Abstract
A new soft coral species, Xeniakonohanasp. nov. (Alcyonacea, Xeniidae), is described from Miyazaki in the warm-temperate region of Japan. This new species has conspicuous and unique spindle sclerites in addition to the simple ellipsoid platelet-shaped sclerites typically found in the genus Xenia. These unique spindles are a specific key morphological characteristic for this new species and for differentiating this species among congeneric species.
Keywords: Alcyonacea, Cnidaria, Miyazaki, new species, Xenia , Xeniidae
Introduction
Species of the family Xeniidae are known as pioneers in tropical coral reefs (Benayahu and Loya 1987), playing an important role for ecological succession in coral reefs. Therefore, knowing how many species of Xeniidae exist, and the range of species diversity will be useful for understanding the coral reef ecosystem.
For species or genus identification of alcyonacean soft corals including xeniids, the shape and arrangement of sclerites are used as key characteristics. Xeniids typically produce minute platelets or corpuscle-like sclerites without tubercular differences among species and genera under light microscopy (Fabricius and Alderslade 2001). The microstructure of sclerites has been shown to be an important character at the genus level of the family Xeniidae. Recently, the type specimens of 21 species in the genus Xenia were rechecked and re-described using sclerite microstructure (Halász et al. 2019). Thus, observation of sclerite microstructure is taxonomically useful for species delimitation, at least in some species of Xenia.
The genus Xenia presently includes 49 valid species (Cordeiro et al. 2021). This genus is characterized by platelet-shaped sclerites with surface microstructure composed of calcite dendritic and sinuous rods (Alderslade 2001; Halász et al. 2019). Koido et al. (2019) reported an undescribed species belonging to Xenia (reported as Xenia sp. 1) from Oshima Island, Miyazaki, in the warm-temperate region (non-coral reef region) of Japan. This previous work emphasized the high species diversity of Xeniidae in Miyazaki, Japan. This study provides a description of this previously undescribed species (Xenia sp. 1) as Xeniakonohana sp. nov., a new species in the genus.
Materials and methods
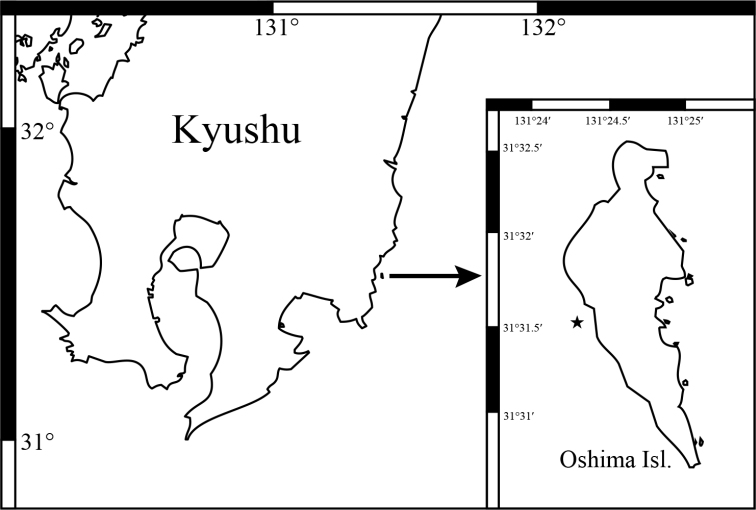
All specimens were collected around Oshima Island (31°31.35'N, 131°24.27'E) (Fig. 1), Miyazaki, Japan, by SCUBA diving and snorkeling. A small piece of tissue (5–10 mm) from each specimen was used for molecular analyses and the remainder was preserved in 99% ethanol for morphological analyses as reported by Koido et al. (2019).
Figure 1.
Collection sites of Xeniakonohana sp. nov. in Miyazaki, Japan.
Specimens were previously deposited in Miyazaki University, Fisheries Sciences (MUFS) but were subsequently transferred and deposited at the Kuroshio Biological Research Foundation, Kochi, Japan (KBF) in the octocoral collection (OA). Morphological characteristics examined under a stereomicroscope included colony height, length and width of the stalk, presence of branches, length and width of polyps, length and width of tentacles, length and width of pinnules, number of rows of pinnules, and number of pinnules in the aboral row. Sclerites from polyps, and ones from the surface and interior of both stalk and branches of each specimen were examined. Sclerite shape, size, and microstructure were examined with light microscopy and scanning electron microscope (SEM) (HITACHI S-4800 and JEOL JSM-6500F).
DNA extraction, amplification, and sequencing
Tissue samples were kept in CHAOS solution for at least a week to dissolve proteins at room temperature as reported by Koido et al. (2019). Total DNA was extracted from CHAOS solutions by conventional phenol/chloroform extraction. The phylogenetic position of X.konohana sp. nov. was inferred using three mitochondrial markers (ND2, mtMutS, COI) (16S647F: 5’-ACA CAG CTC GGT TTC TAT CTA CCA-3’; ND21418R: 5’ -ACA TCG GGA GCC CAC ATA-3’, ND42625F: 5’-TAC GTG GYA CAA TTG CTG-3’, Mut-3458R: 5’-TSG AGC AAA AGC CAC TCC-3’, COII8068F: 5’-CCA TAA CAG GAC TAG CAG CAT C-3’, HC02198: 5’-TAA ACT TCA GGG TGA CCA AAA AAT CA-3’) and a nuclear marker (28S) (28S-Far: 5’-CAC GAG ACC GAT AGC GAA CAA GTA-3’, 28S-Rar: 5’-TCA TTT CGA CCC TAA GAC CTC-3’). PCR reactions for all four markers used 1 μL of DNA solution, 1.6 μL of 2.5 mM dNTP Mixture, 2 μL of 10X Ex Taq buffer, 2 μL of each primer (10 mM), 0.08 μL Ex taq (TaKaRa), and 11.32 μL of sterile distilled water. Amplification of these markers used a GeneQ PCR Thermal Cycler with the following thermal profile; 35 cycles of 90 sec at 94 °C, 60 sec at 58 °C, and 60 sec at 72 °C. Amplicons were checked on 1% agarose gel electrophoresis. All PCR products were treated to remove excess primers and dNTP using Exonuclease I (TaKaRa) and Shrimp Alkaline Phosphatase (TaKaRa). DNA sequences were determined by ABI3000 using a research contract service (Ltd. FASMAC). DNA sequences of 709 bases for mtMutS, 804 for COI, 773 for 28S rDNA, and 673 for ND2 were obtained in this study. DNA sequences for mtMutS, COI, and 28S were combined and analyzed because concatenated DNA sequences using these markers have been recently used for the molecular phylogenetic analyses in the Xeniidae (McFadden et al. 2019; Halász et al. 2019), while sequences for ND2 were analyzed alone because of restricted number of sequences available (McFadden et al. 2006; McFadden and Ofwegen 2012; McFadden et al. 2014b; McFadden et al. 2017). As outgroups for both analyses, we used Paralemnaliathyrsoides (Ehrenberg, 1834) (family Nephtheidae), Rhytismafulvum (Forskål, 1775) (family Alcyoniidae) and Coelogorgiapalmosa Milne Edwards & Haime, 1857 (family Coelogorgiidae), which are all known to be closely related to the Xeniidae (Halász et al. 2019). MEGA6 (Tamura et al. 2013) was used to select appropriate models (T92+G model for the concatenated DNA sequences, including mtMutS, COI, and 28S, and T92 model for ND2) for maximum likelihood (ML) method and to reconstruct the ML phylogenetic trees with 1000 bootstrap replicates. In Bayesian analysis, the concatenated alignment data was treated as a separate data partition with different models of evolution applied to each of the mitochondrial (mtMutS and COI: HKY+G) and nuclear (28S: GTR+G) markers. MrBayes v. 3.2.1 (Ronquist et al. 2012) was run for 50,000,000 generations (until standard deviation of split partitions < 0.01) with a burn-in of 25% and default Metropolis coupling parameters. For phylogenetic analyses, recently published data for three markers (mtMutS, COI, and 28S) from the Xeniidae were also added (Table 1).
