Abstract
Objective:
Lung cancer remains the number one cause of cancer-related mortality worldwide, but less known is that lung cancer patients are among the most psychologically disabled of all cancer groups. Patients with stage-IV non-small cell lung cancer (NSCLC) were studied to test the hypothesis that trajectories of depression and/or anxiety symptoms following diagnosis would show an adverse relationship with survival, beyond relevant controls.
Methods:
Patients with stage-IV NSCLC (N=157) were enrolled (ClinicalTrials.gov Identifier: NCT03199651) at diagnosis and completed validated measures for depressive symptoms (Patient Health Questionnaire-9; PHQ9) and anxiety symptoms (Generalized Anxiety Disorder-7; GAD7). Patients were reassessed every 1-to 2-months thru 24-months (16 assessments; 80% average completion rate) and survival monitored. Joint statistical models provided simultaneous modeling of longitudinal (psychological) and time-to-event (survival) processes. Control variables were age, sex, marital status, education, smoking status, cancer type, and treatment received.
Results:
Depression and anxiety symptoms significantly decreased with time since diagnosis. The two-year trajectory of depressive symptoms was significantly associated with cancer survival after adjustment for covariates [Hazard ratio=1.09 per unit increase in PHQ9, 95% CI 1.03–1.15, p=0.002]. Anxiety was marginally significant in the unadjusted (p=0.053) but not the adjusted (p=0.39) model.
Conclusions:
For the first time, joint model analyses test the interaction of a longitudinal trajectory of psychological symptoms, assessed from diagnosis through 24 months, and cancer survival. New data show the continuation of depressive and anxiety symptoms through treatment and thereafter. Immunotherapy and targeted therapies have dramatically improved survival for patients with advanced NSCLC, however novel data suggest their benefit may be constrained by depressive symptoms.
Keywords: Non-small cell, depression, anxiety, survival, joint model
1. INTRODUCTION
Lung cancer remains the number one cause of all cancer-related mortality worldwide. The most prevalent type, non-small cell lung cancer (NSCLC), accounts for 85% of all cases with most individuals (77%) presenting with advanced (stage-IV) disease.1 Less well-known is that lung cancer patients are among the most psychologically disabled of all cancer groups,2–4 having the greatest prevalence of mood (est. 18%) and anxiety disorders (est. 19%).2,5 Prior studies have found negative emotions, particularly depression, to be associated with mortality in cancer patients. Using Kaplan-Meyer estimates and Cox models,2,6,7 meta-analyses find baseline assessments are associated with shortened survival across cancer types,6 including those with depression and lung cancer [N=4,476; HR=1.39; 95% CI=1.24–1.56].8 It may be that psychological symptoms after diagnosis add to mortality risk, but this possibility has not been examined, nor have patients been studied since the advent of immunotherapy and targeted therapies.
To address these gaps, we test a novel hypothesis that trajectories of depression or anxiety will show added, adverse relationships with survival of patients with advanced NSCLC. It was anticipated that effects would be strongest for depressive symptoms, consistent with prior literature. Anxiety has been less studied, but tested because of the commonality of anxiety disorders, particularly generalized anxiety disorder (GAD),9 and its co-morbidity with depressive disorders.10 Testing these hypotheses with patients with advanced NSCLC is timely, as the introduction of immunotherapies as first line treatment and the increasing use of targeted therapies for oncogenic drivers has dramatically improved overall survival, with 5-year rates as high as 23% in early trials,11 a stark contrast to estimates of 4.2% from the prior decades of chemotherapy use.1 However even with treatment advances, the full impact of their benefit may be constrained by life-threatening negative emotions.
Testing psychological trajectories is a new question requiring different methods. Departing from only baseline to predict survival, post diagnosis assessments were frequent, 16 in 24 months, and largely complete (80% overall compliance). This frequency of data collection enabled the use of joint models (JM) to simultaneously test for associations involving both the psychological and survival processes.12 JMs account for the dynamic characteristics of the trajectory including rate of change in depression scores, cumulative history of depression scores, and deviations from the sample trajectory. This allows the psychological trajectory to be associated with the hazard of the survival event.13 Prognostic variables (age, sex, smoking status, cancer type), and cancer treatment received were controlled in the models. Often omitted from studies of this type, marital status14 and education15 were also included as both are associated with survival. Confirmation of the hypothesis would provide new data showing the added toxicity of anxiety and/or depressive symptom occurrence and continuation as a potential contributor to premature cancer death, even in the context of new cancer therapies for NSCLC.
2. MATERIALS AND METHODS
2.1. Study Design and Participants
From June 2017 to October 2019, individuals were accrued from the thoracic oncology clinics of an NCI-designated Comprehensive Cancer Center (CCC) for participation in an observational study (ClinicalTrials.gov Identifier: NCT03199651; see Figure 1). Inclusion criteria were as follows: newly diagnosed with pathology confirmed stage-IV NSCLC; any Eastern Cooperative Oncology Group (ECOG) performance status; any comorbidity; age ≥ 18 years; English-speaking; willingness to provide access to medical records, biospecimens, and respond to patient reported outcome (PRO) measures. Exclusion criteria were as follows: treatment with definitive chemo-radiotherapy; diagnosis > 90 days at enrollment; receipt of lung cancer treatment for over one month; and presence of disabling hearing, vision, or psychiatric conditions (e.g., schizophrenia) preventing consent or completion of PROs in English. Additionally, second opinion or consult only cases returning to his/her community physician were excluded.
Figure 1.
