Abstract
The ongoing COVID-19 pandemic has left patients with current or past history of cancer facing disparate consequences at every stage of the cancer trajectory. This comprehensive review offers a landscape analysis of the current state of the literature on COVID-19 and cancer including the immune response to COVID-19, risk factors for severe disease, and impact of anticancer therapies. We also review the latest data on treatment of COVID-19 and vaccination safety and efficacy in patients with cancer, as well as impact of the pandemic on cancer care, including the urgent need for rapid evidence generation and real-world study designs.
Keywords: neoplasms, COVID-19, SARS-CoV-2
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has caused an ongoing global pandemic. At the time of writing, over 257 million people have developed the resulting illness, COVID-19, and at least 5.1 million people have died (https://coronavirus.jhu.edu/map.html). Early on in the pandemic, it emerged that patients with certain risk factors or comorbidities including cancer were at heightened risk of severe outcomes.(1–3) In addition to the increased risk of transmission, severity, and risk of death from COVID-19 among patients with cancer, providing cancer care amidst this pandemic has proven to be an extraordinary and unprecedented challenge. Indeed, COVID-19 has affected every aspect of cancer care provision – from screening(4) and diagnosis, to changes in cancer therapies and delays in essential procedures, to lasting psychological consequences brought on by isolation(5) and distress among both patients and caregivers.(6)
These disparate repercussions on people with cancer have rippled deeply and globally. For example, early evidence points to a rise in metastatic cancer incidence, due to a decrease in screening either from healthcare avoidance or funneling of healthcare resources away from preventative care.(7)
This review aims to offer a landscape analysis of the current state of the literature on COVID-19 and cancer. The first section provides a framework to understand the physiologic immune response to SARS-CoV-2 infection with an in-depth view at how this response is affected by immunosuppressed states including cancer. The second section focuses on the spectrum of presentation of COVID-19 in patients with cancer including Post-Acute Sequelae of SARS CoV-2 infection (PASC a.k.a. “long-COVID”) as well as the risk factors for severe disease amongst patients with either a current or past diagnosis of cancer. The next section details the impact of cancer therapies on COVID-19, including therapies with both potential positive and negative effects on COVID-19 severity. We then describe the current best-practice guidelines, evidence, and advances in the treatment of COVID-19 in patients with cancer including general COVID-19 treatment and the importance of palliative care. Next, we summarize the most recent evidence on COVID-19 vaccine efficacy and safety in patients with cancer, followed by an outline on the impact of COVID-19 on cancer care delivery. We conclude with a discussion around real-world data in the COVID-19 era with a specific focus on research design and methodological considerations.
COVID-19 Immune Response in Patients with Cancer
SARS-CoV-2 is an enveloped single-stranded RNA-virus of the β-coronavirus family, the members of which include SARS-CoV and MERS-CoV. (8) At the start of the pandemic, epidemiologic studies identified risk factors associated with severe disease, notably advanced age and male sex(9). Further studies identified additional patient characteristics or underlying medical conditions also associated with poorer prognosis, including race and ethnicity, obesity, and active malignancy.(3,10–17) The early waves of the pandemic identified a “hyper-inflammatory” phenotype particularly among some critically ill patients, similar to known cytokine release syndromes (CRS), prompting numerous clinical studies to target dysregulated inflammation.(18–21) Thus, the demographic profile of the at-risk patient and the clinical syndrome of life-threatening disease were recognized early, although the mechanisms underlying these traits remain obscure. Since late 2020, the clinical complexity of COVID-19 is compounded by the emergence of sets of mutations in the virus, or variants of SARS-CoV-2 that can impact transmissibility and severity of COVID-19.(22)
The biologic basis for individual variability in clinical outcome has been partially clarified through immunologic investigations in vitro, in animal models, and in humans, enabling the construct of a two-step model of pathogenesis (Figure 1).(23,24) Glycosylated spike (S) proteins cover the surface of SARS-CoV-2 and attach to the host cell receptor angiotensin-converting enzyme 2 (ACE2).(25) Infection of the upper respiratory tract’s epithelial cells is mediated by the viral attachment to ACE2 (26–28). At this juncture, “Step 1”, one of the critical determinants of the disease course is the innate type I interferon (IFN) response.(24) Animal models susceptible to, or rendered permissive to, SARS-CoV-2 infection demonstrate early and robust induction of type I IFN following infection of the respiratory mucosa.(29,30) However, SARS-CoV-2 targets specific host proteins to suppress, but not abolish, the type I IFN response. Residual type I IFN response is presumably adequate to clear the infection in most hosts.(31–36) In a complementary series of investigations, pre-existing, neutralizing auto-antibodies to type I IFN have been found in ~15% of patients with severe COVID-19 (without genetic mutations), underscoring the importance of adequate type I IFN immunity at outset in mitigating human infection.(37–43) Thus, insufficient type I IFN at this juncture permits viral replication and propagation to the lungs and to extra-pulmonary sites.(44)
Figure 1: Biological framework for understanding the immune response to COVID-19 in patients with cancer.
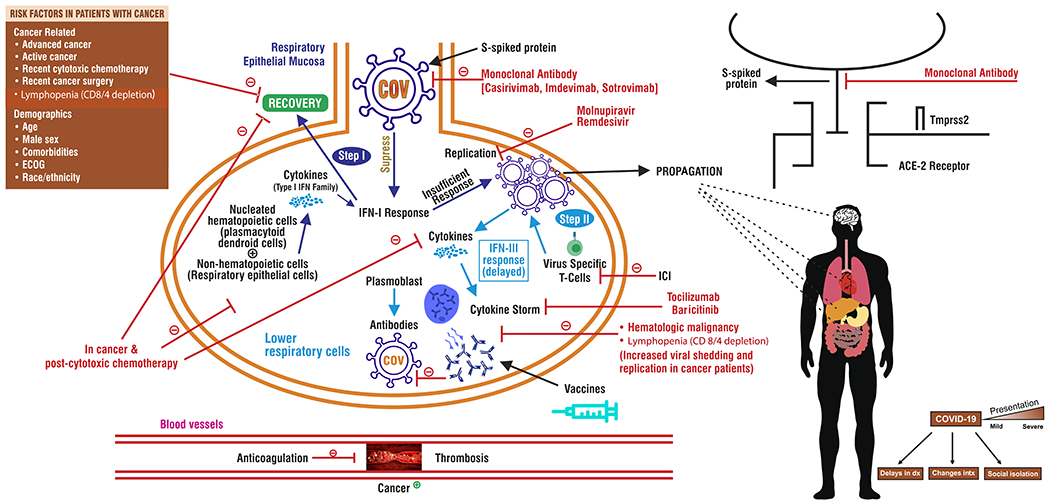
Step 1: Infection of the upper respiratory tract’s epithelial cells
Step 2: Viral replication occurs in the lower airways and alveoli
ACE-2: Angiotensin Converting Enzyme
S-protein: Spike protein
COV: SARS-CoV-2
ECOG: Eastern Cooperative Oncology Group
ICI: Immune Checkpoint Inhibitor
IFN-I: Type-I Interferon response
IFN-II: Type-II Interferon response
VTE: Venous Thromboembolism
In “Step 2”, viral replication occurs in the lower airways and alveoli, 7-14 days following infection. This replication results in progressive recruitment and activation of leukocytes, with excessive production of various cytokines in an attempt to eradicate the virus. After primary exposure, adaptive immunity with development of plasmablasts and neutralizing antibodies, as well as virus-specific T-cells, attempt to clear the infection. Yet when type I IFN responses are impaired, a high viral burden in the lungs overwhelms de novo cell-mediated immunity (CMI).(18,20,28,45–48) In those with intact type I IFN responses, adaptive immunodeficiency (e.g. from iatrogenic immunosuppression) may blunt this process. This results in prolonged viral shedding and replication. However, dysregulation of CMI may produce exuberant responses that are detrimental. Progression to life-threatening disease is marked by significant immunopathology, including epithelial lung damage, endothelial dysfunction, and CRS.(19,21,47,49) Multiple complex interactions between malignant cells, the coagulation cascade, COVID-19 induced proinflammatory cytokines, and stasis secondary to prolonged illness and hospitalization shift hemostatic balance to a procoagulant state associated with a higher incidence of arterial and venous thromboembolism in patients with COVID-19 and active cancer.
Risk factors that define a high risk for severe COVID-19 include patients with past or active malignancy and patients that are post-cytotoxic chemotherapy.(21,50,51) In these patients, immune response is limited by the chronic immunosuppressed state, and one of the consequences of this is reduced plasmacytoid dendritic cells available to respond to infection.(52) Furthermore, these patients are subject to lower levels of adaptive immunity and antibody production in the context of SARS-CoV-2 infection.(53–55) This phenotype is associated with lymphopenias, neutropenia, and decreased types I and III IFN response.(19,28,48,56,57) Consistent with the model currently supported by the data, these findings suggest that patients with cancer are unable to mount an appropriate immune response to clear infection. This above description provides a framework for understanding how immune responses can be aberrantly affected in patients with cancer depending on the viral variant, host factors, type of underlying malignancy, and the impact of certain chemotherapeutic regimens on immunologic axes.
Recent studies have described some of the mechanisms behind the blunted immune response in patients with cancer.(58) In a study of patients with cancer hospitalized with COVID-19, patients with depletion of CD4+ and CD8+ T cells exhibited worse COVID-19 outcomes, and patients with hematologic malignancies had lower B cell immunity.(59) A second study confirmed distinct immune signatures of patients with solid malignancy compared to patients with hematologic malignancy. B cell cytopenia was over-represented in patients with hematologic malignancies. Moreover, patients with hematologic malignancy who recovered from COVID-19 displayed lingering immunological consequences with impaired adaptive lymphocytic and innate myelomonocytic parameters.(60)
An important consequence of this blunted immune response is prolonged viral clearance in patients with cancer, which can result in prolonged illness.26 One study examined the nasopharyngeal swabs from over 1,000 patients with and without cancer to compare duration of viral shedding of SARS-CoV-2 by RT-PCR based cycle threshold (Ct) values and determined that an active malignancy conferred a longer shedding period associated with sustained presence of type 1 IFN.(61) In a study of 20 immunocompromised patients with COVID-19, viable virus could be isolated for up to 63 days post-symptom onset, while viral RNA was detectable for up to 78 days.(62) In clinical practice, prolonged viral shedding, even if the viral particles are no longer viable, usually precludes continuation of cancer therapy, with potential deleterious outcome of cancer progression.
With this background, we now turn to the manifestations of SARS-CoV-2 infection in patients with cancer.
COVID-19 Presentation, Severity, and Resolution in Patients with Cancer
Increased rate of SARS-CoV-2 infection and transmission in patients with cancer
Early reports indicated that patients with cancer have a higher risk of SARS-CoV-2 infection compared to cancer-free controls.(2,63,63) Differences in age, sex, and comorbidities, and increased reliance on the healthcare system have been postulated to account for differences in COVID-19 disease risk.(15,64,65) A large electronic health record (EHR) study of data from 360 hospitals, representing 20% of the United States population, found that patients with a cancer diagnosis within the last year were seven times more likely to develop COVID-19 than patients without cancer, even after adjusting for age, race, sex, comorbidities, transplant status, and nursing home stays.25 This increased risk may be due to immunocompromised state, frequent interactions with the healthcare system, and/or closer monitoring for infection among patients with cancer.21
COVID-19 Presentation
Clinical presentation of SARS-CoV-2 infection in patients with cancer is similar to patients without cancer. Initial symptoms generally include fever, sore throat, fatigue, diarrhea, and anosmia.(64) There is a wide spectrum of presentation of COVID-19, ranging from asymptomatic infection to respiratory failure. In addition to other multiorgan complications, micro and macro vascular thrombosis both venous and arterial is a unique presentation in this infection; see “Anticoagulation” section below.(66) The most frequent symptoms in a report of over 900 patients with cancer were fever, cough, dyspnea, fatigue, and malaise.(3) Low or high absolute lymphocyte count, high absolute neutrophil count, low platelet count, and abnormal creatinine, troponin, lactate dehydrogenase, and C-reactive protein (CRP) levels were all associated with higher COVID-19 severity among hospitalized patients reported to the COVID-19 and Cancer Consortium (CCC19) registry.(16) In addition to these laboratory values, the OnCOVID registry(16,67) found that hypoalbuminemia, high ferritin, and elevated d-dimer were negatively associated with outcome. A recent study evaluated dynamic changes in albumin and lymphocytes (onCOVID inflammatory score) and found that it was independently associated with severe COVID-19.(68,69)
Clinical presentation in patients with cancer is further complicated by several cancer-specific factors. For example, there have been reports that patients with cancer may have increased prevalence of asymptomatic presentation due to reflex screening practices.(70)
Hospitalization and Mortality Rates
In addition to increased susceptibility to SARS-CoV-2 infection, patients with cancer also have higher risks of COVID-19 hospitalization and mortality. For example, among 4,966 patients from the primarily US-based CCC19 registry, 58% were hospitalized (N=2,872) and 14% (N=695) died.(16) A European study of 890 patients found a mortality rate of 33%.(67) A meta-analysis of 110 studies from 10 countries yielded a pooled in-hospital mortality rate among patients with cancer and COVID-19 of 14.1%.(71) Important comparisons were conducted using data from 360 hospitals in the US.(71) Patients with cancer who developed COVID-19 were hospitalized 47.5% of the time and had 14.9% mortality, versus 24.3% hospitalization and 5.3% mortality among COVID-19 patients without cancer, and 12.4% hospitalization and 4.0% mortality among patients with cancer but without COVID-19.(72) As demonstrated by these differing mortality rates, it is important to note the potential influence of geographic heterogeneity. A large study that examined case-fatality rates across the European Union (EU) vs United Kingdom (UK) showed that patients belonging to the UK group had higher case fatality rates which remained significant after multivariable analysis adjusting for known negative COVID-19 prognostic factors.(73)
Moreover, while the general population has seen improvements in COVID-19-related mortality over time, a large study conducted in Europe of more than 195,000 hospitalized patients suggested that mortality in the more than 15,000 patients with a history of cancer and more than 5,000 patients on active cancer treatment may be higher throughout and did not parallel the downward trends seen in patients with no history of cancer.(74) However, a recent report from the European OnCOVID registry recently presented at the ESMO 2021 conference showed improvement in COVID-19 mortality over time.(75)
A separate complicating factor is that the true rate of COVID-19 in patients with cancer remains incompletely quantified because the “full” denominator population is not known, e.g. there is a propensity for many of these studies to evaluate the risk of death in patients admitted to the hospital, and the actual number of all patients with cancer infected with SARS-CoV-2 may not fully reflect the proportion of asymptomatic or minimally symptomatic cases.(76)
Non-Cancer-Specific Clinical Factors Associated with COVID-19 Severity
Patients with cancer represent a heterogeneous population with significant within-group variability. It is important to identify factors associated with worse outcomes to target surveillance and intervention efforts for high-risk patients. Similar to findings from the general public, demographic characteristics associated with worse prognosis in patients with cancer with COVID-19 include advanced age and male sex.(3,16,67,77) Similarly, comorbidities such as cardiovascular, pulmonary, and renal disease as well as their contribution to a higher Klabunde Comorbidity Index have all been associated with higher COVID-19 severity among patients with cancer.(16,78) Moreover, smoking as well as chronic pulmonary disease have been associated with worse outcomes in patients with lung cancer and COVID-19, and increased severity seen in smokers was also found in the lung-cancer specific TERAVOLT study.(79)
Cancer Characteristics and Impact on COVID-19 Severity
Impaired Eastern Cooperative Oncology Group (ECOG) performance status has been linked to higher morbidity and mortality among patients with cancer and COVID-19.(16,78) Several studies have reported that patients with lung cancer or hematological malignancies have worse outcomes than patients with other types of cancer.(3,16,77,80,81) This is likely due to the reduced respiratory capacity and more severe degrees of immunosuppression associated with these malignancies and their associated therapies. Among US Veterans with cancer and COVID-19, male genital cancer and thyroid cancer were also associated with higher mortality.(82) The literature on risks associated with other subtypes of cancer is still maturing. In addition to cancer type, cancer status is also important, as patients with advanced or progressive disease have been reported to have worse outcomes than patients in remission or that have stable disease.(3) Similarly, patients with recently diagnosed cancer have worse outcomes compared to patients with less recent (>6 months) diagnoses; this may be a surrogate for cancer therapy and/or therapy-related immunodeficiency.(1) For a discussion on risk related to specific anti-cancer therapies, please see the “COVID-19 and anti-cancer therapy” section below.