Table 1.
List of specimens of the family Xeniidae examined in this study and accession numbers for 28S, mtMutS, COI and ND2 markers. The origin of the accession number is shown by asterisk (s) in the reference list for each line if more than one reference exists.
Results
Taxonomy
Class Anthozoa Ehrenberg, 1831
Subclass Octocorallia Haeckel, 1866
Order Alcyonacea Lamouroux, 1812
Family Xeniidae Ehrenberg, 1828
Genus. Xenia
Lamarck, 1816
52A3BF13-E181-5B8C-9E48-59C7257C65CE
Type species.
Xeniaumbellata Lamarck, 1816
Emended diagnosis.
(Chiefly after Halász et al. 2019). Colonies are small and soft with cylindrical stalk, undivided or branched, terminating in one or more domed polyp-bearing regions. Polyps are not retractile and are always monomorphic. The dominant sclerites are ellipsoid platelets, usually abundant in all parts of the colony. They are composed of calcite rods, often dendritic or sinuous, mostly radially arranged, at least at the periphery of the sclerites. In addition to ellipsoid platelets, a few species have rods or unique spindles with pointed spear ends.
. Xenia konohana sp. nov.
566DDCE1-F472-55BC-B6E0-211FD5C8311C
http://zoobank.org/D1BD260D-A55D-4A88-9CF6-823E06AF0504
New Japanese name: konohana-umiazami Figs 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10
Figure 3.
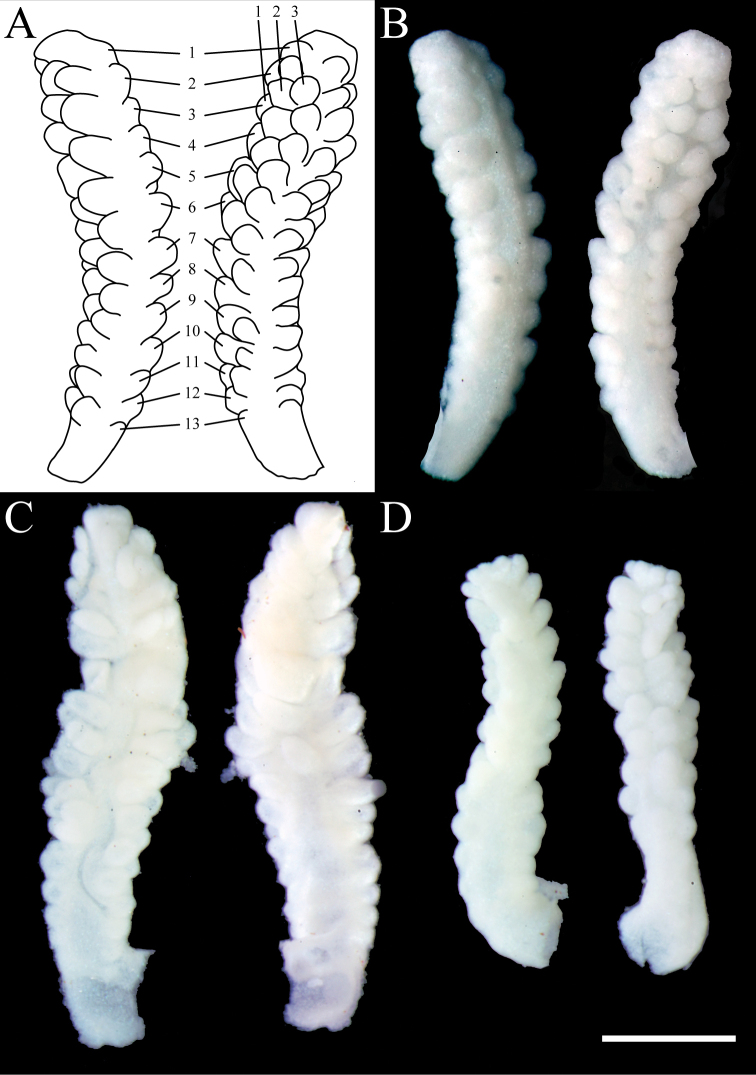
Tentacles of Xeniakonohana sp. nov. aboral (left) and oral sides (right) A schema of holotype KBF-OA-00092: three rows (the number is shown in the upper-right) and 13 pinnules at the outermost row (the number is shown in the center) B holotype KBF-OA-00092 C paratype KBF-OA-00093 D paratype KBF-OA-00094. Scale bar: 1 mm.
Figure 4.
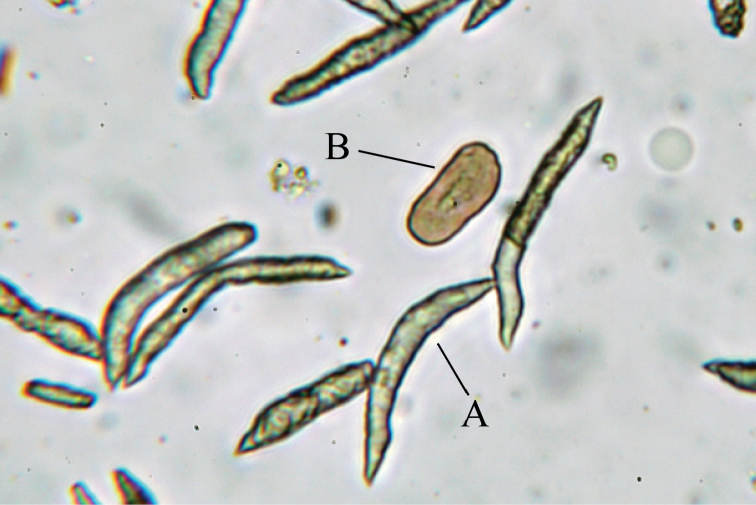
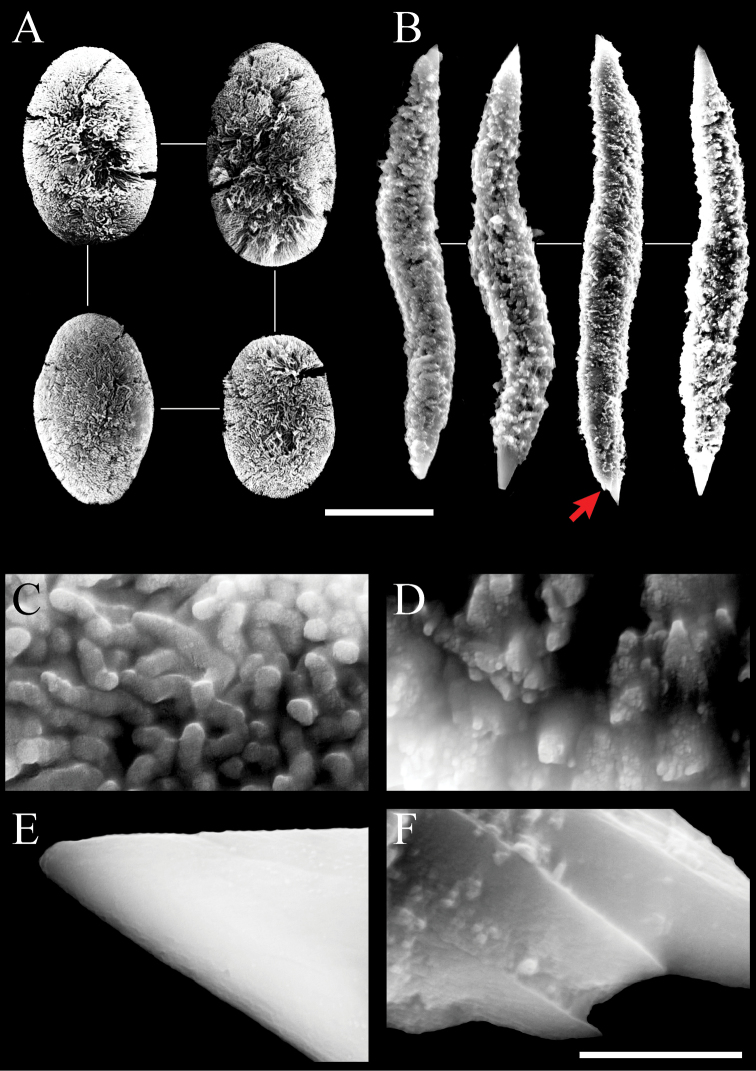
Light microscope images of sclerites in polyps of Xeniakonohana sp. nov., holotype KBF-OA-00092 A spindles B simple platelets.