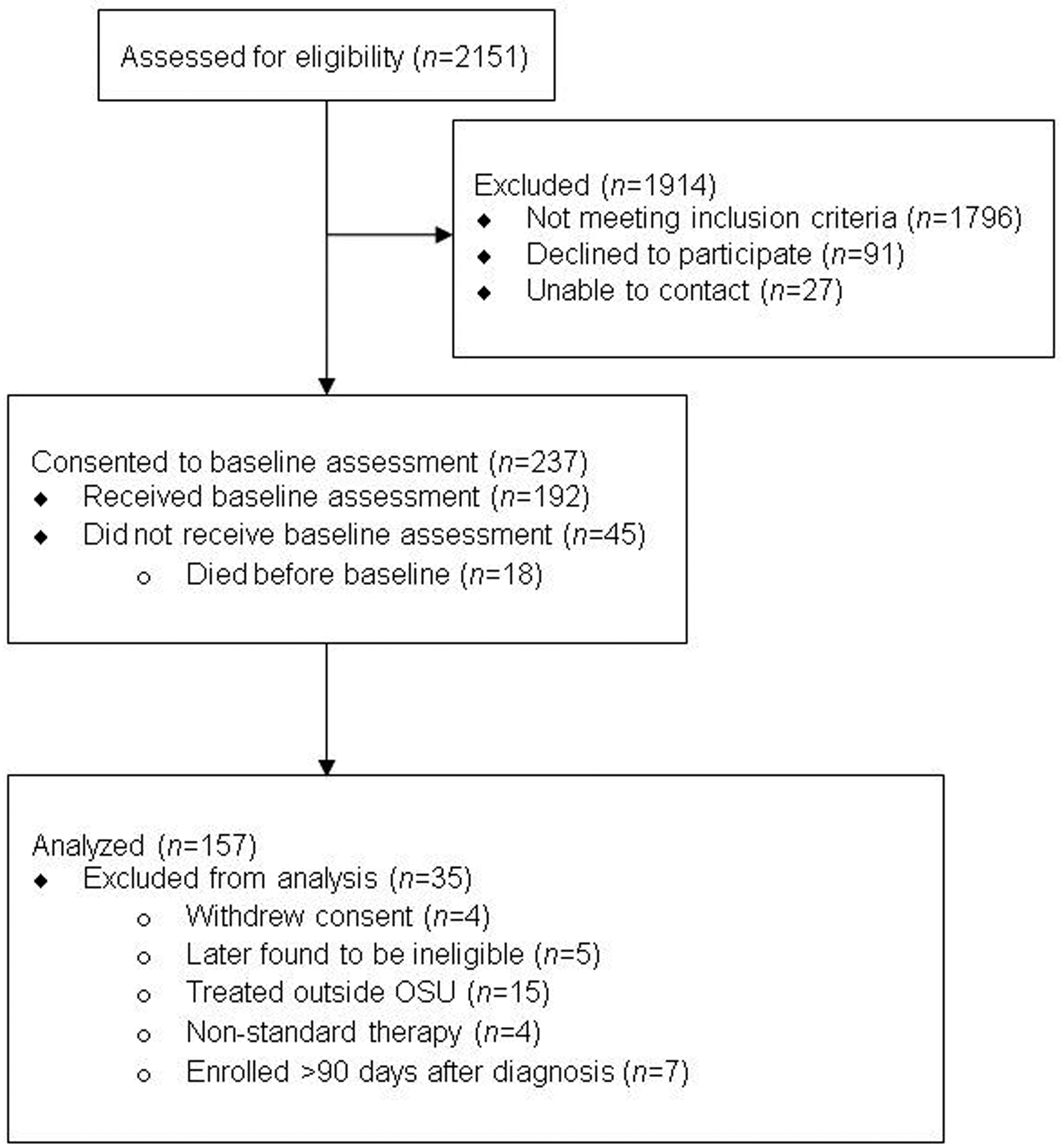
CONSORT Flow Diagram
2.2. Procedures
The Ohio State University Comprehensive Cancer Center Clinical Scientific Review Committee approved the study and procedures in accordance with its ethical standards and the 1964 Helsinki declaration. Research personnel completed informed consent with each patient face-to-face during the first thoracic oncology clinic appointment (see Figure 1 for study flow). Within two weeks of enrollment, trained interviewers from a non-university, professional survey center contacted patients by telephone and PROs were completed (see Measures below). Patients had been provided a “hard copy” PRO booklet to use as reference. Following the baseline interview, patients were assessed monthly for depressive and anxiety symptoms through 8 months, and then bimonthly thru death or 24 months. Patients received $15 per assessment.
2.3. Depression and Anxiety Symptom Measures
American Society of Clinical Oncology (ASCO) recommended measures were used.16 The Patient Health Questionnaire-9 (PHQ9)17 is a 9-item self-report scale assessing the frequency of symptoms of major depressive disorder (MDD).18 Referencing the past 2 weeks, items are rated on a 4-point Likert scale (0=not at all to 3=nearly every day). Total scores range from 0–27. Cutoffs are as follows: none/mild=1–7, moderate=8–14, moderate to severe=15–19, and severe=20–27. For this sample, baseline mean equaled 6.6 (SD=5.1, range 0–24) and internal consistency was α=.806. The Generalized Anxiety Disorder-7 (GAD7)19 is a 7-item measure assessing the frequency of symptoms of generalized anxiety disorder. Referencing the past 2 weeks, items are rated on a 4-point Likert scale (0=not at all to 3=nearly every day). Total scores range from 0–21. Cutoffs are as follows: none=0–4, mild=5–9, moderate=10–14, and moderate to severe/severe=15–21. Baseline mean equaled 5.3 (SD=5.0, range 0–21) and internal consistency was α=.874.
2.4. Covariates
Variables were age, sex, race (White, African American), marital status (yes/no), educational level (<high school, high school, >high school), employment status (yes/no), income level (≤25,000, >25,000), smoking status (never, former, current), lung cancer type (adenocarcinoma, squamous, other), and initial (first line) treatment (chemotherapy, immunotherapy, chemotherapy+immunotherapy, targeted therapy, none). Patients found to have no treatment either declined treatment or had treatment deferred and passed away before being treated.
2.5. Statistical Analysis
To overview, three levels of statistical models were fit.12 First, linear mixed effects models with subject level random effects modeled longitudinal depression and anxiety as a function of baseline covariates. Second, Cox proportional hazards models estimated the association between overall survival and baseline covariates. Thirdly and central to the hypothesis, joint models estimated the association between the longitudinal trajectories and overall survival, controlling for relevant sociodemographics, smoking, disease variables, and cancer treatment. Joint models were fit using the JM package in the R statistical programming software,12 using a relative risk model for survival with a piecewise-constant baseline hazard function.
Overall trends in longitudinal profiles of psychological variables were visualized using locally estimated scatterplot smoothing (LOESS). Longitudinal models included a natural cubic spline basis for flexible estimates of changes in psychological measures over time. Interactions between first line cancer therapy and time were evaluated to determine if trajectories of depression or anxiety varied by therapy.
For JMs for depression (PHQ9) and anxiety (GAD7), three models varying in covariate entry were estimated: Model 1 (no covariates), Model 2 (age, sex, smoking history, cancer type, treatment), and Model 3 (covariates in Model 2 plus education and marital status). Hazard ratios (HR) and 95% confidence intervals (CI) quantify the relative increase in mortality hazard for each unit increase of the psychological measure/covariate. Cox-Snell and Schoenfeld residuals evaluated overall goodness-of-fit and adequacy of the proportional hazard’s assumption, respectively. As the Cox model has been the standard to test the association of baseline symptoms and survival, Cox models with time-varying covariates are provided for the readers’ possible interest.
To visualize the JM results, other analyses illustrate the time-dependent association between longitudinal psychological processes and survival. First, landmark plots show patients’ survival subsequent to relevant landmark times--3, 6, and 12 months--which serve as new time origins. At each time point, survival is conditional, including only patients still alive at the landmark. For the 3-month landmark, for example, only patients who had lived up to the time of 3 months are displayed in the plot. At each time point, patients’ most recent score on the PHQ9/GAD7 prior to the landmark time is considered. For each landmark, the most recent score is then used to classify each patient into a symptom group, i.e., for the PHQ9, groups were patients with 0–7 (none/low) vs. 8–14 (moderate) vs. 15–27 (moderate/severe). Then, Kaplan-Meier survival estimates are done for each symptom group. The same procedure is used for each landmark timepoint.