Impact of Health Disparities
The COVID-19 pandemic has highlighted and exacerbated health disparities due to race and ethnicity.(83) Black and Hispanic patients with cancer are more likely to become infected with COVID-19 and to have severe disease than non-Hispanic White patients.(16,72,82) Early in the pandemic, Black patients with cancer were less likely to receive the experimental COVID-19 treatment remdesivir, likely due to differential access.(84) Furthermore, Black and Hispanic patients were less likely to use or have access to telehealth, and Hispanic patients were more likely to have pandemic-related delays in cancer care compared to White patients.(85) These disparities do not appear to have a biological basis but rather are symptoms of structural racism.(86–89)
Post-acute sequelae of SARS-CoV-2 infection (PASC)
Similar to post-acute viral syndromes described in survivors of other coronavirus epidemics, there are increasing reports of long-term issues after recovery from acute COVID-19.(90–92) The PASC syndrome (colloquially, “long COVID”) is characterized by persistent and prolonged effects that extend beyond 4 weeks from the onset of symptoms; affected patients are sometimes referred to as “COVID long haulers”. While the pathophysiology has not been completely established, it is hypothesized that this long-term syndrome may be due a chronic inflammatory state, persistent viremia, and/or a general hypometabolic state.(93–95) Risk factors for PASC in the general population include older age, self-reported poor health status, and pre-existing comorbidities.(91,93) Based on these risk factors and pathophysiology, it is likely that patients with cancer will also have a higher risk of PASC. Although data in this field remain sparse, early evidence indicates that 15% of patients with cancer and COVID-19 have long term sequelae including respiratory symptoms and chronic fatigue, and that risk factors for PASC in patients with cancer include male sex, age of 65 years or older, 2 or more comorbidities, history of smoking, prior hospitalization for COVID-19, complicated disease, and prior COVID-19 therapy.(76,96) The impact of PASC on patients with cancer is an area of ongoing research and discovery.
Taken together, this section highlights the complexity of interacting factors which increase the risk of severity of COVID-19 in patients with cancer. Next, we will explore the added layer of anti-cancer therapies and their effects—both potentially negative and positive—on COVID-19.
Anti-Cancer Therapies and COVID-19
The potential for exacerbation of COVID-19 severity from systemic anti-cancer therapy has remained a concern throughout the pandemic. This has stemmed from the fact that anti-cancer therapy could either suppress the host immune response (e.g., cytotoxic chemotherapy) or paradoxically exacerbate immune-mediated end organ damage (e.g., immunotherapy). Overall, the data linking COVID-19 severity to active oncologic treatment remains mixed. Elucidating the relationship between COVID-19 outcomes and specific systemic therapies remains challenging due to several factors: the heterogeneity of systemic therapy regimens, timing of therapy relative to COVID-19 exposure; and multiple patient-specific confounders. With a wide variety of single-agent and combinatorial regimens in use, the number of patients receiving any given regimen who go on to develop COVID-19 is relatively small. This limits the analysis of specific treatments. Therefore, studies described below have largely focused on the effect of broad classes of systemic therapy with immunotherapy and cytotoxic chemotherapy as the two most studied examples. Additional targeted agents such as growth factor inhibitors, hormone modulators, or agents which exploit gene mutations have not been as thoroughly investigated.
Immunotherapy
Efforts have focused on the impact of immune checkpoint inhibitors (ICIs) with anti- CTLA-4, PD-1 and PD-L1 as the most commonly prescribed and widely studied.(97) A priori, one could have speculated divergently: whether ICIs might actually be protective against symptomatic COVID-19 (with enhanced protective immunity or more rapid viral clearance), or whether they might exacerbate infection once it is established. Both of these hypotheses stem from their mechanism of action, which is to remove inhibitory signals from cytotoxic T-cells, thus enhancing T-cell function.(98) ICIs have been indeed associated with worse outcome in some studies,(99,100) but other studies have shown no effect on either COVID-19 severity or on the incidence of classical immune-related adverse events in patients who were infected.(82,101–105) A meta-analysis of sixteen studies has similarly shown no effect of recent ICI therapy on disease outcomes.(106) Another meta-analysis suggested a possible increased risk of hospitalization, but not attributing severe disease or mortality with ICI therapy.(97) These varying results may be explained by heterogeneity of the patient population included; ICIs are used in a broad array of cancers, some of which may have underlying predisposition to severe COVID-19, such as lung cancer.(2,79,101) In addition, many of the included studies have limited power due to the relatively low event rate of severe COVID-19 infection, which could miss a smaller but potentially clinically significant effect in certain patients. Amidst this data, no large study has demonstrated a protective effect for immunotherapy.
An additional complex subgroup are those patients with COVID-19 who experience immune-related adverse events as a result of immune checkpoint blockade. (107–109) These events may complicate the diagnosis of COVID-19 or could theoretically compound the effects of COVID-19 infection (e.g., T-cell mediated injury triggered by viral inflammation exacerbated by blockade of T-cell regulators). The interplay of these three conditions (pneumonitis, lung cancer, and COVID-19) and combined management strategy remains unknown and is an active area of study.(110)
Cytotoxic chemotherapy
Cytotoxic chemotherapy has been implicated for increased risk of severe COVID-19, although the data supporting this suggestion is not universally conclusive. With intensely myelosuppressive regimens, there is a risk of impaired immune-mediated viral clearance leading to increased likelihood of severe consequences. Also, as with immunotherapy, there is a risk of chemotherapy-induced pneumonitis with certain agents (e.g., bleomycin, carmustine), compounding the risk of severe COVID-19 by reducing lung functional reserve. However, the evidence so far implicating chemotherapy as a class to increased COVID-19 severity remains mixed. The earliest study to report this was in the Chinese case series by Zhang et al which reported a negative association between any recent anti-cancer therapy and outcome, but sample size of individual therapies, including chemotherapy were small.(111) Since then, there have been multiple studies showing that recent cytotoxic chemotherapy was detrimental.(16,79,100,105,112) Notably, in a large cohort study of risk factors for severe COVID among a general population, recent receipt of chemotherapy was identified as a predictor of severe COVID-19 and the risk increased with degree of myelosuppression.(113) Other studies have failed to find a difference in COVID-19 severity with chemotherapy use,(82,99,102,114) but it is unclear whether these studies were adequately powered. The OnCOVID study found that receipt of active anticancer therapy at the moment of COVID-19 diagnosis was associated with lower risk of complicated disease; however, type of systemic anticancer therapy, including cytotoxic chemotherapy was not associated with COVID-19 severity.(67) Although chemotherapy is the most common treatment modality for cancer management, several factors could explain the divergent results. Chemotherapy as a category is even more heterogeneous than immunotherapy, covering a wide range of cancers and producing varied degrees and duration of myelosuppression, and resulting functional impairment. Given this heterogeneity, conclusions may be particularly sensitive to the set of confounding variables adjusted for, which is inconsistent across studies. Moreover, severity of COVID-19 may be more sensitive to the timing of chemotherapy relative to SARS-CoV-2 exposure than immunotherapy, given the time-dependent nature of chemotherapy-induced myelosuppression (which is not consistently defined across studies). Thus, details of cycle length, numbers of cycles, intensity of regimen, and host responses to chemotherapy may abrogate a signal if one exists – unless specifically studied through appropriately powered subset analyses.
Hematologic malignancy-specific therapies
A specific example concerning patients with hematologic malignancies in a subset analysis of CCC19 registry data has shown a concerningly high mortality rate for patients receiving rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or DNA methyltransferase inhibitors (decitabine, azacitadine).(16) Possible confounders include host factors, as hematologic malignancies have been associated with a higher mortality rate in the pandemic.(81,112,115) In 318 allogeneic stem cell transplant recipients, primarily with underlying hematologic malignancies, development of COVID-19 within 12 months of transplantation was associated with a higher risk of mortality (compared to those without infection).(116) Data with regards to impact of CAR-T therapy in hematologic malignancies are limited. In a series of 57 patients treated with CAR-T therapy (the majority of whom had B-cell neoplasms in complete remission), the authors reported an unadjusted mortality rate of 41%; however, timing of CAR-T therapy did not impact degree of COVID-19 severity.(117)
Targeted anti-cancer therapies
The impact of other therapies remains largely unknown. Interpretation of studies investigating non-chemo/immunotherapy agents grouped together, which alleviates the issue of small sample size, are complicated by the diverse mechanisms of action for these agents. There is very little data uncovering any effects of BRAF/MEK inhibitors, VEGF inhibitors, EGFR inhibitors or anti-estrogen therapy use with COVID-19 severity. However, there are no obvious mechanistic concerns for disease exacerbation for most of these therapies.
Localized anti-cancer therapies
With regards to localized or non-systemic treatment, available data suggests that postoperative mortality rates from SARS-CoV-2 infection are high. An international multicentric observational study of 1128 patients who had surgery during the first viral wave with confirmed SARS-CoV-2 infection within 7 days before or 30 days after surgery demonstrated that 30-day mortality was 24%. Of these, 80% of deaths were due to respiratory complications. In adjusted analyses, in addition to age and sex, cancer-related surgery was independently associated with increased 30-day mortality.(118) In a dedicated radiotherapy study of 350 patients who were infected with SARS-CoV-2 and who received radiation therapy at a single-center, 30-day mortality was 14%.(119) In a multivariate analysis, history of acute renal injury, shorter time between radiotherapy and COVID-19 diagnosis, and higher mean dose of radiation to the heart were associated with worse outcomes. This finding will require replication in a multi-institutional setting.
Potential protective effect of certain anti-cancer therapies
Although the focus for anti-cancer directed systemic therapy has largely been on determining whether there is a detrimental effect, several therapies were hypothesized to play a preventative or ameliorative role. In particular, there were initial reports about a potentially beneficial effect from anti-androgen therapy against COVID-19 infection,(120,121) although subsequent studies have failed to reproduce this finding.(122–125) Some preliminary evidence suggested a potential benefit from the now rarely-used recombinant interferon in two (<80 patients) studies.(126,127) This finding has yet to be replicated in a larger setting. Furthermore, agents used for supportive care (e.g., steroids, tocilizumab) from cancer therapy toxicities may potentially mitigate COVID-19 severity, although any impact would depend on timing of administration relative to infection. A recent randomized controlled trial evaluating ruxolitinib in patients with severe COVID-19 did not meet its primary outcome.(128) A preclinical computational and in-vitro study identified nilotinib as a potential COVID-19 therapeutic.(129) However, overall, there is limited evidence that any systemic oncologic therapies are protective against COVID-19, although several phase 2 and 3 trials are ongoing (Table 1). In terms of potential locally-directed protective therapies, several studies are examining the therapeutic potential of low-dose radiotherapy for the treatment of patients for COVID-19.(130) A preprint version of a trial evaluated low-dose whole lung radiation therapy in 10 patients with COVID-19 who were hospitalized with matched controls. There was a trend for lower median time to clinical recovery as well as shorter hospital stay.(130) This approach remains to be validated as part of an ongoing randomized-controlled trial (ex: NCT04466683).
Table 1.
Systemic anticancer therapies undergoing prospective evaluation in the treatment of COVID-19. 58 of 1243 (5%) ongoing phase 2 or 3 trials registered on ClinicalTrials.gov, as of November 23, 2021, utilize one or more drugs with known anti-cancer effects, many of which have FDA cancer-specific indications. Corticosteroids (dexamethasone, prednisone, etc.) are not included in this list, nor are tofacitinib/baricitinib, which are Janus kinase inhibitors but do not have known anti-cancer activity. Drugs listed only by code name on ClinicalTrials.gov were also not further evaluated for inclusion in this table.
