Abstract
The role of central estrogen in cognitive, metabolic, and reproductive health has long fascinated the lay public and scientists alike. In the last two decades, insight into estrogen signaling in the brain and its impact on female physiology is beginning to catch up with the vast information already established for its actions on peripheral tissues. Using newer methods to manipulate estrogen signaling in hormone-sensitive brain regions, neuroscientists are now identifying the molecular pathways and neuronal subtypes required for controlling sex-dependent energy allocation. However, the immense cellular complexity of these hormone-sensitive brain regions makes it clear that more research is needed to fully appreciate how estrogen modulates neural circuits to regulate physiological and behavioral end points. Such insight is essential for understanding how natural or drug-induced hormone fluctuations across lifespan affect women’s health.
Keywords: female physiology, central estrogen signaling, hypothalamus, ventromedial hypothalamus, arcuate nucleus, sex-dependent neurocircuits, reproduction, menopause, estrogen receptor alpha, brain-bone connection, women’s health
THE FEMALE BRAIN: WAITING FOR A MOLECULAR FRAMEWORK
It has been decades since the first three-dimensional (3D) atomic structures were solved for nuclear hormone receptors, including those that bind sex steroids. Among the major sex steroids, estrogen has emerged as the primary driver of female physiology across different life stages, beginning at birth and ending with its near depletion in the postmenopausal period. To put this in perspective, a quick search for estrogen in PubMed as of 2021 yields well over 250,000 citations. Estrogen is perhaps best studied for its potent effects on reproductive and breast tissues. This latter focus arose primarily because of the proliferative effects of estrogen, especially in hormone-sensitive breast cancer. Despite the known benefits of estrogen on multiple organs, including the brain, findings from the Nurses’ Health Study done nearly 30 years ago, linking hormone replacement therapy and cancer, albeit small, stood out as a pivotal moment in women’s health that dramatically changed how estrogen is viewed (1). Rather than altogether rejecting estrogen therapies, we assert that identifying hormone-responsive pathways and neurocircuits in the brain will likely uncover new strategies to exploit estrogen’s benefits (and minimize the adverse effects).
Before delving into emerging data outlining how central estrogen functions in the female brain, it is worth remembering that estrogen levels in females are constantly changing during different life stages, including puberty, menses, pregnancy, lactation, and menopause (Figure 1a). Here, we focus on the estrogen-sensitive neural circuits and signaling pathways that modulate physiological responses. These pathways enable females to modulate their homeostatic systems and intrinsic behaviors to eventually enable appropriately cued social behaviors. Rather than addressing how estrogen influences cognition or sex-specific social behaviors, we focus on the molecular events that allow central estrogen in the medial basal hypothalamus (MBH) to control female physiology. Similarly, we do not review the role of sex chromosomes in the brain, which can be found elsewhere (2). Instead, we concentrate on how gonadal- and locally generated estrogen affects innate and adaptive responses. Much remains to be discovered as to how central estrogen coordinates neurocircuits to control physiology across lifespan. It is hoped that highlighting current progress in this field will inspire others to help chart the complex molecular blueprint of the female brain.
Figure 1.
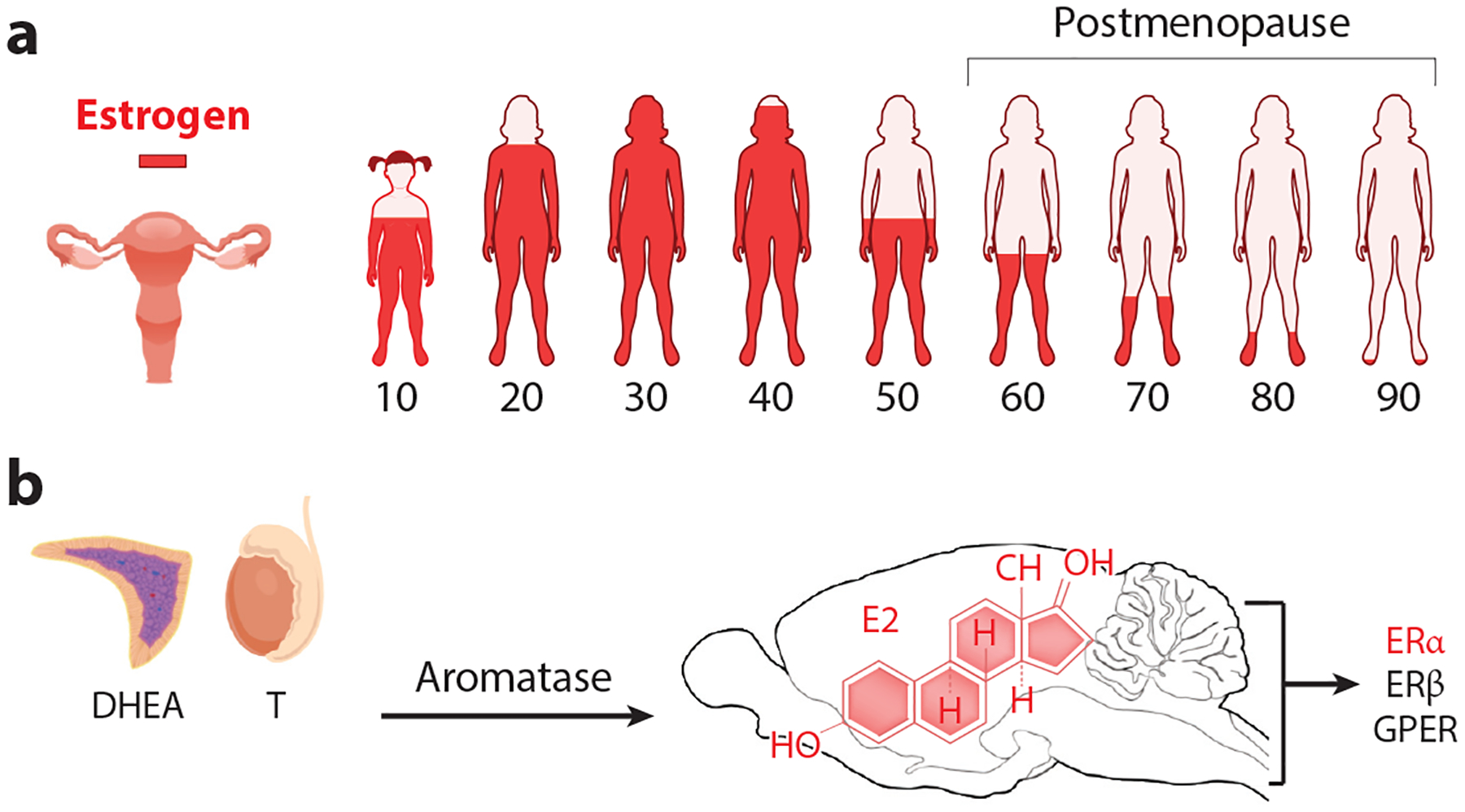
Estrogen signaling in females and the medial basal hypothalamus. (a) Levels of ovarian estrogen during lifespan in women with significant depletion beginning before menopause (~47 years old). (b) Sources of androgens from the testes or adrenals can be converted to estradiol in the gonads, tissues, or in specific brain regions expressing Cyp19A1. E2 binds three estrogen receptors, all of which are found in the brain. Abbreviations: DHEA, dehydroepiandrosterone; E2, estradiol; ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; GPER, G protein–coupled transmembrane estrogen receptor.
STEROIDS IN PRENATAL DEVELOPMENT: SCULPTING CIRCUITS AND BEHAVIOR
Over decades, neuroendocrine-behavioral research suggests that estrogen acts early in the embryonic male brain as a primary driver of masculinization, particularly in rodents. Key regions targeted by estrogens include subcortical regions that are classified as sexually dimorphic, as their molecular signatures diverge based on genetic sex. Unless otherwise noted, the terms sex-differences or sex-specific refer to chromosomal sex assignment as XX (female) or XY (male), rather than gender assignments including genetic and trans-XX males, trans-XY females, or non-binary individuals (Johns Hopkins Medical School of Medicine; https://www.hopkinsmedicine.org/news/articles/glossary-of-terms-1).
The dominant and early effects of estrogens on organizing the male rodent brain are thought to be in place a few weeks after birth, concomitant with a surge in testicular androgen biosynthesis (3). Moving into the brain freely, testosterone is then converted locally by a single enzymatic step via aromatase (P450arom) that is encoded by the gene CYP19A1. However, the notion that local estrogen biosynthesis alone establishes male behavior is not set in stone—active debate still lingers as to the importance of early estrogens on male behavior, especially in humans. In rodents, postnatal estrogen treatment does generate male-specific behaviors, and hypothalamic regions important for sexual behaviors have high Cyp19a1 expression, implying that estrogen is enriched locally in these brain regions (4). Although manipulating the Cyp19a1 gene in all (5) or discrete brain regions alters some sex-specific behaviors in both male and female mice (6), others find that masculine behaviors depend on the X-linked androgen receptor (AR, NR3C4). For example, the global knockout of AR leads to deficits in both sexual (mounting) and aggressive behaviors in male mice (7). Treatment with dihydrotestosterone (DHT), the only androgen unable to be converted to estradiol, successfully rescues sexual behavioral deficits after deleting estrogen receptor alpha (ERα, NR3A1; encoded by Esr1) in male mice, but not when AR is deleted. Eliminating both ERα and estrogen receptor beta (ERβ, NR3A2; encoded by Esr2) reduces male sexual behaviors (8). Interestingly, while genetic ablation of ERα in excitatory or inhibitory neurons using Vglut2-Cre or Vgat-Cre impairs female fertility (9), aggressive behaviors in male mice are diminished only after ablating ERα in inhibitory GABAergic neurons (10). Collectively, these findings suggest that both estrogen and androgen signaling are needed to achieve the entire repertoire of sexual behaviors. Aside from the behavioral changes, others have shown that excess androgens, specifically intracerebroventricular infusion of DHT into female mice, results in long-term metabolic changes, including insulin resistance (11). In humans, others have argued strongly that androgens are the driving force for masculinizing the male brain based on the fact that 46XY individuals with complete androgen insensitivity identify with the female gender and are sexually attracted to males (12). Although extremely rare, 46XY males with mutations in either CYP19A1 or ESR1 who cannot make or respond to estrogen, respectively, self-identify only with the male gender and present clinically with tall stature due to incomplete closure of epiphyseal growth plates in long bones (3, 13, 14). In-depth reviews covering the role of estrogen signaling in male behavior and physiology can be found elsewhere (3, 15).
Any organizational effects of estrogen on the female brain are delayed from those of males owing to the simple fact that the female ovary is thought to remain quiescent, producing few if any steroids in the fetus until the peripubertal stage in mice. For rats, a surge in estrogen begins in both sexes beginning at postnatal day 8, and in humans, a small peak occurs in females during early infancy (16). The presence of alpha fetal protein in female rodents is proposed to occlude estrogen from crossing the blood–brain barrier (BBB) (17). Delayed steroidogenesis in the ovary can be attributed to the sexually dimorphic expression of steroidogenic factor 1 (SF-1, NR5A1), which remains low in the embryonic ovary compared to elevated expression in somatic cells of the testis (18). The thorny issue of how sex steroids molecularly organize hormone-responsive circuits in the MBH to generate sex differences remains unresolved. Studies find that estrogen increases spine density and alters synaptic structure and function (19). Indeed, estradiol benzoate treatment of Purkinje cells cultured from brains lacking Cyp19a1 will reverse lower spine number and dendritic growth (19). Estrogen is also required for the cyclic increase in termini and functional connections in ventral lateral region of the ventromedial hypothalamus (VMHvl) neurons involved in female sexual behavior (20). Data from nonhuman primates suggest that working memory and spine density of pyramidal neurons improve with estradiol treatment (21). Collectively, these data might suggest that cycling hormone levels or the timing of steroid production contribute to sex differences noted in the human brain (22, 23).
GENERATING ESTROGENS IN GONADS AND THE BRAIN
To appreciate how steroids are generated and why their levels are tightly regulated during late embryonic development, it is necessary to discuss another nuclear receptor transcription factor, SF-1. Broadly speaking, SF-1 is essential for the steroidogenesis and biosynthesis of estrogen. Early in development, SF-1 is highly restricted to the bipotential gonad and adrenal gland (24), the anterior pituitary (25), and the primordial MBH (26). As the bipotential gonad and reproductive tracts become morphologically distinct in males and females, expression of SF-1 increases in the testis compared to that of the ovary (18). Essentially, this sex-specific enrichment in the testes helps ensure proper male reproductive differentiation with steroid production peaking just before birth. After birth, steroid production ramps up in females for a short window and then subsides in both sexes until hypothalamic signals awaken a new wave of gonadal steroidogenesis at puberty (16).
