Abstract
This contribution reports on the physical properties of the natural Namibian red Ochre used by the Himba Community in a form of a formulation, so called Otjize as a skin protective and beauty cream. The morphological and crystallographic studies of this red ochre validated its nano-scaled dominating phase of rhombohedral α-Fe2O3 nanocrystals with an additional hydrolized oxide component in a form of γ-FeOOH. The optical investigations showed that such a red ochre exhibits an exceptional UV filtration and a significant IR reflectivity substantiating its effectiveness as an effective UV-blocking & solar heat IR reflector in support of the low skin cancer rate within the Namibian Himba community. In addition, such nanocrystals exhibited a non-negligible antibacterial response against E. Coli & S. Aurus. This study seems confirming the effectiveness of the indigenous Otjize as an effective skin UV protection cream with a sound antimicrobial efficacy against e-Coli & S-Aurus.
Subject terms: Biophysics, Materials science, Nanoscience and technology
Introduction
In addition to the recently discovered Zeta-Fe2O3 structure1, Iron (III) oxide represents one of the most rich and multifunctional oxide families2. It has four standard principal polymorphs, hematite (α-Fe2O3), maghemite (γ-Fe2O3), β-Fe2O3 and ε-Fe2O3; iron (III) oxy-hydroxide also has four polymorphs known as goethite (α-FeOOH), akaganeite (β-FeOOH), lepidocrocite (γ-FeOOH) and feroxyhyte (δ-FeOOH)2. Of a special interest, α-Fe2O3 is a prominent n-type semiconductor with an interesting bandgap Eg = 2.1 eV, conjugated to an excellent chemical stability and low toxicity under ambient conditions. Among these oxides, α-FeOOH and α-Fe2O3 have been commonly investigated for magnetic devices, catalysts, gas sensors and photoelectrodes applications3–10 in addition to pigment production. However, it is worth noting that this latter pigment aspect has been identified as early as the birth of humanity.
Indeed, early human used several marine resources and pigments to express artistically on their natural surroundings & believes ranging from prehistoric, ochre-pigmented images on cave walls to paintings on canvasses in addition to their symbolic behaviors. Its usage in symbolic behavior was found to date164,000 years ago, far earlier than previously documented11.
More precisely, the earliest evidence of the ochre pigment’s usage by ancient humans dates to the Paleolithic, about 285,000 years ago, at a Homo erectus site called GnJh-03 in Kenya. In this location, archaeologists discovered about 70 pieces of ochre weighing about 5 kg12.
Early Homo-sapiens also illustrated extensively with ochre pigment. At Blombos Cave, in South Africa, archaeologists discovered an abalone shell containing finely ground ochre, charcoal as well as fat that may have made up a likely painting kit dating to about 100,000 years ago. The earliest human-made drawing is a red hashtag on small rock flake that dates to about 73,000 years ago, also at Blombos and Klein Kliphuis Caves in South Africa. In addition, about 266,000 years ago, early hominins at a site called Twin Rivers in Zambia collected a type of hematite ochre that has significantly reflective metallic flakes in it making it glittering13–15.
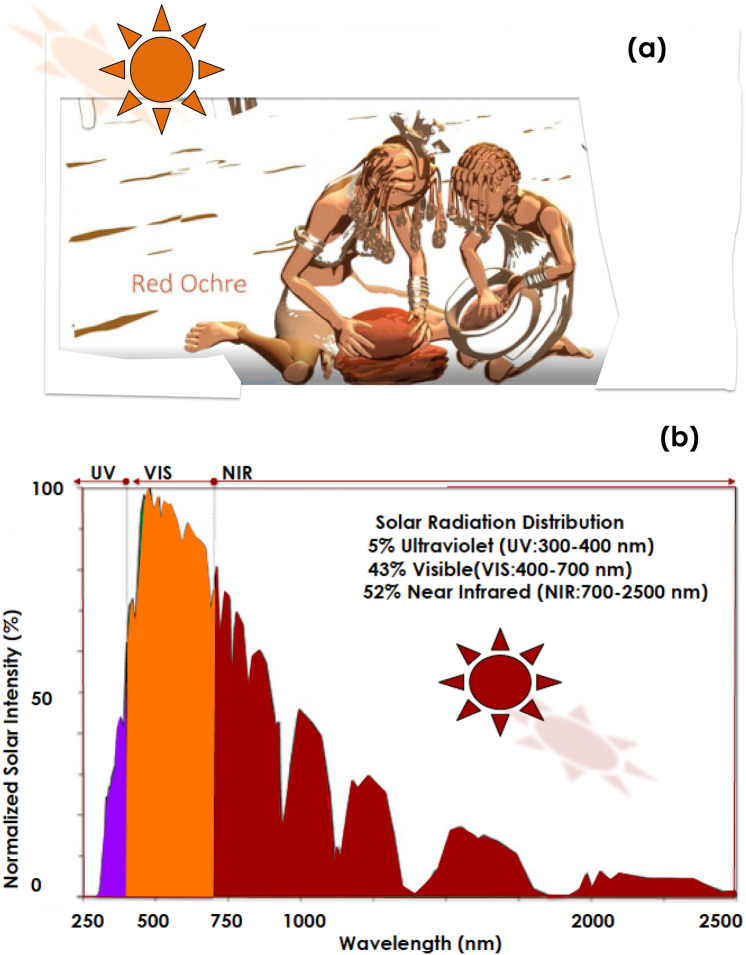
As highlighted previously, while Ochre was used as an effective artistic & behavioral pigment in various locations within the African continent, the Himba ethnic group specifically, has genuinely extended its usage towards cosmetic & skin-protection applications (Fig. 1a). With the Nama & the Herero communities, the Himba society is an additional indigenous social group in Namibia. Living in the northern part within the Kunene region, they are hunter-gatherers, semi-nomadics & pastoralists. Exposed to harsh atmospheric conditions in general and daily solar radiations especially where the Direct Normal irradiation is among the highest globally. In the quasi-absence of clouds, they are exposed through out the year to the standard 5% UV (300–400 nm), 43% VIS (400–700 nm) and 52% NIR-FIR (700–2500 nm) solar radiations (Fig. 1b). Accustomed to a semi-arid climate, the Himba women are famous for covering their skin & hairs with the so called Otjize paste16; a cosmetic mixture of goat-butterfat and ochre pigment.
