Abstract
Background:
Tumefactive demyelination (TD) presents with large inflammatory lesions mimicking tumors or other space-occupying lesions. Limited epidemiology, clinical and radiologic data exist. We report incidence rate, clinical and radiological features in Olmsted County, Minnesota.
Methods:
We retrospectively reviewed patients with CNS inflammatory demyelination-related diagnostic codes (1/1/98–12/31/18) via the Rochester Epidemiology Project database with incidence rates by age and sex adjusted to the 2010 US total population. We used the Expanded Disability Status Scale score (EDSS) to assess outcomes (index attack and last follow-up).
Results:
15/792 multiple sclerosis (MS) patients (8 males,7 females) had Tumefactive MS, representing 1.9% of the MS population. Median attack-onset age was 34.2 years (range 2–61). Tumefactive lesion was the first clinical MS attack in 8/16 patients. CSF oligoclonal bands (OCBs) were present in 8/12 patients. 11/16 patients met Barkhof criteria for dissemination in space. Most remained fully ambulatory (EDSS ≤4 in 13/16 [81%]) after median follow-up duration of 10.5 years (range 1–20.5). Age-adjusted annual incidence rates were 0.46/100,000 [95% CI: 0.12–0.81] for females, 0.66/100,000 [95% CI: 0.23–1.02] for males, and overall, 0.56/100,000 [95% CI: 0.28–0.83]. When age- and sex-adjusted to the 2010 US total population, overall annual incidence rate was 0.57 [95% CI: 0.28–0.84]. Despite aggressive clinical presentation at disease onset, most patients remained fully ambulatory (EDSS≤4 in 13/16) with a relapsing-remitting course.
Conclusions:
Although incidence is rare, TD should be suspected in patients presenting with subacutely progressive neurological deficits associated with MRI findings of ring enhancement, ADC restriction, and OCB on spinal fluid analysis.
INTRODUCTION
Tumefactive demyelination (TD) is an atypical form of demyelination, encompassing various entities such as Marburg disease and Balo’s concentric sclerosis (BCS) (1, 2). Clinically, there is evidence that BCS and TD commonly occur as tumefactive variants of multiple sclerosis (MS) (3).Tumefactive MS (TMS) is characterized by large inflammatory demyelinating lesions with an atypical enhancement pattern, edema, or mass effect (4). This entity poses a diagnostic challenge clinically, radiologically, and even pathologically since these lesions mimic tumors or other space-occupying conditions such as abscesses (5, 6). The differential diagnosis also includes a wide variety of central nervous system inflammatory demyelinating diseases (CNSIDD) such as acute demyelinating encephalomyelitis (ADEM), myelin oligodendrocyte glycoprotein antibody-associated disorder (MOGAD), acute hemorrhagic leukoencephalitis, and aquaporin-4-IgG seropositive neuromyelitis optic spectrum disorders (NMOSD). The diagnosis of these diseases can be challenging. A prior report showed that even histopathology was initially misinterpreted as a non-demyelinating etiology in 31% of cases (7).
The prevalence of tumefactive MS is estimated to be 1.4 to 8.2% of MS patients (8, 9). The incidence of tumefactive MS disease is estimated at 0.3/100,000 people (10, 11). Although TD has been described for over 100 years, little is known about the epidemiology and clinical course of TD. This study aimed to assess the incidence rates of TD using a population-based survey in a well-defined US population and describe clinical and radiological features.
METHODS
Case ascertainment
The medical records of all patients residing in Olmsted County, Minnesota, diagnosed with CNSIDD from January 1, 1998, through December 31, 2018, were retrospectively reviewed to identify incident cases of TD. Patients were identified using the Rochester Epidemiology Project (REP), a linkage system of medical records for all patient-physician encounters among Olmsted County, Minnesota residents (12). Furthermore, we cross-checked the REP identified patients through Mayo Clinic Advanced Cohort Explorer (ACE) Tool. ACE is a clinical data repository including multiple sources of patient information such as patient demographics, diagnosis, hospital notes, laboratory reports, flowsheets, radiology reports, and clinical notes. With ACE’s text search functionality, we queried “large lesion,” “tumefactive,” or “Balo.” Incident cases were required to have established residency at least one year before the onset of TD, eliminating patients who had migrated for medical care. Patients were included if they had a clinical history comprising a demyelinating event along with a cerebral magnetic resonance imaging (MRI) showing one or more large demyelinating plaques (minimum transverse diameter, ≥10 mm). Patients were only included if their first episode of TD occurred during the study period. All patients were reviewed by at least one MS fellowship trained Neurologist. Our group has previously shown that many patients with tumefactive MS do not fulfill MS diagnostic criteria at disease onset (13) . Features suggestive of a diagnosis of tumefactive MS included subacute onset of symptoms, clinical or radiologic response to plasma exchange, other MRI features typical of MS (e.g. spinal lesions, periventricular lesions), persistence of MRI lesions after treatment, optical coherence tomography lesions typical of MS, presence of oligoclonal bands, absent serum testing for MOG-IgG or NMO-IgG and pathological features suggestive of MS on brain biopsy or autopsy. At last follow-up, diagnoses of MS, NMOSD, and MOGAD were made based on the most recent criteria (14–17).
