Abstract
Background
Interfascial plane block can be used to treat postoperative pain after laparoscopic surgery. This study aimed to investigate the effect of ultrasound-guided unilateral rhomboid intercostal and subserratus plane (RISS) block after laparoscopic cholecystectomy on the amount of analgesic consumption.
Methods
Fifty patients who underwent laparoscopic cholecystectomy were included in this quasi-experimental study. Patients fulfilling the inclusion criteria were analyzed in two groups: RISS group (RISS block with 20 ml of 0.25% bupivacaine + intravenous patient-controlled analgesia [IV-PCA] tramadol [n = 25]); and Control group (IV-PCA tramadol [n = 25]). The primary outcome was the total amount of tramadol used over 24 h. Secondary outcomes included side effects, additional analgesic use, and postoperative pain (at rest and during activity) at 2, 6, 12, and 24 h according to numerical rating scale (NRS) scores.
Results
Postoperative tramadol consumption at 24 h was significantly lower in the RISS group than in the Control group (P < 0.001). Resting NRS scores at 2 h and 6 h were significantly lower in the RISS group. NRS scores during movement in the RISS group were significantly lower at 2, 6, and 12 h postoperatively. There was no statistically significant difference in the rate of side effects and additional analgesic use between the groups (P > 0.05).
Conclusions
Unilateral RISS block was an effective method for pain management after laparoscopic cholecystectomy and can be used as a part of multimodal analgesia.
Keywords: Analgesia, Bupivacaine, Laparoscopic cholecystectomy, Nerve block, Pain, Pain management, Postoperative pain, Ultrasonography
Introduction
Laparoscopic gallbladder surgery is the preferred option to open surgical procedures owing to various advantages, including reduced bleeding, lower surgical site infection rates, decreased costs, shorter hospital stay, earlier return to the activities of daily living, and enhanced recovery [1,2]. Despite all the advantages of laparoscopic surgery, however, early postoperative pain can be uncomfortable and even lead to prolonged hospital stay [3]. Pain occurring after abdominal surgery is transmitted by the cutaneous branches of the thoracolumbar (T6–L1) nerves in the anterolateral region [4,5]. Multimodal regimens are used for postoperative analgesia after abdominal surgery, including laparoscopic cholecystectomy. For this purpose, short-acting opioids, non-steroidal anti-inflammatory drugs, and regional anesthesia techniques are used alone and in combination.
The main causes of early pain after laparoscopic cholecystectomy are peritoneum and abdominal wall distension due to pneumoperitoneum and somatic pain at the trocar insertion site(s) [4,5]. Effective use of truncal blocks, such as the erector spinae plane or quadratus lumborum block, has been demonstrated in postoperative pain management in laparoscopic cholecystectomy [6-8]. The rhomboid intercostal and subserratus plane (RISS) block provides analgesia from the third to the 12th thoracic dermatomes and has been used in postoperative pain management for thoracic surgeries [7,8]. Although it is used for postoperative analgesia after upper abdominal surgery, there have been no adequate studies investigating the use of RISS blocks in laparoscopic cholecystectomy [7,8].
Theoretical target dermatomes (T3-T12) for the RISS block may include areas that cause pain in laparoscopic cholecystectomy operations, including trocar insertion sites. Several studies have suggested that incisional pain is more predominant than visceral pain during the first 48 h postoperatively [1,9]. Therefore, the present study aimed to evaluate the effect of unilateral RISS block on postoperative analgesic consumption in patients undergoing laparoscopic cholecystectomy.
Materials and Methods
Patient selection
A quasi-experimental study, involving 120 patients who underwent laparoscopic cholecystectomy between January 2018 December 2020, was conducted with approval of the institutional ethics committee of Bursa Yuksek Ihtisas Training and Research Hospital (IEC # 2020-01-19). Patients 20 to 65 years of age, with American Society of Anesthesiologists (ASA) physical status I and II, were included in the study. Individuals with bleeding disorders, mental incapacity, known allergy to local anesthetics, and body mass index (BMI) of ≥ 35 kg/m2 were excluded. Informed consent was obtained from all participants included in the study. All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee, and with the 2013 Helsinki Declaration and its later amendments or comparable ethical standards.