Figure 1.
(a) Traditional preparation of the Red Ochre pigment by the Himba community, (b) Solar spectral distribution in the north of Namibia to which the Himba group community is exposed.
Otjize cleanses the skin over long periods due to water scarcity and considered as an effective protection from the hot and dry climate, as well as from insect bites. It gives Himba people's skin and hair plaits a distinctive texture, style, and orange or red tinge, and is often perfumed with the aromatic resin of the indigenous omuzumba shrub (Commiphora multijuga)17. From anthropological viewpoint, Otjize is considered foremost a highly desirable aesthetic beauty cosmetic, symbolizing earth's rich red colour and blood, the essence of life, and is consistent with the Himba ideal of beauty (Fig. 1a).
To validate the optical filtering & selectivity as well as the health skin protection of the Ochre pigment used in the Himba Otjize, this contribution reports on its physical properties with a special focus on their optical selectivity in the UV–VIS spectral solar range and their antibacterial efficacy. If so, this would be the originality & novelty of this contribution.
Experiments, results & discussion
For this study, typical Himba Ochre samples from the Kunene region of Namibia were collected from a Himba settlement. They were used without any further physical–chemical treatment. The samples were investigated by High Resolution Scanning Electron microscopy (HRSEM) for their morphological constituency, High Resolution Transmission Electron Microscopy (HRTEM) for the shape & atomic ordering of the elementary crystals if any, Energy Dispersive Spectrometry (EDS) for elemental analysis in addition to room temperature X-Rays Diffraction (XRD) for their crystallographic structure.
These studies were complemented with a full range of optical investigations (Photoluminescence, Infrared & UV–VIS spectroscopies). The antibacterial activity was carried out on 2 standard bacterial species: E-coli & Staphylococus Aurus.
Morphology & crystallographic investigations
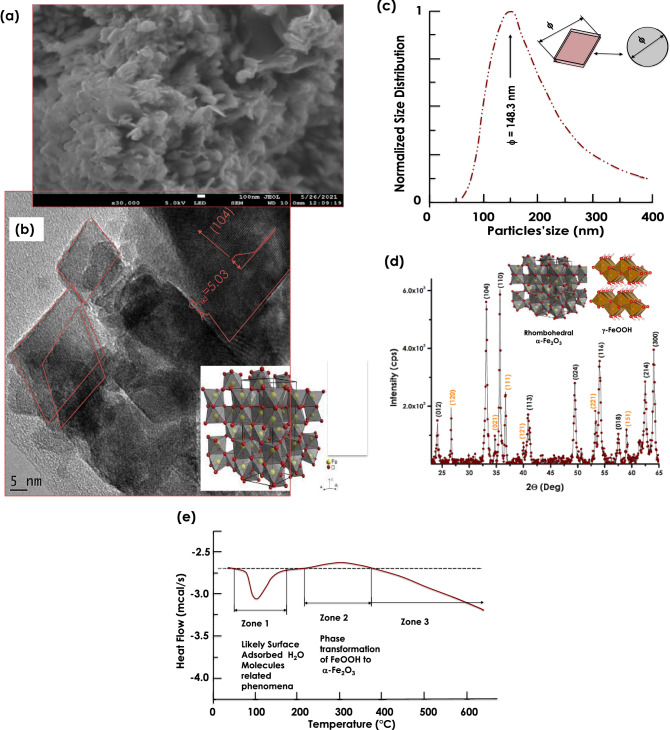
Figure 2a reports the HRSEM of the Himba ochre. As one could notice, it consists of agglomerated lamellar nanoplatelets. Their longitudinal & basal dimensions are relatively wide spread in terms of dimension. The average basal dimension is estimated within the range of 17–31 nm while the longitudinal average dimension is above 100 nm. Figure 2b reports an HRTEM of the Himba Ochre after sonication. The lamellar nano-platelets seem consisting of nanoscale relatively crystalline grains with preferred crystalline orientation and a platelets type morphology. The major observed atomic ordering correponds to an inter-reticular distance of 5.03 Å in agreement with the (104) atomic orientation of pure single phase hematite α-Fe2O3. Figure 2c displays the size distribution of the nanoparticles obtained by standard light scattering. The corresponding profile indicates a relatively polydisperse nanosystem with an average size of <ϕ> = 148.3 nm. In this analysis, the nano-platelets were approximated as spheroids with a diameter ϕ. This latter parameter could be almost be the equivalent of the size of the nanoplatelets.