Figure 5.
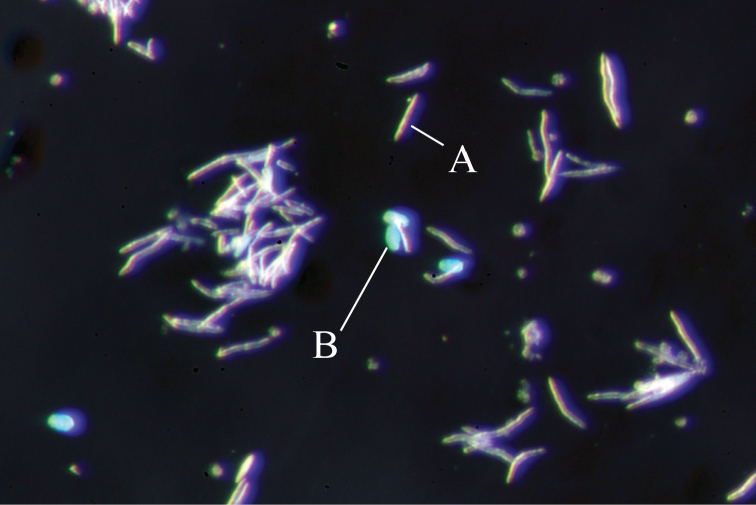
Stereoscopic microscopes images of sclerites in polyps of Xeniakonohana sp. nov., holotype KBF-OA-00092 A spindles B simple platelets.
Figure 6.
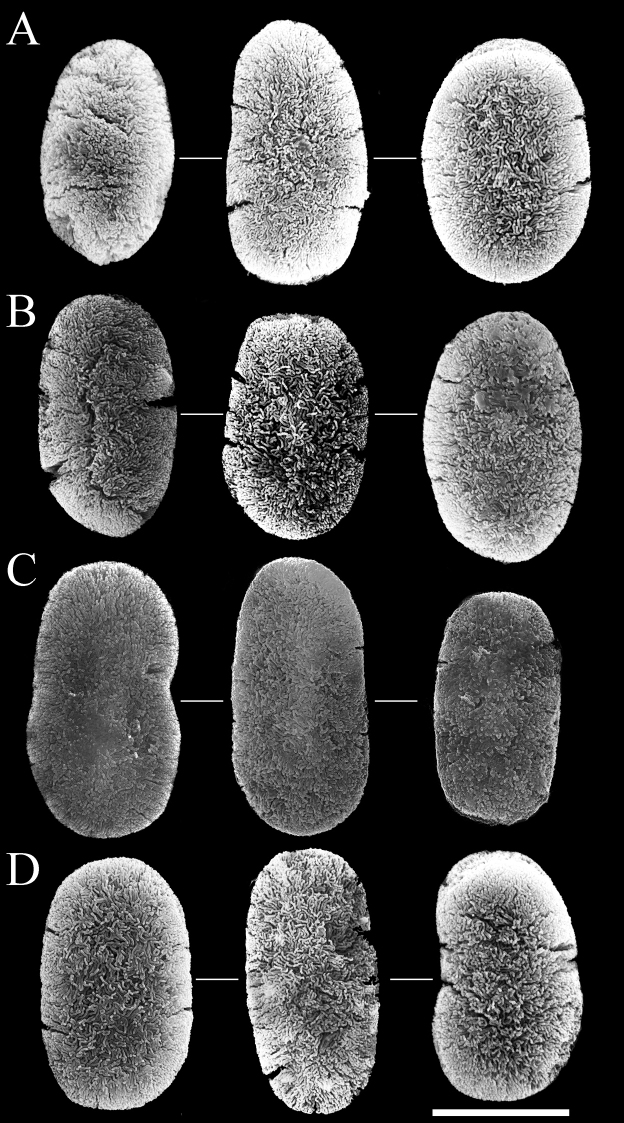
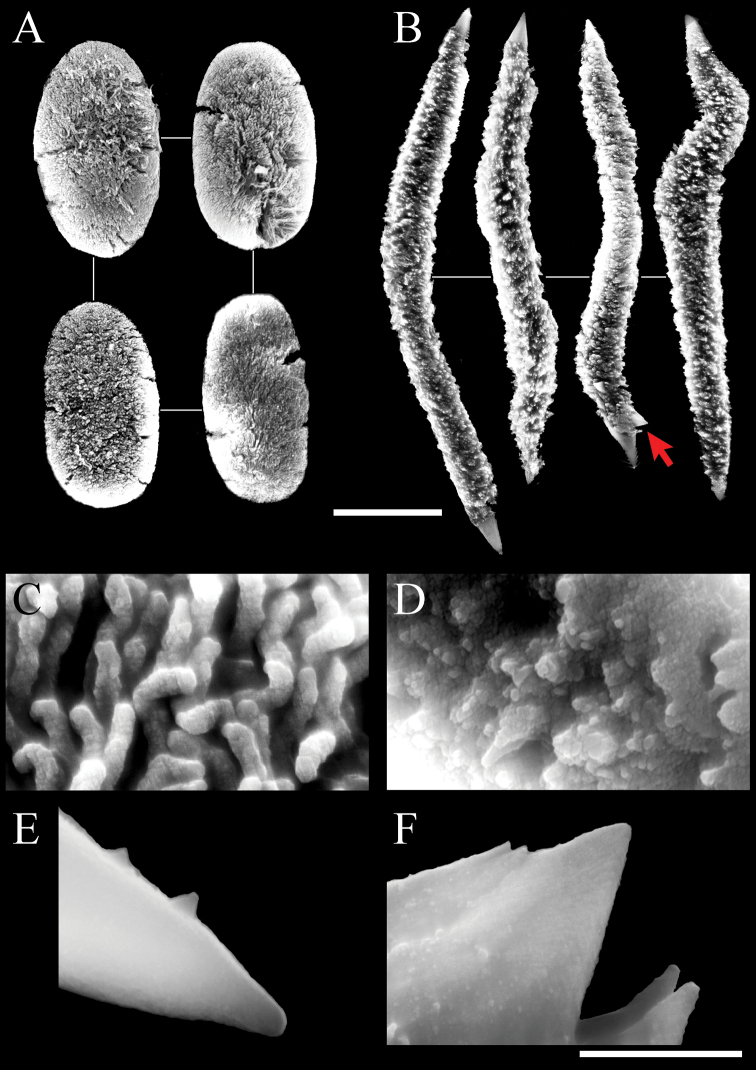
Scanning electron micrographs of platelets of Xeniakonohana sp. nov., holotype KBF-OA-0009 A in tentacles B in polyp body C in stalk surface D in branch surface. Scale bar: 0.010 mm.
