaud N, Péli-Gulli MP, De Virgilio C. Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the Rag family GTPase Gtr1. Sci Signal. 2013;6:ra42. doi: 10.1126/scisignal.2004112. [DOI] [PubMed] [Google Scholar]
- 9.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52:495–505. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen K, Rogala KB, Chou HT, Huang RK, Yu Z, Sabatini DM. Cryo-EM structure of the human FLCN-FNIP2-rag-ragulator complex. Cell. 2019;179:1319–1329.e8. doi: 10.1016/j.cell.2019.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Jeon MJ, Song JS, Chae EJ, Choi JH, Kim GH, et al. Birt-Hogg-Dubé syndrome in Korean: clinicoradiologic features and long term follow-up. Korean J Intern Med. 2019;34:830–840. doi: 10.3904/kjim.2018.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuya M, Yao M, Tanaka R, Nagashima Y, Kuroda N, Hasumi H, et al. Genetic, epidemiologic and clinicopathologic studies of Japanese Asian patients with Birt-Hogg-Dubé syndrome. Clin Genet. 2016;90:403–412. doi: 10.1111/cge.12807. [DOI] [PubMed] [Google Scholar]
- 14.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26:1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 18.Yoshida K, Miyagawa M, Kido T, Ide K, Sano Y, Sugawara Y, et al. Parotid oncocytoma as a manifestation of Birt-Hogg-Dubé syndrome. Case Rep Radiol. 2018;2018:6265175. doi: 10.1155/2018/6265175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindor NM, Kasperbauer J, Lewis JE, Pittelkow M. Birt-Hogg-Dube syndrome presenting as multiple oncocytic parotid tumors. Hered Cancer Clin Pract. 2012;10:13. doi: 10.1186/1897-4287-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahorski MS, Lim DH, Martin L, Gille JJ, McKay K, Rehal PK, et al. Investigation of the Birt-Hogg-Dube tumour suppressor gene (FLCN) in familial and sporadic colorectal cancer. J Med Genet. 2010;47:385–390. doi: 10.1136/jmg.2009.073304. [DOI] [PubMed] [Google Scholar]