SF-1 is a critical factor in regulating a host of steroidogenic enzymes in the adrenal gland and gonads. SF-1 fulfills this role by controlling the rate-limiting first step that initiates the conversion of cholesterol into pregnenolone by cholesterol side-chain cleavage (P450scc, Cyp11A1). When deficient in humans, adrenal insufficiency and impaired gonadal development occur (27). The 21-carbon progesterone steroid generated by p450scc is further modified by designated hydrolases and dehydrogenases to generate endogenous androgens, including androstenedione, testosterone, dehydroepiandrosterone (DHEA), and DHT. As mentioned above, only the first three of these can undergo conversion to estradiol, the most potent of three endogenous estrogens that bind with high affinity to both ERs (Figure 1b). The closest relative of SF-1, liver receptor homolog-1, is also important for generating gonadal steroids, as reviewed in Reference 28.
Despite its pivotal role in producing sex steroids, SF-1 does not adopt the classical features of steroid hormone receptors. Instead, it is constitutively nuclear and functions as a monomer binding with a high affinity to an estrogen response element half-site. SF-1 also fails to bind cholesterol or any other steroid-like molecule. Instead, high-resolution atomic X-ray crystal structures revealed that the rodent and human SF-1 bind to phospholipids with the C16/C18 acyl chains nestled into the large hydrophobic ligand-binding pocket (29, 30). Later, Blind et al. (31) showed that the highest-affinity phospholipid ligand for SF-1 is phosphatidylinositol (3,4,5)-trisphosphate, inducing a conformational change in residues commonly mutated in patients with disorders of sexual development (31, 32). In addition, posttranslational modifications impact SF-1 activity, including phosphorylation (33) and sumoylation, with the latter acting to restrain testosterone production and Leydig cell proliferation in male mice (34). SF-1 also regulates other key genes in male sex determination, including the Y-linked male sex-determining gene, Sry, and the polypeptide anti-Müllerian hormone that destroys the developing female reproductive tract (18, 35).
Unlike the rare ESR1 or CYP19A1 mutations, NR5A1 heterozygous mutations account for a substantial percentage of 46XY disorders of sexual development (36) that can mimic the female phenotype found in SF-1 null mice; these mutant mice ultimately die due to adrenal and gonadal dysgenesis (37). Gene dosage effects are also apparent in mice by the impaired gonadal and adrenal development exhibited by Sf-1 heterozygotes (38). In the brain, SF-1 serves as a defining marker for the ventromedial hypothalamus (VMH, VMN) in the MBH. There, SF-1 appears uncoupled from steroidogenesis and instead is critical for the organization of the VMH. In mice lacking Sf-1, a notable expansion of surrounding GABAergic neurons occurs together with increased Nkx2-1 VMH precursors and loss of projections to the bed nucleus of the stria terminalis (BNST) and the amygdala (39, 40), suggesting that transcriptional targets of SF-1 mediate normal circuitry between the hypothalamus and limbic structures in the telencephalon. Lineage tracing of SF-1 shows that it appears in the earliest born neurons to populate the VMH proper, specifically in the VMHvl (41). The central nervous system (CNS)–restricted pattern of SF-1 expression to the VMH was successfully leveraged by Elmquist and colleagues (42) to genetically manipulate gene expression of the leptin receptor (LepR) gene in the VMH via a Cre-recombinase (Cre) genetic strategy. Since then, scores of studies have used the Sf-1-Cre to manipulate gene expression in all major VMH subregions. Embryonic expression of SF-1 in the VMH anlagen ensures that targeted alleles will be successfully recombined in most but not all VMHvl neurons, ranging from 50% to 80% penetrance depending on the targeted allele and the lab (43, 44). As Sf-1-Cre is also active in peripheral endocrine tissues, it becomes essential to confirm with follow-up studies that phenotypes originate in the brain. A more in-depth discussion of the VMH in coordinating female physiology is presented below.
Hormone-sensitive neurocircuits can also be influenced by the local generation of estrogen due to regional aromatase expression that often colocalizes with ERs (Figure 1b). It was shown that aromatase is highly enriched in the male and female brain, specifically in the BNST, VMHvl, medial amygdala (MeA), and preoptic area (POA), using the Cyp19a1 promoter fused to the placental alkaline phosphatase-1 tracer (4). These regions are highly enriched for ERα and implicated in behaviors that influence reproductive success, including male aggression, female lordosis, pheromone responses, and maternal behaviors. That these Cyp19a1-enriched regions reasonably reflect estrogen production has yet to be shown, as few studies have actually measured local estradiol (E2) levels. Newer data found that eliminating aromatase in Nestin-Cre;Aromfl/fl male mice reduced brain E2 by ~30% as measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) (5). All sexual behaviors were restored in Nestin-Cre;Aromfl/fl gonadectomized (GDX) male mice after replenishing with both testosterone and E2. Testosterone alone only partially restored these behaviors, underscoring the importance of local estrogen production in generating behavioral outputs. Another study found that in aged, GDX marmosets, E2 was lowered in the periphery but not in the hypothalamus after prolonged exposure to the aromatase inhibitor, letrozole; the doses used were similar to those used in breast cancer survivors (45). The only facet affected by this chronic treatment was increased female facial temperatures after a thermal challenge, suggesting that sex-specific thermosensitive neurons are sensitive to local estrogen. In both men and women, positron emission tomography and the aromatase radiotracer [11C]vorozole revealed differences in aromatase availability that correlated with lower self-control and obesity (46). Further efforts that couple accurate E2 measurements with either Cyp19a1 gene manipulations or pharmacological inhibition of aromatase activity are needed to fully define how regional E2 synthesis impacts brain function.
WHICH ESTROGEN RECEPTOR PREDOMINATES IN THE FEMALE BRAIN?
Evolutionary protein reconstruction supports the idea that the original ancestral steroid receptor that evolved hundreds of millions ago is most similar to ERα and ERβ based on ligand-binding domain (LBD) structures (47). Both of these nuclear receptors are highly enriched in subcortical and cortical brain regions, often exhibiting nonoverlapping patterns. As a side note, these estrogen sensors reside primarily in the nucleus in a ligand-independent manner in contrast to other steroid hormone receptors (Figure 2). Using an ERβ fused to red fluorescent protein reporter, reciprocal expression patterns of ERα in the arcuate nucleus (ARC) and VMHvl and ERβ in the paraventricular hypothalamic nucleus (PVN) can be observed (48). Other nuclei, including the medial preoptic area (MPOA), MeA, and BNST, show expression of both receptors in the rat brain (49). Functional comparisons of ERα and ERβ null mice demonstrate a requirement for ERα to maintain metabolic and body weight homeostasis in females and males (50–53), which is mirrored by Cyp19a1 knockout mice. Weight gain in ERα and aromatase loss-of-function mutants results from decreased energy expenditure, defined as the energy necessary to support basic cellular and organ function, thermoregulation, digestion, and energy expended during physical activity, as discussed below.
Figure 2.
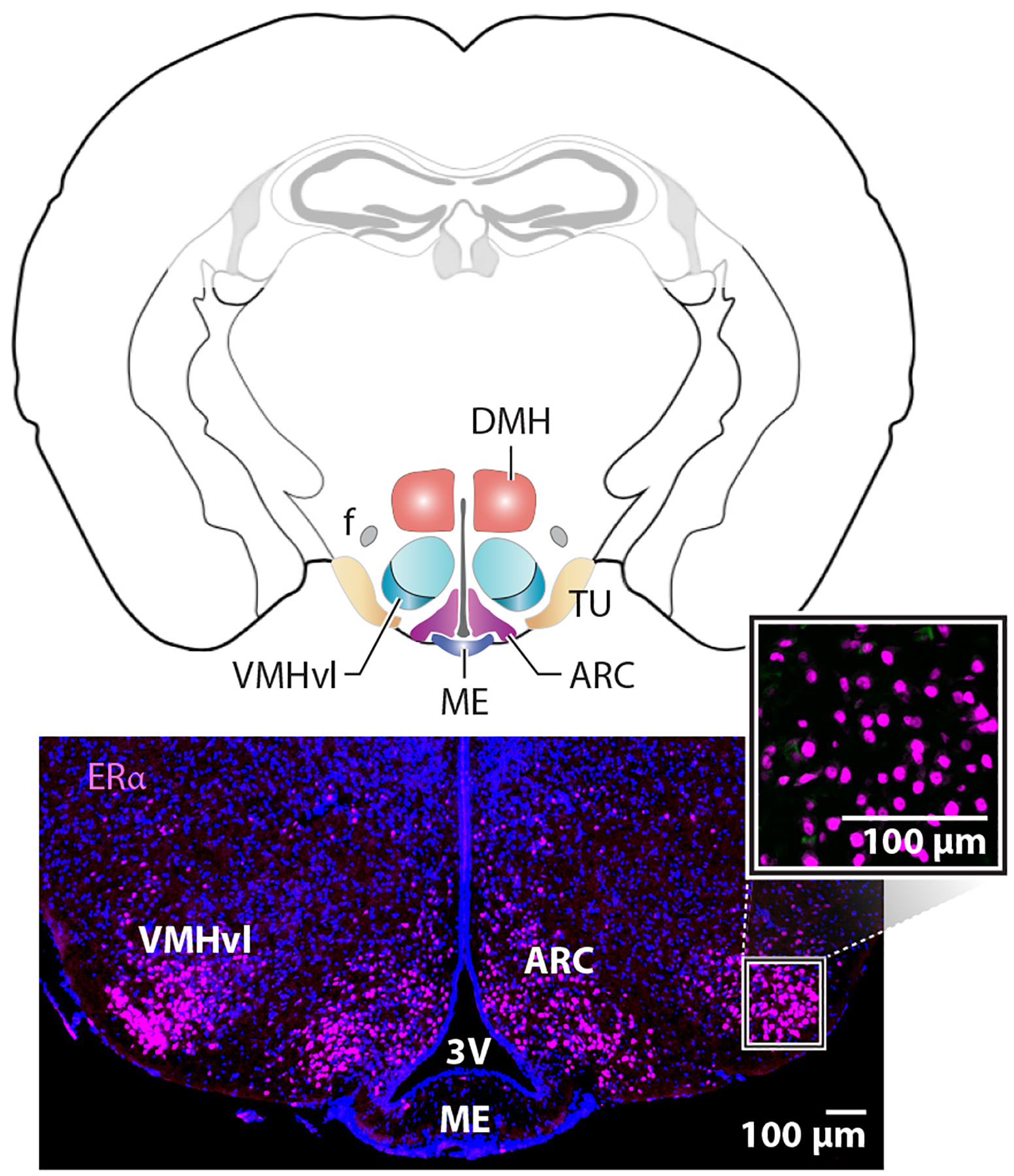
Enriched expression of estrogen receptor alpha (ERα) in the female medial basal hypothalamus is independent of gonadal hormones. Coronal mouse brain schematic with colored regions of interest, including the ventral lateral region of the ventromedial hypothalamus (VMHvl), the arcuate nucleus (ARC), and the tuberal nucleus (TU). Other landmarks include the fornix (f), dorsal medial hypothalamus (DMH), median eminence (ME), and third ventricle (3V). Lower panels show the expression of ERα in the VMHvl and ARC. The higher magnification panel shows nuclear staining at basal hormone levels or in estrogen-depleted ovariectomized females.
Expression of the third estrogen receptor, a G protein–coupled transmembrane estrogen receptor (GPER, GPR30), is reported in the rodent brain but remains understudied compared to ERs (54). That the synthetic ligand GC-1 (Tespria) of GPER reversed some but not all hypometabolic phenotypes and reduced markers of inflammation in obese, ovariectomized females suggest that pharmacological activation of GPER1 partially mitigates the loss of ovarian hormones. Interestingly, hyperphagia, bone loss, and hypoactivity associated with estrogen depletion were unaffected by GC-1 treatment (55). It will be interesting to determine whether the molecularly distinct transduction pathway of GPER regulates unique physiological end points or perhaps functions in non-neuronal brain populations, such as microglia.
Although molecular details of central estrogen signaling are scant compared to those published for peripheral tissues, it is reasonable to assume that ERs function through a classic ligand-dependent model, with endogenous or synthetic ligands inducing conformational changes in the helical bundle of the LBD that then triggers coactivator recruitment (56). Both ERs form homodimers and are recruited to the classical palindromic two half-sites. A big question about the brain is whether there are similar or distinct targets compared to the wealth of data already published for immortalized cultured cells. Several groups have already compiled differentially expressed genes after genetic deletion of ERα and ERβ or after ovariectomy and estrogen treatment (57, 58) and readily identified well-known ERα targets, such as Greb1 and Pgr (59). However, scores of new potential gene targets have also emerged, many of which are key transducers of neuropeptide signaling, dopamine signaling (Slc6a3), prolactin signaling (Prlr), and more.
Obtaining a comprehensive integrative omics map of ERα targets in the VMHvl, BNST, ARC, and other regions faces two substantial technical hurdles. First, immortalized cell lines that reflect the neuronal complexity of estrogen-responsive neuron clusters do not exist; second, standard chromatin immunoprecipitation (ChIP) methods are simply not feasible, requiring a minimum of 5–10 million cells. Tollkuhn’s group (60) successfully adapted CUT&RUN technology (61) to define ERα targets in subcortical brain regions in just 400,000 nuclei—this method designed to be used on a small number of cells revealed some surprising findings. They report that most neuronal ERα targets are distinct from those identified in peripheral tissues or immortalized cells. Moreover, in contrast to ChIP-seq data sets obtained from breast cancer cell lines (62), where ERα cooperates with the forkhead box protein FoxA1 (63), these new CUT&RUN data obtained from the brain reveal that ERα and nuclear factor I X-type are recruited to genes associated with sex-biased psychiatric disorders (60). Defining ERβ and progesterone receptor (PR) brain targets via this method will ultimately generate a comprehensive molecular framework to understand central hormone action.