Fifty patients fulfilling the study criteria were included in the experimental group (RISS block and intravenous patient-controlled analgesia [IV-PCA]), tramadol administered [n = 25]) and the Control group (IV-PCA only; tramadol [n = 25]) using a non-probabilistic sampling method (Fig. 1).
Fig. 1.
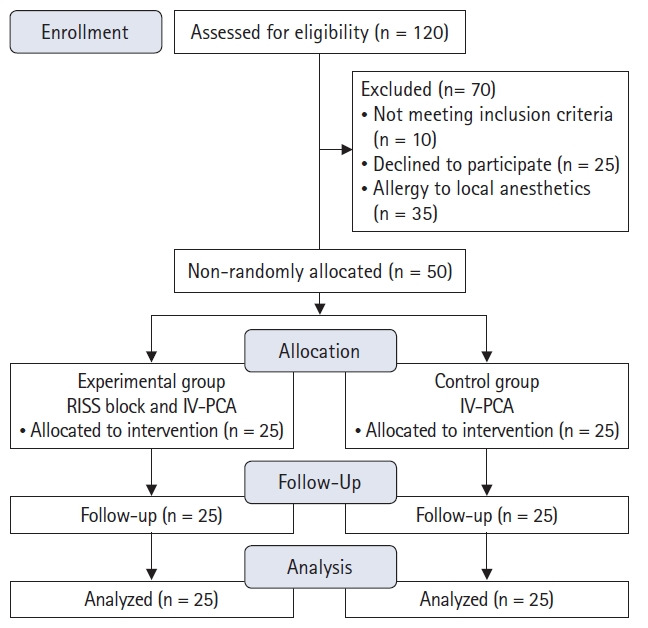
CONSORT flow diagram. RISS: rhomboid intercostal and subserratus plane, IV-PCA: intravenous patient-controlled analgesia.
Surgical protocol
Anesthesia
After routine monitoring in the operating room, general anesthesia was induced using fentanyl (1–2 µg/kg), propofol (1–2 mg/kg), and rocuronium (0.8–1 mg/kg). Anesthesia was maintained using inhaled sevoflurane (3–5%) and an air and oxygen mixture administered at a rate of 2.5–3 L/min. Fentanyl (1-2 µg/kg) was administered during the surgery if needed.
Surgery
The surgical procedure was performed by the same surgical team using the four-port technique. The four ports were placed through the umbilicus, epigastric place (under the xiphoidal process), right lateral subcostal position (the intersection point of the costal arch and anterior axillary line), and right subcostal-midclavicular line. The intra-abdominal pressure never exceeded 14 mmHg.
Pain management
Patients in the preoperative RISS block group underwent procedures with a linear probe (10–18 MHz, MyLab30; Esaote, Italy) in the lateral decubitus position under standard monitoring ASA in the block room, as previously described.
Rhomboid intercostal block
A high-frequency linear probe was placed in the sagittal plane to the medial border of the scapula and then rotated counter-clockwise to acquire a paramedian sagittal oblique image 1–2 cm medial to the scapular edge. A 22-gauge, 100 mm block needle was inserted craniocaudally using an in-plane technique. After confirming the correct placement of the needle tip by hydrodissection, 10 ml of 0.25% bupivacaine was injected into the plane between the rhomboid major and intercostal muscles [7,8] (Fig. 2A).
Fig. 2.
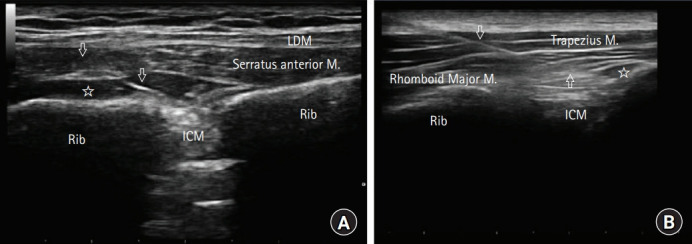
Ultrasound image depicting unilateral rhomboid intercostal and subserratus plane block. (A) rhomboid intercostal block, (B) subserratus plane block. LDM: latissimus dorsi muscle, ICM: intercostal muscle, arrows: ultrasound visible block needle, star: local anesthetic spread under the interfascial plane.
