Abstract
The advantages of laparoscopic resection over open surgery in the treatment of gastric gastrointestinal stromal tumor (GIST) are not conclusive. This study aimed to evaluate the postoperative and oncologic outcome of laparoscopic resection for gastric GIST, compared to open surgery. We retrospectively reviewed the prospectively collected database of 1019 patients with gastric GIST after surgical resection at 13 Korean and 2 Japanese institutions. The surgical and oncologic outcomes were compared between laparoscopic and open group, through 1:1 propensity score matching (PSM). The laparoscopic group (N = 318) had a lower rate of overall complications (3.5% vs. 7.9%, P = 0.024) and wound complications (0.6% vs. 3.1%, P = 0.037), shorter hospitalization days (6.68 ± 4.99 vs. 8.79 ± 6.50, P < 0.001) than the open group (N = 318). The superiority of the laparoscopic approach was also demonstrated in patients with tumors larger than 5 cm, and at unfavorable locations. The recurrence-free survival was not different between the two groups, regardless of tumor size, locational favorableness, and risk classifications. Cox regression analysis revealed that tumor size larger than 5 cm, higher mitotic count, R1 resection, and tumor rupture during surgery were independent risk factors for recurrence. Laparoscopic surgery provides lower rates of complications and shorter hospitalizations for patients with gastric GIST than open surgery.
Subject terms: Gastrointestinal cancer, Surgical oncology
Introduction
Gastrointestinal stromal tumors (GISTs) are the commonest type of mesenchymal tumors of the gastrointestinal tract, with the stomach (50–60%) being the most frequent primary site1. Although tyrosine kinase inhibitors (TKIs) have significantly improved disease-free survival, complete R0 surgical resection remains the standard treatment for primary non-metastatic gastric GIST2–4. Laparoscopic surgery for gastric GIST has been widely performed to meet the current demand for minimal invasiveness. Numerous studies have reported the potential advantages of laparoscopic resection for gastric GISTs, such as earlier return of bowel function, less blood loss, and shorter hospitalization days5,6.
However, the perceived advantages of laparoscopic resection compared with open surgery is not conclusive in the treatment of gastric GIST. Previous studies have lacked reliability and credibility for the following reasons. Since gastric GISTs are rare, with an estimated incidence of 1.5/100,000 per year7,8, no randomized controlled trial (RCT) has clearly demonstrated the beneficial effect of laparoscopic resection of gastric GIST9,10. Therefore, the meta-analyses to date are based on the data extracted from non-RCTs; thus, they have less powerful outcomes11–14. In addition, retrospective observational studies that reported similar or even better postoperative laparoscopy results suffer from selection bias since laparoscopic surgeries are preferred for smaller sized tumors with favorable locations6,15. Several retrospective studies using propensity score matching (PSM) have been conducted to overcome these limitations. However, the small sample sizes have not been sufficient for clear conclusions, and subgroup analyses were not conducted for patients with tumors larger than 5 cm and in unfavorable locations, simultaneously16,17. Although, Xiong et al.18 analyzed 1,027 patients, the largest number ever, only 128 patients were remained for analysis in each group after PSM.
Considering the technical advances in laparoscopic surgery over decades, it is meaningful to prove whether laparoscopic surgery was superior to open resection in gastric GISTs treatment even in the early era of laparoscopic surgery. This multi-center retrospective study enrolled 1019 patients diagnosed with gastric GIST after surgery during the period 2000–2007 at 13 Korean and 2 Japanese institutions, aimed to evaluate whether the laparoscopic resections was superior clinical outcomes and comparable in oncologic safety compared to open surgery, even in the early era of laparoscopic surgery. We also evaluated whether the application of laparoscopic surgery could be feasible and safe in tumors larger than 5 cm and in unfavorable locations.
Results
Before PSM, the laparoscopic group had more patients with a higher propensity score, younger age, tumors ≤ 5 cm, and tumors in a favorable location than the open group. The open group had more cases of gastrectomy, combined resection, higher mitotic count, high-risk by NIH classification, and adjuvant chemotherapy than the laparoscopic group. Except for the missing data, there was no difference between the two groups in immunohistochemical parameters including CD117 and CD34.
After 1:1 PSM, the 318 patients in the laparoscopic group were matched to the 318 patients in the open group in the same period and the distribution of cumulative cases per year has similar distributional tendency with those observed before matching (Supplementary Fig. 1). And, the propensity scores, clinicopathologic variables were all balanced between the laparoscopic and open groups (Table 1). The distribution of the propensity score was more concentrated, and the absolute SMDs of matching covariates decreased near or below 0.1 (Supplementary Fig. 2).
Table 1.