Figure 7.
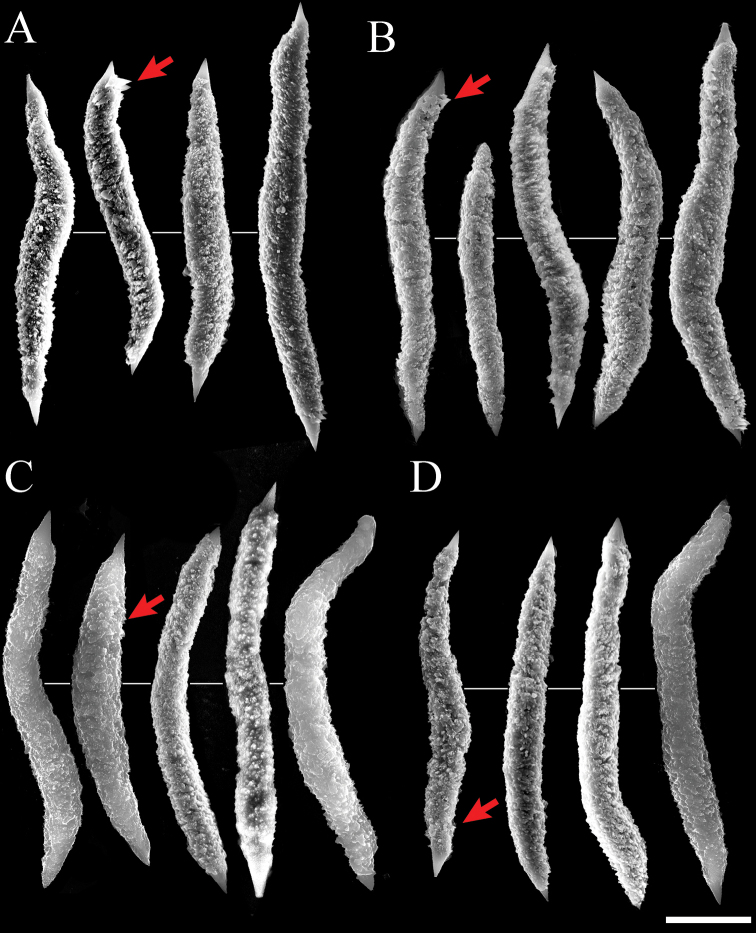
Scanning electron micrographs of spindles of Xeniakonohanasp. nov., holotype KBF-OA-00092 A in tentacles B in polyp body C in stalk surface D in branch surface. Arrow indicates thorns on the surface of spindles. Scale bar: 0.010 mm.
Figure 8.
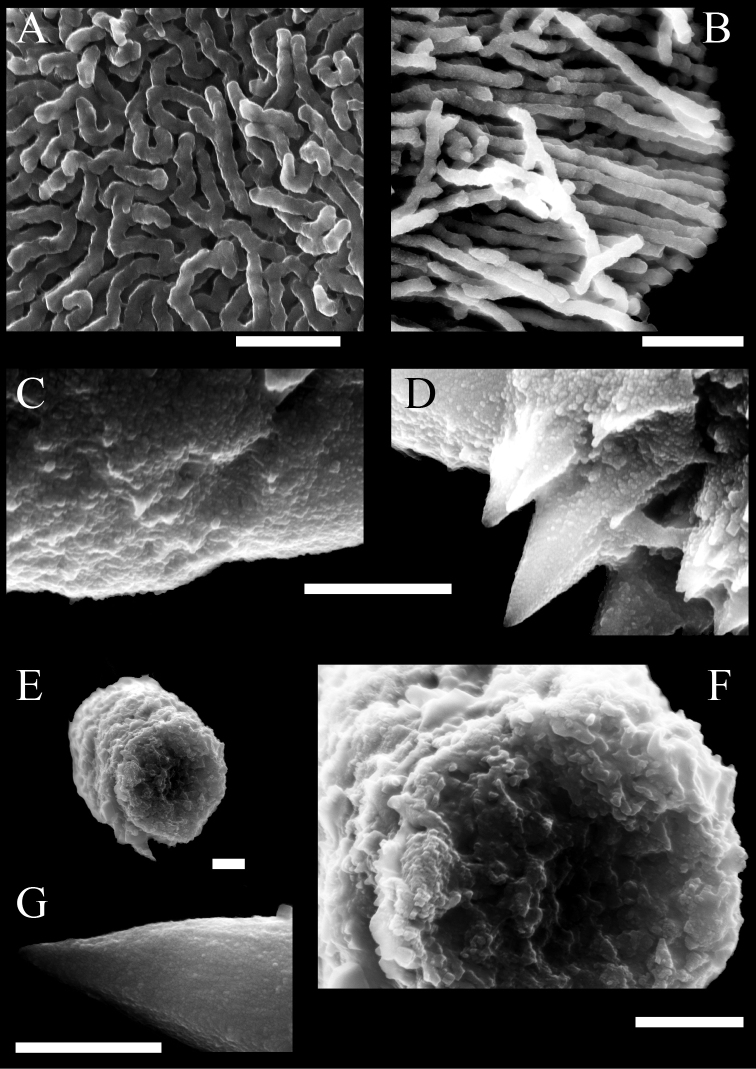
Scanning electron micrographs of the surface of sclerites in tentacles of Xeniakonohana sp. nov., holotype KBF-OA-00092 A surface of platelets covered by minute papillae B broken platelets with radial dendritic rods C central surface of spindle covered by minute granular D thorns on the surface of spindles E broken spindle F close-up view of a broken spindle with fused grain G tip of a spindle. Scale bar: 0.001 mm.
Figure 9.
Scanning electron micrographs of paratype (KBF-OA-00093) of Xeniakonohana sp. nov.: A platelets B spindles (arrow indicates thorns on the surface of spindles) C surface of platelets D central surface of spindle E tip surface of a spindle F thorns on the surface of spindles. Scale bar: 0.01 mm (A, B); 0.001 mm (C–F).
Figure 10.
Scanning electron micrographs of paratype (KBF-OA-00094) of Xeniakonohana sp. nov.: A platelets B spindles (arrow indicates thorns on the surface of spindles) C surface of platelets D central surface of spindle E tip surface of a spindle F thorns on the surface of spindles. Scale bar: 0.01 mm (A, B); 0.001 mm (C–F).
Synonym.
Xenia sp. 1 Koido et al. 2019: Table 1, figs 2J–4J.
Materials.