| Drug | Drug class | Cancer indication1 | Clinical trials |
|---|---|---|---|
| Acalabrutinib | BTK inhibitor | CLL/SLL; Mantle cell lymphoma | Phase 3: NCT04647669 |
| ATRA | Retinoid | Acute promyelocytic leukemia | Phase 2: NCT04568096, NCT05077813 |
| BCG | Non-specific immunotherapy | Bladder cancer | Phase 2: NCT02081326, NCT04659941 Phase 3: NCT04327206, NCT04328441, NCT04350931, NCT04379336, NCT04384549, NCT04461379, NCT04475302, NCT04542330, NCT04648800 |
| Bevacizumab | Anti-VEGF antibody | Breast cancer; Cervical cancer; Colorectal cancer; Glioblastoma; HCC; NSCLC; Ovarian cancer; RCC | Phase 2: NCT04344782 |
| Dasatinib | TKI | CML; Ph+ ALL | Phase 2: NCT04830735 |
| Decitabine | Hypomethylating agent | CMML; MDS | Phase 2: NCT04482621 |
| Duvelisib | PI3K inhibitor | CLL/SLL; Follicular lymphoma | Phase 2: NCT04372602, NCT04487886 |
| Enzalutamide | ARI | Prostate cancer | Phase 2: NCT04456049 |
| Etoposide | Topoisomerase inhibitor | Testicular cancer; SCLC | Phase 2: NCT04356690 |
| Ibrutinib | BTK inhibitor | CLL/SLL; Mantle cell lymphoma; Marginal zone lymphoma, Waldenström macroglobulinemia | Phase 2: NCT04439006, NCT04665115 |
| Imatinib | TKI | CML; DFSP; GIST; MDS; Ph+ ALL; Systemic mastocytosis | Phase 2: NCT04346147, NCT04794088, NCT04953052 Phase 3: NCT04394416, NCT04422678 |
| Interferon2 | Non-specific immunotherapy | CML; Follicular lymphoma; Hairy cell leukemia; Kaposi sarcoma; Melanoma | Phase 2: NCT04379518, NCT04988217, NCT04480138, NCT04356495 Phase 3: NCT04534725 |
| Isotretinoin | Retinoid | None3 | Phase 2: NCT04730895, NCT04577378, NCT04578236, NCT05077813, NCT04389580 Phase 3: NCT04353180 |
| Masitinib | TKI | None4 | Phase 2: NCT05047783 |
| Melphalan | Alkylating agent | Multiple myeloma; Ovarian cancer | Phase 2: NCT04380376 |
| Methotrexate | Antimetabolite | ALL; Breast cancer; GTD; Head & neck cancer; Meningeal leukemia; Mycosis fungoides; NHL; NSCLC; Osteosarcoma; SCLC | Phase 2: NCT04352465, NCT04610567 |
| Nintedanib | TKI | None4 | Phase 2: NCT04338802 Phase 3: NCT04541680 |
| Nivolumab | Anti-PD-1 antibody | Bladder cancer; Colorectal cancer; Esophageal cancer; Gastric cancer; Head & neck cancer; HCC; Hodgkin lymphoma; Melanoma; Mesothelioma; NSCLC; RCC | Phase 2: NCT04343144, NCT04356508, NCT04413838 |
| Pembrolizumab | Anti-PD-1 antibody | Bladder cancer; Cervical cancer; Colorectal cancer; Cutaneous SCC; Endometrial cancer; Esophageal cancer; Gastric cancer; Head & neck cancer; HCC; Hodgkin lymphoma; Melanoma; Merkel cell carcinoma; MSI-H or dMMR solid tumors; NSCLC; PMBCL; RCC; TMB-H solid tumors; TNBC | Phase 2: NCT04335305 |
| Ruxolitinib | JAK inhibitor | Myelofibrosis; Polycythemia vera | Phase 2: NCT04581954, NCT04348695, NCT04403243, NCT04414098 Phase 3: NCT04424056 |
| Selinexor | XPO1 inhibitor | DLBCL; Multiple myeloma | Phase 3: NCT04534725 |
| Sirolimus | mTOR inhibitor | None3 | Phase 2: NCT04341675, NCT04461340 |
| Tamoxifen | SERM | Breast cancer | Phase 2: NCT04389580, NCT04568096 |
| Thalidomide | Immunomodulator (IMiD) | Multiple myeloma | Phase 2: NCT04273529, NCT04273581 |
| Uproleselan | E-selectin antagonist | None4 | Phase 2: NCT05057221 |
US Food and Drug Administration approved indication. Many of these drugs have additional off-label uses, which are not reported here
Includes alpha interferons, but not beta or lambda interferons, which do not have an established role in anti-cancer treatment
No FDA-approved cancer indication; used off-label for some cancer conditions
Not yet FDA-approved for any indication; has preliminary data or non-FDA approval for some cancer conditions
Acronyms: ALL: acute lymphoblastic leukemia; ARI: androgen receptor inhibitor; ATRA: all-trans retinoic acid; BCG: Bacillus Calmette-Guérin; BTK: Bruton tyrosine kinase; CLL: chronic lymphocytic leukemia; CML: chronic myelogenous leukemia; CMML: chronic myelomonocytic leukemia; DFSP: dermatofibrosarcoma protuberans; DLBC: diffuse large B-cell lymphoma; dMMR: mismatch repair deficient; GIST: gastrointestinal stromal tumor; GTD: gestational trophoblastic disease; HCC: hepatocellular carcinoma; JAK: Janus kinase; MDS: myelodysplastic syndrome; MSI-H: microsatellite instability-high; mTOR: mammalian target of rapamycin; NHL: non-Hodgkin lymphoma; NSCLC: non-small cell lung cancer; PD-1: programmed death receptor-1; Ph+ ALL: Philadelphia chromosome-positive acute lymphoblastic leukemia; PI3K: phosphoinositide 3-kinase; PMBCL: primary mediastinal B-cell lymphoma; RCC: renal cell carcinoma; SCC: squamous cell carcinoma; SCLC: small cell lung cancer; SERM: selective estrogen receptor modulator; SLL: small lymphocytic lymphoma; TKI: tyrosine kinase inhibitor; TMB-H: tumor mutational burden-high; TNBC: triple-negative breast cancer; VEGF: vascular endothelial growth factor; XPO1: exportin 1
In the next section, we provide a synthesis of the current best practice evidence and advances in the treatment of COVID-19 in patients with cancer.
Treatment of COVID-19 in Patients with Cancer
The optimal management and therapeutic approach to COVID-19 in patients with cancer has not been fully defined, in part due to their near systematic exclusion from prospective clinical trials.(131) Recommendations for the treatment of COVID-19 for patients with cancer have paralleled guidelines for the general population(132). Generally, guidelines for pharmacologic intervention have been dependent on the severity of symptoms requiring hospitalization, supplemental oxygen, noninvasive versus invasive ventilation, and intensive care unit (ICU) level care. A summary of treatments is provided in Figure 2.
Figure 2: Summary of evidence for COVID-19 treatment in patients with cancer.
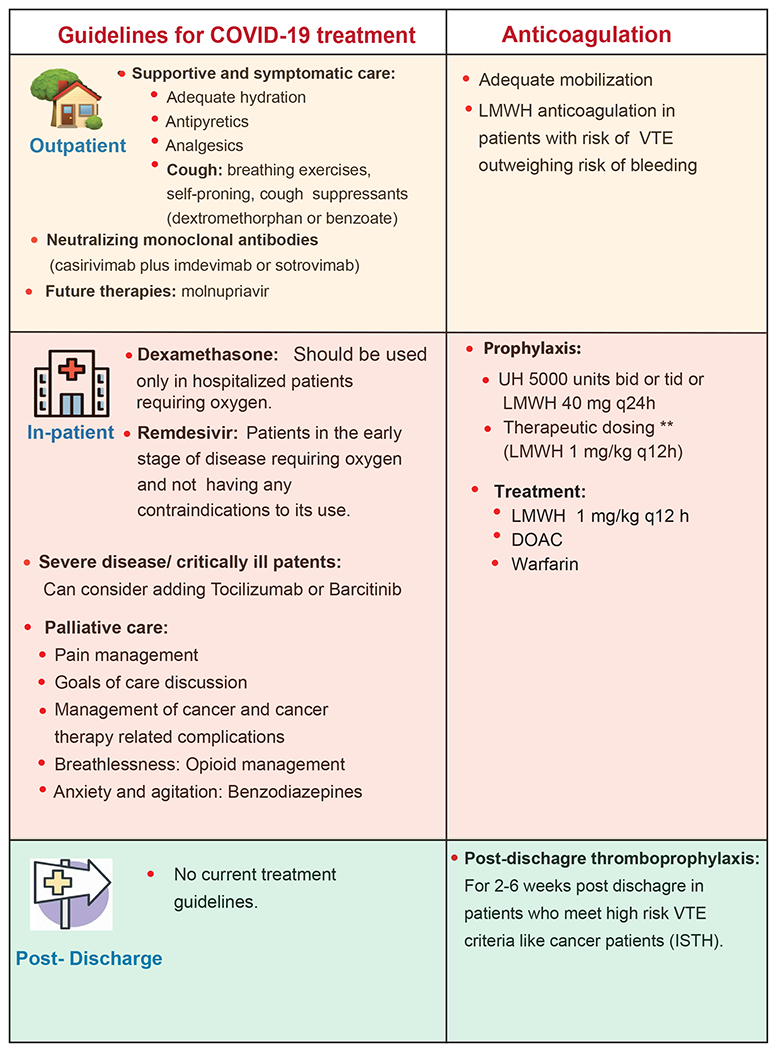
This is a synthesis of much of the available evidence but should not be taken as a guideline or endorsement of a particular clinical strategy. CDC: Center for Disease Control and prevention, WHO: World Health Organization, NICE: National Institute for Health and Care Excellence. ISTH: International Society on Thrombosis and Haemostasis. VTE: Venous thromboembolism. LMWH: Low molecular weight heparin. UH: Unfractionated heparin. DOAC: Direct oral anticoagulant.
General Management for Non-Hospitalized Patients
For patients with mild symptoms not requiring hospitalization or supplemental oxygen, management relies on supportive care. Also, due to the increased risk of thrombosis in these patients (see “COVID-19 Presentation” section) and compounded in patients with active cancer, adequate mobilization is essential.(66,133–135)
Several neutralizing monoclonal antibodies have been developed and are under investigation for the treatment and prevention of COVID-19(136). The majority target the S (spike) protein limiting the ability of the virus to bind and fuse to the target host cell. Guidelines recommend the use of either casirivimab with imdevimab, or sotrovimab to treat non-hospitalized patients with mild/moderate COVID-19 who are at high risk of clinical progression, including patients with cancer on active treatment (https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies). While specific studies in patients with cancer are limited, a retrospective, single-center cohort of 38 patients with COVID-19 treated with bamlanivimab or casirivimab/imdevimab demonstrated that hospitalization and mortality rates due to COVID-19 were low compared to previously described rates among active patients with active cancer.(137) Currently, bamlanivimab plus etesevimab is not recommended given decreased activity against variants of concern, including the Delta variant, which has predominated globally. Additionally, while guideline panels recommend against the use of convalescent plasma to treat all stages of COVID-19, prospective studies conducted in patients with cancer specifically have been lacking. The CCC19 evaluated the utility of inpatient convalescent plasma administration in patients with hematologic cancer.(138) The observational study, which included 966 individuals, of whom 143 received convalescent plasma, demonstrated that convalescent plasma was associated with improved 30-day mortality. Prophylaxis of immunosuppressed patients with the oral antiviral molnupiravir (NCT04405739), ritonavir (NCT04960202) or repurposed medications such as fluvoxamine (NCT04405739), if confirmed effective in clinical trials, may be an alternative to monoclonal antibodies.(139)
General Management for Hospitalized Patients
The approach for the treatment of COVID-19 in hospitalized patients with cancer is evolving, and most prospective trials have not studied cancer populations. Thus far, general clinical trial data support the use of remdesivir, dexamethasone, and tocilizumab or baricitinib (discovered during the pandemic using AI-guided in-silico experiments)(140), with utilization dependent on the degree of respiratory support warranted. It is important to note that none of these trials evaluating these treatments reported the presence of cancer as a pre-existing condition (https://www.covid19treatmentguidelines.nih.gov/management/clinical-1248management/hospitalized-adults--therapeutic-management/) (141) (57) (142) (143) (144). Specific to tocilizumab, there have been reports of its successful use in patients with cancer, (145,146) however, concomitant immunotherapy poses a theoretical risk due to hyperactivation of the immune system causing cytokine storm(147).
Lastly, the COVID-19 pandemic has brought on a separate and dangerous pandemic related to the spread of false information.(148) Several theoretically promising agents with repeatedly negative studies have unfortunately had persistent support on some social media sites, despite the uniform lack of evidence of efficacy. A Cochrane meta-analysis of all randomized-controlled trials evaluating the use of chloroquine or hydroxychloroquine in the treatment of COVID-19 found that they were not effective in reducing COVID-19 mortality or severity.(149) In the same vein, there are no robust data to support the use of ivermectin, azithromycin, or vitamin C, or zinc in the treatment of patients with COVID-19.(150–153)
Given that prospective studies of COVID-19 directed therapies have been limited in patients with cancer, CCC19 investigated the association of hydroxychloroquine, azithromycin, remdesivir, high-dose corticosteroids, and tocilizumab on 30-day all-cause mortality in patients with invasive cancer and COVID-19.(84) In this observational study of 2186 patients, while there was no statistically significant difference in 30-day all-cause mortality with hydroxychloroquine alone, treatment with remdesivir indicated a potential benefit when compared to positive controls. Hydroxychloroquine in combination (most usually with azithromycin) and high-dose corticosteroids alone or in combination were associated with inferior outcomes in this population; further study is necessary to determine why corticosteroids were not associated with benefit in these patients with cancer.
Anticoagulation
Venous thromboembolism (VTE) affects 1-8% of all patients with cancer receiving antineoplastic therapy and is the second most common cause of death in outpatients receiving chemotherapy.(154,155) A study of 2804 patients from CCC19 showed that 9.3% of hospitalized patients with cancer and COVID-19 had a VTE, which increased to 13.4% in 440 patients in the ICU. Apart from ICU admission, recent systemic therapy, active disease, and high-risk VTE cancer subtypes increased the risk of VTE in patients with cancer and COVID-19.(156,156) Prophylactic management recommendations for patients with cancer is hampered by under-representation in anticoagulation-based trials. Guidelines recommend low-molecular-weight heparin (LMWH) anticoagulation in outpatients with risk of VTE outweighing the risk of bleeding,(157,158) or in hospitalized patients with COVID-19. Consensus guidelines recommend initiating standard prophylactic dosing in all hospitalized patients who do not have contraindications for use(159,160). Treatment of acute thrombosis in the context of COVID-19 and cancer should follow those of guidelines (161–163)(164). IL-6 has a potent pro-thrombotic property. Therefore, concurrent use of anticoagulation and cytokine-reducing agents such as steroids and tocilizumab may cause dual blockade of IL-6 induced microvascular thrombosis pathway; however, this needs further investigation in clinical trials.(165) The International Committee on Thrombosis and Haemostasis guidelines recommend extended post-discharge thromboprophylaxis for 2-6 weeks post-discharge in hospitalized patients who meet high VTE risk criteria, such as patients with active cancer.(159,164)
Palliative Care
Studies have demonstrated that while early palliative care is an essential component of care coordination, it is underutilized in patients with cancer and COVID-19.(166) In a preliminary analysis by CCC19 on code status and utilization of palliative care, the majority (79%) of hospitalized patients were full code at the time of admission. Palliative care was involved in only 14% of cases and was associated with a 44% transition in code status to DNR+/− DNI (Do-not Resuscitate +/− Do-not intubate).(167) As patients with COVID-19 can deteriorate rapidly, it is crucial to establish advanced care directives(168) and identify a healthcare proxy early in disease management.(169) (166) Palliative care consultation in patients with cancer and COVID-19 has been shown to facilitate symptom control and improve discharge planning, and therefore should be initiated early-on.(170)Video communication has emerged as a practical, accessible, and acceptable method of communication in the palliative care setting, especially with different visitor restricting policies.(166)
In summary, there are many treatment options for COVID-19, but the data for the subgroup of patients with cancer is nearly completely lacking from prospective studies. Dedicated trials in this population, or at the very least more consistent reporting of cancer as a comorbidity in the major prospective RCTs, is needed to enable informed decision-making. Next, we turn to the prevention of COVID-19 through vaccination.
Safety and Efficacy of COVID-19 Vaccination in Patients with Cancer
The development of highly efficacious COVID-19 vaccines within one year from the identification of SARS-CoV-2 is a remarkable feat of vaccine development.(171,172) The FDA-approved vaccines for COVID-19 have demonstrated safety and efficacy in the general population. Their use is credited to have prevented many COVID-19 deaths.(173,174) However, their efficacy and safety profiles were not established in patients with cancer since participants undergoing active anti-cancer therapy were excluded from the seminal vaccination trials.(175–178) Nevertheless, patients with cancer were prioritized for the COVID-19 vaccine rollout because of higher case fatality rates or the mortality risks among SARS-CoV-2-infected patients with cancer. At the time of this rollout, no data existed for optimal dosing or interactions between active oncologic treatments and the ability of vaccination to induce protective immunity against COVID-19 in patients with cancer.(179–181)
Since the initial FDA EUA for the COVID-19 vaccines in Fall 2020, the safety profile for patients with cancer appears similar to the general population.(182–194) A summary of available data regarding vaccination effectiveness and safety in patients with cancer is provided in Table 2.