OPTIMIZING FERTILITY IN FEMALES
The energetic costs of mammalian reproduction require intricate coordination with metabolic homeostasis. Both under- and overweight deviations affect the onset of fertility and maintenance of the reproductive cycle in females, as reviewed in 64. Furthermore, cyclical changes in the reproductive endocrine axis that support the ovulatory cycle also modulate the balance between energy storage and energy utilization. For females, estrogen production begins at puberty to ensure coordination of egg maturation and release with egg implantation. After puberty, cyclic changes in hormones follow a stereotypic pattern with estrogen exerting positive-feedback regulation resulting in the ovulatory surge. This preovulatory rise in estrogen is coupled with significant physiological and behavioral changes, as Slonaker documented nearly 100 years ago (65). In hindsight, these early descriptive studies set up the hypothesis that intrinsic changes in female physiology direct major adaptive responses in locomotor activity and ingestive behaviors beginning at puberty. Similar behavioral changes were not observed in male rats. Moreover, the periodic bursts of activity dissipate, eventually evolving into a sedentary, inactive, and infertile state in both ovariectomized (OVX) and aged female rats. Aside from the logistics of egg production and fertilization requirements, a host of metabolic and behavioral changes are needed to capitalize on the potential fertilization of oocytes. These changes are all directed by central estrogen signaling.
Studies published over the last decade have begun to provide anatomical and mechanistic insights into the programs that coordinate reproductive physiology, energy homeostasis, and behavioral changes to maximize reproductive success. Understanding these physiological processes will likely impact many facets of human medicine, from understanding how the reproductive system ages with time, how the central circuits are affected by natural or drug-induced menopause, and what pathways might be tapped in the future to circumvent the use of estrogen in hormone replacement therapy. Below, we detail the current work outlining the effects of estrogen in two MBH regions, the ARC and VMHvl.
NEURONAL DIVERSITY AND PRIVILEGED LOCATION OF THE ARCUATE NUCLEUS
Historically, the ARC was characterized simply as a ball of cells near the third ventricle that controlled pituitary cell size. Nearly a century later, researchers discovered that the ARC is a critical sensor of nutrient and hormonal signals and directs a host of physiological responses in metabolism and reproduction (66). ARC development begins in the mouse at embryonic day (E) 10 (67). This process will eventually give rise to at least eight distinct neuron subtypes in the adult rodent, as annotated by the expression of signature neuropeptides or neurotransmitters, including Agouti-related peptide/neuropeptide Y (AgRP/NPY), pro-opiomelanocortin (POMC), kisspeptin (Kiss1/Tac2), kisspeptin and neurokinin B dynorphin (KnDy), somatostatin (SST), growth hormone–releasing hormone (GHRH), tyrosine hydroxylase/dopamine transporter, and cocaine and amphetamine-regulated transcript (CART) neurons (68). All eight of these major neuron subtypes are expressed in both male and female rodents. Recent single-cell RNA-sequencing (scRNA-seq) of the ARC supports further subdivision of these major cell types, underscoring the increasing cellular complexity within this small hypothalamic region (68–70). The developmental pathways contributing to the birth and development of POMC neurons are the most detailed, as reviewed elsewhere (67). Lineage-tracing and fate-mapping experiments determined that ARC Pomc-expressing progenitor cells further develop into NPY, GHRH, and KISS1-expressing neurons (71). For this latter cell type, recent studies find that ARCKiss1 neurons exhibit enriched expression of Esr1, Sox14, NR5a2, and Ar (68), and are detected as early as E13.5 (72).
The ARC resides in an anatomically favorable location in the MBH, just lateral to the third ventricle, where circulating hormones and nutrients can easily pass through the BBB (73, 74). Indeed, some ARC neurons are positioned outside the BBB, with axons projecting through the median eminence, allowing them to sense circulating nutrient cues from blood or cerebral spinal fluid (73, 74). The ARC is also positioned adjacent to tanycytes that line the ventral region of the third ventricle dorsal to the median eminence. These specialized elongated cells with neuron-like processes make synaptic-like contacts with blood, cerebral spinal fluid, and hypothalamic neurons to transmit information about circulating nutrients to effector neurons controlling aspects of energy utilization (75, 76). Studies demonstrate that activated tanycytes induce hyperphagia in rodents in the fed state and alter ARCNPY or ARCPOMC neuron function (77). Interestingly, sex-dependent metabolic phenotypes have emerged after genetic manipulation of tanycytes, consistent with the fact that tanycytes themselves are estrogen-sensitive (78). Recently it was shown that a conditional knockout of tanycytes using the Rax-Cre;ERT2 allele crossed to the Eno2-IsI-DTA allele resulted in increased visceral adiposity and insulin insensitivity in male, but not female mice (79). The juxtaposition of the ARC to these nutrient-sensing cells may facilitate the plasticity needed to coordinate sex-specific energy balance with hormonal fluctuations.
ESTROGEN SIGNALING AS AN ENERGY SENSOR IN THE ARCUATE NUCLEUS
Currently, most neuronal subtypes in the ARC are directly or indirectly sensitive to estrogen signaling. The ARC expresses Cyp19a1, albeit at lower levels than other regions, such as the VMH (80), with higher levels observed in males. It is unclear whether this sex difference leads to elevated local estrogen levels in the male ARC. However, both negative feedback in the hypothalamus-pituitary-gonadal axis (HPG) axis and maintenance of ARCTH (dopaminergic) neurons appear compromised in the global aromatase knockout (5, 81). Bulk RNA-seq of the mouse ARC demonstrates that estrogen signaling regulates genes associated with inflammation, cell signaling, and neurotransmission (58). Newer scRNA-seq data report that each neuronal cluster in the ARC, as defined by their signature neuropeptide/neurotransmitter, expresses differing ERα levels, presumably to tune their output responses (68–70). This fine-tuning would allow ARCERα-responsive neurons to control energy homeostasis and optimize reproductive fitness by changing their sensitivity to oscillating estrogen levels during the estrous cycle. As estrogen levels fluctuate during the estrous cycle, so do energetic outputs such as food intake. For example, high circulating estrogen levels at the apex of follicular maturation and impending ovulation would redirect energetic resources toward activities associated with mate seeking rather than food consumption (43, 44, 82). The molecular details of ERα actions in ARCERα neurons have yet to be fully elucidated. Two downstream ERα targets in the ARC (Kiss1, Tac2) are regulated by direct recruitment to estrogen response elements and indirect mechanisms, possibly by tethering to DNA via cofactor recruitment (58). Nongenomic, fast-acting membrane-associated ERα activity is also implicated, especially in POMC and AgRP/NPY neurons that exhibit an influx of calcium in response to estrogen (83). Regardless of the exact molecular mechanism, sensing and responding to estrogen signaling allows specialized neurons in the ARC to sense available resources and elicit a physiological response (84). Newer experimental approaches have helped solidify concepts initially proposed over 40 years ago on the role of central estrogen signaling in female reproduction (85). Below, we summarize current literature on how ARC neurons deconstruct estrogenic cues to regulate energy homeostasis and discuss new wrinkles that broaden our views on how central estrogen affects food intake and modulates hormonal fluctuations during the reproductive cycle.
Despite the essential role of the ARC in controlling food consumption, it is less certain how ARCERα signaling regulates this process. Conflicting evidence either supports or questions direct control of hunger and satiety pathways by ARCERα neurons. Estrogen signaling in the ARC is proposed to impact food intake by directly modulating communication between ARCPOMC and ARCAgRP neurons or between ARCKiss1 and ARCPOMC/AgRP neurons. Approximately 20–30% of ARCPOMC/CART anorexigenic neurons that signal satiety also express ERα (43) and synapse onto neighboring orexigenic AgRP/NPY neurons (86). These neurons have reciprocal projections back onto POMC neurons presumably for coordinating responses to hunger and satiety cues. Consistent with this idea, genetic deletion of ERα in ARCPOMC neurons using the POMC-Cre driver (POMC-Cre;Esr1fl/fl) elevates Vglut2 transcripts and the spontaneous firing rate of POMC neurons (83). This increase in spontaneous firing rate enhances the release of the excitatory neurotransmitter glutamate and/or the inhibitory neurotransmitter β-endorphin onto neighboring NPY neurons (83). Within ARCNPY neurons, estrogen upregulates Opmr1 (encoding the μ-opioid receptor) and sensitizes responses to β-endorphin released from neighboring ARCPOMC neurons, resulting in a decrease in food intake (83). Estradiol also blunts the orexigenic effects (or hyperphagia) when NPY, but not AgRP, is injected intracerebroventricularly in OVX rats (87). In light of these results, it was unexpected to find that ERα is completely excluded from AgRP/NPY neurons, implying indirect regulation by estrogen, possibly in the liver (88) or by other ERα-positive ARC neurons.
Although food intake increases in POMC-Cre;Esr1fl/fl mice, where estrogen signaling would be blunted in the ARC (43), two studies from our group that abolished all or most estrogen signaling in the ARC failed to find any overt phenotype in food intake during the dark cycle. Indeed, eliminating ERα in the ARC in Nkx2-1Cre;Esr1fl/fl during development (59) or by stereotaxic delivery of AAV-Cre to the ARC of Esr1fl/fl adult mice (57) showed no overt changes in food intake in female mice. Observed differences from these studies could arise from institutional differences in assay parameters and data collection (89) or result from the Cre drivers used. A further complication arises from the expanded expression of POMC-Cre in ARC and non-ARC neurons during development (67, 71). These data open the possibility that ERα signaling in the ARC is not the only driver of estrogen-regulated food intake in female mice. Other estrogen-sensitive brain regions, including the nucleus tractus solitarius and the dorsal raphe nucleus, also express POMC and could easily contribute to the anorexigenic behavior in female rodents (90, 91).
At a cellular level, ERα-sensitive ARCKiss1 neurons excite POMC neurons by three mechanisms: (a) through a sodium/calcium exchanger (92), (b) their regulation of fast-acting glutamatergic synaptic input to POMC and AgRP/NPY neurons (93), and (c) their sensitivity to hunger cues by upregulating the growth hormone secretagogue receptor, GHS-R (94). Interestingly, chemogenetic activation of ARCKiss1 neurons using AAV-DIO-hM3Dq driven by the Kiss1-Cre driver failed to alter feeding during the dark cycle (95). In contrast, silencing ARCKiss1 neurons by Cre-dependent expression of tetanus toxin shifted the circadian rhythm of food intake in female mice from the dark to light phase (96). Clearly, additional studies are needed to understand how estrogen signaling in the ARC and elsewhere successfully resets the drive for food to meet reproductive demands.
KISSPEPTIN NEURONS: ESTROGEN SIGNALING AND REPRODUCTION
Estrogen controls different facets of reproduction by modulating positive and negative feedback from the ovary to the brain using the HPG axis. Arguably, the most well-studied of all estrogen-sensitive ARC neurons are ARCKiss1 neurons. Neurons marked by Kiss1 expression are also sprinkled in the anteroventral periventricular (AVPV) nucleus (AVPVKiss1), the medial amygdala (MeAKiss1), and the preoptic area (POAKiss1). Kiss1 signaling plays a critical role in driving the onset of puberty, as shown by disorders of puberty in mice and humans harboring genetic mutations in either KISS1 or its cognate receptor, G protein–coupled receptor 54 (GPCR54, KISS1R) (97–100). As nearly all ARCKiss1 neurons express ERα (59, 101), they are well positioned to sense and respond to oscillating estrogen levels for controlling pulsatile gonadotropic-releasing hormone (GnRH) (102), which then initiates a chain of events beginning with the pituitary secretion of gonadotropins [follicle-stimulating hormone (FSH) and luteinizing hormone (LH)] and ending with egg maturation and ovarian steroid production. Ovarian estrogen subsequently feeds back to the brain to either generate a surge in hormone levels for ovulation (positive feedback) or maintain constant hormone levels for follicular development and implantation (negative feedback) (Figure 3).
Figure 3.