Holotype: KBF-OA-00092 (MUFS-COMO4 in Koido et al. 2019), Oshima Isl., Nichinan City, Miyazaki Prefecture, depth < 5 m, July 2, 2012. Paratypes: KBF-OA-00093 (MUFS-COMO53 in Koido et al. 2019), Oshima Isl., Nichinan City, Miyazaki Prefecture, depth < 10 m, December 25, 2012; KBF-OA-00094 (One colony with two stems) (MUFS-COMO54 in Koido et al. 2019), Oshima Isl., Nichinan City, Miyazaki Prefecture, depth < 10 m, December 25, 2012.
Descriptions.
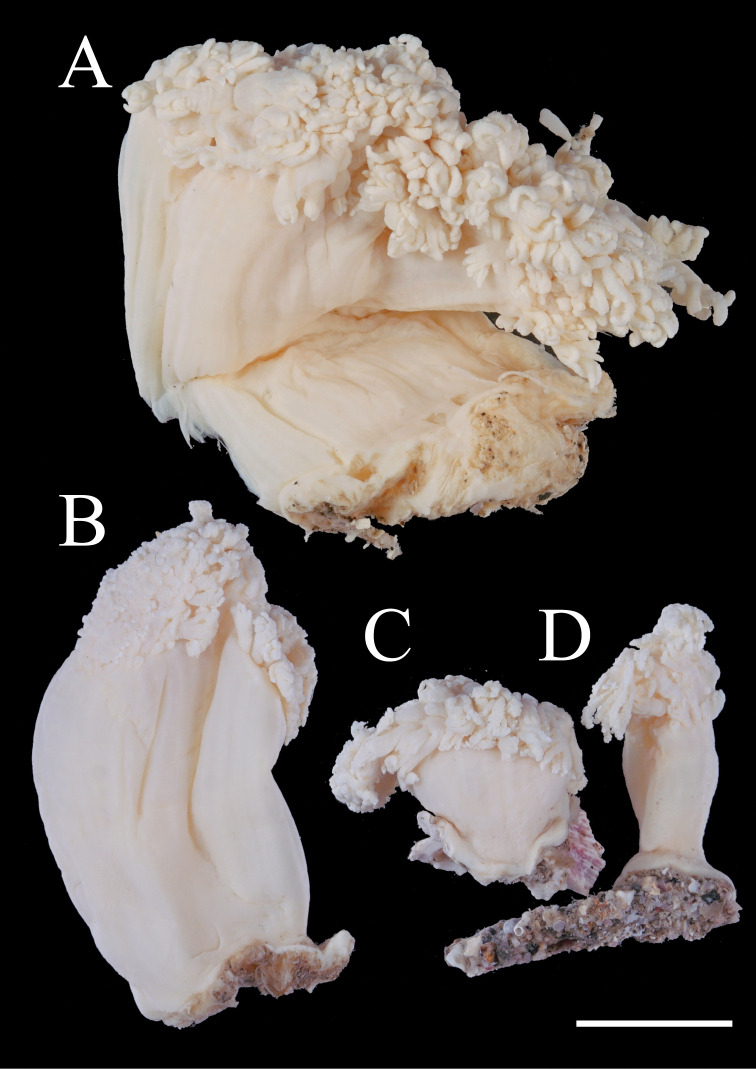
The holotype (Fig. 2A) displays a typical Xenia-style growth form (Alderslade 2001; Benayahu 2010), featuring a distinct cylindrical stalk, 35 mm high and 20 mm wide attached to a rock. The colony possesses three branches 5–7 mm long from a common basal stalk. The whole colony is creamy white in ethanol. Polyps are 4.5–5.0 mm long, excluding tentacles, and 2.0 mm in diameter at their proximal part. Tentacles are 3.0–4.0 mm long and 0.3–0.5 mm wide at their proximal part.
Figure 2.
Fixed specimens of Xeniakonohana sp. nov. A holotype BF-OA-00092 B paratype KBF-OA-00093 C, D paratype KBF-OA-00094. Scale bar: 10 mm.
Pinnules are arranged mostly in three rows along each side of the tentacles, leaving free median space along the oral side. This space is not always visible at the distal part of the longest tentacles. The number of rows of pinnules drops to two toward the proximal part of the tentacle, and occasionally, only a single row can be seen (Fig. 3). The outermost row usually includes 12–16 pinnules each, up to 0.23 mm long and 0.21 mm wide at the proximal part. Typically, no gap between pinnules exists, but in rare cases, a gap of approximately 0.05 mm is observed.
Sclerites are abundant in polyps and surface layers of stalk and branches but absent interior. Under light microscopy, two forms of sclerites are observed – simple platelets (Fig. 4A) and spindles (Fig. 4B). Platelets are brown-red and spindles transparent (Fig. 4) under transmitted illumination. Platelets look pale blue and spindles appear transparent under epi-illumination (Fig. 5).
Polyp sclerites.
Two forms of sclerites, simple platelets and spindles, are seen in polyps (Figs 6A, B, 7A, B). Simple platelets are 0.016–0.021 mm long and 0.009–0.011 mm wide. Spindles, 0.035–0.049 mm long and 0.004–0.006 mm wide, display unique ends with pointed spear tips. Sclerite composition in tentacles (n = 124) is 7.3% simple platelets and 92.7% spindles. In the polyp body (n = 83), these proportions are 4.8% and 95.2%, respectively. Thus, the vast majority of sclerites are spindles. Some spindles have thorns on their surface.
Stalk and branch sclerites.
Two forms of sclerites, simple platelets and spindles, are also found in stalk and branches (Figs 6C, D, 7C, D). Simple platelets, several with an indistinct median waist, are 0.017–0.021 mm long and 0.009–0.011 mm wide. Spindles are 0.038–0.049 mm long and 0.004–0.006 mm wide. All spindles are more or less bent. Sclerite composition in stalk (n = 104) is 7.7% simple platelets and 92.3% spindles. Thus, the vast majority of sclerites are spindles.
Microstructure of sclerites.
The platelets are composed of branched sinuous dendritic rods within the sclerite interior. SEM at 30,000–50,000× magnification shows distal parts of rods that line up almost vertically and parallel to the surface (Fig. 8A, B). The spindles are composed of fused grains with a granular appearance (Fig. 8C, D). Fused grains also exist inside, which can be observed in cross-sections of broken spindles (Fig. 8E, F). Both ends of the spindles are relatively smooth (Fig. 8G). Thorns may form on the surface of spindles (Fig. 7, red arrows indicate the thorn, Fig. 8D shows the thorn expansion).
Variation.
Two preserved paratypes (KBF-OA-00093, KBF-OA-00094) differ in size (Fig. 2B, C). Both paratypes are smaller than the holotype (30 mm high, 15 mm wide of KBF-OA-00093, and 9–16 mm high, 6–9 mm wide of KBF-OA-00094). One paratype (KBF-OA-00094) does not branch but has two stalks connected at the bottom, although this specimen, accidentally, is broken into two pieces (Fig. 2C, D). Tentacle size is 4.0 mm long and 0.5 mm wide for KBF-OA-00093 and 3.0 mm long and 0.5 mm wide for KBF-OA-00094 (Fig. 3C, D). Paratypes display three rows of pinnules along each side of tentacles, consistent with the holotype. Pinnule numbers in the outermost row are 13–16 for KBF-OA-00093, and 12–14 for KBF-OA-00094, compared to 12–16 for the holotype. All paratypes have the two forms of sclerites as well as holotype (Fig. 9, 10), and are similar in the composition. In all parts of all specimens, the vast majority of sclerites are spindles, with the percentages being approximately 83–94% (Table 2).
Table 2.