Figure 2.
(a) High Resolution SEM image of the Red Ochre pigment, (b) its High Resolution TEM observation of the crystalline rhombohedral nanoplatelets like-particles, (c) their Size distribution, (d) its corresponding room temperature X-Ray diffraction pattern, and (e) their differential scanning calorimetry within the temperature range of 25–600 °C.
Figure 2d reports the XRD diffraction pattern of the Ochre sample within the angular range 2θ of 22.5–65 Deg. One can distinguish 2families of diffraction Bragg peaks. The first one is identified & indexed as (012), (104), (110), (113), (024), (116), (018), (214) and (030) of the rhombohedral single phase α -hematite α -Fe2O3. The second family set is characterized by the following diffraction Brag peak. Namely (120), (021), (111), (121), (221), (151). This later set corresponds to γ-FeOOH. It should be noted that (104) diffraction Bragg peak is one of the most intense peak, in agreement with the atomic ordering of the nanocrystals with a preferred lattice atomic ordering of dhkl = 5.04 Å observed in the previous HRTEM studies of Fig. 2b. In a pre-conclusion, the Himba Ochre sample consists of hematite α-Fe2O3 mainly with an amount of a hydrolized form in a form of γ-FeOOH. In view of investigating the thermal stability of such a nano-system, Differential Scanning Calorimetry (DSC) studies under standard air were conducted within the thermal range of 25–650 °C. Figure 2e reports the corresponding DSC profile. This latter exhibits 3 major zones with an exothermic and an endothermic peaks with the first zones. Zone 1 is likely to be related to the melting/evaporation of H2O molecules adsorbed onto the sample surface. Zone 2 corresponds likely to a phase transformation of FeOOH to α -Fe2O318–24.
Elemental investigations
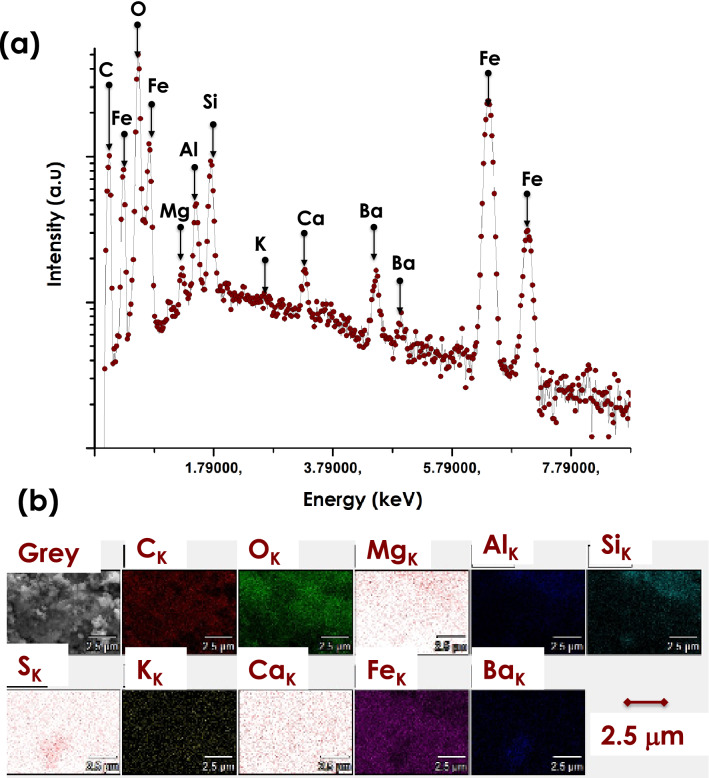
Figure 3a depicts their corresponding elemental EDS spectrum. There are 4 major Fe peaks located at various channels with the O peak as the most intense. The C peak originates from the Carbon coating used to avoid surface charging effect during the HRSEM investigations. Likewise, one can distinguish the presence of several contaminants including Mg, Al, Si, K, Ca & Ba. Figure 3b reports their corresponding 2D scans showing that they are relatively homogeneously distributed within the Ochre sample yet at low concentrations. Their qualitatively values are significantly at low levels i.e. impurities level relatively to Fe & O. The relative atomic ratio of O/Fe is about 1.62 suggesting that the the Himba Ochre is likely hematite Fe2O3 (O/Fe = 1.5) rather than magnetite Fe3O4(O/Fe = 1.3).