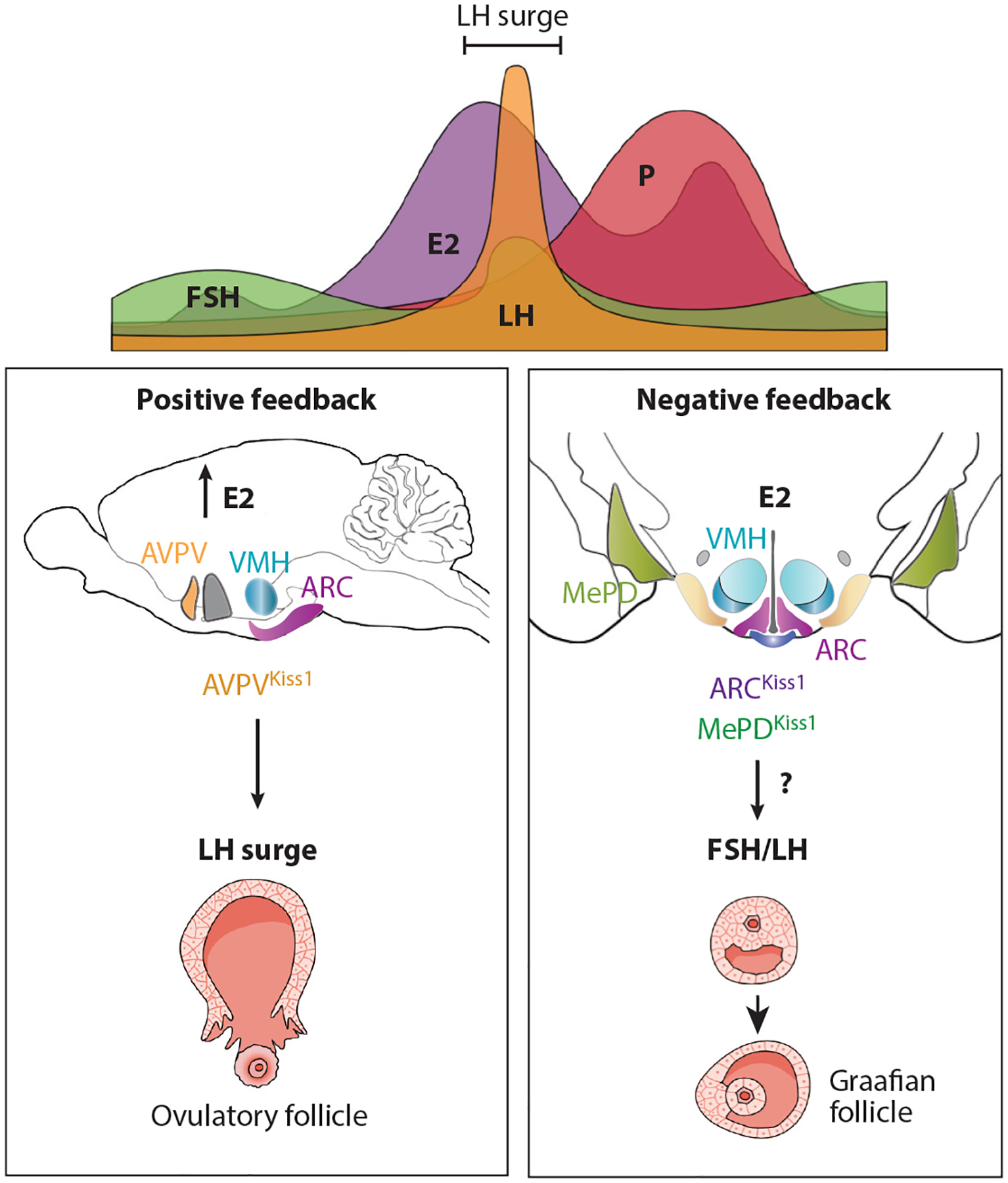
Neurons in the female reproductive cycle. The top panel shows a typical cycle of hormone fluctuations with E2 levels peaking as LH surges to then trigger ovulation. Collective work, including specific targeting of AVPVKiss1 neurons, shows that this population is critical for generating an LH surge during high E2 levels for positive feedback (left lower box). Estrogen signaling in ARCKiss1 neurons is important for maximizing the number of continuous estrous cycles: estrus, diestrus, and proestrus. The number of LH pulses or levels of LH are unchanged after deleting ERα in the ARC or ARCKiss1 neurons. Thus, other estrogen-sensitive brain regions, such as the MeA, must contribute to negative feedback when E2 and LH are low (right lower box). Abbreviations: ARC, arcuate nucleus; AVPV, anteroventral periventricular (nucleus); E2, estradiol; ERα, estrogen receptor alpha; FSH, follicle-stimulating hormone; Kiss, kisspeptin; LH, luteinizing hormone; MeA, medial amygdala; MePD, posterodorsal medial amygdala; P, progesterone; VMH, ventromedial hypothalamus.
Decades of research aimed at unraveling the complex mechanisms exerted by estrogen on the HPG axis suggest that estrogen signaling through ERα in AVPVKiss1 and ARCKiss1 neurons, but not in GnRH neurons, contributes to positive and negative feedback on the HPG (102). As reviewed in Reference 103, positive feedback occurs for a short time. In rodents, this regulatory event requires a critical threshold of circulating estrogen to increase the spontaneous firing of GnRH neurons and generate the LH surge. This period of positive feedback is characterized by increased expression of kisspeptin from AVPVKiss1 neurons in rodent brain slices. Ex vivo brain slice recordings of AVPVKiss1 neurons marked by a green fluorescent protein reporter demonstrate that estrogen increases neuron excitability and burst generation in males and females (104), suggesting that positive feedback mechanisms are conserved between sexes. To better understand positive feedback, ERα was conditionally knocked out using the Vgat-Cre (GABA) or Vglut2-Cre (Glut) for eliminating estrogen signaling in GABAergic and glutamatergic neurons, respectively. Loss of ERα signaling in glutamatergic neurons completely abolished the positive feedback loop (9). Consistent with these data, ERα signaling in all Kiss1 neurons (and other cells) is essential for positive feedback based on the finding that E2 treatment no longer elicited the expected LH surge in mouse models designed to test negative and positive feedback (105). A newer study from Moenter and colleagues (106) points to the importance of AVPVKiss1 neurons in positive feedback (Figure 3) by showing that CRISPR-Cas9 gene editing in these neurons, but not ARCKiss1 neurons, dampened the LH surge generation without affecting the estrous cycle.
Along with positive feedback, estrogen exerts negative feedback on the HPG axis to facilitate the proper maturation of the ovulatory Graafian follicle and maintain constant GnRH pulses (107). Negative feedback disappears after conditionally deleting ERα in most adult neurons using the tamoxifen-inducible CaMKIIa-Cre, resulting in high circulating LH and FSH levels (108). However, deleting ERα in just Kiss1 neurons (Kiss1-Cre;Esr1fl/fl) suggests that while estrogen signaling in ARCKiss1 neurons is critical for restraining pubertal onset owing to suppression of Kiss1 by ERα (98, 108), it appears dispensable for negative feedback (105). Further, when ERα is knocked down in ARCKiss1 neurons using CRISPR-Cas9, estrous cycles are partially disrupted, but surprisingly the number of LH pulses and levels of LH remain unchanged (106). Consistent with this report, we found that negative feedback in mice is not dependent on estrogen signaling in the ARC. Indeed, deleting ERα by stereotaxic delivery of adeno-associated virus (AAV)-Cre to the ARC of adult female Esr1fl/fl mice failed to increase uterine weights or elevate LH and circulating E2 (59), suggesting that facets of negative feedback are driven by estrogen signaling in other hypothalamic or nonhypothalamic nuclei (Figure 3). On that note, the pituitary may also be a critical site of negative feedback based on high LH levels in mouse models that delete ERα using an alphaGSU-Cre driver (109). Recently, chemogenetic activation of posterodorsal medial amygdala (MePDKiss1) neurons in female mice led to an LH surge that involves activation of neurokinin B signaling (110). Collectively, these studies suggest that understanding the precise role of estrogen in negative feedback during the female reproductive cycle will continue to evolve.
KISSPEPTIN NEURONS LINKING REPRODUCTION TO SKELETAL PHYSIOLOGY
Women suffering from a critically low body mass index, anorexia, or morbid obesity often suffer from hypogonadism, infertility, and bone disorders. As mentioned above, ERα-expressing ARCKiss1 neurons are developmentally derived from POMC-expressing precursors and are molecularly competent to sense a variety of nutrients (111) owing to their regulation by orexigenic AgRP neurons (112). Stimulating AgRP neurons by starvation or optogenetic activation inhibits ARCKiss1 neurons and results in infertility and impaired bone mass (112, 113). Recently our lab and others’ identified a female-specific node in the ARC that normally restrains high bone mass, strengthening the relationship between central estrogen signaling and energy allocation (59, 114). Using complementary approaches to conditionally delete ERα in ARCKiss1 neurons, we showed that in both genetic (Nkx2-1Cre;Esr1fl/fl and Kiss1Cre;Esr1fl/fl) and AAV-Cre-directed knockout mouse models, a marked sex-dependent increase in volumetric bone mass resulted (~500% increase) that was accompanied by higher mechanical strength as shown in 3-point bending and L5 vertebrae crush assays. Changes in bone mass were sex specific, occurring in female mice only. No compensatory changes were observed in circulating pituitary hormones, including FSH, a known skeletal effector (59, 115). Importantly, E2, as measured by LC-MS/MS, could not account for the bone phenotype in all three models. The high bone mass phenotype persisted in OVX and aged females (59). These data infer that central estrogen signaling in the brain directly or indirectly places a break on prepubertal bone growth in female rodents (Figure 4). The exact connection between prepubertal longitudinal bone growth, growth hormone, and estrogen signaling is still being worked out. Conditional deletion of ERα in POMC neurons was also found to modestly increase estrogen-sensitive cortical bone mass in females (114), possibly reflecting the fact that ARCKiss1 and ARCPOMC neurons share a common developmental precursor (71).
Figure 4.
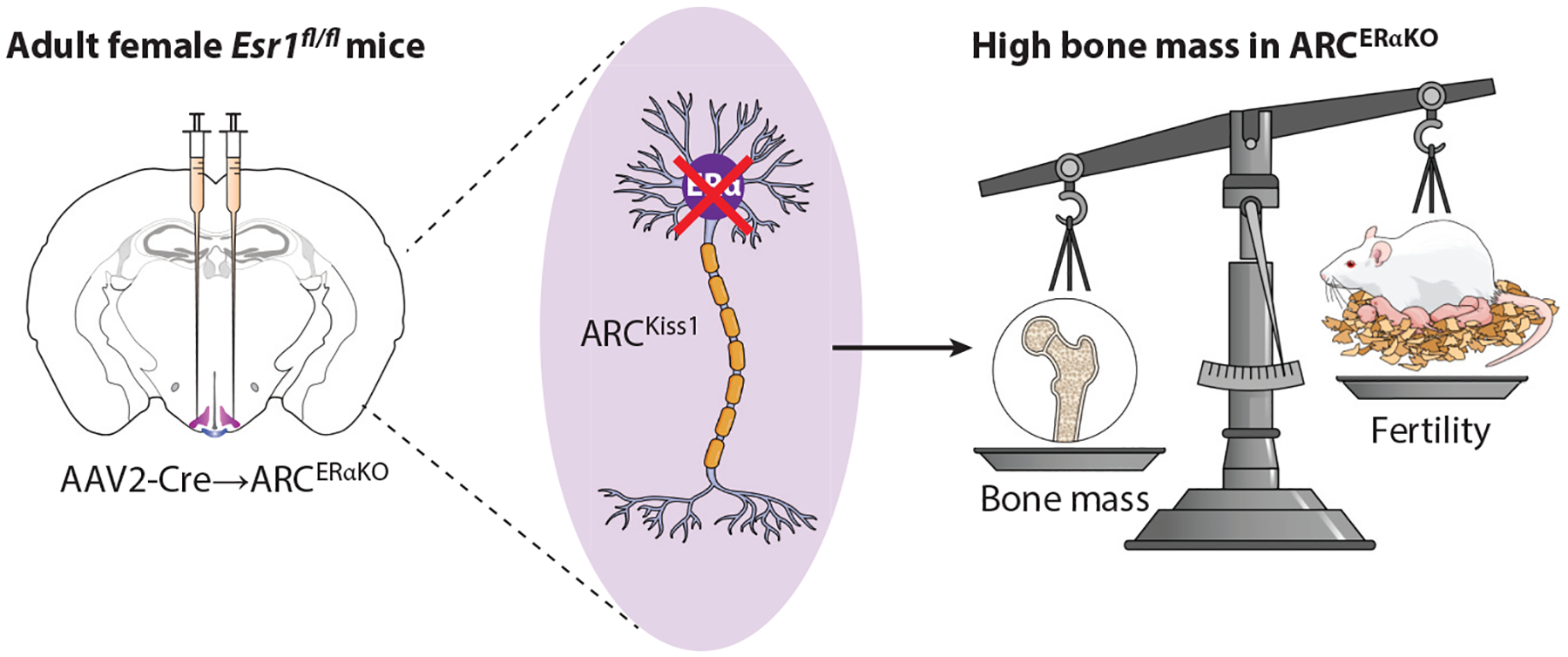
Estrogen signaling in female ARCKiss1 neurons restrains bone. Scheme showing stereotaxic elimination of Esr1fl/fl in the ARC. Other genetic methods have also been used and show a significant increase in bone mass or high bone mass phenotype with drop infertility. The underlying molecular mechanism for this brain-to-bone connection remains to be determined. Abbreviations: AAV, adeno-associated virus; ARC, arcuate nucleus; Kiss, kisspeptin; KO, knockout; VMHvl, ventral lateral region of the ventromedial hypothalamus.