The Mayo Clinic and Olmsted Medical Center (OMC) Institutional Review Boards (IRB) approved the study (IRB number 19–011604, OMC number:052-OMC-19). The study conforms with the WMA Declaration of Helsinki, and patients or their authorized representatives provided informed written consent for their de-identified medical information to be used for research purposes. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Serologic antibody biomarker assessment
In TD cases, serum was tested for MOG antibodies by the live cell-based assay as previously described. Serum in these cases was also tested for aquaporin-4 (AQP-4) antibody by live cell-based assay, inactivated cell-based assay, ELISA, or tissue immunofluorescence as previously described (18, 19).
Data collection
Demographic and clinical data were abstracted from the medical records. Neuroimaging features were reviewed regarding lesion location, size of the lesion, number of fluid-attenuated inversion recovery (FLAIR) lesions, number of gadolinium (Gd)-enhancing lesions, mass effect, contrast enhancement pattern, apparent diffusion coefficient (ADC) pattern, T2-hypointense rim, T1- hypointensity and fulfillment of Barkhof criteria (20).
Statistical analysis
The population data were obtained from the US Census Bureau, US Census 2010. US Total population was used as the denominator to calculate incidence rates. Index attack date (attack associated with the first large demyelinating lesion) was used to determine the age of disease onset. All individuals with a first tumefactive attack between January 1, 1998, and December 31, 2018, were considered incident cases. The person-years-at-risk denominator was calculated from the REP Census data. Incidence rates were estimated as the number of new cases divided by the person-years at risk. Incidence rates were directly adjusted by age and sex to the 2010 US total population. The Poisson distribution was used to estimate standard errors and 95% confidence interval (CI) for the incidence rates.
RESULTS
Demographic and Clinical Characteristics
The main demographic and clinic-radiological features of all patients included in our study are shown in Table 1. Flair MRI at the initial attack is shown in Figure 1. Out of 792 patients with MS, 15 patients had TMS representing 1.9% of the MS population. One patient had MOGAD. No patients with NMOSD were found to have TD lesions. The median age at attack onset was 34.2 years (range 2–61). The presenting event represented the first clinical attack in 8/16 patients. Clinical presentation was polysymptomatic 11/16 patients. Prodromal symptoms, such as malaise and headache, were present in 4/16 patients prior to index attack. The median EDSS score at index attack was 3.5 (range 1.5–9). CSF analysis was performed in 12/16 patients. Of these 12 patients, 3/12 had a spinal tap at the index attack (No.12, No.14, No. 16). CSF unique oligoclonal bands (OCBs) with two or more unique IgG bands were demonstrated in 4/12 patients (No.6, No.8, No.13, No.14). All 4/12 patients with positive OCBs tested for CSF analysis at their first demyelinating attack, and index attack occurred during MS. CSF protein level was normal in 6/12 and elevated in 5/11. The number of CSF nucleated cells was normal in 7/12 and increased in 5/12. AQP4-IgG were tested in 6/12 patients and MOG-IgG in 2/12 (No.8 and No.16) patients with one positive result.