A second illustration uses dynamic predictions from the joint model to estimate expected survival for patients, conditional on their PGQ9/GAD7 scores from baseline thru 5 months.12 This period was chosen to mimic the time during which a psychological intervention might be offered. Then, estimated survival after 5 months is examined for four representative patients, who differ only on whether or not their PHQ9/GAD7 improved or worsened from baseline to 5 months. The survfitJM function in package JM with 1000 Monte-Carlo iterations was used with analyses conducted using R version 4.0.0.
3. RESULTS
3.1. Descriptive
3.1.1. Patients
Table 1 presents baseline demographics, smoking status, treatment, lung cancer type, and first line treatment received for N=157. Patients were diagnosed a median of 34 days prior to the baseline assessment. Initial therapy for the majority of patients was combination chemotherapy+immunotherapy (31.8%), with roughly similar percentages of patients treated with chemotherapy (22.9%), immunotherapy (20.4%), and targeted (18.5%) monotherapies. The median follow-up was 8.7 months, with an inter-quartile range of 4.8 to 15.6 months and a range of 1.1 to 31.4 months. Overall mortality was 51% (80/157).
Table 1. Patient Characteristics.
Baseline demographics, smoking status, treatment, lung cancer type, and first line treatment for the 157 patients enrolled.
| Level | N (%) or Mean (SD) | |
|---|---|---|
| Total Sample | 157 (100) | |
| Age in Years) | 63.76 (10.74) | |
| Sex (%) | Female | 68 (43.3) |
| Male | 89 (56.7) | |
| Race (%) | White | 146 (93.0) |
| Black or African American | 11 ( 7.0) | |
| Married/Partnered (%) | No | 68 (43.3) |
| Yes | 89 (56.7) | |
| Education (%) | < High school | 20 (12.7) |
| High school | 55 (35.0) | |
| > High school | 82 (52.2) | |
| Employed (%) | No | 117 (74.5) |
| Yes | 40 (25.5) | |
| Income (%) | <=25K | 33 (21.0) |
| >25K | 109 (69.4) | |
| Unknown | 15 ( 9.6) | |
| Smoking Status (%) | Never smoker | 21 (13.4) |
| Former smoker | 110 (70.1) | |
| Current smoker | 26 (16.6) | |
| Cancer Type (%) | Adenocarcinoma | 116 (73.9) |
| Squamous | 26 (16.6) | |
| Other | 15 ( 9.6) | |
| Treatment (%) | None | 10 ( 6.4) |
| Chemotherapy | 36 (22.9) | |
| Immunotherapy | 32 (20.4) | |
| Chemo+Immunotherapy | 50 (31.8) | |
| Targeted therapy | 29 (18.5) |
3.1.2. Data integrity
Baseline assessments were completed for 100% of patients. Across follow-ups (16), mean completion rate was 80% of available N. Total number of measurements was 1,116 for both PHQ9 and GAD7. Figure S1, Supplemental Digital Content, provides detailed box-strip plots for each measure at each time point, illustrating that the range of patient scores spanned the full scale for each measure during the first 6 months before gradually decreasing in variability. Overall subject-level standard deviation across all measurements was 4.15 for PHQ9 and 3.89 for GAD7.
3.2. Diagnosis to 24-Months: Trajectories of Psychological Symptoms
Figure 2 shows the trajectories for depression (PHQ9) and anxiety (GAD7) for N=157. Scores on both measures decreased over time. The GAD7 trajectory exhibited some non-linearity, increasing at 6 months before declining. PHQ9 decreased from baseline mean of 6.6 to 4.5 at one year and GAD7 decreased from 5.3 to 2.9.
Figure 2. NSCLC patients (N=157) depression and anxiety symptoms from diagnosis thru 24 months.
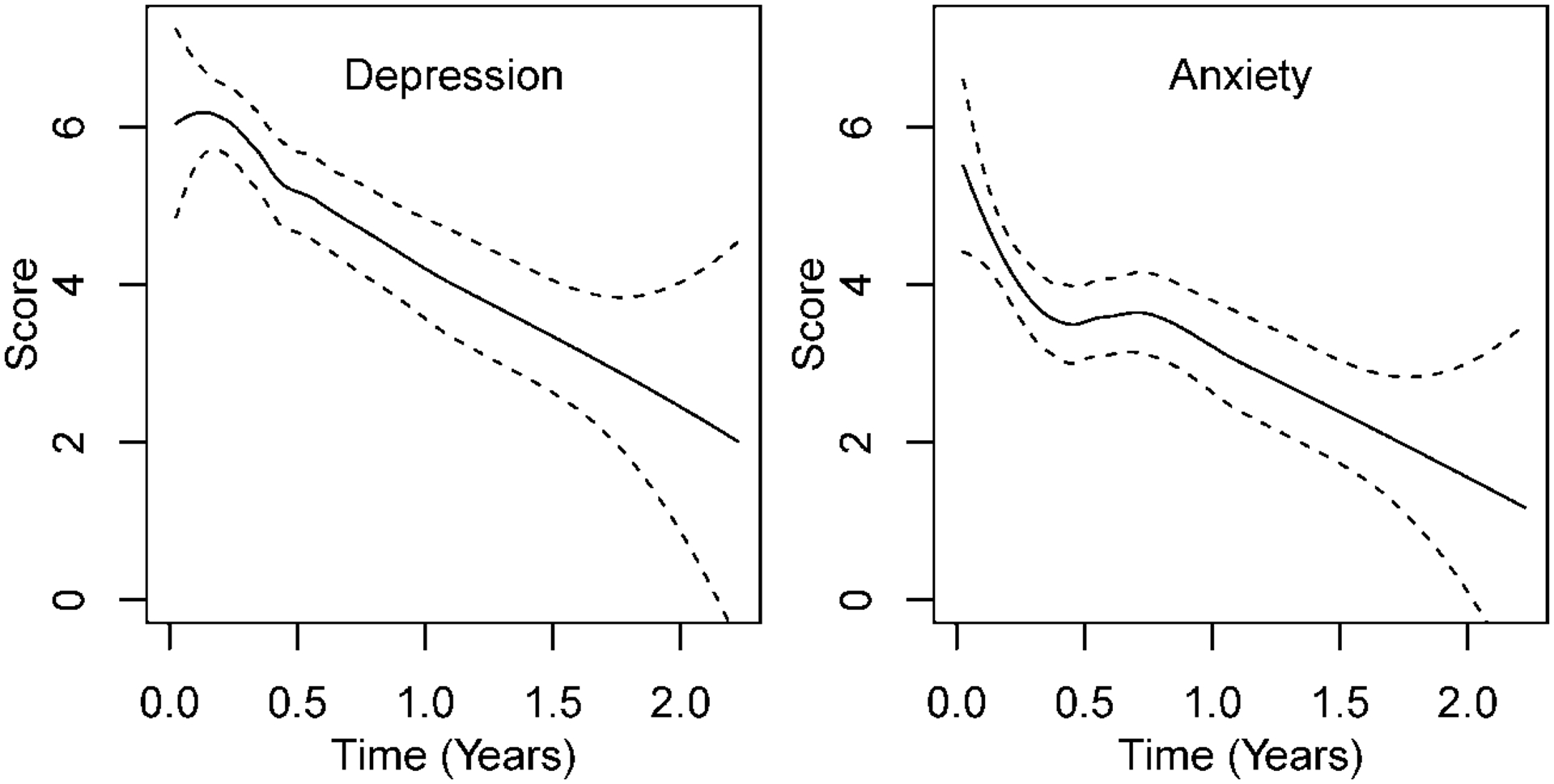
LOESS (locally estimated scatterplot smoothing) for depression (PHQ9) and anxiety (GAD7) scores. Dashed lines indicate 95% confidence intervals.