Another subset in the ARC worth considering is a dorsal medial cluster expressing Slc6a3, which is upregulated by estrogen. ARCKiss1 neurons are thought to inhibit dopaminergic neurons, resulting in an estrogen-dependent release of prolactin (Prl) (116). It will be interesting to determine how these estrogen-responsive ARC modules (ARCKiss1 and ARCSlc6a3) coordinate female bone and energy metabolism, especially during pregnancy. Currently, the limited efficacy of Slc6a3-Cre in the hypothalamus (117) makes it difficult to adequately assess the role of ERα in these ARCSlc6a3/ERα neurons. In summary, we speculate that when needed, estrogen-sensitive ARCKiss1 neurons limit bone formation, allowing for the reallocation of energetic outputs during the preovulatory period or late stages of pregnancy. These normal restraints are gone once this circuit is severed and high bone mass forms (118). The existence of this brain-to-bone node in ARCKiss1 neurons raises an obvious question: Is this increase in bone formation achieved by a neuron- or hormone-based mechanism?
THE VMHvl: A DIVERSE CLUSTER OF ESTROGEN-RESPONSIVE NEURONS
The VMH consists of distinct modules—defined by their cytoarchitecture, gene expression, and function—that control multiple aspects of behavior and physiology in both males and females. Structure-function studies of the VMH collectively support a division in which the dorsome-dial/central VMH subnucleus operates independently of biological sex to regulate energy and glucose homeostasis (119–122) as well as fear and anxiety-like behavior (123–126). In contrast, the VMHvl, defined in part by ERα expression, controls male- and female-specific behaviors (social and asocial), maintains energy expenditure in females, and participates in glucose homeostasis (43, 44, 127) (Figure 5). Functional genetic tools and single-cell transcriptomics are beginning to clarify the regulatory roles of ERα signaling by providing a mechanistic framework for estrogen’s modulation of physiological and behavioral responses across reproductive life stages.
Figure 5.
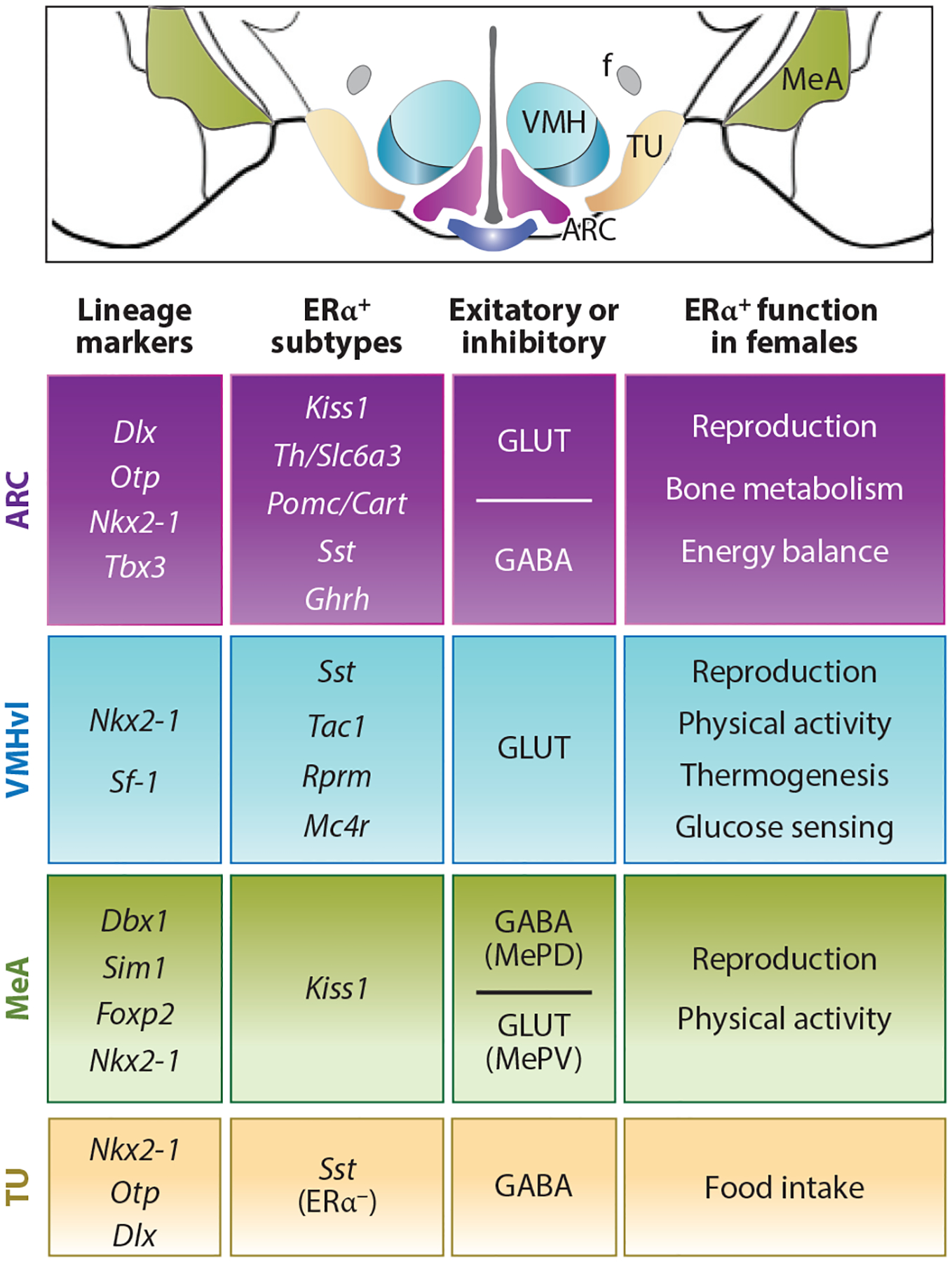
Diversity in estrogen-responsive ARC and the VMHvl neurons. Schematized coronal section through the MBH brain region. Table summarizing lineage markers, defined ERα neuron subtypes, excitatory (GLUT) or inhibitory (GABA) neurotransmitter type, and functional changes in female mice following regional knockout of ERα for the ARC, VMHvl, MeA, and TU. All four areas share Nkx2-1 as a common developmental marker, and as such, Nkx2-1-Cre will eliminate the expression of genes in these and other brain nuclei. Note that Pomc/Cart neurons include both excitatory and inhibitory subtypes. Abbreviations: ARC, arcuate nucleus; Cart, cocaine and amphetamine-regulated transcript; ERα, estrogen receptor alpha; f, fornix; GABA, gamma-aminobutyric acid; Ghrh, growth hormone–releasing hormone; GLUT, glutamate; Kiss, kisspeptin; MBH, medial basal hypothalamus; Mc4r, melanocortin-4 receptor; MeA, medial amygdala; MePD, posterodorsal medial amygdala; MePV, posteroventral medial amygdala; Pomc, proopiomelanocortin; Rprm, Reprimo; Sst, somatostatin; Th, tyrosine hydroxylase; TU, tuberal nucleus; VMHvl, ventral lateral region of the ventromedial hypothalamus.
The VMHvl exhibits several structural and molecular differences between males and females. Among these are male-specific increases in VMHvl volume, resulting from larger cell body size and greater numbers of axonal and dendritic spines (128). However, the VMHvl input/output connectivity is dynamically modulated by changes in circulating steroid hormone levels, including estrogens. Females express more ERα in the VMHvl than males, and this difference is established postnatally (44, 129–131). Further, female-biased ERα expression in the VMHvl corresponds with the emergence of sex-specific DNA methylation patterns in the Esr1 locus (132). Female VMHvl neurons also express higher levels of Pgr [encoding the PR, NR3C3 (133)]. While VMHvlPR neurons are necessary for male-specific aggressive behavior and optimize specific reproductive behaviors in male and female mice, ablating PR-expressing VMHvl neurons fails to influence body weight maintenance in either sex (133). The absence of an overt metabolic phenotype is somewhat remarkable, as nearly all (>90%) of the VMHvlPR neurons, as counted bilaterally, coexpress ERα. Thus, their loss would significantly reduce the number of ERα-positive neurons and implies that the functional segregation of VMHvlPR/ERα and VMHvlERα populations must stem from other molecular or neuroanatomical facets.
Projections from the PR-expressing VMHvl neurons exhibit sex differences. Female VMHvlPR neurons densely innervate the AVPV, a brain region enriched for both ERα and Kiss1 and one critical for female-specific reproductive behaviors, as discussed above (20, 133). This sex difference is unlikely to result from more VMHvlPR neurons in females, as other target regions, such as the MPOA and the periaqueductal gray, do not exhibit a sex bias in VMHvlPR projection densities. It is worth noting that a brain-wide analysis of VMHvlERα neurons failed to identify any notable sex differences in the afferent or efferent projections (134). As more granular approaches are used to precisely map projections from molecularly distinct VMHvlERα subsets, the influence of sex and hormone status on VMHvlERα projections might become more evident.
Sex differences between male and female gene expression programs in the VMHvl appear to depend almost exclusively on gonadal hormones rather than sex chromosomes (135). Indeed, new evidence from scRNA-seq data of VMHvl neurons supports a primary role for gonads in establishing molecular and organizational sex differences. Two separate studies identified a female subset of VMHvl neurons, marked by Reprimo (Rprm) and at least one male-specific subset marked by Prodynorphin (Pdyn) (136, 137). Although Rprm expression is nearly absent in males, paradoxically, its expression in females is independent of circulating estrogens or functional ERα in the VMHvl. Additionally, sex chromosomes are not involved in the sex-specific repression of Rprm in males, as this female-enriched transcript is extinguished in XX female mice harboring male gonads. Thus, the male-specific repression of Rprm expression may arise from early organizational changes in response to gonadal hormones. Furthermore, male-biased Pdyn expression is also hormone dependent, though in this case, ongoing testicular hormone production is required for its male-biased expression in the VMHvl. Finally, the sex-biased gene expression arising from testes-dependent hormones in the VMHvl has been observed for other subcortical brain regions, including the periventricular nucleus, POA, the ventral premammillary nucleus, the amygdala, and the BNST (135). Together, these data underscore the role of steroid hormones rather than sex chromosomes in the organization and maintenance of sex differences in neuropeptide signaling and other neuroeffectors.
Given the variety of reproductive and metabolic functions attributed to VMHvlERα neurons, it is unsurprising that scRNA-seq profiling and targeted functional manipulations would reveal significant molecular heterogeneity among VMHvlERα neurons. Indeed, molecular, functional, and anatomical differences have been identified among the hundreds of ERα+ neurons present in the VMHvl, with the caveat that these signatures depend on the positioning of neurons with a 3D space (134, 136, 138). In some cases, a clear functional distinction can be made within this heterogeneity. For example, more medially located VMHvlERα neurons are active during episodes of female-intruder aggression. In contrast, ERα neurons on the lateral edge of the VMHvl appear to be engaged during reproductive behaviors (138). However, a clear-cut, simple correlation between different molecular clusters with either functional output or anatomical projections has failed to emerge (136). The lack of a simple one-to-one ratio between molecular, anatomical, and functional features suggests that distinct modules within heterogeneous VMHvlERα neurons driving behavioral and physiological subroutines combine to elicit optimal output for a given set of metabolic, reproductive, or social cues.
ESTROGEN CONTROLS MODULES OF ENERGY EXPENDITURE IN VMHvl NEURONS
Several metabolic dysregulations observed in ERα null mice are recapitulated by conditional ERα knockout in the CNS and peripheral nervous system using Nestin-Cre (43). Both male and female Nestin-Cre;Esr1fl/fl mice exhibit increased body weight and visceral fat mass. Similar to ERα null mice, the obesogenic phenotype in these conditional knockout animals is accompanied by decreased energy expenditure, including a drop in spontaneous physical activity. However, unlike ERα null mice, Nestin-Cre;Esr1fl/fl mice are hyperphagic; whether their increased food intake results from a behavioral change or simply reflects an increase in body weight remains to be determined. The obesogenic phenotype in both global ERα knockout and Nestin-Cre;Esr1fl/fl mice occurs with sexual maturity, suggesting that gonadal hormones must maintain and activate CNS circuits that regulate energy balance.
Through a variety of targeted ERα knockdown and knockout studies as well as chemogenetic manipulations, the VMHvl has emerged as a critical estrogen-sensitive node responsible for controlling female energy expenditure, thus relegating the regulation of food intake to ERα-expressing neurons in other brain regions. Manipulating rodent VMHvlERα neurons by various techniques reveals that this cluster controls female energy expenditure by regulating spontaneous physical activity and brown adipose tissue thermogenesis (43, 44, 137, 139, 140). By contrast, MeAERα neurons are essential for normal physical activity levels in both sexes (141), illustrating that central control of energy expenditure by ERα is not limited to female-specific modules, as found for the VMHvl.