Table 2:
Summary of safety and efficacy vaccination studies in patients with cancer
| Cohort | n | Median age years (IQR) | Vaccine studied | Safety | Efficacy | Reference |
|---|---|---|---|---|---|---|
| Single center (London); solid cancers, 88.5% receiving anti-cancer therapy (36.2% parenteral chemotherapy and 15.3% immunotherapy) | 373 | 56 (19-65) | First dose of Pfizer, BioNTech, Oxford, AstraZeneca, Moderna | mild reactogenicity (sore arm, tiredness, and headaches); No grade 4/5 or anaphylaxis | Not reported | (191) |
| Three London hospitals; solid cancer, hematological cancer | 151 | 73.0 (64.5–79.5) | One and two doses of BNT162b2 | fewer adverse symptoms reported compared with healthy controls; No differences between patients with hematological cancer and those with solid cancer. | 38% seroconversion in solid cancer & 18% in hematological cancer after one dose; second dose significantly increased seroconversion in solid cancer within 2 weeks at day 21 after the first dose; better overall T-cell response than antibody response | (182) |
| Single center (Israel) cancer treated with immune checkpoint inhibitors alone or in combination with chemotherapy | 170 | 72 (29-93) | BNT162b2 | Same as healthy controls but patients with cancer had more common muscle pain, none required hospitalization, similar side-effects in treatment groups. | Not reported | (183) |
| Single center (New York City) | 200 | 67 (27–90) | Two doses of the mRNA vaccines (BNT162b2 or mRNA-1273) or one dose of the adenoviral vaccine (AD26.COV2. S) | no adverse effects overall | Seroconversion; significantly lower rate in hematologic malignancies, particularly following immunosuppressive therapies (anti-CD20 therapies, stem cell transplantation) | (215) |
| The Leukemia & Lymphoma Society National Registry; B-cell-derived hematologic malignancies | 1,445 | 68 (16–110 years) | Two doses of mRNA vaccines (mRNA-1273 or BNT162b2) | Not reported | Seroconversion; 75% of all patients with hematologic malignancies produced antibodies | (197) |
| The Leukemia & Lymphoma Society National Registry; B-cell-derived hematologic malignancies | 49 | 66 (31–80) | Heterologous and homologous booster of the mRNA vaccines (BNT162b2 or mRNA-1273) or adenoviral vaccine (AD26.COV2.S) | Not reported | Seroconversion; 55% patients with B-cell malignancies who failed to make anti-S antibodies after full SARS-CoV-2 vaccines seroconverted after booster vaccination | (202) |
| The Leukemia & Lymphoma Society National Registry | 3,574 | Not given | At least one dose COVOD-19 vaccine (BNT162b2 or mRNA-1273, AD26.COV2.S, AZD) | 13% reported no adverse events; distribution similar compared to age-matched healthy individuals | Not reported | https://www.lls.org/news/covid-19-vaccine-safety-among-blood-cancer-patients |
| One site (Pittsburgh); hematologic malignancies | 67 | 70 (62.5 – 73.5) | two doses of either the mRNA-1273 (Moderna) or the BNT162b2 (Pfizer) vaccine | Not reported | Seroconversion; 46% did not produce antibodies and were vaccine non-responders. | (296) |
| Two sites (San Antonio, Geneva) solid and hematological malignancies. | 140 | 63 (55–69) | two doses of mRNA vaccines (mRNA-1273 and BNT162b2) | Not reported | Seroconversion; 94% seroconverted after two vaccine doses. significantly lower seroconversion rates and antibody titers in patients with hematological malignancy than with solid tumors. Patients anti-CD20 treatment 6 months prior did not develop antibodies. | (216) |
| Single center (Italy); multiple myeloma, myeloproliferative malignancies on active treatment | 92 | 70 (28–80) for MPM; 73 (47–78) for MM | Two doses of BNT162b2 | no serious adverse event | Seroconversion; lower titers in MPM and MM patients; particularly those on anti-CD38-based treatment | (205) |
| single center (Israel); solid tumors undergoing active treatment | 102 | 66 (56-72) | Two dose of mRNA vaccine (BNT162b2) | Not reported | Seroconversion; 90% were seropositive after the second vaccine dose; lower titers in patients with cancer, affected by chemotherapy plus immunotherapy | (235) |
| Single center (France); solid tumors; under treatment or within 6 months after treatment | 13 | 17 (16-21) | BNT162b2 mRNA vaccine | Well tolerated; mild pain at the site of injection and fatigue were most frequent systemic symptoms | Seroconversion: 7 of 10 patients developed antibodies before second vaccine dose, and 9 of 10 patients developed antibodies one month after the second injection. | (203) |
| 2 independent cohorts of hematological and solid tumors on active therapy (8.6% were previously infected) | 595 | 67 (19-96) | BNT162b2, mRNA-1273, or AZD1222 COVID-19 vaccines | Not reported | Seroconversion; improved titers after second dose; lower levels in patients on treatment with B-cell–targeting agents | (200) |
| multicenter (US Veterans Health administration); solid or hematologic malignancy | 58,304# | Not available | Any COVID-19 vaccine | Not reported | Laboratory confirmed SARS-CoV-2 infection; 57% for chemotherapy within three months prior to first vaccination dose; 76% for endocrine therapy and 85% for those off systemic therapy for at least six months prior. | (201) |
| lymphoplasmacytic Single patient case study; lymphoma | 1* | 59 | Two doses of the BNT162b2 mRNA vaccine; 1 dose of JNJ-78436735 | mild malaise and headache | seroconversion; No detectable antibodies after 2 doses of BNT162b2; low but detectable antibodies after heterologous booster | (243) |
| Single center; solid tumors on treatment | 53 | 63.7 | two and three doses of the BNT162b2 mRNA vaccine | Not reported | Seroconversion and T-cell assay; all patients seroconverted but had lower overall antibody and T-cell responses in patients with cancer. | (192) |
| Single center; Chronic myelogenous leukemia (CML) on TKI | 16 | 45.6 | first injection of BNT162b2 vaccine | Tolerable; localized inflammation in 56.3% and transient flu-like illness in 23.5% patients | Seroconversion and T-cell response; 87.5% of the patients with CML had detectable antibodies and developed a neutralizing antibody response; 93.3% had T-cell response | (297) |
| hematologic malignancy patients, on treatment | 551 | 65 (22-97) | two doses of the mRNA vaccines (BNT162b2 or mRNA-1273) | Not reported | Seroconversion; positive seroconversion rates (51.5% at 1 month and 68.9% at 3 months), lower antibody titers and diminished neutralizing capacity (26.3% at 1 month and 43.6% at 3 months) | (204) |
| multiple myeloma | 320 | Fully vaccinated (BNT162b2, mRNA-1273, and some unknown); 18.8% had COVID-19 prior to immunization | Not reported | Serology; 81.3% seroconverted after receiving the second vaccine dose; antibody levels highly variable; 15.8% did not have detectable levels | (198) | |
| Single center; Youths and young adults with history of acute lymphoblastic leukemia and allergy to PEG-asparaginase | 32 | 16 (12–29) | BNT162b2, mRNA-1273 | No patients had signs or symptoms of allergic reaction | Not reported | (193) |
| Single center; lymphoma | 67 | 71 (24–90) | BNT162b2, mRNA-1273 | Not reported | Seroconversion; Treatment-naïve lymphoma patients induce IgG antibody response same as healthy controls; but patients on recent anti-CD20 therapy had sub-detection seroconversion. | (206) |
| Chronic lymphocytic leukemia (CLL) | 167 | 71.0 (63.0-76.0) | BNT162b2 | Mild local reactions (pain at the injection site, local erythema or swelling); no statistical difference between the first and second dose of the vaccine; more frequent systemic adverse events after the second dose; no differences in the rate of adverse events for patients on active treatment. | Seroconversion; significantly reduced antibody response rate among patients (39.5%); after treatment (79.2%), in treatment-naive patients (55.2%) and in patients under treatment (16.0%); considerably low response in patients recently treated with Bruton’s tyrosine kinase inhibitors (16%) or venetoclax ± anti-CD20 antibody (13.6%) | (187) |
| myeloproliferative neoplasm patients receiving JAK1 and JAK2 inhibitor (JAKi) ruxolitinib | 30 | 60.8 (36.9-80.3) | first injection BNT162b2 or | Not reported | Seroconversion; significantly lower in patients receiving ruxolitinib; compared to healthy controls only 33.3-38.8% patients seroconverted | (207) |
| Israel nationwide; patients with hematological neoplasms with some on therapy for an active disease | 32,516# | 70 (59-79) | Two doses of mRNA BNT162b2 vaccine | Not reported | Vaccinated patients with hematological neoplasms, compared with vaccinated matched controls, had an increased risk of infections, symptomatic covid-19, covid-19 related hospitalizations, severe covid-19, and covid-19 related death. | (208) |
| multiple myeloma | 44 | 65 | Two doses of mRNA SARS-CoV-2 vaccine (BNT162b2; mRNA-1273 Moderna) | Not reported | B and T cell responses; seronegative MM patients had lower B cells and total CD4+ T cell; only 40% had spike-protein-reactive B cells; Seropositive MM patients had spike reactive B cells and activated CD4+ cells same as healthy controls | (209) |
| CAPTURE (COVID-19 antiviral response in a pan-tumor immune monitoring study) | 357 | 59 years | unvaccinated | Not applicable | detected SARS-CoV-2-specific CD4+ T cells in 77 of 100 patients (77%) and CD8+ T cells in 49 of 100 patients (49%) | (298) |
| SeroNet-CORALE U.S. wide cohort of adult patients with solid tumors or hematological malignancies | 366 | 65 (56-73) years | Two doses of mRNA SARS-CoV-2 vaccine (BNT162b2; mRNA-1273 Moderna) | Not applicable | antibody levels at prior to vaccination; 2-12 weeks; and 16-28 weeks after second dose. Lower antibody levels in cancer | (210) |
| Single center (New York City); hematologic malignancy and solid tumor; 73% on active treatment | 88 | 69 (30–91) years | Booster vaccination with mRNA vaccine (BNT162b2 or mRNA-1273) following previous 2 doses of BNT162b2 | Not reported | 56% of seronegative patients (hematologic malignancies) after previous full vaccination seroconverted after the booster vaccination; Prior anti-CD20/BTK inhibitor therapy was associated with reduced seroconversion even after boosters; patients with prior COVID-19 infection had more robust vaccine responses | (211) |
| Multicenter, Netherlands (Vaccination Against COVID in Cancer (VOICE) trial) | 791 | 55 – 73 years | 2 doses of mRNA-1273 | Fatigue was most prevalent solicited systemic adverse event; fatigue, fever, chills, headache, myalgia, joint pain, and nausea were higher up to 7 days after the second vaccination; serious adverse events included fever, diarrhea, and febrile neutropenia | Seroconversion and spike-specific T-cell response; Most patients of solid tumor cancer developed adequate antibody response to mRNA-1273 vaccination while receiving chemotherapy, immunotherapy, or both. 2% of cohort did not develop antibody or T-cell response | (194) |
| Patients on remission after receiving CD19 or anti-CD22 targeting CAR T-cell treatments (CART | 12 | 53 (16-74) years | 2 doses of mRNA vaccines (BNT162b2 or mRNA-1273) | Not reported | Seroconversion and T-cell response; Significantly lower RBD-IgG but comparable T-cell induction among the CART cohort compared to healthy controls; strong SARS-CoV-2–specific CD4 T-cell responses even after robust B-cell depletion by CAR T cells | (212) |
| Israel, single center, Patients with solid tumors on active therapy | 37 | 67 (43-88) | Booster vaccination with BNT162b2 after 2 doses of mRNA vaccine BNT162b2 | Not reported | Serology; most recipients had enhanced antibodies after 3rd booster, including those with moderate or minimal response following the second vaccine dose | (299) |
| Single site, patients with plasma cell neoplasms | 276 | 74 (62–80) | two doses of the BNT162b2 or one dose of the AZD1222 vaccine | Not reported | Seroconversion, lower production of neutralizing antibodies in patients with MM compared with controls; independent of anti-CD38 or belantamab mafodotin and lymphopenia at the time of vaccination | (300) |
EHR extraction
Single patient case study
Safety of COVID-19 vaccination in patients with cancer
Safety data available from these studies comes with the caveat that rare adverse events are unlikely to be captured at the scale of these smaller studies, and long-term adverse events have not been observed, given the timeframe of the vaccine rollout. Regardless, the data is reassuring against substantially increased risk consistent with safety data from other vaccination trials.(195) Moreover, the mRNA vaccine platform was initially developed for checkpoint-inhibitor-treated melanoma and was previously shown to be safe among patients with cancer.(196)
Antibody response/seroconversion of COVID-19 vaccination in patients with cancer
There is now accumulating evidence for vaccine effectiveness against COVID-19 in patients with cancer.(58,182,184–187,194,197–212) In most patients with cancer, mRNA vaccination leads to seroconversion after the second dose, although antibody titers achieved tend to be inferior to non-cancer controls.(185–187,198,200,207–210,212) There is also emerging evidence that COVID-19 vaccines differ in antigenicity, for example mRNA-1273 (Moderna, Inc.) can generate higher median antibody titers than BNT162b2 (Pfizer and BioNTech).(198,204,213) However, consistent with the observation that patients with hematologic malignancies mount limited immune responses to SARS-CoV-2 infection,(214) seroconversion following COVID-19 vaccination tends to be lower in this group.(190,197–200,215) Patients undergoing chemotherapy and anti-CD20 therapies show further reduction in seroconversion.(185,186,190,198,199,204,215,216)
T and B cell vaccine immunity, and neutralization antibodies in patients with cancer
It is important to note that most studies so far have focused on post-vaccine antibody titers to the viral spike protein (seroconversion) as the assessment of vaccine immunogenicity. Correlates of protection against COVID-19 are not yet fully established but seroconversion is frequently being used as a convenient, measurable and acceptable surrogate of immune protection from symptomatic SARS-CoV-2 infection.(199,217–221) However, antibody titer is an imperfect proxy for overall protection without other aspects of the immune response such as T-cell response being measured.(59,222,223) Despite decline of specific IgG, long-lasting memory T-cells reactive to the nucleocapsid proteins of SARS-CoV-2 can be found up to 17 years after the 2003 outbreak of SARS, suggesting long-lasting and cross-reactive T-cell immunity to this family of coronoviruses.(224) Spike-reactive memory T-cells, but not B-cells or antibodies, can be found in many individuals even before SARS-CoV-2 exposure or vaccination.(225–229) CD4+ T-cells induce antibody response and maintain B-cell memory 6 months after SARS-CoV-2 infection.(225)
In a study of patients with active solid and hematologic malignancies, 1 dose of BNT162b2 yielded a poor T cell response, further strengthening the recommendation of early (day 21) second dose of BNT162b2 vaccination in patients with cancer.(182) In patients with solid cancers on active therapy, after vaccination with two doses of BNT162b2, a T-cell response was observed in most, including nearly half who mounted undetectable neutralizing antibody responses.(192) (192)In a separate study, 77% of patients with hematologic cancer had detectable SARS-CoV-2-specific T-cell responses after COVID-19.(59) In a study of 239 patients with hematologic malignancy, vaccination with two BNT162b2 inocula resulted in only 53% of the patients achieving effective T cellular protection against COVID-19.(230)
Aside from T or B cell immunity, neutralizing antibodies (antibodies which bind to cell-free virus and prevent it from infecting cells) have also emerged as a helpful surrogate for vaccine effectiveness. In a study of nearly 600 patients with cancer, examining protection against SARS-CoV-2 variants of concern, patients with hematological malignancies were more likely to have undetectable neutralizing titers and had lower median titers than those with solid cancers against both variants and WT SARS-CoV-2. By comparison with individuals without cancer, patients with hematological, but not solid, malignancies had reduced neutralizing antibody responses.(58) Of note, patients with inherited agammaglobulinemia or rituximab-induced complete B-cell depletion have recovered from COVID-19 in the absence of neutralizing antibodies.(222,223) It can be assumed that CD8+ T-cells can compensate to some degree for deficient humoral immunity against SARS-CoV-2 infection in some patients.(192)
Risk factors for impaired vaccine response in patients with cancer
From the serology studies, type of malignancy and treatment agents have emerged as two major risk factors for inadequate vaccination response.(231) Since preliminary studies have investigated small cohorts, data on the impact of specific malignancies or treatment regimens is limited. Patients with hematologic malignancies tend to mount inadequate vaccine response, congruent with evidence of decreased humoral response, exhausted T-cell phenotype and prolonged viral shedding after COVID-19.(60) Studies of mixed solid tumor and hematologic cohorts have also independently identified hematologic malignancy as a risk factor for lower efficiency to seroconvert.(182,215,216,232) The risk for inadequate antibody response to vaccination may in part be due to the specific therapies used to treat hematologic malignancies – for example, the B-cell depleting agent rituximab is known to be particularly immunosuppressive.(233) However, hematologic malignancy itself is likely to be an independent contributor in blunted vaccine effectiveness since antibody titers were lower in patients with chronic lymphocytic leukemia even in the absence of therapy.(187,234)
Data so far suggests that immunosuppressive therapies, such as cytotoxic chemotherapy, are a risk factor for reduced antibody response. Multiple studies have demonstrated that although antibody response is preserved in patients with solid malignancies on cytotoxic therapy, the antibody titers in these patients, on average, are reduced compared to age-matched controls.(216,235) Comparatively, ICI therapy and endocrine therapy do not appear to reduce immune response.(215) Importantly, patients on rituximab-based combinations seem to be at the most risk for a reduced response. In a prospective cohort of 131 patients with mixed solid and hematologic malignancy, none of the 4 patients on anti-CD20 therapy developed an antibody response, compared with 15 out of 16 patients on endocrine therapy.(215) A recent study of longitudinal anti-spike and anti-nucleoplasmid antibodies found that B-cell targeted therapies were associated with decreased peak and sustained antibody responses.(210) In patients with leukemia, lymphoma, and multiple myeloma patients, treatment with Bruton tyrosine kinase inhibitors, venetoclax, phosphoinositide 3-kinase inhibitors, anti-CD19/CD20, and anti-CD38/B-cell maturation therapies all hindered vaccination responses.(198,204)
Breakthrough infections, booster doses, and remaining questions
The above preliminary studies reveal immunologic patterns but do not fully guide how they translate to protection from infection. Immunocompromised patients make up a disproportionately higher (40-44%) proportion of vaccinated people hospitalized with breakthrough COVID-19 infections despite making up about 3% of the U.S. adult population.(236–238) Vaccinated patients with hematological malignancies, in particular those on treatment, have a higher risk of severe COVID-19 outcomes than comparable vaccinated healthy people.(208) This has prompted the CDC to recommend an additional dose of an mRNA COVID-19 vaccine in moderately to severely immunocompromised people (https://www.cdc.gov/media/releases/2021/s0813-additional-mRNA-mrna-dose.html), and this may be especially important for patients with hematologic malignancies.(202,239) A phase 1 study in patients with solid tumors on active chemotherapy, examined the role of a third immunization. At 1 week after a third immunization, 16 participants demonstrated a median threefold increase in neutralizing antibody responses, but no improvement was observed in T cell responses.(192) In a prospective The Leukemia & Lymphoma Society National Registry study (NCT04794387), 55% patients with B cell malignancies who failed to make anti-S antibodies after full SARS-CoV-2 vaccines seroconverted after booster vaccination, and the immunogenicity of booster vaccination did not appear to be affected by disease type, vaccine type, homologous or heterologous vaccination pairing, or malignancy-target therapies.(202)
Studies have not yet extensively assessed the duration of vaccination effectiveness against COVID-19 from vaccination in patients with cancer relative to the general population. In a nationwide study of patients with cancer in the US Veterans Health administration, real world effectiveness from vaccination against SARS-CoV-2 infection was investigated.(201) In this retrospective matched cohort study of 58,304 patients with cancer, against the primary outcome of laboratory-confirmed SARS-CoV-2 infection, overall 14-day post-second dose effectiveness, defined as 1 minus the risk ratio of SARS-CoV-2 infection for vaccinated individuals compared to unvaccinated controls, was 57% among the patients receiving chemotherapy versus 76% for those receiving endocrine therapy. Patients who had their last dose of systemic therapy at least 6 months prior to vaccination exhibited 85% vaccine effectiveness. In limited studies, patients with cancer or those receiving anti-CD20 treatment have been shown to be at higher risk of vaccine breakthrough infections compared to the general population.(199,238,240) A more recent claims-based Israeli study examining breakthrough infections reported on 113 patients with hematologic malignancy who experienced COVID-19 infection after vaccination, of whom 70% had severe COVID-19 infection. Despite this high rate of severe infection, COVID-19-related mortality was 13%, which appears to be lower compared to mortality rates of patients with hematologic malignancies reported pre-vaccination rollout.(241) Overall, however, data on protection from severe complications of COVID-19 remains limited and future work on larger cohorts will be needed. The ultimate test of effectiveness of vaccination is protection against symptomatic and severe COVID-19 for which data is still emerging.