3.3. Longitudinal Process Results for PHQ9 and GAD7
Non-linear changes tested by inclusion of natural splines for time were significant for PHQ9 and GAD7. Table S1, Supplemental Digital Content, provides longitudinal process results from the joint model for the PHQ9 and GAD7, including all covariates (Model 3). Other than baseline values, the only covariate significantly associated with survival was education. Patients with a high school education or above had roughly 3 points lower mean PHQ9 and 2 points lower mean GAD7 compared to patients with less than high school education. All other covariates were non-significant, including that for cancer treatment type. As anticipated, there were survival differences between the treatment groups, shown in Figure S2, Supplemental Digital Content. Despite this, the type of cancer treatment patients received did not impact the depression or anxiety trajectories; p-values for treatment-by-time interaction were p=0.18 for PHQ9 and p=0.68 for GAD7).
3.3. Joint Model (JM) Results
Data supporting the JM analyses and HR findings show good model fit (Figure S3, left side, Supplemental Digital Content) and stable parameter estimates (Figure S3, right side). Results from the joint model analyses are provided in Table 2. Inspection of the hazard ratios (HRs) shows the strongest association for the depression (PHQ9) trajectory. All three models had significant associations between PHQ9 and survival, with that for Model 3 (which included age, sex, smoking history, cancer type, treatment, education, and marital status) being HR=1.09, 95% CI 1.03–1.16, p=0.002. Hazard ratios for the anxiety (GAD7) trajectory (HR=1.06, 95% CI 1.0–1.13) were similar for Model 1 (p=0.053) and Model 2 (p=0.071). The HR drops for Model 3 with p=0.39. Standardized hazard ratios (i.e., the relative increase in hazard per standard deviation change in measurement) are given in Table 2, and range from 1.61 (Model 1) to 1.44 (Model 3) for PHQ9 and 1.26 (Model 1) to 1.14 (Model 3) for GAD7.
Table 2.
Joint Model Results Showing Significant Association Between the Depression Trajectory and Survival.
| Measure | Model | HR | 95% CI | SHR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| PHQ9 | Model 1 | 1.122 | (1.068, 1.179) | 1.614 | (1.315, 1.981) | <0.0001 |
| Model 2 | 1.108 | (1.053, 1.166) | 1.533 | (1.241, 1.894) | <0.0001 | |
| Model 3 | 1.092 | (1.033, 1.155) | 1.442 | (1.145, 1.817) | 0.0019 | |
| GAD7 | Model 1 | 1.061 | (0.999, 1.127) | 1.261 | (0.997, 1.594) | 0.053 |
| Model 2 | 1.059 | (0.995, 1.128) | 1.252 | (0.981, 1.597) | 0.071 | |
| Model 3 | 1.035 | (0.967, 1.107) | 1.142 | (0.878, 1.486) | 0.32 |
Association between each longitudinal psychological measure [depression (PHQ9) and anxiety (GAD7)] and overall survival. Estimated hazard ratio (HR), standardized hazard ratio (SHR), 95% confidence intervals (CI), and p-values are provided. HRs are per one-point increase in each measure, while SHRs are per standard deviation increase. Model 1 includes only the PHQ9 or the GAD7. Model 2 adds covariates of age, sex, smoking status, lung cancer type, and treatment type. Model 3 includes all covariates from Model 2 and adds marital status and education.
To aid in the understanding and contribution of these new findings, Cox model results are provided in Table S2, Supplemental Digital Content, for the reader to compare with those of the JM analyses. Cox models have been the biostatistical “gold standard” for testing the relationship between psychological variables measured at baseline and survival.6,8 Cox models identified significant associations between baseline depressive symptoms and mortality, but for anxiety scores also, for both unadjusted and adjusted models. However, these models assume that time-dependent covariates are external, which is violated in this case.12 In contrast, the JM framework explicitly acknowledges the potential underlying relationships between the event and longitudinal process.20 The JM analyses permit the characterization not only of baseline risk associated with depressive symptoms, but the additional mortality risk associated with depressive symptom continuation.
3.4. Illustrations of JM Findings
3.4.1. Landmark Plots
Figure 3 gives landmark plots for survival stratified by patients’ depression and anxiety symptom levels at 3, 6, and 12 months to illustrate the reliability of the findings. Across the three timepoints, the plots show the same effect: patients in the moderate/severe psychological symptom group have lower survival compared to patients with no/mild symptoms. The survival differences between symptom groups are most obvious at the 6- and 12-month landmarks. The plots are also reflective of the JM findings, showing greater and more consistent separation between symptom groups for PHQ9 relative to that for GAD7.
Figure 3. Landmark Survival Plots Showing Differential Survival at 3-, 6-, and 12-Months for Patient Groups Varying in Depression (PHQ9) And Anxiety (GAD7) Symptom Severity at That Time.