Two conditional ERα knockout models, neither of which fully eliminates ERα in the VMHvl (43, 44), revealed an interesting functional dichotomy for VMHvlERα neurons. Initially, Xu et al. (43) utilized Sf-1-Cre to ablate ERα expression specifically from the VMH while sparing its expression in the rest of the CNS (recall that all neurons in the VMH lineage transiently express SF-1). Notably, ERα expression persists in a subset (~50%) of VMHvl neurons in these Sf-1-Cre;Esr1fl/fl mice. Increased visceral adiposity specifically in females mirrors the visceral adiposity seen in the global ERα null mouse models (52), along with underlying hypometabolism resulting from impaired thermogenic capacity. However, spontaneous physical activity was unaffected in this model. In addition, female Sf-1-Cre;Esr1fl/fl knockout mice are infertile. Correa and colleagues (44) used a different approach to indirectly target ERα expression in the VMHvl after eliminating the homeobox transcription factor Nkx2-1, which is critical for early embryonic hypothalamic development, including the VMH. Using the same Sf-1-Cre driver, Sf-1-Cre;Nkx2-1fl/fl mutant females were missing ~26% of the VMHvlERα neurons. Similar to Sf-1-Cre;Esr1fl/fl, these Sf-1-Cre;Nkx2-1fl/fl mice were not hyperphagic. However, the neurons lost in Sf-1-Cre;Nkx2-1fl/fl mice appeared to be functionally distinct from those affected in the Sf-1-Cre;Esr1fl/fl mice, since neither female fertility nor brown adipose tissue thermogenic capacity was affected. Instead, weight gain in Sf-1-Cre;Nkx2-1fl/fl females resulted from a reduction in spontaneous physical activity. That discrete populations of VMHvlERα neurons may contribute to distinct aspects of female energy expenditure are supported by more recent scRNA-seq profiling. Recall that Rprm expression marks a female-specific subset of VMHvlERα neurons, and targeted knockdown of this transcript by delivering small interfering RNA (siRNA) to the VMHvl specifically affected female thermogenesis but not physical activity levels (136, 137). Efforts to further parse VMHvl molecular signatures combined with physiological/behavioral studies will undoubtedly identify new functional subsets within VMHvlERα neurons.
MECHANISMS OF ERα-DEPENDENT REGULATION OF ENERGY HOMEOSTASIS
Despite ample evidence that ERα in VMHvl neurons is necessary to maintain normal energy expenditure levels in female mice, identifying direct, estrogen-sensitive target gene(s) responsible for these functional outputs has been lacking. Krause et al. (57) recently showed that ligand-activated ERα is recruited to the melanocortin-4 receptor gene (Mc4r) to upregulate its expression. This study also answers a long-standing question: How does the preovulatory estrogen surge drive behaviors, such as physical activity, to capitalize on periods of peak reproductive capacity? Using chemogenetics and CRISPR-mediated activation, we discovered that estrogen co-opts MC4R signaling in ~200 VMHvlERα neurons to drive spontaneous activity during female sexual receptivity without affecting thermogenesis or food intake (Figure 6). Together with the fact that estrogen regulates the activity of ARCPOMC neurons, which generate the endogenous ligand for MC4R (83, 84), these findings reinforce the partnership between estrogen and melanocortin signaling and further illustrate how reproductive and metabolic signals converge to satisfy the reproductive drive for females. That VMHvlMC4R neurons project to “speed” and arousal centers in the hippocampal region and hindbrain offers the first hint as to how estrogen acting in the VMHvl causes a flurry of physical activity.
Figure 6.
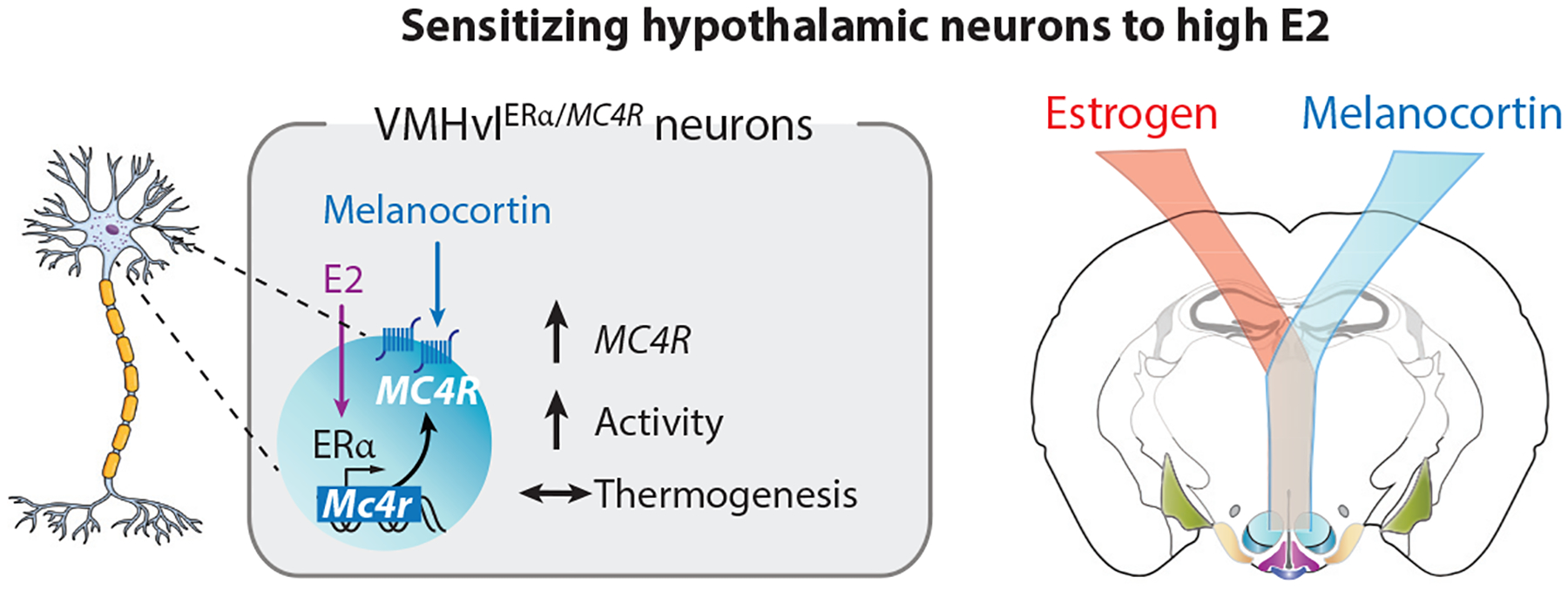
Estrogen signaling in female VMHvlERα/MC4R neurons promotes locomotor activity. High estrogen levels during proestrus or after E2 treatment increase MC4R expression in about 200 VMHvl neurons, which, when stimulated, promote physical activity. This molecular pathway demonstrates how the estrogen receptor can sensitize adaptive behaviors to prioritize energy utilization in service of reproductive fitness. Abbreviations: E2, estradiol; ERα, estrogen receptor α; MC4R, melanocortin-4 receptor; VMHvl, ventral lateral region of the ventromedial hypothalamus.
Defining the molecular consequences of ERα in the VMHvl and other brain regions is further complicated because an ERα variant lacking a functional DNA binding domain can rescue some but not all metabolic phenotypes of ERα null mice (53). Others report that the benefits of estrogens on metabolism depend solely on the activation function 2 in the LBD, inferring that coregulators rather than the DNA binding domain recruit ERα to target genes (142). As these models manipulate ERα globally, it is unclear whether these mechanisms are operational in the VMHvl. In addition, ERα has been shown to influence signal transduction cascades directly. Multiple studies have linked high E2 (natural or pharmacological) with phosphatidyl inositol-3 kinase (PI3K) and AMPK signaling pathways in the VMHvl (53, 57, 127, 140), which in turn modulates synaptic plasticity (143). Inhibiting PI3K signaling largely recapitulates phenotypes observed following the loss of estrogen signaling or ERα neurons in the VMH (43, 44) and prevents the estrogen-dependent excitement of VMHvlERα neurons (127). In addition, brain-derived neurotrophic factor (BDNF), an upstream activator of PI3K, is expressed in the VMH and particularly enriched in the VMHvl (144). VMH-targeted Bdnf knockout does not impact energy expenditure or physical activity (145). Still, conditional knockout of glutamatergic components downstream of BDNF impairs neuronal excitability and glucose homeostasis, specifically in female mice (146). Such nongenomic pathways may underlie estrogen’s ability to rapidly alter VMHvl membrane conductance and sensitivity to excitatory neurotransmitters (147).
In characterizing the impact of VMHvlERα neurons on female metabolism, it is worth considering the potential contribution of the tuberal nucleus (TU) that lies just outside the lateral edges of the shell encasing the VMH proper (Figure 5). Anatomically, TU and VMHvlERα neurons are often difficult to disentangle, especially in the more posterior regions, when the TU encroaches on the lateral edge of the VMHvl. In fact, in rats, it has been suggested that TU neurons express ERα and project to many of the same target brain regions as VMHvl neurons (148). As a result, ERα-expressing neurons in the VMHvl and TU are sometimes considered a single unit (134). However, other lines of evidence argue that the TU and VMHvl are molecularly and functionally distinct. TU neurons downregulate Nkx2-1 in the postnatal period (44, 149), which continues to be enriched in the VMHvl. TU neurons are also overwhelmingly GABAergic (150), while VMHvl neurons are glutamatergic (41, 121, 149). Moreover, targeting ERα neurons using the Vglut2-Cre ablates all ERα in the VMHvl, whereas targeting ERα with Vgat-Cre leaves it fully intact in the VMHvl (10). This study establishes that TU neurons do not contribute to the pool of ERα-expressing neurons in the VMHvl region. Finally, TU and VMHvl neurons differentially affect food intake and activity. Whereas stimulating or ablating somatostatin-expressing TU neurons drives or reduces food intake, respectively, while leaving energy expenditure unchanged (151), VMHvlERα neurons affect spontaneous activity and energy expenditure without changing food intake (44, 57, 137). In conclusion, VMHvl and TU neurons appear to represent two distinct neuronal populations that should be considered as separate anatomical and functional entities.
OTHER ESTROGEN-RESPONSIVE BRAIN REGIONS YET TO BE FULLY EXPLORED
The diverse functions of central estrogen signaling in two regions of the MBH, the ARC and VMHvl, are now beginning to come into sharp focus. With newer technologies, we have come to appreciate the diversity of neuronal subtypes within these two well-studied MBH nuclei. Following the molecular and functional annotation of hormone-responsive neurons, it becomes crucial to ask how estrogen modulates afferent/efferent projections and neuronal plasticity and how the quick pace of neurotransmission is influenced by the slower time course inherent in classic genomic signaling. More effort is needed to gain the level of clarity that has been brought to bear on well-studied cortical regions, such as the hippocampus. In addition, other brain regions are sure to contribute to sex differences in energy homeostasis and deserve further study. Among the hormone-sensitive brain regions worth considering, two of the most tantalizing sexually dimorphic regions include the preoptic area of the hypothalamus, the MPOA, and the central amygdala region, the MeA. Although these two brain regions have not yet received the same attention given to the ARC and VMHvl, three studies in the last year established that excitatory and inhibitory neurons in the MPOA and periventricular nucleus, some of which are estrogen responsive, control core body temperature (152–154). It remains to be determined whether all or just some of these thermoregulatory neurons are directly modulated by estrogen, perhaps accounting for the higher core body temperature observed in C57BL/6 female mice that is also hormone dependent (155). On a similar note, most studies seeking to classify the molecular properties of neurons and circuits that modulate female physiology have used intact or OVX adult female mice. On occasion, aged intact female mice are included. Casting a broader net to ask how estrogen-responsive neurons in the ARC or VMHvl directly or indirectly affect the late stages of pregnancy or lactation when estrogen surges or drops, respectively, will be of interest.
Finally, while much of this review is focused on estrogen signaling in neuronal function, another understudied but exciting area is the role of estrogen in central neuroinflammatory responses and metabolism, as reviewed in Reference 156. Related to this discussion, others propose that estradiol protects the female brain from inflammation by maintaining the integrity of the BBB (157). Depleting neuron-derived E2 in the forebrain by targeting Cyp19a1 increased neuroinflammation and damage and resulted in cognitive deficits in the Barnes test in a two-vessel occlusion model for generating global ischemia (158). Dietary excess easily triggers the morphological hallmarks of microgliosis in the MBH before overt obesity, but only in male mice, as reviewed elsewhere (159). This sex difference begs the question as to whether estrogen signaling in adjacent MBH neurons or microglia confers protection in females. On that note, neuroinflammatory gene markers were found depressed in microglia harvested from female brains. However, when transplanted into a focal cerebral ischemia model, their protective effects do not depend on circulating sex steroids (160, 161). Future efforts in this research area are highly relevant to understanding the onset of neurodegenerative diseases as females age.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
The identification of ERα-dependent changes in gene expression and circuit architecture is clarifying the impact of hormone signaling across cell types and timescales. Ultimately, it becomes important to know if estrogen-responsive brain nodes discovered in animal models will translate to women’s health. Two life conditions are worth considering in this context: (a) natural hormone fluctuations across lifespan that affect health ranging from metabolism to mental disorders and (b) frequently prescribed drugs that profoundly change hormone levels. An example of the first condition is human menopause, which often leads to metabolic decline, including decreased lean mass, increased visceral adiposity, and poor blood glucose regulation (162); every one of these symptoms mimics phenotypes in female rodents lacking central estrogen signaling. The widening time gap between the age of menopause onset and today’s extended longevity in women exacerbates health issues and raises a fundamental question: How can postmenopausal women achieve healthy aging in an estrogen-depleted state? This concern is further compounded for younger women subjected to premature menopause induced by hormone therapy. Examples of the second condition that involve drug therapies are plentiful. While widely used today for minimizing postmenopausal symptoms and for feminizing hormone treatment in transgender medicine, estrogen replacement therapy is not without risks. Oral contraceptives taken by millions of women override hormonal fluctuations during the menstrual cycle, masking the normal ebb and flow of estrogen on brain function.