Subserratus block
The ultrasound probe was slid down inferolateral to identify the serratus anterior muscle at the level of T6–T9. After confirming the needle position, 10 ml of 0.25% bupivacaine was injected between the serratus anterior and intercostal muscles [7,8] (Fig. 2B). All patients received tenoxicam 20 mg intravenously 30 min before the end of the surgery. Postoperative pain management in the surgical ward was maintained using an IV-PCA device with the same setting for all patients. The PCA device delivered a 25 mg bolus dose of tramadol on demand (maximum dose, 400 mg/day), with a lock time of 30 min and no basal infusion. Paracetamol (1 g) was administered as rescue analgesia. Pain was assessed using a 10-point numerical rating scale (NRS), ranging from 0 (no pain) to 10 (worst pain imaginable).
Outcome measure
The primary outcome measure of the study was total tramadol consumption at 24 h postoperatively. Secondary outcome measures included assessment of the total amount of opioids administered intraoperatively, postoperative NRS scores at rest and during movement (2, 6, 12, and 24 h), sensorial dermatomal block level (30 min after block administration and at 2 h postoperatively), rescue analgesic consumption, and postoperative nausea and vomiting. During movement, the postoperative NRS scores were evaluated while coughing or performing in-bed movements at 2 h and after taking five steps forward at 6 h. Patients were asked if they experienced pain at the port sites 2 h postoperatively.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corp., USA) for Windows 2013 (Microsoft Corp., USA). Data normality was evaluated using the Kolmogorov-Smirnov test. The Chi-squared test was used for inter-group comparisons of categorical data and the Mann-Whitney U test was used for continuous variables. Differences with P value of < 0.05 were considered to be statistically significant.
Power analysis
In previous studies, tramadol consumption at 6 h postoperatively was found to be 59.7 ± 13.7 mg in patients who used IV-PCA (tramadol) [10]. In this study, a 20% decrease in tramadol consumption at 6 h postoperatively was expected in the RISS block group. To obtain a study power of 85% (α = 0.05), 25 patients per group was required; as such, a total of 50 patients was calculated.
Results
Demographic variables are summarized in Table 1. There were no statistically significant differences between the groups in terms of age, BMI, and surgery duration. Postoperative tramadol consumption at 24 h was significantly lower in the RISS group than in the Control group (89 mg [range, 50, 175 mg] vs. 142 mg [range, 5, 275 mg]; respectively, P < 0.001) (Fig. 3). Resting NRS scores at 2 h and 6 h postoperatively were significantly lower in the RISS group. There were no significant differences between the other measurements (Table 2). NRS scores during movement in the RISS group were significantly lower at 2, 6, and 12 h postoperatively (Table 3). There were no differences of intraoperative opioid requirements, postoperative nausea and vomiting, and additional analgesic consumption (paracetamol) (Table 4).
Table 1.
Comparison of Demographic Characteristics of the Patients
| Characteristic | RISS group (n = 25) | Control group (n = 25) | P value |
|---|---|---|---|
| Age (yr) | 51.6 (36, 62) | 51 (35, 65) | 0.784 |
| BMI (kg/m2) | 24.1 (21.6, 28.0) | 23.4 (21.3, 27.3) | 0.234 |
| Sex M/F n(%) | 17 (68)/8 (32) | 15 (60)/10 (40) | 0.769 |
Values are presented as median (Q1, Q3) or number (%). RISS: rhomboid intercostal and subserratus plane, BMI: body mass index.
Fig. 3.