Table 1:
Demographic, Clinical and Radiological Features of the Olmsted County patients with Tumefactive Multiple Sclerosis
| No | Sex | Race | Age at index attack | EDSS at index attack | EDSS at last follow-up | MRI characteristics at index attack | Course classification | Index attack treatment | DMTs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lesion location | Lesion size (in mm) | Number of FLAIR lesions | Number of Gad-enhancing lesions | Barkhof Criteria | ADC Parameters | Enhancement Pattern | Mass effect | |||||||||
| 1 | F | White | 37 | 2 | 1 | Occipital | 10.3 | ≥10 | 3 | No | N/A | Incomplete ring | None | RRMS | Corticosteroid | IFN- |
| 2 | M | White | 28 | 2.5 | 1 | Frontal | 22.5 | 2 | 1 | Yes | Facilitation | None | Mild | RRMS | Corticosteroid | None |
| 3 | F | White | 41 | 3 | 0 | Frontal | 16.2 | 3 | 2 | Yes | Facilitation, Isointense | Central only | None | RRMS | Corticosteroid | Natalizumab |
| 4 | M | White | 61 | 8 | 10 | Frontal | 17.2 | ≥10 | 16 | Yes | Restriction, Facilitation | concentric ring | None | SPMS | Corticosteroid ,Mitoxantrone | IFN- |
| 5 | F | White | 43 | 1.5 | 7 | Frontal | 10.3 | ≥10 | 3 | Yes | N/A | Central only | None | SPMS | Corticosteroid | Fingolimod , IFN |
| 6 | F | White | 28 | 5 | 7.5 | Frontal | 22 | ≥10 | 9 | No | Facilitation | Incomplete ring | Mild | RRMS | Corticosteroid, PLEX | Alemtuzumab, Natalizumab, Rituximab, IFN, Teriflunomide |
| 7 | F | White | 54 | 4.5 | 2 | Brain stem | 12.2 | ≥10 | 1 | Yes | N/A | Central only | None | RRMS | Corticosteroid | None |
| 8 | M | Unknown | 41 | 3.5 | 3 | Cerebellum | 11 | 8 | 3 | Yes | Facilitation | Single ring | Mild | RRMS | Corticosteroid | Natalizumab, Ocrelizumab, Glatiramer acetate, IFN |
| 9 | M | White | 41 | 5 | 0 | Occipital | 12.4 | ≥10 | 2 | Yes | N/A | Heterogeneous | None | RRMS | Corticosteroid PLEX | IFN |
| 10 | M | Unknown | 21 | 9 | 2 | Frontal | 49 | 1 | 1 | No | Restriction, Facilitation | Heterogeneous | Moderate | monophasic | Corticosteroid, PLEX | None |
| 11 | M | White | 20 | 3.5 | 2 | Brainstem | 16.6 |
≥10 | 4 | Yes | N/A | None | None | RRMS | Corticosteroid | IFN |
| 12 | M | White | 38 | 3 | 1 | Cerebellum | 18.1 | 5 | 3 | No | Restriction | Heterogeneous | None | RRMS | Corticosteroid, PLEX | IFN |
| 13 | F | White | 23 | 7.5 | 2 | Temporal | 12.2 | ≥10 | 17 | Yes | N/A | Incomplete ring | None | RRMS | Corticosteroid | IFN |
| 14 | M | White | 20 | 1.5 | 0 | Frontal | 17.5 | 3 | 2 | Yes | Restriction | Concentric ring | None | RRMS | Corticosteroid | Dimethyl fumarate |
| 15 | F | Unknown | 31 | 6 | 1 | Frontal | 18 | 2 | 1 | No | Restriction | Heterogeneous | None | RRMS | Corticosteroid, PLEX | IFN |
| 16 | M | Hispanic | 3 | 0 | 0 | Cerebellum | 14.9 | ≥10 | 2 | Yes | Facilitation | Heterogeneous | None | MOG-IgG associated demyelinating disease | Corticosteroid | None |
No: Patient number; F: Female; M: Male; OCBs: Oligoclonal Bands; PLEX: Plasma Exchange; EDSS: Expanded Disability Status Scale; Gad: Gadolinium; RR: relapsing-remitting; NR: not reported; DMT: Disease-Modifying Therapy
Figure 1.