Sclerite composition of Xeniakonohana sp. nov.
| Tentacles | Polyp body | Stalk | |||||
|---|---|---|---|---|---|---|---|
| platelets | spindles | platelets | spindles | platelets | spindles | ||
| KBF-OA-00092 (holotype) | Fig. 2A | n = 124 | n = 83 | n = 104 | |||
| 7.3% | 92.7% | 4.8% | 95.2% | 7.7% | 92.3% | ||
| KBF-OA-00093 (paratype) | Fig. 2B | n = 123 | n = 132 | n = 85 | |||
| 5.7% | 94.3% | 10.6% | 89.4% | 7.1% | 92.9% | ||
| KBF-OA-00094 (paratype) | Fig. 2C | n = 138 | n = 103 | n = 91 | |||
| 10.1% | 89.9% | 5.8% | 94.2% | 6.6% | 93.4% | ||
| Fig. 2D | n = 92 | n = 152 | n = 96 | ||||
| 12.0% | 88.0% | 17.1% | 82.9% | 7.3% | 92.7% | ||
Locality.
The species is common in waters around Oshima Island, Miyazaki, Japan, at depths from 5 to 10 m. Specimens exist attached to the surface of rocks or rock debris.
Etymology.
Konohana is named after a goddess in Japanese mythology, “Konohanasakuya-hime” (“hime” is “princess” in English). Her shrine is in Miyazaki Prefecture. The present study also proposes a standard Japanese name “konohana-umiazami” for X.konohana sp. nov. The specimen KBF-OA-00092 is designated as the standard specimen for this new Japanese name.
Remarks.
Most Xenia species have only ellipsoid platelets or spheroid sclerites (Halász et al. 2019). Although only two species, X.membranacea Schenk, 1896 and X.depressa Kükenthal, 1909 have been reported to display rod-shaped sclerites in their original descriptions, this type of sclerite has not been found in the syntype of X.membranacea (Halász et al. 2019), and X.depressa has never been re-described and the existence of the type materials are unknown. Therefore, we treated the existence of rod-shaped sclerites as either incorrect for X.membranacea or unverified for X.depressa in this study. On the other hand, X.konohana sp. nov. (= Xenia sp. 1 by Koido et al. 2019) has unique spindle sclerites in addition to ellipsoid platelets (Figs 4–10). This combination does not occur in other species in the genus. Moreover, it is clear that spindles are the majority sclerites in tentacles, polyp body and stalks for all three specimens (KBF-OA-00092 to KBF-OA-00094).
All three specimens (KBF-OA-00092 to KBF-OA-00094) were nearly identical in sclerite shape, size and composition of two types of sclerite forms (xeniid platelets and unique spindles), number of pinnules, and molecular phylogenetic position. Eight species of Xenia (X.blumi Schenk, 1896, X.crassa Schenk, 1896, X.cylindricaRoxas 1933, X.fisheri Roxas, 1933, X.garciae Bourne, 1895, X.hicksoni Ashworth, 1899, X.ternatana Schenk, 1896, and X.viridis Schenk, 1896), which partly overlap with X.konohana sp. nov. in exhibiting platelet sclerites, 3–4 rows of pinnules and 12–23 outermost row of pinnules, are distinguishable by the absence of the specific sclerite form, “unique spindle” (Table 3). A variation of pinnules has been reported in many species in xeniid genera, and the number of pinnules is likely to be unreliable as a character to determine the species boundaries (Halász et al. 2019; McFadden et al. 2017). Therefore, the information on sclerites is more important than ever as a character for identifying species boundaries.
Table 3.
Morphological comparison with congeneric species. *including oval, round, circles, discs, and biscuit-like shapes. Dashes means absent. Question marks mean unverified. NR means not reported. Note that morphological data were referred from the re-description paper by Halász et al. (2019) rather than the original descriptions for some species.
| Species | Rows of pinnules | Pinnules in the outermost row | Sclerites | Crest on the sclerites | Main branch | Secondary branches | References | ||
|---|---|---|---|---|---|---|---|---|---|
| platelets* | rods | Spindles | |||||||
| X.bauiana | 4 | 26–30 | present | – | – | – | NR | NR | Halász et al. 2019 |
| X.blumi | 3 | 18–20 | present | – | – | – | NR | NR | Halász et al. 2019 |
| X.crassa | 3–4 | 13–18 | present | – | – | present | NR | NR | Halász et al. 2019 |
| X.cylindricacy | 3 | 18–20 | present | – | – | NR | 2 | – | Roxas 1933 |
| X.depressa | 2 | 18–26 | present | ? | – | NR | NR | NR | Kükenthal 1909 |
| X.delicata | 3–4 | 18–23 | – | – | – | – | 0–5 | 0–3 | Halász et al. 2019 |
| X.elongata | 3–4 | 20–24 | present | – | – | NR | 2–3 | – | Dana 1846, Imahara 1992 |
| X.fimbriata | 3 | 8–15 | – | – | – | NR | 2–3 | present | Utinomi 1955 |
| X.fisheri | 3 | 18–22 | present | – | – | NR | – | – | Roxas 1933 |
| X.flexibilis | 4 | 14–32 | present | – | – | – | NR | NR | Halász et al. 2019 |
| X.fusca | 4(3–5) | 14–22 | present | – | – | – | NR | NR | Halász et al. 2019 |
| X.garciae | 3 | 16–22 | present | – | – | present | – | – | Halász et al. 2019 |
| X.grasshoffi | 4 | 15–24 | present | – | – | present | NR | NR | Halász et al. 2019 |
| X.hicksoni | 3 | 12–20 | present | – | – | NR | usually branched | 2 | Ashworth 1899, |
| Utinomi 1950 | |||||||||
| X.kuekenthali | 1 | 8–10 | – | – | – | – | 5 | 0–2 | Halász et al. 2019 |
| X.kusimotoensis | 2 | 10–12 | present | – | – | NR | 2 | – | Utinomi 1955 |
| X.lepida | 3 | 28–34 | – | – | – | – | present | 3rd branches | Halász et al. 2019 |
| X.mayi | 5 | 24–32 | present | – | – | NR | single or divided | – | Roxas 1933 |
| X.membranacea | 4 | 20–25 | present | – | – | present | 8 | NR | Halász et al. 2019 |
| X.multipinnata | 3–4 | 40–50 | – | – | – | NR | present | – | Tixier-Durivault 1966 |
| X.multispiculata | 2–3 | 26–30 | present | – | – | NR | present | – | Kükenthal 1909 |
| X.mucosa | 4 | 30–42 | – | – | – | – | 2 | 0–2 | Halász et al. 2019 |
| X.novaebritanniae | 2 | 9–10 | present | – | – | – | NR | NR | Halász et al. 2019 |
| X.rubens | 4(3–5) | 12–19 | present | – | – | – | 2 | – | Halász et al. 2019 |
| X.sansibariana | 4 | 26–33 | – | – | – | – | NR | NR | Halász et al. 2019 |
| X.stellifera | 4–9 | <9 | present | – | – | NR | present | present | Verseveldt 1977 |
| X.ternatana | 3 | 15–23 | present | – | – | present | NR | NR | Halász et al. 2020 |
| X.tripartita | 3 | 5–6 | present | – | – | NR | – | – | Roxas 1933 |
| X.tumbatuana | 3 | NR | – | – | – | NR | present | – | May 1898 |
| X.umbellata | 3 | 19–22 | present | – | – | – | – | – | Halász et al. 2019 |
| X.viridis | 3 | 15–22 | present | – | – | present | NR | NR | Halász et al. 2019 |
| X.konohana sp. nov. | 3 | 12–18 | present | – | Present | – | 2–3 | – | This study |
Molecular phylogenetic results
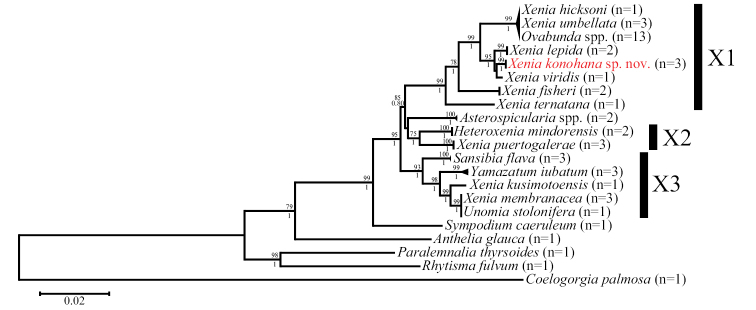
Molecular phylogenetic trees using the ML and Bayes methods showed very similar topologies. Therefore, in this study, only ML trees are shown (Figs 11, 12). As pointed out in previous studies (Halász et al. 2019; Benayahu et al. 2021), the genus Xenia is paraphyletic and polyphyletic with some other taxa, and separated into three clades (clades X1–X3) in the mtMutS+COI+28S tree (Fig. 11). All three clades were supported by high bootstrap values (75 to 99%) and posterior probabilities (1). Asides from Xenia, clade X1 included Ovabunda, clade X2 included Heteroxenia, and clade X3 included Sansibia, Yamazatum and Unomia. All three specimens of X.konohana sp. nov., which had the same DNA sequences for all four markers, belonged to clade X1 forming a sister clade with Ovabunda spp., X.umbellata and X.hicksoni, and united with X.lepida Verseveldt, 1971 and X.viridis within a strongly supported subclade (bootstrap values: 95%, posterior probability: 1).