Figure 3.
(a) EDS elemental spectrum of the Red Ochre pigment & (b) its corresponding 2-D disctribution scan of the various detected elements.
Vibrational spectroscopy investigations
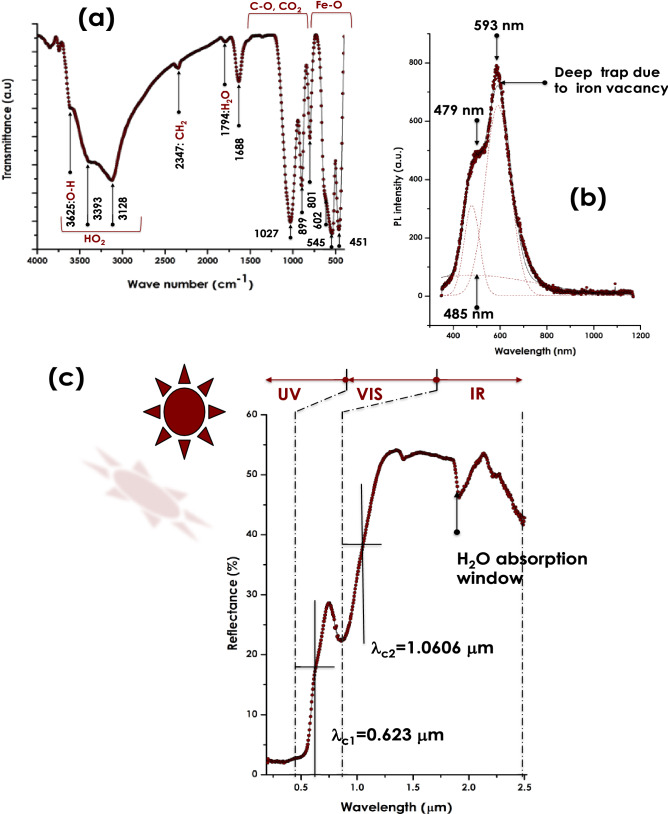
Figure 4a reports the Attenuated Total Reflection (ATR)-Fourier Transform InfraRed (FTIR) spectrum of the Himba Ochre within the 400–4000 cm-1 spectral range. One can notice the relatively sharp mode at 451 cm-1 & the convoluted peaks at 545 & 602 cm-1 which are characteristic of the Fe–O vibrational modes. The various absorption modes from 801–1688 cm-1 are assigned to C=O, C–O–C stretching modes related to adsorbed CO2 molecules on the surface of the α-Fe2O3 crystals. In addition to the 2 low intensity vibrational modes of 1794 & 2347 cm−1 assigned to H2O & CH2 respectively, there is a broad band stretching from 2750–3700 cm−1 which may rise from various vibrational modes of H2O molecules. The latter molecules are likely due to atmospheric water molecules adsorbed on the surface of the α-Fe2O3 nano-crystals or those of the γ-FeOOH.
Figure 4.
(a) ATR-FTIR vibrational modes of the Red Ochre pigment, (b) its Photoluminescence emission spectrum under an excitation wavelength λexc = 270 nm, and (c) its UV–VIS-NIR reflectance in the spectral range of 190–2500 nm.
Defects & luminescence investigations
From luminescence viewpoint in defects free α -Fe2O3, luminescence emissions would originates from the valence band of the Fe (3d) and O (2p) states, to the conduction band dominated by Fe (4 s) states. Because the local configuration of the d-band, α-Fe2O3 does not exhibit photoluminescence emission in its bulk form25. Figure 4b reports the luminescence spectrum at room temperature of the Otjize ochre sample under an excitation of 270 nm. It shows a relatively intense emission with a shoulder. More precisely, The simulation of the emission profile consists of 1 broad & 2 sharp emissions centered at 485, 479 and 593 nm respectively. The origin of these luminescence emissions could be due to two major factors: (i)the Oxygen surface atomic coordination as per the Pauling & Hendrick’s model for α -Fe2O326 (ii) An increase in the F-O bonding separation inducing an enhancement in the magnetic coupling between Fe3+ ions neighbours as reported by Han et al.27 and Blake et al.28,, (iii)surface defects which may arise from deep traps caused by Fe vacancies28.