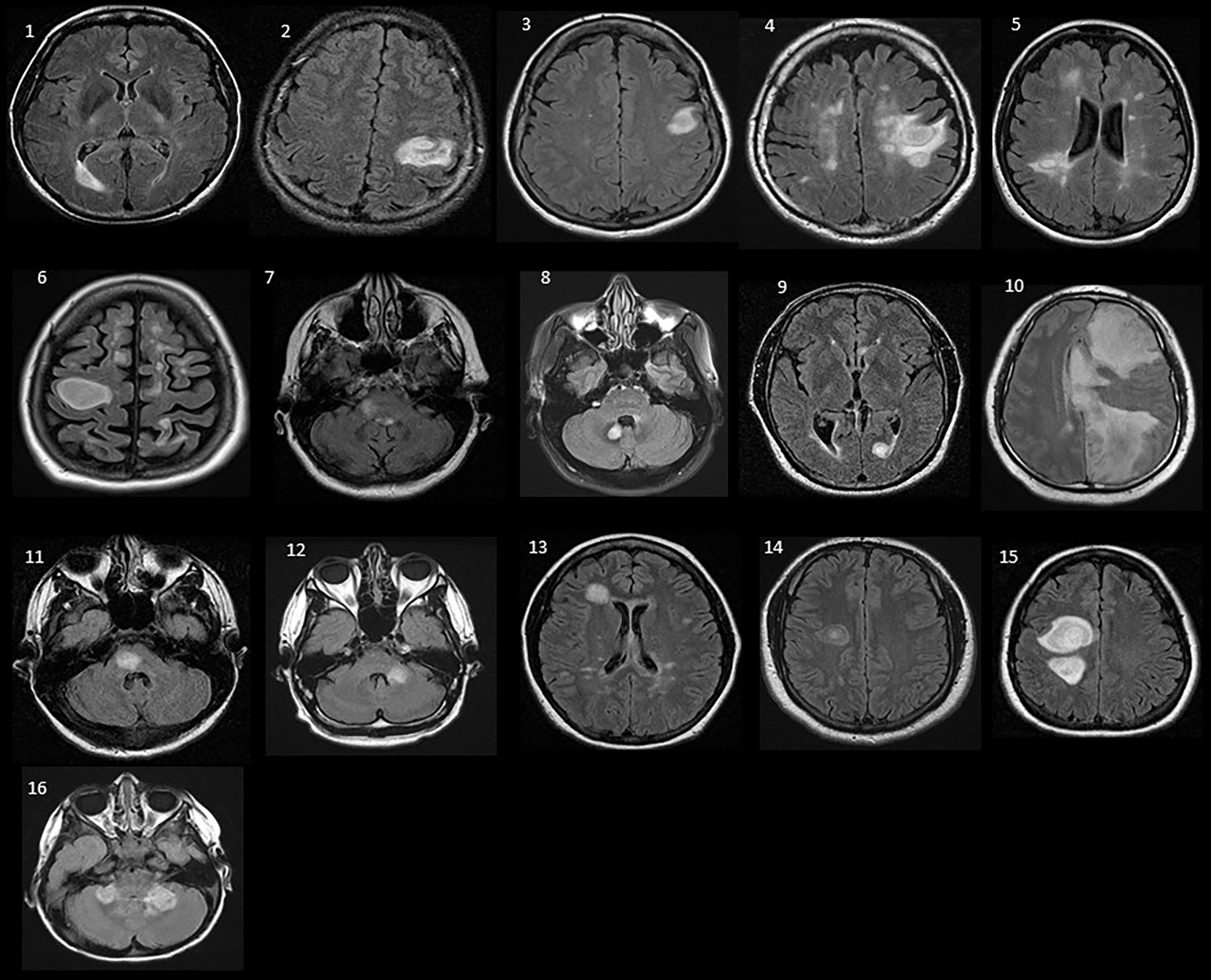
The index brain MRI axial fluid-attenuated inversion recovery (FLAIR) sequences of all patients (1–16) with tumefactive demyelination.
Brain biopsy was performed in 2/16 patients (No. 2 and No.10), and pathological evaluation was consistent with MS. McDonald criteria for MS (2017) were fulfilled in 4/16 patients at the time of index attack (patient No.11, No.12, No.13, No.14). Patient No.15 was diagnosed with MS 10 months after the index attack. Patient No.16 with MOGAD had relapsing ADEM, encephalomyelitis, and optic neuritis.
The median duration of follow-up was 10.5 years (range 1–20.5). At the last follow-up, the median EDSS score was 1.5 (range 0–10); the course of the disease was relapsing-remitting MS in 12/16 patients, secondary progressive MS in 2/16 patients, and monophasic in 1/16 patients. Patients No. 3 and No. 15 with BCS had favorable outcomes with an EDSS score of 1 at the last follow-up. Patient No. 4 with BCS died due to progressive dementia related to MS 2 years after tumefactive lesion onset. All patients were initially treated with high-dose corticosteroids. Plasma exchange (PLEX) was commenced in 5/16 cases after an ineffective response to corticosteroids. Disease-modifying therapy (DMT) was initiated in 11/16 patients.
Neuroimaging
Solitary TD lesions were present in 8/16 patients, BCS in 3/16 patients, and multiple tumefactive lesions in 5/16 patients. Lesions were mainly located in the frontal (8/16), followed by occipital (2/16) lobes, the brainstem (2/16), cerebellum (3/16), and temporal lobe (1/16). At the initial presentation, 11/16 patients met the Barkhof criteria for dissemination in space. “Butterfly lesions,” bihemispheric lesions crossing the corpus callosum [Figure 2], were present in 1/16. A hypointense T2W rim was observed in 1/16 patients. T1 hypointensity in the acute lesion was seen in 14/16 patients. ADC imaging was available in 10/16 patients. Of these 10 patients, tumefactive lesions showed a variety of ADC patterns. Restricted diffusion in any part of the lesion was seen in 5/10, facilitation in 6/10, and isointense pattern in 2/10. Post-gadolinium enhancement was present in 14/16 tumefactive lesions, with central enhancement in 4/14, incomplete open-ring enhancement in 3/14, heterogeneous pattern in 5/14, and concentric ring enhancement in 2/14.