The clinicopathologic characteristics between the laparoscopic group and open group.
| Variables | Before PSM | P value | After PSM | P value | ||
|---|---|---|---|---|---|---|
| Laparoscopic group (n = 373) |
Open group (n = 542) |
Laparoscopic group (n = 318) |
Open group (n = 318) |
|||
| Propensity scores | ||||||
| 0.5 ± 0.1 | 0.3 ± 0.2 | < 0.001 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.056 | |
| Age, years (mean ± SD) | ||||||
| 57.1 ± 11.4 | 60.0 ± 12.0 | 0.019 | 57.4 ± 11.3 | 58.4 ± 11.3 | 0.245 | |
| Sex | ||||||
| Male | 175 (46.9) | 272 (50.2) | 0.346 | 148 (46.5) | 150 (47.2) | 0.937 |
| Female | 198 (53.1) | 270 (49.8) | 170 (53.5) | 168 (52.8) | ||
| BMI, kg/m2 (mean ± SD) | ||||||
| 24.3 ± 3.1 | 24.0 ± 3.1 | 0.179 | 24.2 ± 3.0 | 24.2 ± 3.1 | 0.859 | |
| Underlying disease | ||||||
| Hypertension | 98 (26.4) | 170 (31.2) | 0.121 | 84 (26.4) | 103 (32.4) | 0.117 |
| Cardiovascular | 17 (4.6) | 23 (4.2) | 0.870 | 13 (4.1) | 15 (4.7) | 0.847 |
| Cerebrovascular | 3 (0.8) | 3 (0.6) | 0.691 | 2 (0.6) | 1 (0.3) | 0.563 |
| Pulmonary disease | 19 (5.1) | 26 (4.8) | 0.877 | 15 (4.7) | 19 (6.0) | 0.598 |
| Hepatic disease | 22 (5.9) | 25 (4.6) | 0.366 | 27 (8.5) | 36 (11.3) | 0.288 |
| Diabetes mellitus | 30 (8.1) | 64 (11.8) | 0.077 | 19 (6.0) | 17 (5.3) | 0.864 |
| Renal disease | 2 (0.5) | 1 (0.2) | 0.569 | 2 (0.6) | 1 (0.3) | 0.563 |
| Radicality of resection | ||||||
| R0 resection | 368 (98.7) | 533 (98.3) | 0.790 | 313 (98.4) | 313 (98.4) | 1.000 |
| R1 resection | 5 (1.3) | 9 (1.7) | 5 (1.6) | 5 (1.6) | ||
| Extent of resection | ||||||
| Wedge resection | 343 (92.0) | 370 (68.3) | < 0.001 | 291 (91.5) | 288 (90.6) | 0.950 |
| Partial gastrectomy | 21 (5.6) | 88 (16.2) | 21 (6.6) | 22 (6.9) | ||
| Total gastrectomy | 2 (0.5) | 78 (14.4) | 2 (0.6) | 3 (0.9) | ||
| Enucleation | 7 (1.9) | 6 (1.1) | 4 (1.3) | 5 (1.6) | ||
| Combined resection | ||||||
| Total | 29 (7.8) | 83 (15.3) | < 0.001 | 27 (8.5) | 20 (6.3) | 0.363 |
| Cholecystectomy | 23 (6.2) | 19 (3.5) | 21 (6.6) | 12 (3.8) | ||
| Liver resection | 2 (0.5) | 5 (0.9) | 2 (0.5) | 1 (0.3) | ||
| Colon resection | 1 (0.3) | 17 (3.1) | 1 (0.3) | 2 (0.5) | ||
| Adrenalectomy | 1 (0.3) | 7 (1.3) | 1 (0.3) | 1 (0.3) | ||
| SB resection | 1 (0.3) | 3 (0.6) | 1 (0.3) | 1 (0.3) | ||
| Splenectomy | 0 (0) | 40 (7.4) | 0 (0) | 1 (0.3) | ||
| Pancreatectomy | 0 (0) | 20 (3.7) | 0 (0) | 1 (0.3) | ||
| Others | 3 (0.8) | 1 (0.3) | 3 (0.8) | 1 (0.3) | ||
| Tumor size, mean (mm) | ||||||
| 37.3 ± 18.6 | 65.2 ± 46.5 | < 0.001 | 38.3 ± 19.5 | 40.1 ± 17.0 | 0.217 | |
| Tumor size category (cm) | ||||||
| > 0, ≤ 2 | 62 (16.6) | 42 (7.7) | < 0.001 | 52 (16.4) | 38 (11.9) | 0.554 |