Multiple questions on the future of vaccination for patients with cancer remain unanswered. It is unclear how effective current vaccines will remain against future SARS-CoV-2 variants, and what strategies will be most effective to protect a vulnerable patient population. Booster vaccinations recommendations are still evolving, and preliminary evidence suggests that booster vaccinations are safe,(242,243) and potentially effective against new variants.(244) A trial in adults with solid tumors showed that a third immunization with BNT162b2 boosted neutralizing antibody titers in most participants to protective level, but the improvements were fairly modest and circulating spike-specific T-cell frequencies did not change.(192) Data are lacking on how effective booster vaccinations will be in the heterogeneous cancer patient population, whether “mix-and-match” approaches with in-class and across-class vaccines are effective(245), and how booster vaccinations should be optimally timed with anticancer therapy. These questions remain important directions for future studies.
In the final sections of this review, we turn to the effects of COVID-19 on the whole population with or at risk of cancer, followed by a discussion of the methodologic challenges of studying COVID-19 in patients with cancer.
The Impact of COVID-19 on Cancer Care Delivery
At the onset of the COVID-19 pandemic, inaccessible testing and protective personal equipment (PPE) shortages were widespread. Therefore, recommendations of adaptive care strategies (aimed at maintaining continuity and quality of cancer care while mitigating risk of infection transmission within the contingencies of the healthcare system) were made by expert panels,(246,247) and oncology societies.(248–251) This resulted in widespread temporary suspensions of essential cancer services such as screening, diagnostic procedures, and treatments.(4) The reorganization of cancer management had unintended consequences of significant decreases in cancer screening, cancer management visits, cancer surgeries, access to healthcare delivery, and cancer research.(252) Frequent determinants for disruptions in cancer care were provider- or systems-based due to reduction in service availability with impact on treatment, diagnosis, or general health service.(253) It is important to consider the patient perspective in the midst of all of these and other changes (Figure 3).
Figure 3:
Perspectives from a cancer survivor and patient advocate.
Cancer Screening and Prevention
Due in part to the decreased capacity for non-COVID care and decrease in primary care visits, both primary and secondary prevention of cancer were negatively impacted.(252,254) In contrast to 2019, a cross-sectional study suggests that the diagnosis of cancer decreased by 46% overall in 2020. Examples range from a 25% drop in pancreatic cancer diagnoses to a 52% decrease for new breast cancer diagnoses, early in the pandemic.(255) Data from US central cancer registries will further inform this observation but will not be available until 2022 at the earliest, given the normal lag in registry reporting. During the pandemic, routine cancer screening rates have also declined. In the United Kingdom screening declined across all studied cohorts, most notably breast cancer screening by 90% and in colorectal cancer screening by 85%.(256) In the U.S. screenings for breast, colon, prostate, and lung cancers in older adults were lower by 85%, 75%, 74%, and 56%, respectively,(257) and there were reduced cervical cancer screenings for women aged 21 to 65 years.(258) Studies tracking observed versus expected cancer cases(259) and modeling studies(7,260,261) suggest a significant reservoir of undiagnosed cancer due to pandemic-related decreases in screening. Compared to pre-pandemic figures, a UK-based population modeling study estimates increased mortality and avoidable deaths ranging from 4% to 17%, depending on tumor type, due to pandemic-related diagnostic delays.(7)
The impact of delayed diagnosis is disproportionately profound in vulnerable populations, which will result in widening disparities.(258) Researchers in Canada and Scotland reported on the negative impact of the pandemic on all cancer screening programs and identified older age and low neighborhood income as factors associated with diagnostic delays.(262,263) DeGroff et al. further reported decreases in screening and recovery among women from underrepresented minority populations.(264) In another study, disparities seen at the onset of the pandemic remained persistent when screening resumed.(265)
Cancer Therapy
Around the world, and even within locales, there has been variability in individual treatment decision-making in an attempt to maintain evidenced-based cancer care during the pandemic while ensuring patient safety. In addition to delays and cancellations of surgeries, modifications included the use of local or regional anesthesia in place of general anesthesia when feasible(266), change in surgical technique to decrease aerosol generation(267), and enhanced recovery protocols to decrease hospital stays(268). Within radiation therapy there are often multiple dose/fractionation regimens that can have clinical parity for a particular disease entity, allowing for the consideration of truncated treatment times.(269) In some cases a modality change was recommended; for example, consensus panels often eschewed surgical therapies in favor of radiation or chemoradiation.(246) Systemic regimens were altered with prolonged dosing intervals,(270) intravenous regimens being replaced by oral or subcutaneous agents(271) or the type of systemic therapy was chosen to decrease the likelihood of hematologic toxicity.(272) In an example of an extreme modification, stem cell transplants in hematologic malignancies were replaced with radiotherapy.(266) In the OnCOVID registry, among 466 patients who had recovered from COVID-19 who were on systemic anti-cancer therapy, 15% permanently discontinued therapy, and 38% resumed treatment with a dose or regimen adjustment; permanent treatment discontinuation was independently associated with an increased risk of death, while dose or regimen adjustments were not associated with worse outcome.(76)
For patients with cancer, a delay in surgery has the potential to increase the likelihood of advanced disease and decreased survival.(7) Data from an observational modeling study examining the effect of COVID-19 on surgery delays and outcomes showed that delays by 3 to 6 months reduced life-years gained by surgery by 19% and 43% respectively.(273) Another study reported a 6-8% increase in the risk of death for every four-week delay across surgical, systemic therapy, and radiotherapy indications for seven analyzed cancers.(274) A large international prospective study of over 20,000 people awaiting surgery found that 10% of patients did not receive surgery for a COVID-19-related reason, with moderate and full lockdowns being associated with non-operation.(275) The aggregate effects of these alterations to standard of care therapy will be the subject of ongoing study for several years and may lead to insights to guide practice in future pandemics. Early estimates of the 1-year impact of reduced supply and demand of cancer services by 40% resulted in 78% excess deaths in survivors of cancer.(275)
Telehealth
Driven by the need to preserve PPE, minimize physical contact within healthcare facilities, and reduce potential exposure to infection, COVID-19 has propelled the use of telemedicine. There have been mixed reactions among patients to the telehealth experience, with an appreciation for the accessibility, ease, and convenience that it allows, against the challenges of available access to internet/technology.(276,277)
The increased use of virtual consultation ushers in a new set of concerns including maintenance of cybersecurity, confidentiality, delivery of medications, and documentation.(278) The pivot to telemedicine(279) highlights challenges mediated by sociodemographic factors(280) potentially deepening the divide for disadvantaged and marginalized groups. In particular, despite increased telehealth visits during the pandemic, Black and Hispanic patients were less likely to have an increase in telehealth utilization.(85) In addition to changes in direct patient contact, videoconferencing has been adopted by many centers for multidisciplinary tumor boards. This has the advantage of improved ease of attendance for participants, but with mixed results in regard to efficiency because of potential audiovisual sharing difficulties and delays in supporting information like pathology slides and imaging.(4,281) The rapid and successful deployment of virtual medicine has transformed cancer care and will likely become a permanent aspect of its delivery, although this outcome is highly dependent on regulatory decisions.
Access to Care, Social Isolation, and Rationing
Additional effects of the pandemic have been omnipresent and have affected all patients with cancer. These include the barriers of accessing in-person and telemedicine care during provider shortages and re-assignments, cancellation of procedures due to healthcare system capacity issues, social isolation due to visitor limitations at healthcare facilities, and general avoidance of social outings. Preliminary research suggests that the “fear of COVID” is common in patients with cancer and can lead to debilitating anxiety.(282) In a study of 1000 patients with cancer carried out during the early and late pandemic, fear of COVID-19 was linked to psychological distress and persisted throughout the pandemic among under-resourced patients with cancer. The authors concluded that timely psychosocial support is critical to meet increased care needs experienced by patients with cancer during the COVID-19 pandemic. Early on in the pandemic, in exceptional circumstances, certain patients with cancer were not being offered potentially life-saving therapies such as ECMO or intubation.(96)
Statistical and Research Design Considerations
Underlying all aspects of COVID-19 research—from disease characterization to potential treatment and vaccination evaluation to appropriate public health communication—is data generation. The abundance of data on COVID-19 has been very valuable. However, when muddled or inaccurate, data can be detrimental. Because of the urgency to answer public health questions amid a dearth of randomized trial data early in the pandemic, real-world data (RWD) have emerged as useful tools to understand and contextualize emerging situations. This provides an opportunity to rapidly characterize natural history including disease progression and risk factors, examine health equity, and evaluate treatments.
The rapid rise in use of RWD applied to COVID-19 is accompanied by the inherent challenges to the use of observational data that vary in their purpose, type, completeness, and granularity. Data quality is a salient—if not the greatest—challenge in the use of RWD for generating inference. Measures of data quality can include plausibility, reliability, conformance, completeness, accuracy, and reliability. Assessment of whether a dataset is fit to answer a research question requires a high level of data familiarity. Furthermore, transparency and reproducibility in methods is integral to interpretation (https://www.strobe-statement.org/ ).(283,284)
Observational studies are subject to the potential for inherent confounding and bias in the absence of randomization, including selection bias, measured and unmeasured confounding, residual confounding, and collider bias. For example, collider bias can infer associations between two or more variables which affect the likelihood of an individual being sampled, misrepresenting the true associations between these variables in the sample.(285) In COVID-19 research designs, confounding by indication (e.g., evaluating ACE inhibitor and angiotensin receptor blocker treatment effects on COVID-19) and confounding by severity (e.g., differential nature of underlying comorbidities or more severe disease) are particularly relevant. Moreover, temporal and geographic biases can arise from the spatiotemporal patterns in the pandemic, public health mitigation strategies (i.e., masking), available treatments and authorizations, testing, as well as vaccine uptake. These time and geographical-varying aspects have created measurement challenges in data capture— for example inaccurate estimates of vaccine exposure or severity outcomes which could lead to the potential for information bias, where the exposure may be misclassified due to incomplete capture.