Optical investigations & UV selectivity
In terms of optical absorbance, standard hematite α-Fe2O3 exhibits several absorbance bands centered at about 347 nm, 543 nm assigned to metal charge transfer direct transitions and to the double excitation processes 6A1(6 s) + 6A1(6 s) to 4T1(4G) + 4T1(4G) respectively29. Those in the range of 478–550 nm are caused by 6A1(6 s) to 4E, 4A1(4G) ligand field transitions30. In addition to these various absorbance bands, there is a strong absorbance at 543 nm which at the origin of the red color of hematite. Figure 4c reports the Reflectance of the Otjize ochre sample in the spectral range of 190–2500 nm. As one can notice, there is a sharp spectral cut-off at about 570 nm. The reflectance is minimal below such a cut-off; < 3%. Because of the opacity of the sample, the spectral range in the UV & part of the VIS is fully absorbed by the Otjize ochre sample in the measured spectral range of 190–570 nm. Hence, it can be concluded that the Otjize ochre sample is an exceptional UVA & UVB selective filter & hence an effective shield against solar UV radiations induced skin cancer. Following a dip in the reflectance centered at 855 nm, there is a plateau-like with an average of 53.6% suggesting that the Otjize ochre coating minimizes the skin overheating by reflecting-back at least half of the solar heat in the IR region. While the dip centered at 855 nm is due to of 6A1(6 s) to 4T1(4G) ligand field transition31, the one at about 1950 nm corresponds to a H2O absorption window. This latter absorption is likely to be caused by atmospheric H2O molecules adsorbed on the α-Fe2O3 Otjize ochre sample. One should mention that this optical cut-off behavior of hematite which makes it of a special interest in solar water photo-splitting applications7,8,32–34.
Antibacterial studies
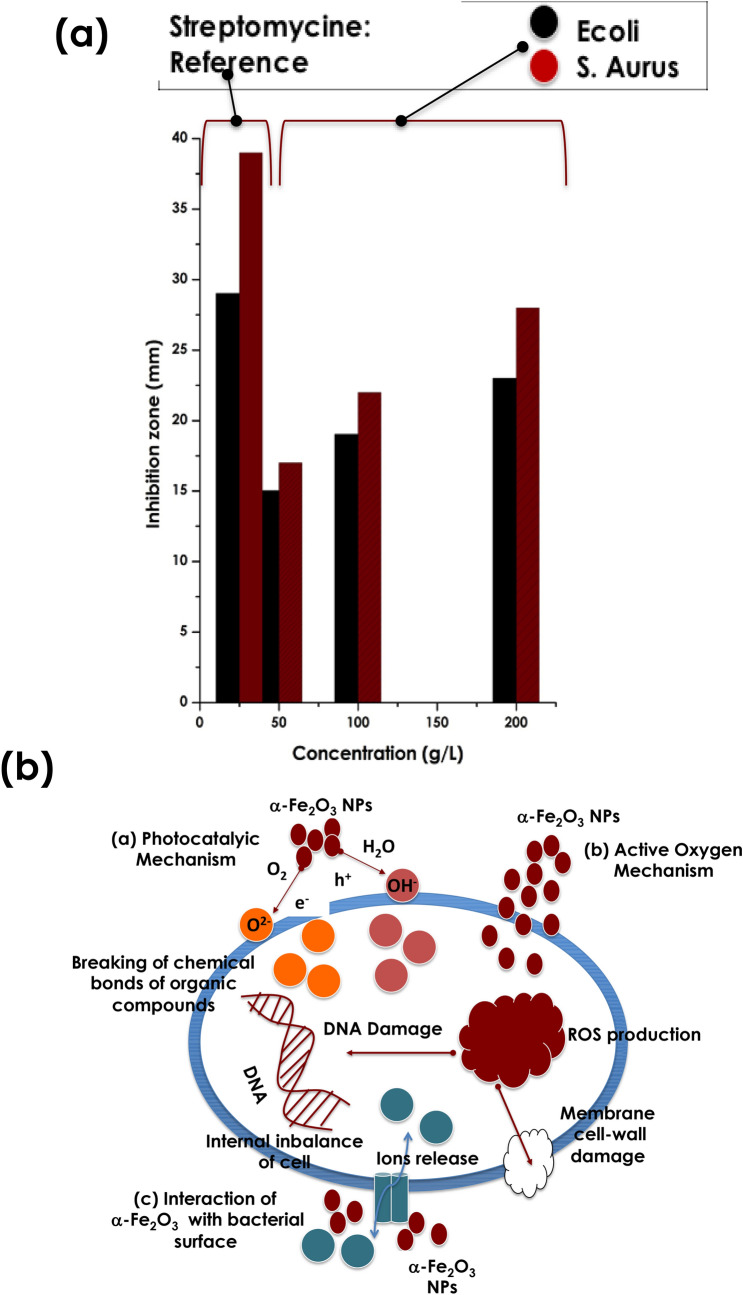
Relatively to the standard nano-TiO2 & nano ZnO possessing cut-off wavelengths at the UV-Bleu spectral range at the vicinity of 380 & 390 nm respectively, the one of the current Himba Otjize red Ochre is by contrast in the visible range at the vicinity of 570 nm as per (Fig. 4c). Consequentially, the nano-scaled α -Fe2O3 of the Himba Otjize red Ochre should exhibit an antibacterial response. Indeed, as reported in Fig. 5a, the antibacterial activity against E-coli & staphylococcus-aurus. More precisely, it reports the inhibition zone against E-coli & staphylococcus-aurus at various Himba Otjize red Ochre α-Fe2O3 concentrations;50, 100 & 200 μg/μl. One can notice that there is a crystal clear an antibacterial against both species yet relatively low compared to the standard Streptomycine. However, the efficacy of the Himba Otjize red Ochre α-Fe2O3 nanoparticles become effective at higher concentrations. This trend is comparable to those obtained by Rufus et al.35 on pure hematite bio-synthesized using natural extract of Psidium guajava’s leaves as an effective chelating agent.