Figure 2.
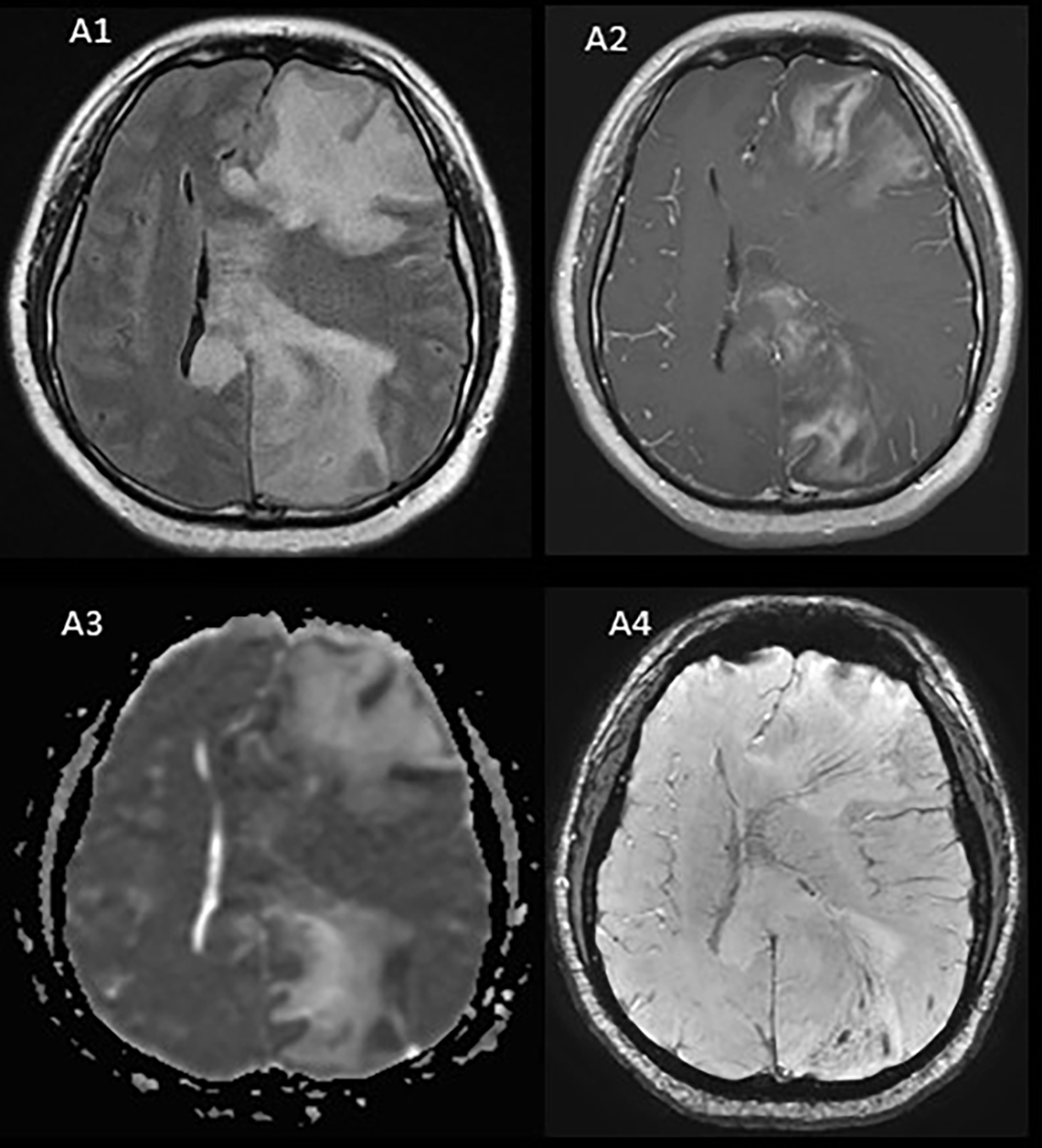
Patient 10 (A1-A4) Brain MRI axial FLAIR demonstrates a large heterogeneous left hemisphere lesion with extensive edema and prominent involvement of the corpus callosum (A1) with heterogenous contrast enhancement on axial- T1 post-contrast MRI (A2). Apparent diffusion coefficient (ADC) indicated mild diffusion restriction, predominantly along the margin of the T2 hyperintensity in the frontal and parietal lobes (A3). Susceptibility weighted imaging (A4) demonstrates the presence of central veins running through the lesion, consistent with demyelination.