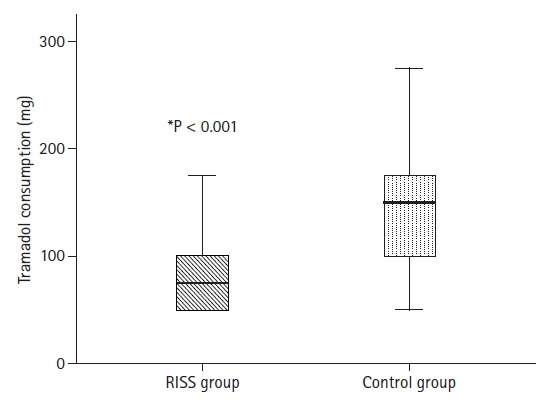
Total tramadol consumption at 24 h postoperatively. RISS: rhomboid intercostal and subserratus plane.
Table 2.
Comparison of NRS Scores at Rest between the Groups
| NRS (at rest) | RISS group (n = 25) | Control group (n = 25) | P value* |
|---|---|---|---|
| 2 h | 1.7 (0, 6) | 2.7 (1, 6) | 0.004† |
| 6 h | 1.6 (0, 5) | 2.5 (1, 6) | 0.022† |
| 12 h | 1.96 (0, 5) | 2 (1, 6) | 0.635 |
| 24 h | 0.96 (0, 6) | 1 (1, 6) | 0.621 |
Values are presented as median (Q1, Q3).
Mann-Whitney U test for the inter-group comparisons. Values marked with †indicate statistically significant differences (i.e., P < 0.05).
RISS: rhomboid intercostal subserratus plane, NRS: numerical rating scale.
Table 3.
Comparison of NRS Scores on Movement between Groups
| NRS (on movement) | RISS group (n = 25) | Control group (n = 25) | P value* |
|---|---|---|---|
| 2 h | 2.7 (0, 6) | 5.1 (3, 7) | < 0.001† |
| 6 h | 2 (0, 5) | 4 (0, 7) | < 0.001† |
| 12 h | 1.8 (0, 3) | 3.2 (0, 6) | 0.014† |
| 24 h | 1.9 (0, 4) | 1.9 (0, 5) | 0.861 |
Values are presented as median (Q1, Q3).
Mann-Whitney U test for the inter-group comparisons. Values marked with †indicate statistically significant differences (i.e., P < 0.05).
RISS: rhomboid intercostal and subserratus plane, NRS: numerical rating scale.
Table 4.
Side Effects, Additional Analgesic Requirement(s), Duration of Surgery
| Characteristic | RISS group (n = 25) | Control group (n = 25) | P value |
|---|---|---|---|
| Postoperative nausea and vomiting | – | – | NS |
| Additional analgesic requirement(s) (n) | 1 | 3 | 0.186 |
| Duration of surgery (min) | 51 (40, 67) | 49 (40, 60) | 0.497 |
| Opioid administered during surgery (µg) | 76 (60, 110) | 81 (60, 150) | 0.726 |
Values are presented as median (Q1, Q3), or numbers. RISS: rhomboid intercostal and subserratus plane.
Evaluation of RISS block levels
The preoperative sensory block level was tested according to the loss of sensation to cold 30 min after the RISS block using alcohol-soaked cotton swabs. Loss of sensation was achieved at the T4-T12 dermatomes in 5 patients, T5-T10 in 13, T6-T9 in 5, and T7-T10 in 2. Five patients underwent sensory blocks that reached the anterior midline (Fig. 4). Postoperative evaluation revealed that 10 patients experienced discomfort at the umbilical port insertion site, and 13 experienced pain at the epigastric port insertion site (Fig. 4).
Fig. 4.
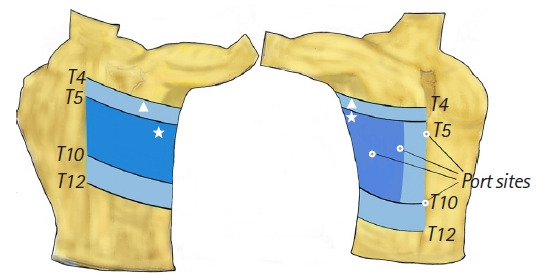
Dermatomal dispersion of sensorial block to the cold stimulus. White triangle (light blue area): the widest area of the sensory extent of block T4-T12 in 5 patients and anterior midline in 5 patients, white star (dark blue area): the most detected sensory level; T5-T10 in 13 patients, white circle: port insertion sites.