Additionally, to prevent the reoccurrence of ER-positive breast cancer, millions of survivors are urged to take antihormonal therapy for 5–10 years. Commonly prescribed drugs include the selective ER modulator tamoxifen or aromatase inhibitors, such as anastrozole, exemestane, and letrozole. In addition, newer selective ER degraders have recently emerged that block estrogen signaling and induce degradation of ERα (56). Given that some of these drugs pass readily through the BBB, knowing how they regulate hormone-sensitive brain regions could ultimately address unwanted side effects, thereby improving premature termination of these cancer therapies. To date, only a handful of studies have addressed this issue, and more basic research in this understudied area is needed.
In the future, investigators should consider incorporating experimental parameters that better address the notable sex differences in male and female rodents. For example, using thermoneutral housing temperatures in female mice can provide a much more robust experimental model that more closely mimics the human lifestyle (163). Alternatively, leveraging new techniques, such as CRISPR-based gene editing to retool the classic lab rat model would allow one to leverage their reliable and robust physiological responses, especially when assessing reproduction. Finally, to fully appreciate how central estrogen signaling elicits appropriate metabolic and behavioral responses, the field must also work out how the slow course of hormone action effectively modulates neurotransmission. In meeting these challenges, our research community will continue to actively reshape the science of hormone action in the brain.
ACKNOWLEDGMENTS
This research is supported by the US National Institutes of Health (NIH) (R01DK121657, R01AG062331, U01 NS113869) and a GCRLE Senior Scholar Award (0320) to H.A.I., an American Heart Association (AHA) Postdoctoral Fellowship to W.C.K. (16POST27260361), and AHA and NIH funding to C.B.H. (F32 DK107115-01A1, AHA Postdoctoral Fellowship 16POST29870011, K01AG065916). We apologize in advance to all investigators and trainees whose research could not be cited because of space limitations.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, et al. 1995. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N. Engl. J. Med 332:1589–93 [DOI] [PubMed] [Google Scholar]
- 2.Arnold AP. 2004. Sex chromosomes and brain gender. Nat. Rev. Neurosci 5:701–8 [DOI] [PubMed] [Google Scholar]
- 3.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. 2017. Estrogens in male physiology. Physiol. Rev 97:995–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, et al. 2009. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks DC, Coon VJ, Ercan CM, Xu X, Dong H, et al. 2020. Brain aromatase and the regulation of sexual activity in male mice. Endocrinology 161:bqaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger EK, Burke KJ Jr., Yang CF, Bender KJ, Fuller PM, Shah NM. 2015. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 10:453–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, et al. 2004. Brain masculinization requires androgen receptor function. PNAS 101:1673–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, et al. 2000. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (αβERKO). PNAS 97:14737–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheong RY, Czieselsky K, Porteous R, Herbison AE. 2015. Expression of ESR1 in glutamatergic and GABAergic neurons is essential for normal puberty onset, estrogen feedback, and fertility in female mice. J. Neurosci 35:14533–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu MV, Tollkuhn J. 2017. Estrogen receptor alpha is required in GABAergic, but not glutamatergic, neurons to masculinize behavior. Horm. Behav 95:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro G, Allard C, Morford JJ, Xu W, Liu S, et al. 2018. Androgen excess in pancreatic β cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 3:e98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hines M, Ahmed SF, Hughes IA. 2003. Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Arch. Sex. Behav 32:93–101 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Hamilton KJ, Perera L, Wang T, Gruzdev A, et al. 2020. ESR1 mutations associated with estrogen insensitivity syndrome change conformation of ligand-receptor complex and altered transcriptome profile. Endocrinology 161:bqaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard V, Kherra S, Francou B, Fagart J, Viengchareun S, et al. 2017. Familial multiplicity of estrogen insensitivity associated with a loss-of-function ESR1 mutation. J. Clin. Endocrinol. Metab 102:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juntti SA, Coats JK, Shah NM. 2008. A genetic approach to dissect sexually dimorphic behaviors. Horm. Behav 53:627–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell MR. 2018. Comparing postnatal development of gonadal hormones and associated social behaviors in rats, mice, and humans. Endocrinology 159:2596–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, et al. 2006. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat. Neurosci 9:220–26 [DOI] [PubMed] [Google Scholar]
- 18.Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. 1994. Nuclear receptor steroidogenic factor 1 regulates the Müllerian inhibiting substance gene: a link to the sex determination cascade. Cell 77:651–61 [DOI] [PubMed] [Google Scholar]
- 19.Hara Y, Waters EM, McEwen BS, Morrison JH. 2015. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev 95:785–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue S, Yang R, Tantry A, Davis CH, Yang T, et al. 2019. Periodic remodeling in a neural circuit governs timing of female sexual behavior. Cell 179:1393–408.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, et al. 2007. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. PNAS 104:11465–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, et al. 2014. Sex differences in the structural connectome of the human brain. PNAS 111:823–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, et al. 2018. Sex differences in the adult human brain: evidence from 5216 UK Biobank participants. Cereb. Cortex 28:2959–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda Y, Shen WH, Ingraham HA, Parker KL. 1994. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol 8:654–62 [DOI] [PubMed] [Google Scholar]
- 25.Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, et al. 1994. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev 8:2302–12 [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. 1995. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol. Endocrinol 9:478–86 [DOI] [PubMed] [Google Scholar]
- 27.Buonocore F, Achermann JC. 2020. Primary adrenal insufficiency: New genetic causes and their long-term consequences. Clin. Endocrinol 92:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meinsohn MC, Smith OE, Bertolin K, Murphy BD. 2019. The orphan nuclear receptors steroidogenic factor-1 and liver receptor homolog-1: structure, regulation, and essential roles in mammalian reproduction. Physiol. Rev 99:1249–79 [DOI] [PubMed] [Google Scholar]
- 29.Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, et al. 2005. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–55 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Choi M, Cavey G, Daugherty J, Suino K, et al. 2005. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol. Cell 17:491–502 [DOI] [PubMed] [Google Scholar]
- 31.Blind RD, Sablin EP, Kuchenbecker KM, Chiu HJ, Deacon AM, et al. 2014. The signaling phospholipid PIP3 creates a new interaction surface on the nuclear receptor SF-1. PNAS 111:15054–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, et al. 2009. Structure of SF-1 bound by different phospholipids: evidence for regulatory ligands. Mol. Endocrinol 23:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, et al. 1999. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell 3:521–26 [DOI] [PubMed] [Google Scholar]
- 34.Lee FY, Faivre EJ, Suzawa M, Lontok E, Ebert D, et al. 2011. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev. Cell 21:315–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekido R, Lovell-Badge R. 2008. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453:930–34 [DOI] [PubMed] [Google Scholar]
- 36.Suntharalingham JP, Buonocore F, Duncan AJ, Achermann JC. 2015. DAX-1 (NR0B1) and steroidogenic factor-1 (SF-1, NR5A1) in human disease. Best Pract. Res. Clin. Endocrinol. Metab 29:607–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo X, Ikeda Y, Parker KL. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–90 [DOI] [PubMed] [Google Scholar]
- 38.Bland ML, Jamieson C, Akana S, Dallman M, Ingraham HA. 2000. Gene dosage effects of steroidogenic factor 1 (SF-1) in adrenal development and the stress. Endocr. Res 26:515–16 [DOI] [PubMed] [Google Scholar]
- 39.Dellovade TL, Young M, Ross EP, Henderson R, Caron K, et al. 2000. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J. Comp. Neurol 423:579–89 [DOI] [PubMed] [Google Scholar]
- 40.Tran PV, Lee MB, Marin O, Xu B, Jones KR, et al. 2003. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol. Cell. Neurosci 22:441–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung CC, Kurrasch DM, Liang JK, Ingraham HA. 2013. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. J. Comp. Neurol 521:1268–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, et al. 2006. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, et al. 2011. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14:453–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, et al. 2015. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep 10:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gervais NJ, Remage-Healey L, Starrett JR, Pollak DJ, Mong JA, Lacreuse A. 2019. Adverse effects of aromatase inhibition on the brain and behavior in a nonhuman primate. J. Neurosci 39:918–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biegon A, Alia-Klein N, Alexoff DL, Fowler JS, Kim SW, et al. 2020. Relationship of estrogen synthesis capacity in the brain with obesity and self-control in men and women. PNAS 117:22962–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eick GN, Colucci JK, Harms MJ, Ortlund EA, Thornton JW. 2012. Evolution of minimal specificity and promiscuity in steroid hormone receptors. PLOS Genet 8:e1003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagoshi S, Maejima S, Morishita M, Takenawa S, Otubo A, et al. 2020. Detection and characterization of estrogen receptor beta expression in the brain with newly developed transgenic mice. Neuroscience 438:182–97 [DOI] [PubMed] [Google Scholar]
- 49.Shughrue PJ, Lane MV, Merchenthaler I. 1999. Biologically active estrogen receptor-β: evidence from in vivo autoradiographic studies with estrogen receptor α-knockout mice. Endocrinology 140:2613–20 [DOI] [PubMed] [Google Scholar]
- 50.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-α knockout mice. PNAS 97:12729–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. 2003. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology 144:230–39 [DOI] [PubMed] [Google Scholar]
- 52.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, et al. 2000. Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem. Biophys. Res. Commun 278:640–45 [DOI] [PubMed] [Google Scholar]
- 53.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, et al. 2011. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J. Clin. Investig 121:604–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. 2009. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol 202:223–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma G, Hu C, Staquicini DI, Brigman JL, Liu M, et al. 2020. Preclinical efficacy of the GPER-selective agonist G-1 in mouse models of obesity and diabetes. Sci. Transl. Med 12:eaau5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fanning SW, Greene GL. 2019. Next-generation ERα inhibitors for endocrine-resistant ER+ breast cancer. Endocrinology 160:759–69 [DOI] [PubMed] [Google Scholar]
- 57.Krause WC, Rodriguez R, Gegenhuber B, Matharu N, Rodriguez AN, et al. 2021. Oestrogen engages brain MC4R signalling to increase physical activity in female mice. Nature 599:131–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang JA, Stires H, Belden WJ, Roepke TA. 2017. The arcuate estrogen-regulated transcriptome: estrogen response element-dependent and -independent signaling of ERα in female mice. Endocrinology 158:612–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herber CB, Krause WC, Wang L, Bayrer JR, Li A, et al. 2019. Estrogen signaling in arcuate Kiss1 neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nat. Commun 10:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gegenhuber B, Wu MV, Bronstein R, Tollkuhn J. 2021. Regulation of neural gene expression by estrogen receptor alpha. BioRxiv 349290. 10.1101/2020.10.21.349290 [DOI] [Google Scholar]
- 61.Skene PJ, Henikoff JG, Henikoff S. 2018. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc 13:1006–19zzzz [DOI] [PubMed] [Google Scholar]
- 62.Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, et al. 2007. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol. Cell. Biol 27:5090–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, et al. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 64.Della Torre S, Benedusi V, Fontana R, Maggi A. 2014. Energy metabolism and fertility: a balance preserved for female health. Nat. Rev. Endocrinol 10:13–23 [DOI] [PubMed] [Google Scholar]
- 65.Slonaker JR. 1924. The effect of pubescence, oestruatlon and menopause on the voluntary activity in the albino rat. Am. J. Physiol 68:294–315 [Google Scholar]
- 66.Andermann ML, Lowell BB. 2017. Toward a wiring diagram understanding of appetite control. Neuron 95:757–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toda C, Santoro A, Kim JD, Diano S. 2017. POMC neurons: from birth to death. Annu. Rev. Physiol 79:209–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huisman C, Cho H, Brock O, Lim SJ, Youn SM, et al. 2019. Single cell transcriptome analysis of developing arcuate nucleus neurons uncovers their key developmental regulators. Nat. Commun 10:3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam BYH, Cimino I, Polex-Wolf J, Nicole Kohnke S, Rimmington D, et al. 2017. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol. Metab 6:383–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, et al. 2017. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci 20:484–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Padilla SL, Carmody JS, Zeltser LM. 2010. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med 16:403–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U. 2014. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J. Neurosci 34:3756–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haddad-Tovolli R, Dragano NRV, Ramalho AFS, Velloso LA. 2017. Development and function of the blood-brain barrier in the context of metabolic control. Front. Neurosci 11:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yulyaningsih E, Rudenko IA, Valdearcos M, Dahlen E, Vagena E, et al. 2017. Acute lesioning and rapid repair of hypothalamic neurons outside the blood-brain barrier. Cell Rep 19:2257–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, et al. 2013. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun 4:2049. [DOI] [PubMed] [Google Scholar]