Figure 11.
Phylogenetic relationships of species in the Xeniidae based on the concatenated mtMutS, COI and 28S sequences. Numbers above main branches show percentages of bootstrap values (> 50%) in maximum likelihood analysis; numbers below main branches show Bayesian posterior probabilities. X1, X2 and X3 denote clades defined by McFadden et al. (2014b). Xeniakonohana sp. nov. is shown in red.
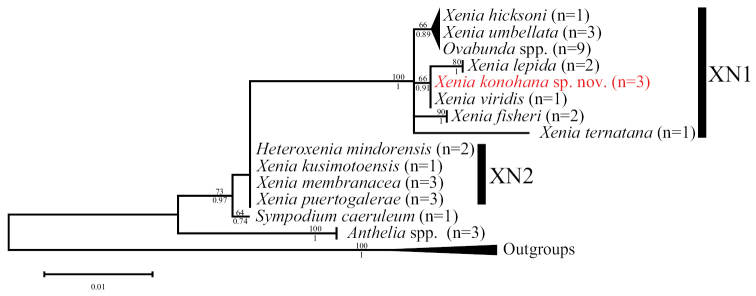
Figure 12.
Phylogenetic relationships of species in the Xeniidae based on ND2 sequences. Numbers above main branches show percentages of bootstrap values (> 50%) in maximum likelihood analysis; numbers below main branches show Bayesian posterior probabilities. Xeniakonohana sp. nov. is shown in red.
On the other hand, in the ND2 tree, Xenia was separated into only two clades (XN1 and XN2) (Fig. 12). Clade XN1 was strongly supported by high bootstrap value (100%) and posterior probability (1), and included the same members with all three specimens of X.konohana sp. nov. in clade X1 in the mtMutS+COI+28S tree. For clade XN2, this clade was not supported by bootstrap values and posterior probabilities, but three Xenia species and Heteroxeniamindorensis in this clade were genetically identical. Clade XN2 included members belonging to both clades X2 and X3 in the mtMutS+COI+28S tree.
Although X.viridis was not genetically separated from X.konohana sp. nov. in the ND2 tree (Fig. 12), they were clearly separated from each other in the mtMutS+COI+28S tree (Fig. 11). Thus, the molecular phylogenetic tree based on the concatenated DNA sequences of mtMutS, COI, and 28S, and the tree based on ND2 support the phylogenetic position of X.konohana sp. nov. in the genus Xenia (Figs 11, 12).
Discussion
The genus Xenia is polyphyletic and paraphyletic with other xeniid genera such as Ovabunda, Heteroxenia, Sansibia, Asterospicularia, Unomia, and Yamazatum based on molecular studies (Janes et al. 2014; McFadden et al. 2014b; Benayahu et al. 2018a; Halász et al. 2019; Benayahu et al. 2021). In the present study, Xenia was also polyphyletic as well as paraphyletic with some other genera (Figs 11, 12), but X.konohana sp. nov. formed a clade with two congeneric species, X.lepida and X.viridis, and was closely related to a sister clade with Ovabunda spp., X.hicksoni, and X.umbellata. These four Xenia species are similar to X.konohana sp. nov. in the number of rows and the outermost row of pinnules, but they do not exhibit spindle sclerites. Ovabunda exhibits only simple platelets like Xenia, but it also displays a corpuscular surface microstructure on platelet surfaces. Xenia, including X.konohana sp. nov., exhibits a dendritic microstructure on these surfaces of simple platelets. Further taxonomic revision of Xenia and related genera such as Ovabunda, Heteroxenia, Sansibia, Asterospicularia, Unomia, and Yamazatum may be necessary due to these phylogenetic relationships. Still, we conclude that Xeniakonohana sp. nov. is a new member of Xenia based on molecular phylogenetic relationships and the presence of unique spindles along with Xenia-specific ellipsoid platelets with dendritic surface microstructure.
Supplementary Material
Acknowledgements
We thank T. Mezaki (Kuroshio Biological Research Foundation) and S. Nakachi (Natural History Lab.) for their assistance with microstructure analyses and sampling. We also thank Y. Oku (Okinawa Churaumi Aquarium) and Coral Lab. of University of Miyazaki for their assistance with sampling; and Y. Goto (Bio-Imaging Lab, University of Miyazaki), K. Arai, T. Matsuzaki and T. Okumura (Center for Advanced Marine Core Research, Kochi University) for assistance with SEM operations. This study was funded by a grant from the Kuroshio Biological Research Foundation to T. Koido, and by JSPS KAKENHI (No.18K06423) to H. Fukami.