Figure 5.
(a) Antibacterial response of the Red Ochre pigment against the E-coli & S-Aurus compared to standard reference Streptomycine (b), Schematic representation of the 3 potential mechanisms inducing the antibacterial activity of the Ochre.
In terms of the antibacterial activity of the nano-scaled α-Fe2O3 of the Himba Otjize red Ochre, one can distinguish 3 potentials mechanisms which are schematically represented in Fig. 5b36–43;
(i) Photocatalytic mechanism, (ii)Reactive Oxygen Species mechanism & or (iii)Cell surface interaction with the α-Fe2O3 nanoparticles mechanism. In the first mechanism, once the the α-Fe2O3 nanoparticles are excited with a light energy larger than the bandgap, an e_−H+ excitonic pair is created. This e_–H+ pair reacts with O2, hydroxyl groups as well as the adsorbed H2O molecules to produce sturdy oxidative compounds such as OH_, O2_ and H2O2. The H+ and OH_ are characterized by strong oxidative properties, able to break the chemical bonds of various compounds inducing the degradation of the micro-organisms and therefore antibacterial effectiveness. The second potential mechanism involves Reactive oxygen species. Once diffused in the cell, the nanoscaled α-Fe2O3 nanoparticles produce ROS species inducing a severe damage to bacterial membranes and hence bacteriolysis & aggregation of α-Fe2O3 nanoparticles within the bacteria leading their death. In the third mechanism, and because of the high surface to volume ratio of the α-Fe2O3 nanoparticles, their interaction with the cell surface leads to a significant bacterial membrane damage and the release of ion channels, resulting in internal ionic imbalance of the cells, and eventually death43,44.
As a major pre-conclusion of this study, it is worth pointing that the effective UV filtration of the red ochre used by the Himba Women could explain the low skin cancer within such a community in Namibia45.
Conclusion
This study was geared towards the investigation of the bio-physical properties of Red Ochre pigment used by the Himba in their Otjize formulation to protect their skin from solar radiations. It was found that the Red Ochre pigment consisted of nanoscaled particles of mainly α-Fe2O3 with γ-FeOOH nanoparticles. Such a Red Ochre pigment exhibited a significant UVA & UVB blocking optical properties hence is an effective skin cancer protection. In addition, it exhibits an effective reflectivity in the IR region hence minimizing solar heat burning of the Himba bodies in the harsh Namibian desertic climate. In addition, it was found that such a red Ochre pigment exhibit additional antibacterial efficacy against E-coli & Staphylococcus Aurus. As a major conclusion of this study, it is worth pointing that the effective UV filtration of the red ochre used by the Himba Women could explain the low skin cancer within such a community in Namibia.
Acknowledgements
This research was supported by the German DAAD, the French CNRS, the National Research Foundation of South Africa, the University of South Africa, iThemba-LABS as well as the UNESCO UNISA ITLABS-Africa Chair in Nanosciences & Nanotechnologies (U2ACN2). Ms. Adele Teymouri is specially acknowledged for her sound advices & fruitful discussions on pigments. Likewise, we wish to acknowledge Dr. UGRO for assisting with the background of Figure 1a.