Consistent definitions for data elements and outcomes, such as the ordinal WHO clinical progression scale,(286) could better facilitate replication and synthesis of results across studies and populations. While advanced statistical methods are available that attempt to generate causal inference from observational data, these methods require additional, sometimes untestable, assumptions that must be considered in each individual context.” (287) At minimum, selection of appropriate comparators for both positive and negative controls are essential.(288) Because the total eligible population for a convenience sample is not easily estimable, the definition of a denominator remains elusive meaning that population inferences are challenging. For example, a commonly reported statistic is the percentage of inpatients with SARS-CoV-2 infection or that are vaccinated versus unvaccinated. However, the more logical statistic for public health communication to explain risk perception would be the percentage of vaccinated people that are hospitalized. This illustrates a more general problem that is widespread in an incomplete data ecosystem.Because of these intricate methodological considerations, RWD design and analysis requires a collaborative, multidisciplinary approach to ensure appropriate data selection and analysis to provide the best possible evidence for patient and clinician decision-making. Attention to data quality assessment, protocol development, and a priori statistical analysis plans are necessary.(289) RWD can contribute meaningfully to rapid categorization and understanding of broader patient populations than those included in trials, which is particularly important in evaluating COVID-19 treatment and vaccination effectiveness in patients with cancer. While randomized controlled trials remain the gold standard, efforts during the COVID-19 pandemic have demonstrated the potential opportunity for registry-based studies, observational cohort studies, and pragmatic trials to improve care delivery and clinical research to provide for more inclusive improvement of patient health outcomes.(290)
In addition to challenges related to RWD during this health crisis, several ethical concerns related to informed consent for participation in research have been highlighted. While many institutions have adopted innovative methods such as e-consent, implementation has not always been seamless due to lack of personnel and infrastructure (especially in underserved communities), user-friendly interfaces, and availability of translators for non-English language speaking individuals.(291,292) These hurdles have highlighted the need to improve technology and accessibility for e-consent, assure presence of translators, and to simplify the e-consent process by reducing the lengths or number of forms required.(293)
Conclusion
This landscape analysis provides the reader with the current state of knowledge along with many of the challenges faced in determining evidence for patients with COVID-19 and cancer. Overall, our collective understanding is remarkably advanced less than two years into a generational pandemic, but many gaps and unanswered questions remain. In comparison to the early years of the last generational pandemic (HIV/AIDS), the number of basic, translational, and clinical researchers tackling COVID-19 has been nothing short of remarkable. Many of these researchers pivoted from pre-existing programs, and the effects on their unrelated cancer and infectious diseases research remain to be determined. International grassroots efforts have been catalyzed by social media in a way that would have been unimaginable before the internet.(290,294,295) The next chapter of the pandemic is yet to be written, but it is clear that much remains to be learned so that the direct and indirect effects of the pandemic on patients with cancer are mitigated to the fullest extent possible.
Statement of Significance:
Patients with cancer have faced severe consequences at every stage of the cancer journey due to the COVID-19 pandemic. This comprehensive review offers a landscape analysis of the current state of the field regarding COVID-19 and cancer. We cover the immune response, risk factors for severe disease, and implications for vaccination in patients with cancer, as well as the impact of the COVID-19 pandemic on cancer care delivery. Overall, this review provides an in-depth summary of the key issues facing patients with cancer during this unprecedented health crisis.
Funding
National Cancer Institute grant P30 CA068485 to Alicia Beeghly-Fadiel, Benjamin French, Sanjay Mishra & Jeremy L. Warner
Conflict of Interest Disclosures
Dr. Elkrief reports salary support from Canadian Institute of Health Research, the Royal College of Physicians and Surgeons of Canada (Detweiler Travelling Fellowship), and the Henry R. Shibata Fellowship outside the submitted work.
Dr. Jhawar declares grant from Varian Medical Systems; outside the submitted work.
Dr. Johnson declares Advisory boards/consulting for BMS, Catalyst, Jansen, Iovance, Merck, Mosaic ImmunoEngineering, Oncosec, Pfizer, Targovax, and research funding from BMS and Incyte.
Dr. McKay received research funding from Bayer, Pfizer, Tempus; serves on Advisory Board for AstraZeneca, Bayer, Bristol Myers Squibb, Calithera, Exelixis, Janssen, Merck, Novartis, Pfizer, Sanofi, Tempus; is a consultant for Dendreon, Myovant, Sorrento Therapeutics, Vividion; serves on the molecular tumor board at Caris.
Dr. Vinh declares salary support through the clinician-scientist scholar Junior 2 program from Fonds de la recherche en santé du Québec (FRQS); Grants or contracts from Canadian Institutes of Health Research, COVID Immunity Task Force, Jeffrey Modell Foundation to his institution; Consulting fees from Qu Biologics, UCB Biosciences GmbH; Payment or honoraria from CSL Behring, Merck Canada, Novartis Canada, Shire; Support for attending meetings and/or travel from CSL Behring; Patents pending (PCT/US2021/042875; United States Provisional Application No. 63/280,983); Leadership or fiduciary role as Chair of AMMI Canada Guidelines committee; member of Clinical Immunology Society Education Committee; ass outside the submitted work.
Dr. Mishra reports support from National Cancer Institute (P30 CA068485), and the International Association for the Study of Lung Cancer to his institution) during the conduct of the study; personal fees from National Geographic for writing articles outside the submitted work.
Dr. Warner reports grants from NIH and the International Association for Study of Lung Cancer during the conduct of the study; grants from AACR; personal fees from Westat, Melax Tech, Flatiron Health, and Roche; equity in HemOnc.org LLC, outside the submitted work.
Drs. Beeghly-Fadiel, Enriquez, French, Glover, Jani, Reuben, Rivera, Surbhi Shah, Mansi Shah, Shaikh, Wu; and Stacey Tinianov has nothing to disclose.
References
- 1.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alom S, Chiu CM, Jha A, Lai SHD, Yau THL, Harky A. The Effects of COVID-19 on Cancer Care Provision: A Systematic Review. Cancer Control J Moffitt Cancer Cent. United States; 2021;28:1073274821997425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kampen JJA, van de Vijver DAMC, Fraaij PLA, Haagmans BL, Lamers MM, Okba N, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun. 2021;12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Momenimovahed Z, Salehiniya H, Hadavandsiri F, Allahqoli L, Günther V, Alkatout I. Psychological Distress Among Cancer Patients During COVID-19 Pandemic in the World: A Systematic Review. Front Psychol. 2021;12:682154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–5. [DOI] [PubMed] [Google Scholar]
- 10.Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, et al. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buikema AR, Buzinec P, Paudel ML, Andrade K, Johnson JC, Edmonds YM, et al. Racial and ethnic disparity in clinical outcomes among patients with confirmed COVID-19 infection in a large US electronic health record database. EClinicalMedicine. 2021;39:101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen A, Miller AD, Zambrano LD, Wu MJ, Oster ME, Godfred-Cato S, et al. Demographic and Clinical Factors Associated With Death Among Persons <21 Years Old With Multisystem Inflammatory Syndrome in Children-United States, February 2020-March 2021. Open Forum Infect Dis. 2021;8:ofab388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev Off J Int Assoc Study Obes. 2020;21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkrief A, Desilets A, Papneja N, Cvetkovic L, Groleau C, Lakehal YA, et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: A multicentre observational cohort study. Eur J Cancer Oxf Engl 1990. 2020;139:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grivas P, Khaki AR, Wise-Draper TM, French B, Hennessy C, Hsu C-Y, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID-19. Am J Pathol. 2021;191:4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Ye Q. Cellular Immune Response to COVID-19 and Potential Immune Modulators. Front Immunol. 2021;12:646333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng Z, Zhang M, Zhu T, Zhili N, Liu Z, Xiang R, et al. Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;98:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell. 2020;38:629–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinh DC, Abel L, Bastard P, Cheng MP, Condino-Neto A, Gregersen PK, et al. Harnessing Type I IFN Immunity Against SARS-CoV-2 with Early Administration of IFN-β. J Clin Immunol. 2021;41:1425–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Yang C, Xu X-F, Xu W, Liu S-W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salahudeen AA, Choi SS, Rustagi A, Zhu J, van Unen V, de la O SM, et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature. 2020;588:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the Nervous System. Cell. 2020;183:16–27.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe. 2021;29:1052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Vries RD, Rockx B, Haagmans BL, Herfst S, Koopmans MP, de Swart RL. Animal models of SARS-CoV-2 transmission. Curr Opin Virol. 2021;50:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Maglorius Renkilaraj MRL, et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6:eabl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Made CI, Simons A, Schuurs-Hoeijmakers J, van den Heuvel G, Mantere T, Kersten S, et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA. 2020;324:663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solanich X, Vargas-Parra G, van der Made CI, Simons A, Schuurs-Hoeijmakers J, Antolí A, et al. Genetic Screening for TLR7 Variants in Young and Previously Healthy Men With Severe COVID-19. Front Immunol. 2021;12:719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smieszek SP, Polymeropoulos VM, Xiao C, Polymeropoulos CM, Polymeropoulos MH. Loss-of-function mutations in IFNAR2 in COVID-19 severe infection susceptibility. J Glob Antimicrob Resist. 2021;26:239–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–8. [DOI] [PubMed] [Google Scholar]
- 37.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann H-H, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troya J, Bastard P, Planas-Serra L, Ryan P, Ruiz M, de Carranza M, et al. Neutralizing Autoantibodies to Type I IFNs in >10% of Patients with Severe COVID-19 Pneumonia Hospitalized in Madrid, Spain. J Clin Immunol. 2021;41:914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez SE, Bastard P, Kelly K, Gervais A, Norris PJ, Dumont LJ, et al. Neutralizing Autoantibodies to Type I Interferons in COVID-19 Convalescent Donor Plasma. J Clin Immunol. 2021;41:1169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goncalves D, Mezidi M, Bastard P, Perret M, Saker K, Fabien N, et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Transl Immunol. 2021;10:e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. England; 2021;595:283–8. [DOI] [PubMed] [Google Scholar]
- 42.van der Wijst MGP, Vazquez SE, Hartoularos GC, Bastard P, Grant T, Bueno R, et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med. United States; 2021;13:eabh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol. United States; 2021;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hillyer P, Mane VP, Schramm LM, Puig M, Verthelyi D, Chen A, et al. Expression profiles of human interferon-alpha and interferon-lambda subtypes are ligand- and cell-dependent. Immunol Cell Biol. 2012;90:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Remy KE, Mazer M, Striker DA, Ellebedy AH, Walton AH, Unsinger J, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffy ME. Health promotion in the family: current findings and directives for nursing research. J Adv Nurs. England; 1988;13:109–17. [DOI] [PubMed] [Google Scholar]
- 47.Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen M-C, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5:917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. United States; 2020;26:1623–35. [DOI] [PubMed] [Google Scholar]
- 50.García LF. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front Immunol. 2020;11:1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tembhare PR, Sriram H, Chatterjee G, Khanka T, Gokarn A, Mirgh S, et al. Comprehensive immune cell profiling depicts an early immune response associated with severe coronavirus disease 2019 in cancer patients. Immunol Cell Biol [Internet]. John Wiley & Sons, Ltd; 2021. [cited 2021 Dec 2];n/a. Available from: 10.1111/imcb.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon G, Kim HJ, Park SJ, Lee HE, Woo H, Ahn YJ, et al. Anxiolytic-like effect of danshensu [(3-(3,4-dihydroxyphenyl)-lactic acid)] in mice. Life Sci. 2014;101:73–8. [DOI] [PubMed] [Google Scholar]
- 54.Esperança-Martins M, Gonçalves L, Soares-Pinho I, Gomes A, Serrano M, Blankenhaus B, et al. Humoral Immune Response of SARS-CoV-2-Infected Patients with Cancer: Influencing Factors and Mechanisms. The Oncologist. 2021;26:e1619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansi L, Spehner L, Daguindau E, Bouiller K, Almotlak H, Stein U, et al. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur J Cancer Oxf Engl 1990. 2021;150:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fendler A, Au L, Shepherd S, Byrne F, Cerrone M, Boos L, et al. Functional antibody and T-cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Res Sq. 2021;rs.3.rs-916427. [DOI] [PubMed] [Google Scholar]
- 59.Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27:1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdul-Jawad S, Baù L, Alaguthurai T, Del Molino Del Barrio I, Laing AG, Hayday TS, et al. Acute Immune Signatures and Their Legacies in Severe Acute Respiratory Syndrome Coronavirus-2 Infected Cancer Patients. Cancer Cell. 2021;39:257–275.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goubet A-G, Dubuisson A, Geraud A, Danlos F-X, Terrisse S, Silva CAC, et al. Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis. Cell Death Differ. 2021;1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med. 2020;383:2586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Dam PA, Huizing M, Mestach G, Dierckxsens S, Tjalma W, Trinh XB, et al. SARS-CoV-2 and cancer: Are they really partners in crime? Cancer Treat Rev. 2020;89:102068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barba M, Krasniqi E, Ciliberto G, Vici P. Cancer patients and coronavirus disease 2019: evidence in context. J Transl Med. 2020;18:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinato DJ, Zambelli A, Aguilar-Company J, Bower M, Sng CCT, Salazar R, et al. Clinical Portrait of the SARS-CoV-2 Epidemic in European Patients with Cancer. Cancer Discov. 2020;10:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correction: Systemic pro-inflammatory response identifies patients with cancer with adverse outcomes from SARS-CoV-2 infection: the OnCovid Inflammatory Score. J Immunother Cancer. 2021;9:e002277corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dettorre GM, Patel M, Gennari A, Pentheroudakis G, Romano E, Cortellini A, et al. The systemic pro-inflammatory response: targeting the dangerous liaison between COVID-19 and cancer. ESMO Open. 2021;6:100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hempel D, Milani V, Kleespies A, Hempel L, Ebner F, Zehn D, et al. 1680P SARS-CoV-2 infections in outpatients with cancer: Most infected patients are asymptomatic carriers without impact on chemotherapy. Ann Oncol. 2020;31:S995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarifkar P, Kamath A, Robinson C, Morgulchik N, Shah SFH, Cheng TKM, et al. Clinical Characteristics and Outcomes in Patients with COVID-19 and Cancer: a Systematic Review and Meta-analysis. Clin Oncol R Coll Radiol G B. 2021;33:e180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Q, Berger NA, Xu R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA Oncol. 2021;7:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinato DJ, Scotti L, Gennari A, Colomba-Blameble E, Dolly S, Loizidou A, et al. Determinants of enhanced vulnerability to coronavirus disease 2019 in UK patients with cancer: a European study. Eur J Cancer Oxf Engl 1990. 2021;150:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmieri C, Turtle L, Drake T, Harrison E, Docherty A, Greenhalf B, et al. LBA60 Prospective data of >20,000 hospitalised patients with cancer and COVID-19 derived from the International Severe Acute Respiratory and emerging Infections Consortium WHO Coronavirus Clinical Characterisation Consortium: CCP-CANCER UK. Ann Oncol. 2021;32:S1337. [Google Scholar]
- 75.Pinato DJ, Patel M, Lambertini M, Colomba E, Pommeret F, Van Hemelrijick M, et al. 1565MO Time-dependent improvement in the clinical outcomes from COVID-19 in cancer patients: An updated analysis of the OnCovid registry. Abstr Book ESMO Congr 2021 16 – 21 Sept 2021. 2021;32:S1132. [Google Scholar]