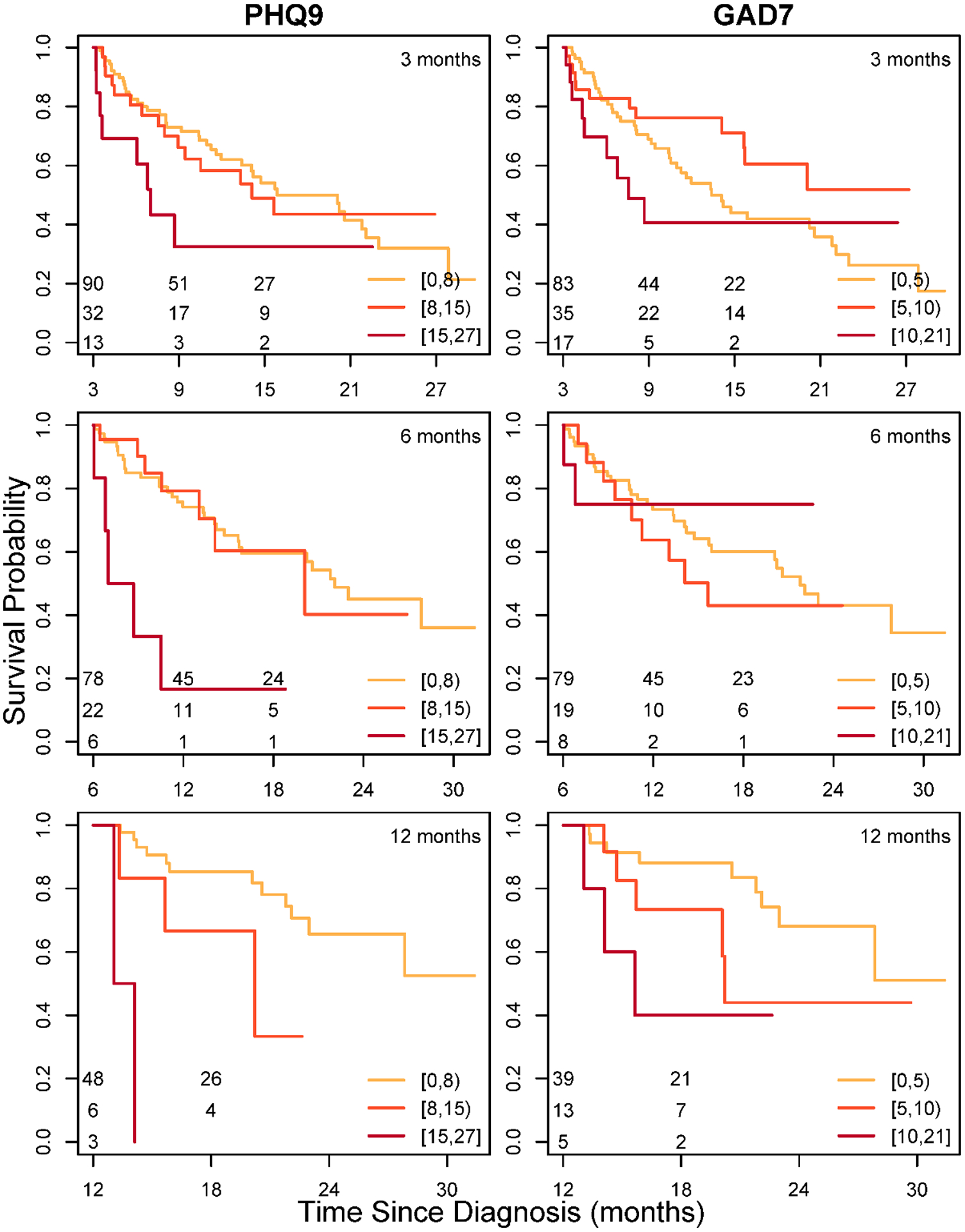
For each landmark (3, 6, and 12 months), survival is conditional on patients being alive at the landmark. Symptom level classification is based on an individual’s most recent symptom score prior to the landmark time. Symptom levels for PHQ9 are 1–7 (none/mild), 8–14 (moderate), and 15–27 (moderately severe / severe), with higher scores indicating worse symptoms. GAD7 symptom levels are 0–4 (none), 5–9 (mild), and 10–14 (moderate) and 15–21 (moderate/severe), with higher scores indicating worse symptoms. The number at risk in each symptom group is indicated in the bottom corner, left, of each figure.
3.4.2. Contrast of Patients With Improving Versus Worsening 5-month Depression/Anxiety Trajectories and Resultant Differences in Survival.
Patients in this study were not randomized to receipt of a psychological intervention versus not. However, Figure 4 is informative, illustrating differential survival arising from a shift in the post diagnosis depression/anxiety trajectory. Actual data from four patients with similarly high baseline PHQ9 or GAD7 symptom values (2 patients for each measure) were used. Within each PHQ9/GAD7 pair, one patient shows declining (i.e., improving) symptoms from baseline to 5 months, whereas the other patient shows increasing (worsening) symptoms. Based on the 1-to 5-month trajectories, joint models provide a predicted survival estimate for each patient (right-hand side of each plot). Covariates for each patient were held the same so that only the patients’ PHQ9/GAD7 measurements from baseline to 5 months differed. The detrimental association between these differing 5-month PHQ9 trajectories and survival is provided with a projected 1-year survival. For Patient 1, whose depression symptoms improved, projected 1 year survival is 64% versus Patient 2, whose depression symptoms worsened, having only 42% survival projected. A similar scenario is seen for patients 3 and 4 who differ in GAD trajectories (bottom panel, Figure 4). Here, Patient 3 (left plot) reports a decline to no symptoms (0) at 5 months and has projected 1-year survival of 74%, whereas Patient 4 reports steadily increasing anxiety (21, severe) by 5 months and has projected 1-year survival of 53%. In summary, these per patient examples show the relationship between depressive or anxiety symptom improvement and lengthened survival time, versus depressive or anxiety symptoms continuing or worsening and shortened survival.
Figure 4. Patients With Improving Versus Worsening Short-Term Depression/Anxiety Trajectories And Resultant Differences In Predicted Survival. Procedures.