Author contributions
DH: Data collection, analysis & manuscript writing, RA: Data collection & analysis, SA: Data collection & analysis, LM: EDS analysis, & Data collection & Analysis JS: Data Collection & Analysis NS: Research project management, review from the anthropological perspective AG: Manuscript review& various inputs MM: project inception, data analysis, interpretation & manuscript writing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. Havenga, Email: havengads@gmail.com
M. Maaza, Email: Maazam@unisa.ac.za
References
- 1.Tuček J, Machala M, Ono S, Namai A, Yoshikiyo M, Imoto K, Tokoro S, Ohkoshi S, Zbořil R. Zeta-Fe2O3: A new stable polymorph in iron(III) oxide family. Sci. Rep. 2015;5:1509. doi: 10.1038/srep15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornell RM, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses. Wiley-VCH Publishers; 2003. [Google Scholar]
- 3.Hanslık T, Tlaskal J, Subrt J, Zapletal V. Behaviour of iron (III) oxide hydroxides and iron (III) oxides in concentrated solutions of alkali hydroxides. Mater. Chem. 1981;6:267–276. [Google Scholar]
- 4.Song L, Zhang S. Formation of α-Fe2O3/FeOOH nanostructures with various morphologies by a hydrothermal route and their photocatalytic properties. Colloids Surf. A. 2009;348:217–220. [Google Scholar]
- 5.Faust BC, Hoffmann MR, Bahnemann DW. Photocatalytic oxidation of sulfur dioxide in aqueous suspensions of alpha-iron oxide (Fe2O3) J. Phys. Chem. 1989;93:6371–6381. [Google Scholar]
- 6.Mohapatra SK, John SE, Banerjee S, Misra M. Water Photooxidation by Smooth and Ultrathin α-Fe2O3 Nanotube Arrays. Chem. Mater. 2009;21:3048–3055. [Google Scholar]
- 7.Sivula K, Le Formal F, Gratzel M. Solar water splitting: Progress using hematite (α -Fe2O3) photoelectrodes. Chemsuschem. 2011;4:432–449. doi: 10.1002/cssc.201000416. [DOI] [PubMed] [Google Scholar]
- 8.Sivula K. Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J. Am. Chem. Soc. 2010;132:7436–7444. doi: 10.1021/ja101564f. [DOI] [PubMed] [Google Scholar]
- 9.Hermanek M, Zboril R, Medrik I, Pechousek J, Gregor C. Catalytic efficiency of iron(III) oxides in decomposition of hydrogen peroxide: Competition between the surface area and crystallinity of nanoparticles. J. Am. Chem. Soc. 2007;129:10929–10936. doi: 10.1021/ja072918x. [DOI] [PubMed] [Google Scholar]
- 10.Mohsan B, Shaista A. Green synthesis of Fe2O3 nanoparticles from orange peel extract and a study of its antibacterial activity. J. Korean Phys. Soc. 2020;76(9):848–854. [Google Scholar]
- 11.Bu K, Cizdziel JV, Russ J. The source of iron-oxide pigments used in pecos river style rock paints. Archaeometry. 2013;55(6):1088–1100. [Google Scholar]
- 12.Roebroeks W, Sier MJ, Kellberg-Nielsen T, De Loecker D, Pares JM, Arps CES, Mucher HJ. Use of red ochre by early Neandertals. Proc. Natl. Acad. Sci. 2012;109(6):1889–1894. doi: 10.1073/pnas.1112261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henshilwood C, D'Errico F, Van Niekerk K, Coquinot Y, Jacobs Z, Lauritzen SE, Menu M, Garcia-Moreno R. A 100,000-year-old ochre-processing workshop at blombos cave, South Africa. Science. 2011;334:219–222. doi: 10.1126/science.1211535. [DOI] [PubMed] [Google Scholar]
- 14.Dayet L, Le Bourdonnec FX, Daniel F, Porraz G, Texier PJ. Ochre Provenance and Procurement Strategies During The Middle Stone Age at Diepkloof Rock Shelter, South Africa. Archaeometry. 2015;5:807–829. [Google Scholar]
- 15.Dayet L, Texier PJ, Daniel F, Porraz G. Ochre resources from the Middle Stone Age sequence of Diepkloof Rock Shelter, Western Cape, South Africa. J. Archaeol. Sci. 2013;40(9):3492–3505. [Google Scholar]
- 16.Bollig M, Heinemann H. Ochre people and heroic herders—visual presentations of the Himba of Namibia’s Kaokoland. Vis. Anthropol. 2002;15(3):267–312. [Google Scholar]
- 17.Crandall DP. Himba flora taxonomy and herbal medicines. Anthropos. 2004;99(1):200–207. [Google Scholar]
- 18.Music S, Krehula S, Popovic S. Thermal decomposition of γ-FeOOH. Mater. Lett. 2004;58:444–448. [Google Scholar]
- 19.Bashir M. Solution-processed two-dimensional materials for next-generation photovoltaics. J. Korean Phys. Soc. 2020;76(9):848–854. [Google Scholar]
- 20.Jiao H, Jiao G. Hydrothermal synthesis and characterization of monodisperse α-Fe2O3 nanoparticles. Mater. Lett. 2009;63:2725–2727. [Google Scholar]
- 21.Tong G, Wu W, Guan J, Qian H, Yuan J, Li W. Rapid preparation of a-FeOOH and a-Fe2O3 nanostructures by microwave heating and their application in electrochemical sensors. J. Alloys Compd. 2011;509:4320–4326. [Google Scholar]