| > 2, ≤ 5 | 247 (66.2) | 241 (44.5) | 202 (63.5) | 206 (64.8) | ||
| > 5, ≤ 7 | 49 (13.1) | 96 (17.7) | 49 (15.4) | 58 (18.2) | ||
| > 7, ≤ 10 | 10 (2.7) | 67 (12.4) | 10 (3.1) | 11 (3.5) | ||
| > 10, ≤ 15 | 5 (1.3) | 69 (12.7) | 5 (1.6) | 5 (1.6) | ||
| > 15, ≤ 20 | 0 (0) | 17 (3.1) | 0 (0) | 0 (0) | ||
| > 20, ≤ 30 | 0 (0) | 9 (1.7) | 0 (0) | 0 (0) | ||
| > 30 | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | ||
| Longitudinal location | ||||||
| GEJ to Cardia | 59 (15.8) | 81 (14.9) | 0.540 | 53 (16.7) | 40 (12.6) | 0.417 |
| Upper third | 153 (41.0) | 234 (43.2) | 129 (40.6) | 146 (45.9) | ||
| Upper to middle | 1 (0.3) | 2 (0.4) | 1 (0.3) | 0 (0) | ||
| Middle third | 67 (18.0) | 96 (17.7) | 57 (17.9) | 47 (14.8) | ||
| Middle to lower | 0 (0) | 5 (0.9) | 1 (0.3) | 1 (0.3) | ||
| Lower third | 93 (24.9) | 123 (22.7) | 77 (24.2) | 83 (26.1) | ||
| Entire length | 0 (0) | 1 (0.2) | 0 (0) | 1 (0.3) | ||
| Circumferential location | ||||||
| Greater | 110 (29.5) | 141 (26.0) | 0.001 | 96 (30.2) | 89 (28.0) | 0.231 |
| Lesser | 80 (21.4) | 139 (25.6) | 68 (21.4) | 78 (24.5) | ||
| Anterior | 93 (24.9) | 90 (16.6) | 74 (23.3) | 58 (18.2) | ||
| Posterior | 90 (24.1) | 163 (30.1) | 80 (25.2) | 91 (28.6) | ||
| Entire | 0 (0) | 9 (1.7) | 0 (0) | 2 (0.6) | ||
| Locational preference | ||||||
| Favorable | 176 (47.2) | 203 (37.5) | 0.004 | 145 (45.6) | 130 (40.9) | 0.262 |
| Unfavorable | 197 (52.8) | 339 (62.5) | 173 (54.4) | 188 (59.1) | ||
| Mitotic rate (per 50 HPF) | ||||||
| ≤ 5 | 276 (74.0) | 352 (64.9) | < 0.001 | 242 (76.1) | 242 (76.1) | 0.225 |
| > 5, ≤ 10 | 71 (19.0) | 96 (17.7) | 56 (17.6) | 46 (14.5) | ||
| > 10 | 26 (7.0) | 94 (17.3) | 20 (6.3) | 30 (9.4) | ||
| NIH risk stratification | ||||||
| Very low | 49 (13.1) | 36 (6.6) | < 0.001 | 41 (12.9) | 32 (10.1) | 0.232 |
| Low | 183 (49.1) | 183 (33.8) | 157 (49.4) | 160 (50.3) | ||
| Intermediate | 100 (26.8) | 128 (23.6) | 85 (26.7) | 76 (23.9) | ||
| High | 41 (11.0) | 195 (36.0) | 35 (11.0) | 50 (15.7) | ||
| CD 117 | ||||||
| Yes | 306 (97.1) | 366 (98.4) | 0.303 | 260 (96.7) | 202 (99.0) | 0.125 |
| No | 9 (2.9) | 6 (1.6) | 9 (3.3) | 2 (1.0) | ||
| Unidentified | 58 cases | 170 cases | 49 case | 114 case | ||
| CD 34 | ||||||
| Yes | 281 (98.6) | 333 (97.9) | 0.762 | 241 (98.8) | 186 (98.4) | 0.752 |
| No | 4 (1.4) | 7 (2.1) | 3 (1.2) | 3 (1.6) | ||
| Unidentified | 88 case | 202 case | 74 case | 129 case | ||
| Adjuvant treatment (Gleevec) | ||||||
| Yes | 7 (1.9) | 52 (9.6) | < 0.001 | 7 (2.2) | 7 (2.2) | 1.000 |
| No | 366 (98.1) | 490 (90.4) | 311 (97.8) | 311 (97.8) | ||
GEJ gastroesophageal junction, HPF high power field, PSM propensity score matching, SD standard deviation, SB small bowel.