- 76.Pinato DJ, Tabernero J, Bower M, Scotti L, Patel M, Colomba E, et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021;S1470-2045(21)00573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Joode K, Dumoulin DW, Tol J, Westgeest HM, Beerepoot LV, van den Berkmortel FWPJ, et al. Dutch Oncology COVID-19 consortium: Outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer Oxf Engl 1990. 2020;141:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lièvre A, Turpin A, Ray-Coquard I, Le Malicot K, Thariat J, Ahle G, et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur J Cancer Oxf Engl 1990. 2020;141:62–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garassino MC, Whisenant JG, Huang L-C, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nader Marta G, Colombo Bonadio R, Nicole Encinas Sejas O, Watarai G, Mathias Machado MC, Teixeira Frasson L, et al. Outcomes and Prognostic Factors in a Large Cohort of Hospitalized Cancer Patients With COVID-19. JCO Glob Oncol. 2021;7:1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fillmore NR, La J, Szalat RE, Tuck DP, Nguyen V, Yildirim C, et al. Prevalence and Outcome of COVID-19 Infection in Cancer Patients: A National Veterans Affairs Study. J Natl Cancer Inst. 2021;113:691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shiels MS, Haque AT, Haozous EA, Albert PS, Almeida JS, García-Closas M, et al. Racial and Ethnic Disparities in Excess Deaths During the COVID-19 Pandemic, March to December 2020. Ann Intern Med. 2021;M21–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivera DR, Peters S, Panagiotou OA, Shah DP, Kuderer NM, Hsu C-Y, et al. Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discov. 2020;10:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmidt AL, Bakouny Z, Bhalla S, Steinharter JA, Tremblay DA, Awad MM, et al. Cancer Care Disparities during the COVID-19 Pandemic: COVID-19 and Cancer Outcomes Study. Cancer Cell. 2020;38:769–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wadhera RK, Wadhera P, Gaba P, Figueroa JF, Joynt Maddox KE, Yeh RW, et al. Variation in COVID-19 Hospitalizations and Deaths Across New York City Boroughs. JAMA. 2020;323:2192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SJ, Bostwick W. Social Vulnerability and Racial Inequality in COVID-19 Deaths in Chicago. Health Educ Behav Off Publ Soc Public Health Educ. 2020;47:509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and Racial/Ethnic Disparities. JAMA. 2020;323:2466–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simons RL, Lei M-K, Beach SRH, Barr AB, Simons LG, Gibbons FX, et al. Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Dev Psychol. 2018;54:1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. United States; 2021;27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114:428–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bell ML, Catalfamo CJ, Farland LV, Ernst KC, Jacobs ET, Klimentidis YC, et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PLOS ONE. Public Library of Science; 2021;16:e0254347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: An overview. Diabetes Metab Syndr. 2021;15:869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paul BD, Lemle MD, Komaroff AL, Snyder SH. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2021;118:e2024358118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matta J, Wiernik E, Robineau O, Carrat F, Touvier M, Severi G, et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern Med. 2021; doi: 10.1001/jamainternmed.2021.6454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cortellini A, Roldán E, Garcia MC, Berardi R, Sánchez A, Martinez C, et al. 1560O Prevalence and impact of COVID-19 sequelae on treatment pathways and survival of cancer patients who recovered from SARS-CoV-2 infection. Ann Oncol. 2021/09/21 ed. European Society for Medical Oncology. Published by Elsevier Ltd.; 2021;32:S1130–S1130. [Google Scholar]
- 97.Qian W, Ye Y, Zuo L, Song T, Xu Q, Wang Y, et al. Immune checkpoint inhibitors use and effects on prognosis of COVID-19 infection: a systematic review and meta-analysis. Immunotherapy. Future Medicine; 2021;13:1271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. Cancer Discov. 2020;10:1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee LY, Cazier J-B, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. The Lancet. Elsevier; 2020;395:1919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rogiers A, Pires da Silva I, Tentori C, Tondini CA, Grimes JM, Trager MH, et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9:e001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klebanov N, Pahalyants V, Murphy WS, Theodosakis N, Zubiri L, Klevens RM, et al. Risk of COVID-19 in Patients with Cancer Receiving Immune Checkpoint Inhibitors. The Oncologist. 2021;26:e898–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharafeldin N, Bates B, Song Q, Madhira V, Yan Y, Dong S, et al. Outcomes of COVID-19 in Patients With Cancer: Report From the National COVID Cohort Collaborative (N3C). J Clin Oncol Off J Am Soc Clin Oncol. 2021;39:2232–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer Oxf Engl 1990. 2020;141:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA. 2018;320:1702–3. [DOI] [PubMed] [Google Scholar]
- 108.Dirienzo W, Ciprandi G, Caria M, Scordamaglia A, Bagnasco M, Canonica GW, et al. T cell activation surface markers and autologous mixed lymphocyte reaction do not differ in true and pseudo food allergy. Int Arch Allergy Appl Immunol. 1987;83:193–7. [DOI] [PubMed] [Google Scholar]
- 109.Johnson DB, Taylor KB, Cohen JV, Ayoubi N, Haugh AM, Wang DY, et al. Anti-PD-1-Induced Pneumonitis Is Associated with Persistent Imaging Abnormalities in Melanoma Patients. Cancer Immunol Res. 2019;7:1755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Naidoo J, Reuss JE, Suresh K, Feller-Kopman D, Forde PM, Mehta Steinke S, et al. Immune-related (IR)-pneumonitis during the COVID-19 pandemic: multidisciplinary recommendations for diagnosis and management. J Immunother Cancer. 2020;8:e000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee LYW, Cazier J-B, Starkey T, Briggs SEW, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jee J, Foote MB, Lumish M, Stonestrom AJ, Wills B, Narendra V, et al. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38:3538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spanjaart AM, Ljungman P, de La Camara R, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet Lond Engl. 2020;396:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.LaPlant Q, Thor M, Shaverdian N, Shin JY, Gilbo P, Luo J, et al. Association of prior radiation dose to the cardiopulmonary system with COVID-19 outcomes in patients with cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;161:115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol Off J Eur Soc Med Oncol. 2020;31:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel VG, Zhong X, Liaw B, Tremblay D, Tsao C-K, Galsky MD, et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol Off J Eur Soc Med Oncol. 2020;31:1419–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klein EA, Li J, Milinovich A, Schold JD, Sharifi N, Kattan MW, et al. Androgen Deprivation Therapy in Men with Prostate Cancer Does Not Affect Risk of Infection with SARS-CoV-2. J Urol. 2021;205:441–3. [DOI] [PubMed] [Google Scholar]
- 123.Karimi A, Nowroozi A, Alilou S, Amini E. Effects of Androgen Deprivation Therapy on COVID-19 in Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. Urol J. 2021;6691. [DOI] [PubMed] [Google Scholar]
- 124.Koskinen M, Carpen O, Honkanen V, Seppänen MRJ, Miettinen PJ, Tuominen JA, et al. Androgen deprivation and SARS-CoV-2 in men with prostate cancer. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmidt AL, Tucker MD, Bakouny Z, Labaki C, Hsu C-Y, Shyr Y, et al. Association Between Androgen Deprivation Therapy and Mortality Among Patients With Prostate Cancer and COVID-19. JAMA Netw Open. 2021;4:e2134330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alavi Darazam I, Shokouhi S, Pourhoseingholi MA, Naghibi Irvani SS, Mokhtari M, Shabani M, et al. Role of interferon therapy in severe COVID-19: the COVIFERON randomized controlled trial. Sci Rep. 2021;11:8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Q, Chen V, Shannon CP, Wei X-S, Xiang X, Wang X, et al. Interferon-α2b Treatment for COVID-19. Front Immunol. 2020;11:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137–146.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Banerjee S, Yadav S, Banerjee S, Fakayode SO, Parvathareddy J, Reichard W, et al. Drug Repurposing to Identify Nilotinib as a Potential SARS-CoV-2 Main Protease Inhibitor: Insights from a Computational and In Vitro Study. J Chem Inf Model. 2021;61:5469–83. [DOI] [PubMed] [Google Scholar]
- 130.Hess CB, Buchwald ZS, Stokes W, Nasti TH, Switchenko JM, Weinberg BD, et al. Low-dose whole-lung radiation for COVID-19 pneumonia: Planned day 7 interim analysis of a registered clinical trial. Cancer. 2020;126:5109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Friese CR, Choueiri TK, Duma N, Farmakiotis D, Grivas P, Rini BI, et al. Care without a compass: Including patients with cancer in COVID-19 studies. Cancer Cell. 2021;39:895–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mouffak S, Shubbar Q, Saleh E, El-Awady R. Recent advances in management of COVID-19: A review. Biomed Pharmacother Biomedecine Pharmacother. 2021;143:112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma L, Sahu SK, Cano M, Kuppuswamy V, Bajwa J, McPhatter J, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. BioRxiv Prepr Serv Biol. 2021;2021.02.22.432177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21:382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shaya J, Lee A, Cabal A, McKay RR. Outcomes of active cancer patients with COVID-19 infection treated with COVID-19 neutralizing monoclonal antibodies. J Clin Oncol. Wolters Kluwer; 2021;39:3137–3137. [Google Scholar]
- 138.Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, et al. Association of Convalescent Plasma Therapy With Survival in Patients With Hematologic Cancers and COVID-19. JAMA Oncol. 2021;7:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haidar G, Mellors JW. Improving the Outcomes of Immunocompromised Patients With Coronavirus Disease 2019. Clin Infect Dis. 2021;73:e1397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet Lond Engl. 2020;395:e30–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.ACTIV-3/TICO LY-CoV555 Study Group, Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, et al. A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Lond Engl. 2021;397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Velasco Puyó P, Moreno L, Díaz de Heredia C, Rivière JG, Soler Palacín P. Tocilizumab in a child with acute lymphoblastic leukaemia and COVID-19-related cytokine release syndrome. An Pediatr. 2020;93:132–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bonomi M, Maltese M, Brighenti M, Muri M, Passalacqua R. Tocilizumab for COVID-19 Pneumonia in a Patient With Non-Small-cell Lung Cancer Treated With Chemoimmunotherapy. Clin Lung Cancer. 2021;22:e67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bersanelli M Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Agley J, Xiao Y. Misinformation about COVID-19: evidence for differential latent profiles and a strong association with trust in science. BMC Public Health. 2021;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2:CD013587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Popp M, Stegemann M, Metzendorf M-I, Gould S, Kranke P, Meybohm P, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev. 2021;7:CD015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kamel AM, Monem MSA, Sharaf NA, Magdy N, Farid SF. Efficacy and safety of azithromycin in Covid-19 patients: A systematic review and meta-analysis of randomized clinical trials. Rev Med Virol. 2021;e2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baladia E, Pizarro AB, Ortiz-Muñoz L, Rada G. Vitamin C for COVID-19: A living systematic review. Medwave. 2020;20:e7978. [DOI] [PubMed] [Google Scholar]
- 153.Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw Open. 2021;4:e210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. [DOI] [PubMed] [Google Scholar]
- 155.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost JTH. 2007;5:632–4. [DOI] [PubMed] [Google Scholar]
- 156.Li A, Kuderer NM, Hsu C-Y, Shyr Y, Warner JL, Shah DP, et al. The CoVID-TE risk assessment model for venous thromboembolism in hospitalized patients with cancer and COVID-19. J Thromb Haemost JTH. 2021;19:2522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.COVID-19 rapid guideline: reducing the risk of venous thromboembolism in over 16s with COVID-19 [Internet]. London: National Institute for Health and Care Excellence (NICE); 2020. [cited 2021 Sep 20]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK566720/ [PubMed] [Google Scholar]
- 158.Langer F, Kluge S, Klamroth R, Oldenburg J. Coagulopathy in COVID-19 and Its Implication for Safe and Efficacious Thromboprophylaxis. Hamostaseologie. 2020;40:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Horowitz NA, Brenner B. Thrombosis and Hemostasis Issues in Cancer Patients with COVID-19. Semin Thromb Hemost. 2020;46:785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leentjens J, van Haaps TF, Wessels PF, Schutgens REG, Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021;8:e524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med. Massachusetts Medical Society; 2020;382:1599–607. [DOI] [PubMed] [Google Scholar]
- 162.Young A, Phillips J, Hancocks H, Hill C, Joshi N, Marshall A, et al. OC-11 - Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism. Thromb Res. 2016;140 Suppl 1:S172–173. [DOI] [PubMed] [Google Scholar]
- 163.Bona RD, Hickey AD, Wallace DM. Efficacy and safety of oral anticoagulation in patients with cancer. Thromb Haemost. 1997;78:137–40. [PubMed] [Google Scholar]
- 164.Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost JTH. 2020;18:1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hadid T, Kafri Z, Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2021;47:100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ting R, Edmonds P, Higginson IJ, Sleeman KE. Palliative care for patients with severe covid-19. BMJ. 2020;370:m2710. [DOI] [PubMed] [Google Scholar]
- 167.Loggers ET, Wulff-Burchfield EM, Subbiah IM, Khaki AR, Egan P, Farmakiotis D, et al. Code status and outcomes in patients with cancer and COVID-19: A COVID-19 and cancer consortium (CCC19) registry analysis. J Clin Oncol. Wolters Kluwer; 2021;39:12035–12035. [Google Scholar]
- 168.Singhai P, Rao K, Rao S, Salins N. Palliative care for advanced cancer patients in the COVID-19 pandemic: Challenges and adaptations. Cancer Res Stat Treat. 2020;3:127–32. [Google Scholar]
- 169.Cheng HWB. Palliative care for cancer patients with severe COVID-19: the challenge of uncertainty. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2021;29:1153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Soosaipillai G, Wu A, Dettorre GM, Diamantis N, Chester J, Moss C, et al. Specialist palliative and end-of-life care for patients with cancer and SARS-CoV-2 infection: a European perspective. Ther Adv Med Oncol. 2021;13:17588359211042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wherry EJ, Jaffee EM, Warren N, D’Souza G, Ribas A, AACR COVID-19 and Cancer Task Force. How Did We Get a COVID-19 Vaccine in Less Than 1 Year? Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27:2136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fauci AS. The story behind COVID-19 vaccines. Science. 2021;372:109. [DOI] [PubMed] [Google Scholar]
- 173.Gupta S, Cantor J, Simon KI, Bento AI, Wing C, Whaley CM. Vaccinations Against COVID-19 May Have Averted Up To 140,000 Deaths In The United States. Health Aff Proj Hope. 2021;40:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bartsch SM, Wedlock PT, O’Shea KJ, Cox SN, Strych U, Nuzzo JB, et al. Lives and Costs Saved by Expanding and Expediting Coronavirus Disease 2019 Vaccination. J Infect Dis. 2021;224:938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Corti C, Curigliano G. Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol Off J Eur Soc Med Oncol. 2021;32:569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID-19 Vaccination for Patients with Cancer while Vaccine Supply Is Limited. Cancer Discov. 2021;11:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Desai A, Gainor JF, Hegde A, Schram AM, Curigliano G, Pal S, et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Waldhorn I, Holland R, Goshen-Lago T, Shirman Y, Szwarcwort-Cohen M, Reiner-Benaim A, et al. Six-Month Efficacy and Toxicity Profile of BNT162b2 Vaccine in Cancer Patients with Solid Tumors. Cancer Discov. 2021;11:2430–5. [DOI] [PubMed] [Google Scholar]
- 185.Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021;7:1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, et al. Immunogenicity and Safety of the BNT162b2 mRNA COVID-19 Vaccine Among Actively Treated Cancer Patients. JNCI J Natl Cancer Inst. 2021;djab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Roeker LE, Knorr DA, Thompson MC, Nivar M, Lebowitz S, Peters N, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Benjamini O, Rokach L, Itchaki G, Braester A, Shvidel L, Goldschmidt N, et al. Safety and efficacy of BNT162b mRNA Covid19 Vaccine in patients with chronic lymphocytic leukemia. Haematologica [Internet]. 2021. [cited 2021 Dec 2]; Available from: https://haematologica.org/article/view/haematol.2021.279196 doi: 10.3324/haematol.2021.279196 [DOI]
- 190.Herzog Tzarfati K, Gutwein O, Apel A, Rahimi-Levene N, Sadovnik M, Harel L, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96:1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.So ACP, McGrath H, Ting J, Srikandarajah K, Germanou S, Moss C, et al. COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers. 2021;13:3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27:2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mark C, Gupta S, Punnett A, Upton J, Orkin J, Atkinson A, et al. Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatr Blood Cancer. 2021;68:e29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans A-MC, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Garassino MC, Ribas A. At the Crossroads: COVID-19 and Immune-Checkpoint Blockade for Cancer. Cancer Immunol Res. 2021;9:261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–12. [DOI] [PubMed] [Google Scholar]