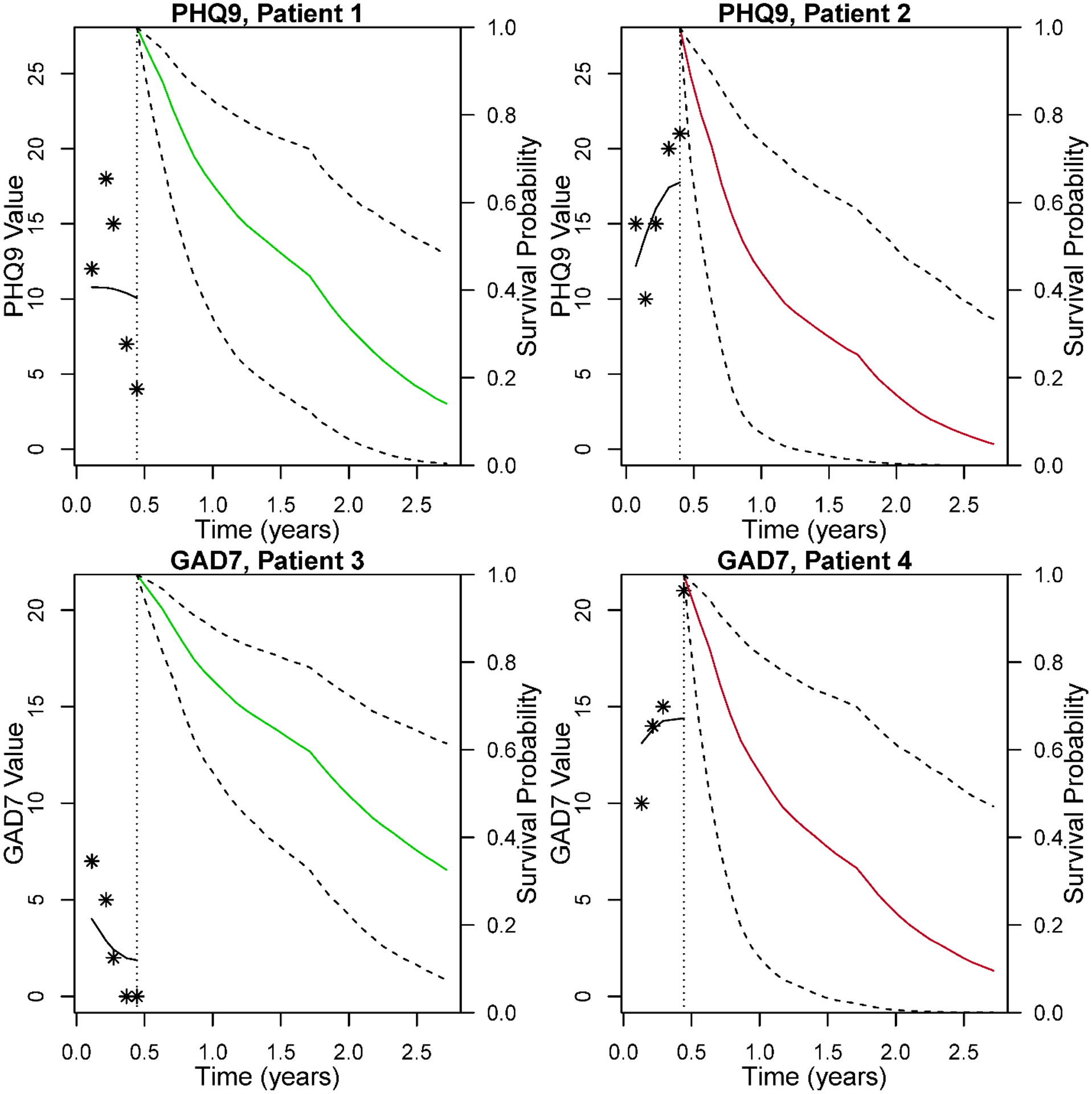
Each plot illustrates a single patient with and his/her psychological symptom score (displayed as asterisks) from baseline through 5 months on the left-hand side of each plot; the black line shows the smoothed model estimate from baseline to 5 months for depression (PHQ9; top) and anxiety (GAD7; bottom). On the right-hand sides for each patient, survival curves are projected based on the PHQ9 or GAD7 scores through 5 months, using dynamic predictions from the full joint model (JM) with all covariates. The four patients differed only in their respective PHQ9/GAD7 scores thru 5 months, as covariates were identical (male, ageapp 65, married, high school education, former smoker, adenocarcinoma, immunotherapy). The dashed lines indicate 95% pointwise confidence intervals for the survival curves. Top PHQ9 panel JM predictions show Patient 1’s 5-month improvements in PHQ-9 followed by more favorable estimated survival (green line), in comparison to Patient 2’s worse projected survival (red line), which was preceded by worsening PHQ9 scores. Bottom GAD7 panel: Similarly, JM predictions showing 5-month anxiety declines for Patient 3 versus 5-month anxiety worsening for Patient 4. Correspondingly, Patient 3 has more favorable projected survival (green line) compared to estimated survival for Patient 3 (red line).
Note: PHQ9 (Patient Health Questionnaire-9), GAD7 (Generalized Anxiety Disorder-7), JM (joint model)
4. DISCUSSION
Novel use of joint model analyses (JMs) provided valid inferences to discover the association between the longitudinal psychological data and lung cancer survival. A key element to the analyses were the frequency of psychological data collection--obtained bi/monthly for two years--and its integrity, with 80% compliance. Patients with advanced NSCLC have a high frequency of stress, depression and anxiety symptoms at diagnosis, but would significant symptom levels thereafter be associated with increased mortality risk? JMs provided a rigorous answer to this question, showing premature mortality for advanced NSCLC patients associated with the post diagnosis depressive symptom trajectory, beyond any contributions of baseline values, demographics, prognostic variables, cancer type, and receipt of new cancer treatments. Alone, cancer treatment predicted survival, but receiving new therapies did not interact with or mute the association of depressive symptom trajectories with survival.
Novel findings quantify, for the first time, the psychological course for patients with advanced NSCLC, and moreover, the psychological course as patients receive new therapies (see Fig. 2). Compared to other high incidence cancers (breast, prostate, colon), lung cancer patients have received little psychosocial study generally, despite lung patients having greater psychological morbidity than all other cancer groups. When studied intensively at diagnosis, patients from the cohort showed 28% with moderate and 8% with moderate to severe depressive symptom levels and 6% with moderate and 18% with moderate to severe levels of anxiety symptoms.21 But statistical patterns of symptoms thereafter, essentially from diagnosis thru successful treatment or death, have been unknown.
Joint modeling (JM) is a superior analytic strategy for this question and will likely become the gold standard for future studies. If subjects die or are too sick to complete an assessment this non-random dropout is ignored in standard longitudinal models (e.g., mixed models), but JMs can properly account for the nonrandom dropout common to longitudinal studies of NSCLC patients or others with severe illness. JMs also provide valid inference for the association between the longitudinal psychological data and survival by incorporating shared subject-level random effects to account for the psychological/survival inter-dependency.22 However, limitations are still inherent with observational data including that which is longitudinal. Causal relationships between psychological symptom trajectories and patient survival, of either direction, cannot be made based on these results. Changes in health status and disease progression increase mortality risk.7, 11 Follow-up studies are needed to explore longitudinal association of these factors with survival. These limitations are shared by all studies examining association between cancer mortality risk and patient reported outcomes such as quality of life.7
The importance and reliability of the findings in Table 2 may be difficult to fully appreciate when considered alone. Thus, two types of novel illustrations were provided to “make real” the relationship of depression symptom levels and survival by viewing patients grouped by their symptom severities (Figure 3) or viewed as individuals (Figure 4). Landmark plots (Figure 3) illustrate the uniformity and reliability of poorer survival for patients with moderate to severe depressive symptoms, even seen when patients survive as long as 3, 6, or even 12 months. The three GAD7 plots provide a conceptual replication of the effect.