Incidence of tumefactive demyelination in Olmsted County
During the 10-year period from January 1, 1998, to December 1, 2018, 16 incident cases of TD were diagnosed. The age-adjusted annual incidence rates were 0.46/100,000 (95% CI: 0.12–0.81) for females, 0.66/100,000 for males (95% CI: 0.23–1.02), and overall 0.56/100,000 (95% CI: 0.28–0.83). When age- and sex-adjusted to the 2010 US total population, the overall annual incidence rate was 0.57 (95% CI: 0.28–0.84). The crude and adjusted incidence rates for each six age group are presented in Table 2.
Table 2:
Incidence* of Tumefactive Multiple Sclerosis in Olmsted County, 1998–2018
| Stratum (age-group) | Females | Males | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Population** | Cases | Rate | Population | Cases | Rate | Population | Cases | Rate | |
| 0–19 | 420701 | 0 | 0 | 438792 | 1 | 0.22 | 859493 | 1 | 0.11 |
| 20–29 | 249032 | 2 | 0.80 | 208663 | 4 | 1.92 | 457695 | 6 | 1.31 |
| 30–39 | 226080 | 2 | 0.88 | 211740 | 1 | 0.47 | 437820 | 3 | 0.68 |
| 40–49 | 214948 | 2 | 0.93 | 199986 | 2 | 1.00 | 414934 | 4 | 0.96 |
| 50–59 | 196611 | 1 | 0.50 | 177519 | 0 | 0.00 | 374130 | 1 | 0.26 |
| 60–70 | 141005 | 0 | 0.00 | 125513 | 1 | 0.80 | 266518 | 1 | 0.37 |
| Total | 1027676 | 7 | 0.68 | 923421 | 8 | 0.87 | 1951097 | 15 | 0.77 |
| Adjusted Rates *** | Age adjusted Incidence rate: 0.46 S.E.: 0.17 95% CI: [0.12–0.81] |
Age adjusted Incidence rate: 0.66 S.E.: 0.22 95% CI: [0.23–1.02] |
Age adjusted Incidence rate: 0.56 S.E.: 0.14 95% CI: [0.28–0.83] Age and sex adjusted rate: 0.57 SE: 0.14 95% CI: [0.28–0.84] |
||||||
Incidence rate per 100,000 population.
Population expressed as person-years from 1998–2018
Rates are adjusted to the 2010 US total population.
CI: Confidence Interval; S.E.: Standard Error
DISCUSSION
Epidemiology
The occurrence of TD resembling brain tumors is well recognized and mainly described in case reports and case series. To our knowledge, the current report represents the first population-based incidence study to date. The adjusted incidence rate of TD for all ages was estimated at 0.57 per 100,000 inhabitants per year. This result confirms previous assumptions that TD is a rare entity (21).
The differential diagnosis of patients presenting with TD lesions encompasses a broad spectrum of diseases. In the same geographic population, the adjusted incidence rate of glioma for all ages is 5.51 per 100,000 inhabitants, ten times higher than TD incidence (22, 23). Moreover, an incidence rate of 11.1 per 100,000 population per year was reported for brain metastases over 34 years from 1935 through 1968 in our region, which is higher than the incidence of MS, glioma, and TD in Olmsted County (24). The central brain tumor registry of the United States reported an incidence of 0.43 per 100,000 for primary CNS lymphoma, which is close to the incidence rate of TD in our study (25). This highlights the need for long-term careful clinical and radiographic monitoring of patients diagnosed with TD lesions to avoid any misdiagnosis.
TMS accounts for almost 1.9% (15/792) of all MS patients in Olmsted County; this is similar to the rate reported by Sanchez et al. in 2017 (26), showing a higher-than-expected prevalence in MS patients, which is estimated at 1–2/1000 cases when compared to other CNS-demyelinating conditions (27). This likely reflects changes in MS diagnostic criteria over time, allowing more patients to fulfill MS diagnosis. One patient with MOGAD was found in our cohort. TD disease has been reported in other CNSIDD conditions such as NMOSD and MOGAD (4). The fact that we did not find any patients with NMOSD likely reflects the relatively lower prevalence of these conditions. The population-based approach used in this study overcomes the inherent referral bias of other clinic-based cohorts.