Citation
Koido T, Imahara Y, Fukami H (2022) Xenia konohana sp. nov. (Cnidaria, Octocorallia, Alcyonacea), a new soft coral species in the family Xeniidae from Miyazaki, Japan. ZooKeys 1085: 29–49. https://doi.org/10.3897/zookeys.1085.77924
References
- Alderslade P. (2001) Six new genera and six new species of soft corals, and some proposed familial and subfamilial changes within the Alcyonacea (Coelenterata: Octocorallia). Bulletin of the Biological Society of Washington 10: 15–65. [Google Scholar]
- Ashworth JH. (1899) The structure of Xeniahicksoni, nov. sp., with some observations on Heteroxeniaelizabethae, Kölliker. Quarterly Journal of Microscopical Science 42(3): 245–304. [pls 23–27] 10.1242/jcs.s2-42.167.245 [DOI] [Google Scholar]
- Benayahu Y, Loya Y. (1987) Long-term recruitment of soft-corals (Octocorallia: Alcyonacea) on artificial substrata at Eilat (Red Sea). Marine Ecology Progress Series 38: 161–167. 10.3354/meps038161 [DOI] [Google Scholar]
- Benayahu Y. (2010) A new genus of a soft coral of the family Xeniidae (Cnidaria: Octocorallia) from Japan. Galaxea 12: 53–64. 10.3755/galaxea.12.53 [DOI] [Google Scholar]
- Benayahu Y, van Ofwegen LP, McFadden CS. (2018a) Evaluating the genus Cespitularia Milne Edwards & Haime, 1850, with descriptions of new genera of the family Xeniidae (Octocorallia, Alcyonacea). ZooKeys 754: 63–101. 10.3897/zookeys.754.23368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayahu Y, van Ofwegen LP, Dai CF, Jeng MS, Soong K, Shlagman A, Du SW, Hong P, Imam NH, Chung A, Wu T, McFadden CS. (2018b) The octocorals of Dongsha Atoll (South China Sea): an iterative approach to species identification using classical taxonomy and molecular barcodes. Zoological Studies 57: e50. [DOI] [PMC free article] [PubMed]
- Benayahu Y, Ekins M, McFadden CS. (2021) Overview of the genus Sympodium Ehrenberg, 1834 (Octocorallia, Alcyonacea, Xeniidae), with the description of new species, revealing regional endemism. Zootaxa 5072(4): 324–350. 10.11646/zootaxa.5072.4.2 [DOI] [PubMed] [Google Scholar]
- Brockman SA, McFadden CS. (2012) The mitochondrial genome of Paraminabeaaldersladei (Cnidaria: Anthozoa: Octocorallia) supports intramolecular recombination as the primary mechanism of gene rearrangement in octocoral mitochondrial genomes. Genome Biology and Evolution 4: 994–1006. 10.1093/gbe/evs074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro R, McFadden C, van Ofwegen L, Williams G. (2021) World List of Octocorallia. Xeniidae Ehrenberg, 1828. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=125271 [Accessed on 2021–10–20]
- Dana JD. (1846) Zoophytes. United States Exploring Expedition during the years 1838–1842. Lea and Blanchard, Philadelphia 7: 1–740. [61 pls] [Google Scholar]
- Fabricius K, Alderslade P. (2001) Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science, Townsville, 264 pp. [Google Scholar]
- Halász A, McFadden CS, Toonen R, Benayahu Y. (2019) Re-description of type material of Xenia Lamarck, 1816 (Octocorallia: Xeniidae). Zootaxa 4652: 201–239. 10.11646/zootaxa.4652.2.1 [DOI] [PubMed] [Google Scholar]
- Imahara Y. (1992) Octocorallia (Stolonifera, Telestacea and Alcyonacea). In: Nishimura S. (Ed.) , Guide to seashore animals of Japan with color pictures and key. Hoikusha Publishing Co. Ltd., Osaka 1: 69–91. [pls 13–19]
- Janes MP, McFadden CS, Chanmethakul T. (2014) A new species of Ovabunda (Octocorallia, Xeniidae) from the Andaman Sea, Thailand with notes on the biogeography of this genus. ZooKeys 431: 1–17. 10.3897/zookeys.431.7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koido T, Imahara Y, Fukami H. (2019) High species diversity of the soft coral family Xeniidae (Octocorallia, Alcyonacea) in the temperate region of Japan revealed by morphological and molecular analyses. ZooKeys 862: 1–22. 10.3897/zookeys.862.31979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kükenthal W. (1909) Diagnosen neuer Alcyonarien. (7. Mitteilung). Zoologischer Anzeiger 35(1/2): 46–53.
- May W. (1898) Die von Dr. Stuhlmann im Jahre 1889 gesammelten ostafrikanischen Alcyonaceen des Hamburgers Museums. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten 15(2): 1–38. 10.5962/bhl.title.23020 [DOI] [Google Scholar]
- McFadden CS, France SC, Sanchez JA, Alderslade P. (2006) A molecular phylogenetic analysis of the Octocorallia (Cnidaria: Anthozoa) based on mitochondrial protein-coding sequences. Molecular Phylogenetics and Evolution 41: 513–527. 10.1016/j.ympev.2006.06.010 [DOI] [PubMed] [Google Scholar]
- McFadden CS, Benayahu Y, Pante E, Thoma JN, Nevarez PA, France SC. (2011) Limitations of mitochondrial gene barcoding in Octocorallia. Molecular Ecology Resources 11(1): 19–31. 10.1111/j.1755-0998.2010.02875.x [DOI] [PubMed] [Google Scholar]
- McFadden CS, van Ofwegen LP. (2012) Stoloniferous octocorals (Anthozoa, Octocorallia) from South Africa, with descriptions of a new family of Alcyonacea, a new genus of Clavulariidae, and a new species of Cornularia (Cornulariidae). Invertebrate Systematics 26: 331–356. 10.1071/IS12035 [DOI] [Google Scholar]
- McFadden CS, Brown AS, Brayton C, Hunt CB, van Ofwegen LP. (2014a) Application of DNA barcoding in biodiversity studies of shallow-water octocorals: Molecular proxies agree with morphological estimates of species richness in Palau. Coral Reefs 33: 275–286. 10.1007/s00338-013-1123-0 [DOI] [Google Scholar]
- McFadden CS, Reynolds AM, Janes MP. (2014b) DNA barcoding of xeniid soft corals (Octocorallia: Alcyonacea: Xeniidae) from Indonesia: species richness and phylogenetic relationships. Systematics & Biodiversity 12: 247–257. 10.1080/14772000.2014.902866 [DOI] [Google Scholar]
- McFadden CS, Haverkort-Yeh R, Reynolds AM, Halász A, Quattrini AM, Forsman ZH, Benayahu Y, Toonen RJ. (2017) Species boundaries in the absence of morphological, ecological or geographical differentiation in the Red Sea Octocoral genus Ovabunda (Alcyonacea: Xeniidae). Molecular Phylogenetics and Evolution 112: 174–184. 10.1016/j.ympev.2017.04.025 [DOI] [PubMed] [Google Scholar]
- McFadden CS, Gonzalez A, Imada R, Shi SS, Hong P, Ekins M, Benayahu Y. (2019) Molecular operational taxonomic units reveal restricted geographic ranges and regional endemism in the Indo-Pacific octocoral family Xeniidae. Journal of Biogeography 46: 992–1006. 10.1111/jbi.13543 [DOI] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxas HA. (1933) Philippine Alcyonaria. The families Cornulariidae and Xeniidae. The Philippine Journal of Science 50: 49–110. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tixier-Durivault A. (1966) Quelques Octocoralliaires Australiens. Bulletin de la Museum Nationale d’Histoire naturelle Paris, Ser. 2, 37(4): 705–716.
- Utinomi H. (1950) Some xeniid alcyonarians from Japan and adjacent localities. Publications of the Seto Marine Biological Laboratory 1(3): 81–91. 10.5134/174440 [DOI] [Google Scholar]
- Utinomi H. (1955) Two new species of Xenia from Kusimoto (Coelenterata, Alcyonaria). Publications of the Seto Marine Biological Laboratory 4: 105–109. [Google Scholar]
- Verseveldt J. (1977) Australian Octocorallia (Coelenterata). Australian Journal of Marine and Freshwater Research 28: 171–240. 10.1071/MF9770171 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.