The process data (Figure 4) illustrates the powerful effect of shift in the symptom trajectory for individuals. Prior research with breast cancer patients (N=116) assessed intensively and followed for 5 years showed individual differences among patients in the level of stress, depressive symptoms, and immunity (Natural killer cell lysis, T cell blastogenesis) at baseline, but relevant to these data were additional findings showing individual differences among patients in the slope of change across all measures in the next 5 years, i.e., the trajectories.23 This phenomenon of slope differences is illustrated in Figure 4, however here the effect is illustrated for specific persons and the resultant outcomes provided. The differential survival among individuals is due to the preceding baseline to five-month symptom trajectories. Holding all covariates constant excepting the short term (1- to 5-month) symptom change, individual patients differed in survival. If symptoms remitted (Patients 1, 3) survival was favorable, compared to symptoms maintained or worsening (Patients 2, 4). Psychological interventions delivered during this period--the early months following diagnosis--have shown robust biobehavioral effects and reduced risk for recurrence in other cancer patients.24
Research is needed to discover the mechanism(s) by which psychological symptoms, particularly depression, have continuing toxicity in association with lung cancer survival. A commonality among depression,25,26 lung cancer,27 and immune checkpoint inhibiting therapies,28,29 are their inflammatory inducing effects,30 occurring in an immune system already vulnerable from smoking31 and age-related cellular function decline.32,33 This may be the perfect storm, as evidenced by advanced lung cancer patients having, compared to all other cancer patients, the 1) highest levels of depression at diagnosis; 2) highest maintenance of depression compared to all others, as evidenced in post diagnosis cross sectional and longitudinal designs, 3) highest levels of suicide; and as shown here, 4) a lingering association of depression and survival, beyond the effects of precision medicine therapies. Intensive study of NSCLC patients combining robust measures of depression, cell biology, and inflammation are need to directly explore biobehavioral mechanisms driving depression toxicity.
Contextual aspects of the study are considered. Methodologic recommendations for tests of the prognostic value of PROs were followed.7 The ASCO recommended measures used are conceptually grounded with long records of acceptability, reliability, and validity. The sample size was smaller than some but larger than others in survival sturdies.2,6,7 In addition to sample size, equally relevant is a having sufficient number of events to predict, with 50% being optimal, and sufficient follow up duration, which was the case here. Power was enhanced with the month-by-month frequency of measurements, completeness of the data, and patients’ compliance. Few longitudinal behavioral studies of cancer patients have done trajectory measurements as shown here. Regarding patient accrual, there were no age or functional status exclusions, unlike clinical trials.34 Worldwide lung cancer patients share common features, having a disease of smoking and aging. The state from which these patients came has among the highest smoking rates in the U.S. (21.1% vs. 14%) and also has the 44th lowest lung cancer mortality rate. Also, 50% of the patients were from rural Ohio Appalachia counties, a predominantly Caucasian region with multiple health disparities and negative social determinants of health,35 including those for lung cancer survival. Nevertheless, the patients were accrued from a single institution and further, generalizability of findings to lung cancer groups diverse on other health determinants is unknown.
5. Conclusion
New data show post diagnosis trajectories of psychological symptoms predicted risk of premature mortality from advanced NSCLC, even controlling for the survival benefits of immune- and targeted therapies. Substantial evidence underscores the need for psychological therapies to address the common comorbidities of stress, depression, and anxiety with advanced NSCLC.2,8,21 Without interventions, worries will remain regarding patients having suboptimal understanding of their disease and its treatment,36 impaired decision-making and engagement in treatment,37,38 lowered tolerance of symptoms and treatment side effects,39,40 and poor motivation to maintain functional status.41,42 Attention to and improvement of patients’ psychological status may provide added benefit that prolongs a life of quality and comfort and potentially improves overall survival.
Supplementary Material
ACKNOWLEDGEMENTS.
The authors acknowledge the patients for their participation and Jonathan Covarrubias, Sarah Reisinger, and the Strategic Research Group for research assistance.
Funding.
This work was supported by the Ohio State University Comprehensive Cancer Center Pelotonia; National Institutes of Health K12 CA133250, R03 AG064374-01, and P30CA016058.
Conflicts of Interest.
B.L. Andersen, J. McElroy, R.M. Smith have no conflicts of interests to declare. D.P. Carbone reports personal fees from the following: Abbvie, Adaptimmune, Agenus, Amgen, Ariad, AstraZeneca, Biocept, Boehringer Ingelheim, Celgene, Clovis, Daiichi Sankyo, Inc. (DSI), EMD Serono, Flame Biosciences, Foundation Medicine, G1Therapeutics/ Intellisphere, GenePlus, Genentech/Roche, Glaxo-Smith-Kline, Gloria Biosciences, Gritstone, Guardant Health, Humana, Incyte, Inivata, Inovio, Janssen, Kyowa Kirin, Loxo Oncology, Merck, MSD, Nexus Oncology, Novartis, Oncocyte, Palobiofarma, Pfizer, prIME Oncology, Stemcentrx, Takeda Oncology, and Teva, grants and personal fees from Bristol Myers-Squibb (BMS) outside the submitted work during the conduct of the study. C.J. Presley reports grants from National Institute of Aging and the National Cancer Institute during the conduct of the study P.G. Shields reports grants from National Cancer Institute during the conduct of the study. G. N. Brock reports grants from the National Cancer Institute and National Center for Advancing Translational Sciences during the conduct of the study.