Clinical features and outcomes
Despite an aggressive clinical presentation (EDSS ≥ 4 in 11/16 [69%]) at disease onset, most of our patients remained fully ambulatory (EDSS ≤ 4 in 13/16 [81%]) with a relapsing-remitting course after a median 10-year follow-up. This is in line with the previously reported cohort of biopsy-proven TD, where 73% were ambulatory at the last follow-up (28).
The data on the female-to-male ratio differ in the literature. Two cohorts of patients have indicated female preponderance, with 62 to 68% of patients being females (29, 30), while the previous biopsy cohort of TD in our center showed a roughly equal ratio of 1.3:1 (7). In our series, the prevalence was higher in men with the general male-to-female ratio of 1.14:1. The median age at tumefactive attack in our cohort was 34.2 years, consistent with previous reports, indicating TD occurs most frequently between the ages of 20 and 40 (7, 26, 28, 30). In our report, 8/16 (50%) patients presented with TD as a first demyelinating attack, while this rate has been reported to be 53 to 78% in previous studies (7, 29, 30). Discrepancies could be due to recall bias.
The clinical presentation of TD is often polysymptomatic for demyelinating disease given the size, location, and potential mass effect of the lesion. In our study, clinical presentations at index attack were polysymptomatic in most patients (11/16) with motor and sensory predominance. Despite the typical symptoms and signs of MS in the current series, atypical presentations, including seizure, abulia, and aphasia, were also observed (3/16). This supports previous studies, likely reflective of the size of the lesions and cortical involvement (7, 26, 29, 31).
Study limitations
The main limitation of this study is its retrospective nature with a small number of patients. Formal cognitive assessment of patients was not collected in a standardized way; thus, although motor disability assessed by the EDSS looks favorable, the long-term cognitive outcome is uncertain.
CONCLUSION
TD is rare but can present de novo without prior symptoms of demyelination. Most patients presenting with TD have MS as their underlying diagnosis.TD should be suspected in patients presenting with subacutely progressive neurological deficits associated with MRI findings of ring enhancement, ADC restriction, and OCB on spinal fluid analysis.
FUNDING
Dr. Fereidan-Esfahani was funded by fellowship from Mayo Clinic Center for MS and Autoimmune Neurology. Dr. Tobin has received research funding from Mallinckrodt Inc. and from the Mayo Clinic Center for MS and Autoimmune Neurology. This study was supported by grant funding from NIH 1R01NS113803-01A1 and grant R01NS113828.
DECLARATION OF CONFLICTING INTERESTS
Dr. Flanagan is a site principal investigator in a randomized placebo-controlled clinical trial of Inebilizumab (A CD19 inhibitor) in neuromyelitis optica spectrum disorders funded by MedImmune/Viela Bio. He receives no personal compensation and just receives reimbursement for the research activities related to the trial. He also has received funding from the NIH (R01NS113828).
REFERENCES
- 1.Balo J Encephalitis periaxialis concentrica. Archives of Neurology & Psychiatry 1928;19(2):242–64. [Google Scholar]
- 2.Johnson M, Lavin P, Whetsell W. Fulminant monophasic multiple sclerosis, Marburg’s type. Journal of Neurology, Neurosurgery & Psychiatry. 1990;53(10):918–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolliffe EA, Guo Y, Hardy TA, Morris PP, Flanagan EP, Lucchinetti CF, et al. Clinical and Radiologic Features, Pathology, and Treatment of Balo Concentric Sclerosis. Neurology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy TA, Reddel SW, Barnett MH, Palace J, Lucchinetti CF, Weinshenker BG. Atypical inflammatory demyelinating syndromes of the CNS. The Lancet Neurology. 2016;15(9):967–81. [DOI] [PubMed] [Google Scholar]
- 5.Seewann A, Enzinger C, Filippi M, Barkhof F, Rovira A, Gass A, et al. MRI characteristics of atypical idiopathic inflammatory demyelinating lesions of the brain. Journal of Neurology. 2008;255(1):1–10. [DOI] [PubMed] [Google Scholar]
- 6.Kaeser MA, Scali F, Lanzisera FP, Bub GA, Kettner NW. Tumefactive multiple sclerosis: an uncommon diagnostic challenge. Journal of chiropractic medicine. 2011;10(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131(7):1759–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frederick MC, Cameron MH. Tumefactive demyelinating lesions in multiple sclerosis and associated disorders. Current neurology and neuroscience reports. 2016;16(3):26. [DOI] [PubMed] [Google Scholar]