Discussion
The present study investigated the analgesic effect of unilateral RISS block after laparoscopic cholecystectomy and revealed less tramadol consumption in the RISS block group during the 24 h postoperative follow-up. The RISS block group demonstrated lower NRS scores at rest up to 6 h and lower NRS scores with movement at the 12 h follow-up.
Pain after laparoscopic cholecystectomy necessitates the use of multimodal analgesia methods due to its somatic and visceral components [1,9]. Visceral pain due to laparoscopic cholecystectomy emerges due to surgical manipulation of the pneumoperitoneum and gallbladder bed. Thus, visceral pain can be reduced with shorter surgery times and creating a pneumoperitoneum with lower pressure [11]. However, somatic pain due to trocar entry incisions has been suggested to be the main cause of early pain after laparoscopic gallbladder surgery [9].
The rhomboid intercostal block has previously been used for pain management in thoracic wall surgery. However, it was later modified by Elsharkawy et al. [7,8,12] and used together with the subserratus plane block in the treatment of post-abdominal surgery pain, and the combination of the two blocks was subsequently renamed the “RISS block”. RISS block using 30 ml of local anesthetic has been successfully used in transapical transcatheter aortic valve implantation [13]. In another case series, RISS block using 20 ml of local anesthetic was used for pain management for multiple rib fractures, and the authors indicated that local anesthetic could spread to the ventral and dorsal radices of the intercostal nerves [14]. Elsharkawy et al. [7] described RISS blocks performed on 6 fresh non-embalmed cadavers and on 15 live patients with different indications, including upper abdominal surgeries. They demonstrated that the lateral branches of the intercostal nerves were dyed from T4 to T8 in all cadavers and, in the clinical part of their study, they observed that the most cephalocaudal extent of the sensory loss to cold was from T2 to T12 [7]. In a study involving 21 patients undergoing abdominal surgery, RISS blocks provided analgesia at dermatomes varying from T3 to T12 with a high patient satisfaction rate [8].
The nerves targeted by the RISS block include the lateral cutaneous branches of the ventral branches of the thoracic intercostal nerves, located between the rhomboid muscle and the intercostal muscles, and deep into the scapula serratus anterior muscle. In addition, it has been reported that 2 different mechanisms may be operative during analgesia. First, local anesthetic agents may affect the dorsal rami of the thoracic intercostal nerves at the point where the erector spinae muscle originates from the thoracic transverse processes at the level of T3–T9 through medial spread in the tissue plane. Second, the authors hypothesized that local anesthesia may also spread into the paravertebral space because of its spread under the erector spinae muscle [8].
In our study, the dermatomal coverage of RISS blocks was consistent with the literature. The most cephalad extent of the block was T4, and the most caudal extent was the T12 dermatome. Sensory loss was present in the medial and lateral areas, whereas only five patients experienced a sensory block at the midline of the abdominal wall. A limited number of studies have reported the efficacy of unilateral regional blocks after laparoscopic abdominal surgeries. In another study, subcostal transversus abdominis plane blocks were found to result in significantly lower postoperative opioid consumption than local anesthetic infiltration to port sites after laparoscopic cholecystectomy [15].
In the present study, unilateral RISS blocks were effective in reducing pain scores and opioid consumption after laparoscopic surgery. RISS blocks appear to be good choices as part of a multimodal analgesia regimen for both thoracic and upper abdominal surgeries. Compared with central blocks, RISS blocks are less invasive and associated with fewer complications such as nerve damage, hemodynamic instability, and bleeding [8].