- 22.Zhang G-Y, Xu Y-Y, Gao D-Z, Sun Y-Q. Materials development and potential applications of transparent ceramics: A review. J. Alloys Compd. 2011;509:885–890. [Google Scholar]
- 23.Hao Q, Liu S, Yin X, Wang Y, Li T, Wang Q. Fabrication of LDH nanosheets on β-FeOOH rods and applications for improving the fire safety of epoxy resin. Solid State Sci. 2010;12:2125–2129. [Google Scholar]
- 24.Diamandescu L, Mihaila-Tarabasanu D, Calogero S, Popescu-Pogrion N, Feder M. Hydrothermal synthesis and characterization of some polycrystalline α-iron oxides. Solid State Ionics. 1997;101:591–596. [Google Scholar]
- 25.Jahagirdar AA, Dhananjaya N, Monika DL, Kesavulu CR, Nagabhushana H, Sharma SC, Nagabhushana BM, Shivakumara C, Rao JL, Chakradhar RPS. Structural, EPR, optical and magnetic properties of α-Fe2O3. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2013;104:512–518. doi: 10.1016/j.saa.2012.09.069. [DOI] [PubMed] [Google Scholar]
- 26.Pauling L, Hendricks B. “The crystal structures of Hematite & Corundum. J. Am. Chem. Soc. 1925;47(3):781–790. [Google Scholar]
- 27.Han Q, Liu Z, Xu Y, Chen Z, Wang T, Zhang T. Low temperature synthesis and photocatalytic activity of rutile TiO2 nanorod superstructures. J. Phys. Chem. C. 2007;111:5034–5038. [Google Scholar]
- 28.Blake R, Hessevick R, Zoltai T, Finger L. Refinement of the hematite structure. Am. Mineral. 1966;51:123–129. [Google Scholar]
- 29.Marusak LA, Messier R, White WB. Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. J. Phys. Chem. Solids. 1980;41:981–984. [Google Scholar]
- 30.Wheeler DA, Wang G, Ling Y, Li Y, Zhang JZ. Nanostructured hematite: Synthesis, characterization, charge transfer. Energy Environ. Sci. 2012;5:6682–6702. [Google Scholar]
- 31.He YP, Miao YM, Li CR, Wang SQ, Cao L, Xie SS, Yang GZ, Zou BS. Size and structure effect on optical transitions of iron oxide nanocrystals. Phys. Rev. 2005;71:125411–125419. [Google Scholar]
- 32.Beermann N, Vayssieres L, Lindquist SE, Hagfeldt A. Photoelectrochemical studies of oriented nanorod thin films of hematite. J. Electrochem. Soc. 2000;147:2456–2461. [Google Scholar]
- 33.Ozer N, Tepehan F. Optical and electrochemical characteristics of sol–gel deposited iron oxide films. Sol. Energy Mater. Sol. Cells. 1999;56:141–152. [Google Scholar]
- 34.Vayssieres L, Sathe C, Butorin SM, Shuh DK, Nordgren J, Guo JH. Characterization of α-Fe2O3 nano powders synthesized by emulsion precipitation-calcination route and rheological behaviour of α-Fe2O3. Adv. Mater. 2005;17:2320–2323. [Google Scholar]
- 35.Rufus A, Sreeju N, Philip D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RCS Adv. 2016;6:1–26. [Google Scholar]
- 36.Dutta RK, Nenavathu BP, Gangishetty MK. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfac. 2012;94:143–150. doi: 10.1016/j.colsurfb.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 37.Kuang HJ, Yang L, Xu HY. Antibacterial properties and mechanism of zinc oxide nanoparticles: Research progress. Chin. J. Pharmacol. Toxicol. 2015;29(1):153–157. [Google Scholar]
- 38.Yamamoto Y, Imai N, Mashima R, Konaka R, Inoue M, Dunlap WC. Singlet oxygen from irradiated titanium dioxide and zinc oxide. Methods Enzymol. 2000;319:29–37. doi: 10.1016/s0076-6879(00)19005-6. [DOI] [PubMed] [Google Scholar]
- 39.Chu LC, Zhou ZW, Duan XF, Tang LL. Materials development and potential applications of transparent oxides. Mater. Rev. 2003;17:84–85. [Google Scholar]
- 40.Fang M, Chen JH, Xu XL, Yang PH, Hildebrand HF. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int. J. Antimicrob. Agents. 2006;27:513–517. doi: 10.1016/j.ijantimicag.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008;279:71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 42.Touati D. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 2000;373:1–6. doi: 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]
- 43.Makhluf S, Dror R, Nitzan Y, Abramovich Y, Jelinek R, Gedanken A. Antimicrobial activity of SnO2 nanoparticles against Escherichia coli and Staphylococcus aureus and conventional antibiotics. Adv. Funct. Mater. 2005;15:1708–1715. [Google Scholar]
- 44.Mei XJ, Wang ZW, Zheng X. Soluble microbial products in membrane bioreactors in the presence of ZnO nanoparticles. J. Memb. Sci. 2014;451:169–176. [Google Scholar]
- 45.Zietsman A, Gariseb B, Rautenbach N, Pontac J, Roii T, Taapopi L. Cancer in Namibia 2006–2009. Namibian Cancer Registry. 2009;2:2. [Google Scholar]