- 9.Patriarca L, Torlone S, Ferrari F, Di Carmine C, Totaro R, di Cesare E, et al. Is size an essential criterion to define tumefactive plaque? MR features and clinical correlation in multiple sclerosis. The Neuroradiology Journal. 2016;29(5):384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brod SA, Lindsey JW, Nelson F. Tumefactive demyelination: Clinical outcomes, lesion evolution and treatments. Mult Scler J Exp Transl Clin. 2019;5(2):2055217319855755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balloy G, Pelletier J, Suchet L, Lebrun C, Cohen M, Vermersch P, et al. Inaugural tumor-like multiple sclerosis: clinical presentation and medium-term outcome in 87 patients. J Neurol. 2018;265(10):2251–9. [DOI] [PubMed] [Google Scholar]
- 12.Rocca WA, Yawn BP, Sauver JLS, Grossardt BR, Melton LJ, editors. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings; 2012: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobin WO, Kalinowska-Lyszczarz A, Weigand SD, Guo Y, Tosakulwong N, Parisi JEE, et al. Clinical Correlation of Multiple Sclerosis Immunopathologic Subtypes. Neurology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology. 2018;17(2):162–73. [DOI] [PubMed] [Google Scholar]
- 15.Sechi E, Buciuc M, Pittock SJ, Chen JJ, Fryer JP, Jenkins SM, et al. Positive Predictive Value of Myelin Oligodendrocyte Glycoprotein Autoantibody Testing. JAMA Neurol. 2021;78(6):741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78(9):665–71; discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majed M, Fryer JP, McKeon A, Lennon VA, Pittock SJ. Clinical utility of testing AQP4-IgG in CSF: Guidance for physicians. Neurol Neuroimmunol Neuroinflamm. 2016;3(3):e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sastre-Garriga J, Tintoré M, Rovira A, Nos C, Río J, Thompson AJ, et al. Specificity of Barkhof criteria in predicting conversion to multiple sclerosis when applied to clinically isolated brainstem syndromes. Archives of neurology. 2004;61(2):222–4. [DOI] [PubMed] [Google Scholar]
- 21.Paty D, Oger J, Kastrukoff L, Hashimoto S, Hooge J, Eisen A, et al. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology. 1988;38(2):180-. [DOI] [PubMed] [Google Scholar]
- 22.Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology. 2003;61(10):1373–7. [DOI] [PubMed] [Google Scholar]
- 23.Ryan CS, Juhn YJ, Kaur H, Wi C-I, Ryu E, King KS, et al. Long-term incidence of glioma in Olmsted County, Minnesota, and disparities in postglioma survival rate: a population-based study. Neuro-Oncology Practice. 2020;7(3):288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liigant A, Asser T, Kulla A, Kaasik AE. Epidemiology of Primary Central Nervous System Tumors in Estonia. Neuroepidemiology. 2000;19(6):300–11. [DOI] [PubMed] [Google Scholar]
- 25.Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro-oncology. 1999;1(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez P, Meca-Lallana V, Barbosa A, Manzanares R, Palmí I, Vivancos J. Tumefactive demyelinating lesions of 15 patients: Clinico-radiological features, management and review of the literature. Journal of the Neurological Sciences. 2017;381:32–8. [DOI] [PubMed] [Google Scholar]
- 27.Poser S, Luer W, Bruhn H, Frahm J, Bruck Y, Felgenhauer K. Acute demyelinating disease. Classification and non-invasive diagnosis. Acta Neurol Scand. 1992;86(6):579–85. [DOI] [PubMed] [Google Scholar]
- 28.Pittock SJ, McClelland R, Achenbach S, Konig F, Bitsch A, Brück W, et al. Clinical course, pathological correlations, and outcome of biopsy proved inflammatory demyelinating disease. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(12):1693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altintas A, Petek B, Isik N, Terzi M, Bolukbasi F, Tavsanli M, et al. Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Multiple Sclerosis Journal. 2012;18(10):1448–53. [DOI] [PubMed] [Google Scholar]
- 30.Wallner-Blazek M, Rovira A, Fillipp M, Rocca MA, Miller DH, Schmierer K, et al. Atypical idiopathic inflammatory demyelinating lesions: prognostic implications and relation to multiple sclerosis. Journal of neurology. 2013;260(8):2016–22. [DOI] [PubMed] [Google Scholar]
- 31.Jeong IH, Kim S-H, Hyun J-W, Joung A, Cho H-J, Kim HJ. Tumefactive demyelinating lesions as a first clinical event: Clinical, imaging, and follow-up observations. Journal of the Neurological Sciences. 2015;358(1):118–24. [DOI] [PubMed] [Google Scholar]