Abbreviations:
- PHQ9
Patient Health Questionnaire-9
- GAD7
Generalized Anxiety Disorder-7
- ECOG
Eastern Cooperative Oncology Group
- JMs
joint models
- CCC
Comprehensive Cancer Center
- NSCLC
Non-Small Cell Lung Cancer
- PRO
Patient Reported Outcome
- HR
Hazard Ratio
- PH
Proportional Hazards
- ASCO
American Society of Clinical Oncology
- MDD
Major Depressive Disorder
- LOESS
locally estimated scatterplot smoothing
REFERENCES
- 1.SEER Preliminary Cancer Incidence Rate Estimates for 2017, and diagnosis years 2000 to 2017, SEER 18, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/statistics/preliminary-estimates/, based on the February 2019 SEER data submission and the November 2018 SEER data submission. Posted to the SEER website, September 2019. [Google Scholar]
- 2.Sullivan DR, Forsberg CW, Ganzini L, Au DH, Gould MK, Provenzale D, Slatore CG. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol 2016;34:3984–3991. doi:10.1200%2FJCO.2016.66.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol 2008;26:4731–4738. doi:10.1200%2FJCO.2007.13.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linden W, Vodermaier A, MacKenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics 2009;50:383–391. doi: 10.1176/appi.psy.50.4.383. [DOI] [PubMed] [Google Scholar]
- 6.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Rev Clin Oncol 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 7.Mierzynska J, Piccinin C, Pe M, Martinelli F, Gotay C, Coens C, Mauer M, Eggermont A, Groenvold M, Bjordal K, Reijneveld J, Velikova G, Bottomley A. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: a systematic review. Lancet Oncol 2019;20:e685–e698. doi: 10.1016/S1470-2045(19)30656-4. [DOI] [PubMed] [Google Scholar]
- 8.Walker J, Mulick A, Magill N, Symeonides S, Gourley C, Burke K, Belot A, Quartagno M, van Niekerk M, Toynbee M, Frost C, Sharpe M. Major depression and survival in people with cancer. Psychosom Med 2021;83:410–416. doi: 10.1097/psy.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remes O, Brayne C, van der Linde R, Lafortune L. A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav 2016;6:e00497. doi: 10.1002/brb3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush J, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 11.Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn M-J, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, Patnaik A, Gubens M, Ramalingam SS, Felip E, Goldman JW, Scalzo C, Jensen E, Kush DA, Hui R. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518–2527. doi:10.1200%2FJCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizopoulos D Joint models for longitudinal and time-to-event data: with applications in R. Boca Raton, FL: CRC Press, 2012. [Google Scholar]
- 13.Ediebah DE, Galindo-Garre F, Uitdehaag BM, Ringash J, Reijneveld JC, Dirven L, Zikos E, Coens C, van den Bent MJ, Bottomley A, Taphoorn MJB. Joint modeling of longitudinal health-related quality of life data and survival. Qual Life Res 2015;24:795–804. doi: 10.1007/s11136-014-0821-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Yin K, Zheng D, Gu J, Luo J, Wang S, Chen H. Marital status independently predicts non-small cell lung cancer survival: a propensity-adjusted SEER database analysis. J Cancer Res Clin Oncol 2020;146:67–74. doi: 10.1007/s00432-019-03084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willen L, Berglund A, Bergstrom S, Bergqvist M, Ojdahl-Boden A, Wagenius G, Lambe M. Educational level and management and outcomes in non-small cell lung cancer. A nationwide population-based study. Lung Cancer 2019;131:40–46. doi: 10.1016/j.lungcan.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, Rowland JH. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol 2014;32:1605–1619. doi:10.1200%2FJCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 20.Tsiatis AA, Davidian M. Joint modeling of longitudinal and time-to-event data: an overview. Stat Sinica 2004;14:809–834. https://www.jstor.org/stable/24307417. [Google Scholar]
- 21.Andersen BL, Valentine TR, Lo SB, Carbone DP, Presley CJ, Shields PG. Newly diagnosed patients with advanced non-small cell lung cancer: A clinical description of those with moderate to severe depressive symptoms. Lung Cancer 2020;145:195–204. doi: 10.1016/j.lungcan.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol 2010;28:2796–2801. 10.1200/JCO.2009.25.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen BL, Goyal NG, Westbrook TD, Bishop B, Carson WE. Trajectories of stress, depressive symptoms, and immunity in cancer survivors: Diagnosis to 5 years. Clin Cancer Res 2017;23:52–61. 10.1158/1078-0432.CCR-16-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol 2004;22:3570–3580. 10.1200%2FJCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009;71:171–186. doi: 10.1097/psy.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 27.Brown D, Zingone A, Yu Y, Zhu B, Candia J, Cao L, Ryan BM. Relationship between circulating inflammation proteins and lung cancer diagnosis in the National Lung Screening Trial. Cancer Epidemiol Biomarkers Prev 2019;28:110–118. doi: 10.1158/1055-9965.epi-18-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postow MA, Hellmann MD. Adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. doi: 10.1056/nejmra1703481. [DOI] [PubMed] [Google Scholar]
- 29.Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018;360:k793. doi: 10.1136/bmj.k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitharouli MC, Hagenaars SP, Glanville KP, Coleman JRI, Hotopf M, Lewis CM, Pariante CM. Elevated C-reactive protein in patients with depression, independent of genetic, health, and psychosocial factors: Results from the UK Biobank. Am J Psychiatry (in press). doi: 10.1176/appi.ajp.2020.20060947. [DOI] [PubMed] [Google Scholar]
- 31.Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, Sharma S, Dubinett SM. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc 2008;5:811–815. doi: 10.1513/pats.200809-100th. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vadasz Z, Haj T, Kessel A, Toubi E. Age-related autoimmunity. BMC Med 2013;11:94. doi: 10.1186/1741-7015-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes M, Teixeira AL, Coelho A, Araujo A, Medeiros R. The role of inflammation in lung cancer. Adv Exp Med Biol 2014;816:1–23. doi: 10.1007/978-3-0348-0837-8_1. [DOI] [PubMed] [Google Scholar]
- 34.Al-Baimani K, Jonker H, Zhang T, Goss GD, Laurie SA, Nicholas G, Wheatley-Price P. Are clinical trial eligibility criteria an accurate reflection of a real-world population of advanced non-small-cell lung cancer patients? Curr Oncol 2018;25:e291–e297. doi:10.3747%2Fco.25.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins GT, Kim T, Munson J. Residence in rural areas of the United States and lung cancer mortality: Disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc 2017;14:403–411. doi: 10.1513/AnnalsATS.201606-469OC. [DOI] [PubMed] [Google Scholar]
- 36.Khrystolubova N, Shieh M, Patel AJ, Bailey R. Pharmacist-led patient education and adverse event management in patients with non-small cell lung cancer receiving afatinib in a community-based, real-world clinical setting. J Oncol Pharm Pract 2020;26:13–22. doi: 10.1177/1078155219833441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kneuertz PJ, Jagadesh N, Perkins A, Fitzgerald M, Moffat-Bruce SD, Merritt RE, D’Souza DM. Improving patient engagement, adherence, and satisfaction in lung cancer surgery with implementation of a mobile device platform for patient reported outcomes. J Thorac Dis 2020;12:6883–6891. doi: 10.21037/jtd.2020.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mokhles S, Nuyttens J, de Mol M, Aerts JGJV, Maat APWM, Birim O, Bogers AJJC, Takkenberg JJM. Treatment selection of early stage non-small cell lung cancer: the role of the patient in clinical decision making. BMC Cancer 2018;18:1–10. doi: 10.1186/s12885-018-3986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison EJ, Novotny PJ, Sloan JA, Yang P, Patten CA, Ruddy KJ, Clark MM. Emotional problems, quality of life, and symptom burden in patients with lung cancer. Clin Lung Cancer 2017;18:497–503. doi: 10.1016/j.cllc.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung MR, Patel MV, Djalalov S, Le LW, Shepherd FA, Burkes RL, Feld R, Lin S, Tudor R, Leighl NB. Evolution of symptom burden of advanced lung cancer over a decade. Clin Lung Cancer 2017;18:274–280.e6. doi: 10.1016/j.cllc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Avancini A, Sartori G, Gkountakos A, Casali M, Trestini I, Tregnano D, Bria E, Jones LW, Milella M, Lanza M, Pilotto S. Physical activity and exercise in lung cancer care: will promises be fulfilled? Oncologist 2020;25:e555–e569. doi: 10.1634/theoncologist.2019-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Talor S, Rosario DJ, Thaha MA, Bourke L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev 2018;9:CD010192. doi: 10.1002/14651858.CD010192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