- 76.Lee DA, Bedont JL, Pak T, Wang H, Song J, et al. 2012. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci 15:700–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bolborea M, Pollatzek E, Benford H, Sotelo-Hitschfeld T, Dale N. 2020. Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. PNAS 117:14473–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prevot V, Dehouck B, Sharif A, Ciofi P, Giacobini P, Clasadonte J. 2018. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev 39:333–68 [DOI] [PubMed] [Google Scholar]
- 79.Yoo S, Cha D, Kim S, Jiang L, Cooke P, et al. 2020. Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia 68:1987–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beyer C, Green SJ, Barker PJ, Huskisson NS, Hutchison JB. 1994. Aromatase-immunoreactivity is localised specifically in neurones in the developing mouse hypothalamus and cortex. Brain Res 638:203–10 [DOI] [PubMed] [Google Scholar]
- 81.Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC. 2004. Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice. Mol. Cell. Neurosci 27:466–76 [DOI] [PubMed] [Google Scholar]
- 82.Gonzalez-Mariscal G, Melo AI, Jimenez P, Beyer C, Rosenblatt JS. 1996. Estradiol, progesterone, and prolactin regulate maternal nest-building in rabbits. J. Neuroendocrinol 8:901–7 [DOI] [PubMed] [Google Scholar]
- 83.Stincic TL, Grachev P, Bosch MA, Ronnekleiv OK, Kelly MJ. 2018. Estradiol drives the anorexigenic activity of proopiomelanocortin neurons in female mice. eNeuro 5:ENEURO.0103–18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stincic TL, Ronnekleiv OK, Kelly MJ. 2018. Diverse actions of estradiol on anorexigenic and orexigenic hypothalamic arcuate neurons. Horm. Behav 104:146–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodman RL, Knobil E. 1981. The sites of action of ovarian steroids in the regulation of LH secretion. Neuroendocrinology 32:57–63 [DOI] [PubMed] [Google Scholar]
- 86.Wang D, He X, Zhao Z, Feng Q, Lin R, et al. 2015. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santollo J, Eckel LA. 2008. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav. Brain Res 191:173–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benedusi V, Della Torre S, Mitro N, Caruso D, Oberto A, et al. 2017. Liver ERα regulates AgRP neuronal activity in the arcuate nucleus of female mice. Sci Rep 7:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corrigan JK, Ramachandran D, He Y, Palmer CJ, Jurczak MJ, et al. 2020. A big-data approach to understanding metabolic rate and response to obesity in laboratory mice. eLife 9:e53560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asarian L, Geary N. 2007. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148:5656–66 [DOI] [PubMed] [Google Scholar]
- 91.Cao X, Xu P, Oyola MG, Xia Y, Yan X, et al. 2014. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J. Clin. Investig 124:4351–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu LY, van den Pol AN. 2008. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J. Neurosci 28:5433–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu J, Rivera HM, Bosch MA, Padilla SL, Stincic TL, et al. 2018. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife 7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frazao R, Dungan Lemko HM, da Silva RP, Ratra DV, Lee CE, et al. 2014. Estradiol modulates Kiss1 neuronal response to ghrelin. Am. J. Physiol. Endocrinol. Metab 306:E606–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, et al. 2017. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat. Neurosci 20:42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Padilla SL, Perez JG, Ben-Hamo M, Johnson CW, Sanchez REA, et al. 2019. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr. Biol 29:592–604.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stafford LJ, Xia C, Ma W, Cai Y, Liu M. 2002. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res 62:5399–404 [PubMed] [Google Scholar]
- 98.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, et al. 2010. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. PNAS 107:22693–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci 29:11859–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. 2016. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology 157:4021–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frazao R, Cravo RM, Donato J Jr., Ratra DV, Clegg DJ, et al. 2013. Shift in Kiss1 cell activity requires estrogen receptor α. J. Neurosci 33:2807–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lindzey J, Jayes FL, Yates MM, Couse JF, Korach KS. 2006. The bi-modal effects of estradiol on gonadotropin synthesis and secretion in female mice are dependent on estrogen receptor-α. J. Endocrinol 191:309–17 [DOI] [PubMed] [Google Scholar]
- 103.Moenter SM, Chu Z, Christian CA. 2009. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J. Neuroendocrinol 21:327–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rønnekleiv OK, Zhang C, Bosch MA, Kelly MJ. 2015. Kisspeptin and gonadotropin-releasing hormone neuronal excitability: molecular mechanisms driven by 17β-estradiol. Neuroendocrinology 102:184–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dubois SL, Acosta-Martinez M, DeJoseph MR, Wolfe A, Radovick S, et al. 2015. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology 156:1111–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L, Vanacker C, Burger LL, Barnes T, Shah YM, et al. 2019. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife 8:e43999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dorling AA, Todman MG, Korach KS, Herbison AE. 2003. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology 78:204–9 [DOI] [PubMed] [Google Scholar]
- 108.Greenwald-Yarnell ML, Marsh C, Allison MB, Patterson CM, Kasper C, et al. 2016. ERα in Tac2 neurons regulates puberty onset in female mice. Endocrinology 157:1555–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, et al. 2008. Pituitary gonadotroph estrogen receptor-α is necessary for fertility in females. Endocrinology 149:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fergani C, Leon S, Padilla SL, Verstegen AM, Palmiter RD, Navarro VM. 2018. NKB signaling in the posterodorsal medial amygdala stimulates gonadotropin release in a kisspeptin-independent manner in female mice. eLife 7:e40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dudek M, Ziarniak K, Sliwowska JH. 2018. Kisspeptin and metabolism: the brain and beyond. Front. Endocrinol 9:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, et al. 2017. AgRP to Kiss1 neuron signaling links nutritional state and fertility. PNAS 114:2413–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim JG, Sun BH, Dietrich MO, Koch M, Yao GQ, et al. 2015. AgRP neurons regulate bone mass. Cell Rep 13:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farman HH, Windahl SH, Westberg L, Isaksson H, Egecioglu E, et al. 2016. Female mice lacking estrogen receptor-α in hypothalamic proopiomelanocortin (POMC) neurons display enhanced estrogenic response on cortical bone mass. Endocrinology 157:3242–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, et al. 2006. FSH directly regulates bone mass. Cell 125:247–60 [DOI] [PubMed] [Google Scholar]
- 116.Szawka RE, Ribeiro AB, Leite CM, Helena CV, Franci CR, et al. 2010. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology 151:3247–57 [DOI] [PubMed] [Google Scholar]
- 117.Doan KV, Kinyua AW, Yang DJ, Ko CM, Moh SH, et al. 2016. FoxO1 in dopaminergic neurons regulates energy homeostasis and targets tyrosine hydroxylase. Nat. Commun 7:12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herber CB, Ingraham HA. 2019. Should we make more bone or not, as told by Kisspeptin neurons in the arcuate nucleus. Semin. Reprod. Med 37:147–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coutinho EA, Okamoto S, Ishikawa AW, Yokota S, Wada N, et al. 2017. Activation of SF1 neurons in the ventromedial hypothalamus by DREADD technology increases insulin sensitivity in peripheral tissues. Diabetes 66:2372–86 [DOI] [PubMed] [Google Scholar]
- 120.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, et al. 2014. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab 20:1030–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, et al. 2007. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5:383–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. 1995. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44:180–84 [DOI] [PubMed] [Google Scholar]
- 123.Cheung CC, Krause WC, Edwards RH, Yang CF, Shah NM, et al. 2015. Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Mol. Metab 4:857–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kunwar PS, Zelikowsky M, Remedios R, Cai H, Yilmaz M, et al. 2015. Ventromedial hypothalamic neurons control a defensive emotion state. eLife 4:e06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, et al. 2013. Independent hypothalamic circuits for social and predator fear. Nat. Neurosci 16:1731–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viskaitis P, Irvine EE, Smith MA, Choudhury AI, Alvarez-Curto E, et al. 2017. Modulation of SF1 neuron activity coordinately regulates both feeding behavior and associated emotional states. Cell Rep 21:3559–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saito K, He Y, Yang Y, Zhu L, Wang C, et al. 2016. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Sci. Rep 6:23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flanagan-Cato LM. 2011. Sex differences in the neural circuit that mediates female sexual receptivity. Front. Neuroendocrinol 32:124–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brock O, De Mees C, Bakker J. 2015. Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice. J. Neuroendocrinol 27:264–76 [DOI] [PubMed] [Google Scholar]
- 130.Yokosuka M, Okamura H, Hayashi S. 1997. Postnatal development and sex difference in neurons containing estrogen receptor-α immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J. Comp. Neurol 389:81–93 [DOI] [PubMed] [Google Scholar]
- 131.Cao J, Patisaul HB. 2011. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats. J. Comp. Neurol 519:2954–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cisternas CD, Cortes LR, Golynker I, Castillo-Ruiz A, Forger NG. 2020. Neonatal inhibition of DNA methylation disrupts testosterone-dependent masculinization of neurochemical phenotype. Endocrinology 161:bqz022. [DOI] [PubMed] [Google Scholar]
- 133.Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, et al. 2013. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153:896–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lo L, Yao S, Kim DW, Cetin A, Harris J, et al. 2019. Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. PNAS 116:7503–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, et al. 2012. Modular genetic control of sexually dimorphic behaviors. Cell 148:596–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim DW, Yao Z, Graybuck LT, Kim TK, Nguyen TN, et al. 2019. Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell 179:713–28.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Veen JE, Kammel LG, Bunda PC, Shum M, Reid MS, et al. 2020. Hypothalamic estrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat. Metab 2:351–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, et al. 2017. Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci 20:1580–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, et al. 2007. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. PNAS 104:2501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, et al. 2014. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu P, Cao X, He Y, Zhu L, Yang Y, et al. 2015. Estrogen receptor-α in medial amygdala neurons regulates body weight. J. Clin. Investig 125:2861–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Handgraaf S, Riant E, Fabre A, Waget A, Burcelin R, et al. 2013. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes 62:4098–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Taxier LR, Gross KS, Frick KM. 2020. Oestradiol as a neuromodulator of learning and memory. Nat. Rev. Neurosci 21:535–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA. 2006. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J. Comp. Neurol 498:637–48 [DOI] [PubMed] [Google Scholar]
- 145.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. 2007. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci 27:14265–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fagan MP, Ameroso D, Meng A, Rock A, Maguire J, Rios M. 2020. Essential and sex-specific effects of mGluR5 in ventromedial hypothalamus regulating estrogen signaling and glucose balance. PNAS 117:19566–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kow LM, Easton A, Pfaff DW. 2005. Acute estrogen potentiates excitatory responses of neurons in rat hypothalamic ventromedial nucleus. Brain Res 1043:124–31 [DOI] [PubMed] [Google Scholar]
- 148.Canteras NS, Simerly RB, Swanson LW. 1994. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J. Comp. Neurol 348:41–79 [DOI] [PubMed] [Google Scholar]
- 149.Ma T, Wong SZH, Lee B, Ming GL, Song H. 2021. Decoding neuronal composition and ontogeny of individual hypothalamic nuclei. Neuron 109:1150–67.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mickelsen LE, Bolisetty M, Chimileski BR, Fujita A, Beltrami EJ, et al. 2019. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci 22:642–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luo SX, Huang J, Li Q, Mohammad H, Lee CY, et al. 2018. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361:76–81 [DOI] [PubMed] [Google Scholar]
- 152.Hrvatin S, Sun S, Wilcox OF, Yao H, Lavin-Peter AJ, et al. 2020. Neurons that regulate mouse torpor. Nature 583:115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takahashi TM, Sunagawa GA, Soya S, Abe M, Sakurai K, et al. 2020. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature 583:109–14 [DOI] [PubMed] [Google Scholar]
- 154.Zhang Z, Reis FMCV, He Y, Park JW, DiVittorio JR, et al. 2020. Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice. Nat. Commun 11:6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sanchez-Alavez M, Alboni S, Conti B. 2011. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age 33:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Villa A, Della Torre S, Maggi A. 2019. Sexual differentiation of microglia. Front. Neuroendocrinol 52:156–64 [DOI] [PubMed] [Google Scholar]
- 157.Sohrabji F 2007. Guarding the blood-brain barrier: a role for estrogen in the etiology of neurodegenerative disease. Gene Expr 13:311–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu Y, Sareddy GR, Wang J, Zhang Q, Tang FL, et al. 2020. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. J. Neurosci 40:7355–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, et al. 2017. Microglial inflamma-tory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab 26:185–97.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, et al. 2018. Sex-specific features of microglia from adult mice. Cell Rep 23:3501–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, et al. 2014. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep 9:633–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goossens GH, Jocken JWE, Blaak EE. 2021. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol 17:47–66 [DOI] [PubMed] [Google Scholar]
- 163.Fischer AW, Cannon B, Nedergaard J. 2018. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol. Metab 7:161–70 [DOI] [PMC free article] [PubMed] [Google Scholar]