- 197.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39:1031–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:e583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mair MJ, Berger JM, Berghoff AS, Starzer AM, Ortmayr G, Puhr HC, et al. Humoral Immune Response in Hematooncological Patients and Health Care Workers Who Received SARS-CoV-2 Vaccinations. JAMA Oncol. 2021;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu JT-Y, La J, Branch-Elliman W, Huhmann LB, Han S, Parmigiani G, et al. 1562MO Effectiveness of COVID-19 vaccination in cancer patients: A nationwide Veterans Affairs study. Ann Oncol. 2021;32:S1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;S1535-6108(21)00490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, et al. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: A monocentric experience. Eur J Cancer Oxf Engl 1990. 2021;154:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chung DJ, Shah GL, Devlin SM, Ramanathan LV, Doddi S, Pessin MS, et al. Disease- and Therapy-Specific Impact on Humoral Immune Responses to COVID-19 Vaccination in Hematologic Malignancies. Blood Cancer Discov. 2021;2:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol OncolJ Hematol Oncol. 2021;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jurgens EM, Ketas TJ, Zhao Z, Joseph Satlin M, Small CB, Sukhu A, et al. Serologic response to mRNA COVID-19 vaccination in lymphoma patients. Am J Hematol. 2021;96:E410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guglielmelli P, Mazzoni A, Maggi L, Kiros ST, Zammarchi L, Pilerci S, et al. Impaired response to first SARS-CoV-2 dose vaccination in myeloproliferative neoplasm patients receiving ruxolitinib. Am J Hematol. 2021;96:E408–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader A, et al. Effectiveness of the BNT162b2mRNA Covid-19 Vaccine in Patients with Hematological Neoplasms. Blood. 2021;blood.2021013768. doi: 10.1182/blood.2021013768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Aleman A, Upadhyaya B, Tuballes K, Kappes K, Gleason Charles R, Beach K, et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell. 2021;39:1442–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Figueiredo JC, Merin NM, Hamid O, Choi SY, Lemos T, Cozen W, et al. Longitudinal SARS-CoV-2 mRNA vaccine-induced humoral immune responses in cancer patients. Cancer Res. 2021;canres.CAN-21-3554-E.2021. doi: 10.1158/0008-5472.CAN-21-3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2021;S1535610821006061. doi: 10.1016/j.ccell.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Parvathaneni K, Torres-Rodriguez K, Meng W, Hwang W-T, Frey N, Naji A, et al. SARS-CoV-2 Spike-Specific T-Cell Responses in Patients With B-Cell Depletion Who Received Chimeric Antigen Receptor T-Cell Treatments. JAMA Oncol [Internet]. 2021. [cited 2021 Dec 2]; Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2786409 doi: 10.1001/jamaoncol.2021.6030 [DOI] [PMC free article] [PubMed]
- 213.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA. 2021;326:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–1090.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, Di Marco M, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. [DOI] [PubMed] [Google Scholar]
- 219.Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Post N, Eddy D, Huntley C, van Schalkwyk MCI, Shrotri M, Leeman D, et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PloS One. 2020;15:e0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. American Association for the Advancement of Science; 0:eab3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Soresina A, Moratto D, Chiarini M, Paolillo C, Baresi G, Focà E, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2020;31:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wurm H, Attfield K, Iversen AK, Gold R, Fugger L, Haghikia A. Recovery from COVID-19 in a B-cell-depleted multiple sclerosis patient. Mult Scler Houndmills Basingstoke Engl. 2020;26:1261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–62. [DOI] [PubMed] [Google Scholar]
- 225.Ni L, Ye F, Cheng M-L, Feng Y, Deng Y-Q, Zhao H, et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52:971–977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mateus J, Grifoni A, Tarke A, Sidney J, Ramirez SI, Dan JM, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schulien I, Kemming J, Oberhardt V, Wild K, Seidel LM, Killmer S, et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nat Med. United States; 2021;27:78–85. [DOI] [PubMed] [Google Scholar]
- 228.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Malard F, Gaugler B, Gozlan J, Bouquet L, Fofana D, Siblany L, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021;11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Griffiths EA, Segal BH. Immune responses to COVID-19 vaccines in patients with cancer: Promising results and a note of caution. Cancer Cell. 2021;39:1045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Thakkar A, Pradhan K, Jindal S, Cui Z, Rockwell B, Shah AP, et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Houot R, Levy R, Cartron G, Armand P. Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? Eur J Cancer Oxf Engl 1990. 2020;136:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Parry H, McIlroy G, Bruton R, Ali M, Stephens C, Damery S, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Massarweh A, Eliakim-Raz N, Stemmer A, Levy-Barda A, Yust-Katz S, Zer A, et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021;7:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tenforde MW, Patel MM, Ginde AA, Douin DJ, Talbot HK, Casey JD, et al. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. MedRxiv Prepr Serv Health Sci. 2021;2021.07.08.21259776. [Google Scholar]
- 237.Tenforde MW, Patel MM, Ginde AA, Douin DJ, Talbot HK, Casey JD, et al. Effectiveness of Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccines for Preventing Coronavirus Disease 2019 Hospitalizations in the United States. Clin Infect Dis. 2021;ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27:1652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McKenzie DR, Muñoz-Ruiz M, Monin L, Alaguthurai T, Lechmere T, Abdul-Jawad S, et al. Humoral and cellular immunity to delayed second dose of SARS-CoV-2 BNT162b2 mRNA vaccination in patients with cancer. Cancer Cell. 2021;39:1445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Juthani PV, Gupta A, Borges KA, Price CC, Lee AI, Won CH, et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis. 2021;21:1485–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pagano L, Salmanton-García J, Marchesi F, Lopez-Garcia A, Lamure S, Itri F, et al. COVID-19 in vaccinated adult patients with hematological malignancies. Preliminary results from EPICOVIDEHA. Blood. 2021;blood.2021014124. doi: 10.1182/blood.2021014124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021;385:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hill JA, Ujjani CS, Greninger AL, Shadman M, Gopal AK. Immunogenicity of a heterologous COVID-19 vaccine after failed vaccination in a lymphoma patient. Cancer Cell. 2021;39:1037–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Heterologous SARS-CoV-2 Booster Vaccinations: Preliminary Report. medRxiv. 2021;2021.10.10.21264827. [Google Scholar]
- 246.van de Haar J, Hoes LR, Coles CE, Seamon K, Fröhling S, Jäger D, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26:665–71. [DOI] [PubMed] [Google Scholar]
- 247.Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A War on Two Fronts: Cancer Care in the Time of COVID-19. Ann Intern Med. 2020;172:756–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Curigliano G, Banerjee S, Cervantes A, Garassino MC, Garrido P, Girard N, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:1320–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jazieh AR, Chan SL, Curigliano G, Dickson N, Eaton V, Garcia-Foncillas J, et al. Delivering Cancer Care During the COVID-19 Pandemic: Recommendations and Lessons Learned From ASCO Global Webinars. JCO Glob Oncol. 2020;6:1461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Guckenberger M, Belka C, Bezjak A, Bradley J, Daly ME, DeRuysscher D, et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2020;146:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bartlett DL, Howe JR, Chang G, Crago A, Hogg M, Karakousis G, et al. Management of Cancer Surgery Cases During the COVID-19 Pandemic: Considerations. Ann Surg Oncol. 2020;27:1717–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wallis CJD, Catto JWF, Finelli A, Glaser AW, Gore JL, Loeb S, et al. The Impact of the COVID-19 Pandemic on Genitourinary Cancer Care: Re-envisioning the Future. Eur Urol. 2020;78:731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Riera R, Bagattini ÂM, Pacheco RL, Pachito DV, Roitberg F, Ilbawi A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob Oncol. 2021;7:311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Helsper CW, Campbell C, Emery J, Neal RD, Li L, Rubin G, et al. Cancer has not gone away: A primary care perspective to support a balanced approach for timely cancer diagnosis during COVID-19. Eur J Cancer Care (Engl). 2020;29:e13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open. 2020;3:e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin Cancer Inform. 2020;4:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin Cancer Inform. 2020;4:1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Miller MJ, Xu L, Qin J, Hahn EE, Ngo-Metzger Q, Mittman B, et al. Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21-65 Years in a Large Integrated Health Care System - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dinmohamed AG, Visser O, Verhoeven RHA, Louwman MWJ, van Nederveen FH, Willems SM, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. [DOI] [PubMed] [Google Scholar]
- 261.Ward ZJ, Walbaum M, Walbaum B, Guzman MJ, Jimenez de la Jara J, Nervi B, et al. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: a simulation-based analysis. Lancet Oncol. 2021;S1470-2045(21)00426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Walker MJ, Meggetto O, Gao J, Espino-Hernández G, Jembere N, Bravo CA, et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: A provincial, population-based study. Prev Med. 2021;151:106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Campbell C, Sommerfield T, Clark GRC, Porteous L, Milne AM, Millar R, et al. COVID-19 and cancer screening in Scotland: A national and coordinated approach to minimising harm. Prev Med. 2021;151:106606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.DeGroff A, Miller J, Sharma K, Sun J, Helsel W, Kammerer W, et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev Med. 2021;151:106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Marcondes FO, Cheng D, Warner ET, Kamran SC, Haas JS. The trajectory of racial/ethnic disparities in the use of cancer screening before and during the COVID-19 pandemic: A large U.S. academic center analysis. Prev Med. 2021;151:106640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wilkinson E How cancer services are fighting to counter covid-19’s impact. BMJ. 2020;370:m2747. [DOI] [PubMed] [Google Scholar]
- 267.Civantos AM, Carey RM, Lichtenstein GR, Lukens JN, Cohen RB, Rassekh CH. Care of immunocompromised patients with head and neck cancer during the COVID-19 pandemic: Two challenging and informative clinical cases. Head Neck. 2020;42:1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chiang J, Yang VS, Han S, Zhuang Q, Ooi G, Sin IH, et al. Minimizing transmission of COVID-19 while delivering optimal cancer care in a National Cancer Centre. J Cancer Policy. 2020;25:100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Thomson DJ, Yom SS, Saeed H, El Naqa I, Ballas L, Bentzen SM, et al. Radiation Fractionation Schedules Published During the COVID-19 Pandemic: A Systematic Review of the Quality of Evidence and Recommendations for Future Development. Int J Radiat Oncol Biol Phys. 2020;108:379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Elkrief A, Kazandjian S, Bouganim N. Changes in Lung Cancer Treatment as a Result of the Coronavirus Disease 2019 Pandemic. JAMA Oncol. 2020;6:1805–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Mulvey TM, Jacobson JO. COVID-19 and Cancer Care: Ensuring Safety While Transforming Care Delivery. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38:3248–51. [DOI] [PubMed] [Google Scholar]
- 272.Rodler S, Apfelbeck M, Stief C, Heinemann V, Casuscelli J. Lessons from the coronavirus disease 2019 pandemic: Will virtual patient management reshape uro-oncology in Germany? Eur J Cancer Oxf Engl 1990. 2020;132:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lai AG, Pasea L, Banerjee A, Hall G, Denaxas S, Chang WH, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10:e043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jiang CY, El-Kouri NT, Elliot D, Shields J, Caram MEV, Frankel TL, et al. Telehealth for Cancer Care in Veterans: Opportunities and Challenges Revealed by COVID. JCO Oncol Pract. United States; 2021;17:22–9. [DOI] [PubMed] [Google Scholar]
- 277.van der Lee ML, Schellekens MPJ. Bridging the distance: Continuing psycho-oncological care via video-consults during the COVID-19 pandemic. Psychooncology. 2020;29:1421–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shachar C, Engel J, Elwyn G. Implications for Telehealth in a Postpandemic Future: Regulatory and Privacy Issues. JAMA. United States; 2020;323:2375–6. [DOI] [PubMed] [Google Scholar]
- 279.Wosik J, Fudim M, Cameron B, Gellad ZF, Cho A, Phinney D, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc JAMIA. 2020;27:957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Greenhalgh T, Shaw S, Wherton J, Vijayaraghavan S, Morris J, Bhattacharya S, et al. Real-World Implementation of Video Outpatient Consultations at Macro, Meso, and Micro Levels: Mixed-Method Study. J Med Internet Res. 2018;20:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Dharmarajan H, Anderson JL, Kim S, Sridharan S, Duvvuri U, Ferris RL, et al. Transition to a virtual multidisciplinary tumor board during the COVID-19 pandemic: University of Pittsburgh experience. Head Neck. 2020;42:1310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lawhon V, Caston NE, Smith KL, Gallagher KD, Huang C-H, Azuero A, et al. Fear of COVID-19: Effects on mental health in under-resourced patients with cancer. J Clin Oncol. Wolters Kluwer; 2021;39:152–152. [Google Scholar]
- 283.Blacketer C, Defalco FJ, Ryan PB, Rijnbeek PR. Increasing Trust in Real-World Evidence Through Evaluation of Observational Data Quality. medRxiv. 2021;2021.03.25.21254341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pratt NL, Mack CD, Meyer AM, Davis KJ, Hammill BG, Hampp C, et al. Data linkage in pharmacoepidemiology: A call for rigorous evaluation and reporting. Pharmacoepidemiol Drug Saf. John Wiley & Sons, Ltd; 2020;29:9–17. [DOI] [PubMed] [Google Scholar]
- 285.Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pearl J An introduction to causal inference. Int J Biostat. 2010;6:Article 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Thompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J Med. 2021;385:1355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Shyr Y, Berry LD, Hsu C-Y. Scientific Rigor in the Age of COVID-19. JAMA Oncol. 2021;7:171–2. [DOI] [PubMed] [Google Scholar]
- 290.Desai A, Mohammed TJ, Duma N, Garassino MC, Hicks LK, Kuderer NM, et al. COVID-19 and Cancer: A Review of the Registry-Based Pandemic Response. JAMA Oncol [Internet]. 2021. [cited 2021 Sep 2]; Available from: 10.1001/jamaoncol.2021.4083 [DOI] [PMC free article] [PubMed]
- 291.Barwise A, Sharp R, Hirsch J. Ethical Tensions Resulting from Interpreter Involvement in the Consent Process. Ethics Hum Res 2019;41:31–5. [DOI] [PubMed] [Google Scholar]
- 292.Hunt LM, de Voogd KB. Are good intentions good enough? Informed consent without trained interpreters. J Gen Intern Med. 2007;22:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ramsetty A, Adams C. Impact of the digital divide in the age of COVID-19. J Am Med Inform Assoc JAMIA. 2020;27:1147–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Desai A, Kuderer NM, Lyman GH. Crowdsourcing in Crisis: Rising to the Occasion. JCO Clin Cancer Inform. United States; 2020;4:551–4. [DOI] [PubMed] [Google Scholar]
- 295.Desai A, Warner J, Kuderer N, Thompson M, Painter C, Lyman G, et al. Crowdsourcing a crisis response for COVID-19 in oncology. Nat Cancer. 2020;1:473–6. [DOI] [PubMed] [Google Scholar]
- 296.Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. MedRxiv Prepr Serv Health Sci. 2021;2021.04.06.21254949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Harrington P, Doores KJ, Radia D, O’Reilly A, Lam HPJ, Seow J, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol. 2021;194:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Fendler A, Au L, Shepherd STC, Byrne F, Cerrone M, Boos LA, et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Nat Cancer [Internet]. 2021. [cited 2021 Dec 2]; Available from: https://www.nature.com/articles/s43018-021-00275-9 doi: 10.1038/s43018-021-00275-9 [DOI] [PubMed]
- 299.Rottenberg Y, Grinshpun A, Ben-Dov IZ, Oiknine Djian E, Wolf DG, Kadouri L. Assessment of Response to a Third Dose of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Patients With Solid Tumors Undergoing Active Treatment. JAMA Oncol [Internet]. 2021. [cited 2021 Dec 2]; Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2786532 doi: 10.1001/jamaoncol.2021.6764 [DOI] [PMC free article] [PubMed]
- 300.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11:138. [DOI] [PMC free article] [PubMed] [Google Scholar]