The broadest analgesic efficacy detected for the RISS block was between the T3 and T12 dermatomes. Trocar insertion sites for laparoscopic cholecystectomy concern the T6 and T10 dermatomes. It was believed that analgesia could be provided in these dermatomes using the RISS block. In addition, we believe that the potential mechanisms of action of RISS block, such as the paravertebral spread of local anesthetics, ventral rami blockade of intercostal nerves, and neuronal structures in the anatomical structure of the fascia, which we have attempted to address in the study, may be effective for analgesia. Several reports have commented on the RISS block mechanism; however, its paravertebral extension remains controversial. A notable result of the present study was that five patients experienced sensory blocks extending to the abdominal midline. Nevertheless, except for these 5 patients in the RISS block group, 10 patients with no sensory block in the midline were not troubled by pain from the umbilical port site. We believe this may be due to the anatomical structure of the fascia, which is considered to play a role in the efficacy of interfascial blocks. It is believed that, apart from the intercostal nerve block, the sensory innervation of the fascia and the presence of sympathetic nerve endings may play a role in the efficacy of interfascial blocks [16,17]. Animal studies have immunohistochemically identified free nerve endings of the thoracolumbar fascia and the presence of Ruffini and Pacinian corpuscles [18]. Another animal study found that dorsal horn neurons became prominent after stimulation of these receptors, and a different study involving humans found that interfascial injection of saline (0.9%) created burning and throbbing-like symptoms known to be transmitted by A- and C-fiber nociceptors [19,20]. Similar results and considerations have been shared in studies using the interfascial injection technique for the treatment of myofascial pain syndrome [21-23]. Stecco et al. [24] described the proprioception and nociception properties of the fascial system through Aδ, C, and postganglionic sympathetic fibers. Despite the limited literature, the current data indicate that the anatomical structure of the fascia may play a role in the efficacy of interfascial blocks.
The present study was limited by its lack of randomization and the small cohort of subjects. Additionally, no adjuvant drugs were used. RISS block procedures are new techniques; as such, long-term follow-up data remain lacking. Although there are published studies that have used adjuvants, we did not add adjuvants to local anesthetic drugs in the present study because they were not used in our clinical practice.
In conclusion, unilateral RISS block was an effective method for pain management after laparoscopic cholecystectomy and may be used as a component of multimodal analgesia regimens.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Korgün Ökmen (Conceptualization; Data curation; Investigation; Methodology; Writing – original draft)
Hande Gürbüz (Supervision; Writing – review & editing)
Hakan Özkan (Investigation; Methodology; Visualization)
References
- 1.Siddiqui NA, Azami R, Murtaza G, Nasim S. Postoperative port-site pain after gall bladder retrieval from epigastric vs. umbilical port in laparoscopic cholecystectomy: a randomized controlled trial. Int J Surg. 2012;10:213–6. doi: 10.1016/j.ijsu.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Kim SS, Kim SH, Mun SP. Should subcostal and lateral trocars be used in laparoscopic cholecystectomy? A randomized, prospective study. J Laparoendosc Adv Surg Tech A. 2009;19:749–53. doi: 10.1089/lap.2009.0159. [DOI] [PubMed] [Google Scholar]
- 3.Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90:261–9. doi: 10.1016/S0304-3959(00)00406-1. [DOI] [PubMed] [Google Scholar]
- 4.Børglum J, Maschmann C, Belhage B, Jensen K. Ultrasound-guided bilateral dual transversus abdominis plane block: a new four-point approach. Acta Anaesthesiol Scand. 2011;55:658–63. doi: 10.1111/j.1399-6576.2011.02430.x. [DOI] [PubMed] [Google Scholar]
- 5.Ure BM, Troidl H, Spangenberger W, Dietrich A, Lefering R, Neugebauer E. Pain after laparoscopic cholecystectomy. Intensity and localization of pain and analysis of predictors in preoperative symptoms and intraoperative events. Surg Endosc. 1994;8:90–6. doi: 10.1007/BF00316616. [DOI] [PubMed] [Google Scholar]
- 6.Elsharkawy H, Pawa A, Mariano ER. Interfascial plane blocks: back to basics. Reg Anesth Pain Med. 2018;43:341–6. doi: 10.1097/AAP.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 7.Elsharkawy H, Maniker R, Bolash R, Kalasbail P, Drake RL, Elkassabany N. Rhomboid intercostal and subserratus plane block: a cadaveric and clinical evaluation. Reg Anesth Pain Med. 2018;43:745–51. doi: 10.1097/AAP.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 8.Elsharkawy H, Hamadnalla H, Altinpulluk EY, Gabriel RA. Rhomboid intercostal and subserratus plane block -a case series. Korean J Anesthesiol. 2020;73:550–6. doi: 10.4097/kja.19479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee IO, Kim SH, Kong MH, Lee MK, Kim NS, Choi YS, et al. Pain after laparoscopic cholecystectomy: the effect and timing of incisional and intraperitoneal bupivacaine. Can J Anaesth. 2001;48:545–50. doi: 10.1007/BF03016830. [DOI] [PubMed] [Google Scholar]
- 10.Ökmen K, Metin Ökmen B, Topal S. Ultrasound-guided posterior quadratus lumborum block for postoperative pain after laparoscopic cholecystectomy: a randomized controlled double blind study. J Clin Anesth. 2018;49:112–7. doi: 10.1016/j.jclinane.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Michaloliakou C, Chung F, Sharma S. Preoperative multimodal analgesia facilitates recovery after ambulatory laparoscopic cholecystectomy. Anesth Analg. 1996;82:44–51. doi: 10.1097/00000539-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Elsharkawy H, Saifullah T, Kolli S, Drake R. Rhomboid intercostal block. Anaesthesia. 2016;71:856–7. doi: 10.1111/anae.13498. [DOI] [PubMed] [Google Scholar]
- 13.Ueshima H. Rhomboid intercostal and subserratus plane block for transapical transcatheter aortic valve implantation. J Clin Anesth. 2019;54:146. doi: 10.1016/j.jclinane.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Yayik AM, Aydin ME, Tekin E, Ulas AB, Ahiskalioglu A. An alternative plane block for multiple rib fractures: rhomboid intercostal and sub-serratus block (RISS) Am J Emerg Med. 2019;37:2263. doi: 10.1016/j.ajem.2019.158429. [DOI] [PubMed] [Google Scholar]
- 15.Arık E, Akkaya T, Ozciftci S, Alptekin A, Balas Ş. Unilateral transversus abdominis plane block and port-site infiltration: comparison of postoperative analgesic efficacy in laparoscopic cholecystectomy. Anaesthesist. 2020;69:270–6. doi: 10.1007/s00101-020-00746-1. [DOI] [PubMed] [Google Scholar]
- 16.Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221:507–36. doi: 10.1111/j.1469-7580.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco R. The mechanism of the quadratus lumborum block: a peripheral sympathetic field block? Br J Anaesth. 2016;117:EL_13593. [Google Scholar]
- 18.Yahia L, Rhalmi S, Newman N, Isler M. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63:195–7. doi: 10.3109/17453679209154822. [DOI] [PubMed] [Google Scholar]
- 19.Schilder A, Hoheisel U, Magerl W, Benrath J, Klein T, Treede RD. Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain. 2014;155:222–31. doi: 10.1016/j.pain.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi T, Hoheisel U, Mense S. Dorsal horn neurons having input from low back structures in rats. Pain. 2008;138:119–29. doi: 10.1016/j.pain.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Ganjaei KG, Ray JW, Waite B, Burnham KJ. The fascial system in musculoskeletal function and myofascial pain. Curr Phys Med Rehabil Rep. 2020;8:364–72. [Google Scholar]
- 22.Domingo T, Blasi J, Casals M, Mayoral V, Ortiz-Sagristá JC, Miguel-Pérez M. Is interfascial block with ultrasound-guided puncture useful in treatment of myofascial pain of the trapezius muscle? Clin J Pain. 2011;27:297–303. doi: 10.1097/AJP.0b013e3182021612. [DOI] [PubMed] [Google Scholar]
- 23.Kongsagul S, Vitoonpong T, Kitisomprayoonkul W, Tantisiriwat N. Ultrasound-guided physiological saline injection for patients with myofascial pain. J Med Ultrasound. 2019;28:99–103. doi: 10.4103/JMU.JMU_54_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stecco C, Stern R, Porzionato A, Macchi V, Masiero S, Stecco A, et al. Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat. 2011;33:891–6. doi: 10.1007/s00276-011-0876-9. [DOI] [PubMed] [Google Scholar]