Surgical outcomes and complications before and after PSM
The rates of overall complications were significantly lower in the laparoscopic group than in the open group, either before (4.0% vs. 8.3%, P = 0.010) or after PSM (3.5% vs. 7.9%, P = 0.024). The complication requiring major intervention were lower in the laparoscopic group than in the open group, either before (2.1% vs. 5.5%, P = 0.011) or after PSM (1.9% vs. 5.7%, P = 0.020). The laparoscopic group had lower wound complications than the open group (0.6% vs. 3.1%, P = 0.037) after PSM. Intestinal motility disorder was not found in the laparoscopic group but was found in the open group. The hospitalization days of the laparoscopic group were significantly shorter than those of the open group, either before (6.7 ± 4.9 vs. 10.2 ± 7.7, P < 0.001) or after PSM (6.7 ± 5.0 vs. 8.8 ± 6.5, P < 0.001). The mortality rate within 30 days was not different between the two groups, regardless of PSM (Table 2).
Table 2.
Operative outcomes and surgical complications of gastric GIST patients treated by laparoscopic versus open resection.
| Variable | Before PSM | P value | After PSM | P value | ||
|---|---|---|---|---|---|---|
| Laparoscopic group (N = 373) |
Open group (N = 542) |
Laparoscopic group (N = 318) |
Open group (N = 318) |
|||
| Operation time (minute) | ||||||
| Overall | 113.9 ± 58.6 | 125.5 ± 71.3 | 0.011 | 114.0 ± 57.0 | 100.8 ± 54.5 | 0.003 |
| Wedge resection or enucleation | 112.0 ± 58.6 | 103.4 ± 59.7 | 0.052 | 111.7 ± 56.9 | 97.4 ± 53.7 | 0.002 |
| Gastrectomy | 143.7 ± 51.1 | 175.4 ± 70.2 | 0.014 | 143.1 ± 50.3 | 143.6 ± 46.3 | 0.973 |
| Conversion to open surgery | 14 (3.8) | – | – | 11 (3.5) | – | – |
| Tumor rupture during surgery | 0 (0) | 13 (2.4) | 0.001 | 0 (0) | 6 (1.9) | 0.031 |
| Hospital days (mean ± SD) | ||||||
| Overall | 6.7 ± 4.9 | 10.1 ± 7.7 | < 0.001 | 6.7 ± 5.0 | 8.8 ± 6.5 | < 0.001 |
| Wedge resection or enucleation | 6.5 ± 4.8 | 9.2 ± 7.8 | < 0.001 | 6.4 ± 4.9 | 8.7 ± 6.7 | < 0.001 |
| Gastrectomy | 9.8 ± 5.6 | 12.0 ± 7.2 | 0.094 | 8.8 ± 2.6 | 9.4 ± 2.3 | 0.389 |
| Postoperative complications | ||||||
| Total number of cases with complication | 15 (4.0) | 45 (8.3) | 0.010 | 11 (3.5) | 25 (7.9) | 0.024 |
| Wound | 4 (1.1) | 13 (2.4) | 0.212 | 2 (0.6) | 10 (3.1) | 0.037 |
| Fluid collection | 1 (0.3) | 6 (1.1) | 0.251 | 1 (0.3) | 1 (0.3) | 1.000 |
| Intraabdominal bleeding | 3 (0.8) | 6 (1.1) | 0.746 | 3 (0.9) | 3 (0.9) | 1.000 |
| Luminal bleeding | 3 (0.8) | 2 (0.4) | 0.403 | 2 (0.6) | 1 (0.3) | 0.563 |
| Stenosis | 2 (0.5) | 4 (0.7) | 0.718 | 2 (0.7) | 1 (0.3) | 0.563 |
| Intestinal motility disorder | 0 (0) | 6 (1.1) | 0.087 | 0 (0) | 5 (1.6) | 0.062 |
| Leakage | 1 (0.3) | 2 (0.4) | 0.799 | 0 (0) | 1 (0.3) | 0.317 |
| Fistula | 0 (0) | 2 (0.4) | 0.517 | 0 (0) | 1 (0.3) | 0.317 |
| Pulmonary complications | 0 (0) | 5 (0.9) | 0.085 | 0 (0) | 4 (1.3) | 0.124 |
| Urinary complications | 1 (0.3) | 2 (0.4) | 0.793 | 1 (0.3) | 1 (0.3) | 1.000 |
| Hepatic complications | 0 (0) | 2 (0.4) | 0.517 | 0 (0) | 0 (0) | – |
| Clavien–Dindo classification | ||||||
| Grade I | 3 (0.3) | 6 (1.1) | 0.745 | 2 (0.6) | 4 (1.3) | 0.686 |
| Grade II | 4 (1.1) | 14 (2.6) | 0.146 | 3 (0.9) | 6 (1.9) | 0.505 |
| Grade IIIa | 8 (2.1) | 17 (3.1) | 0.415 | 6 (1.9) | 8 (2.5) | 0.788 |
| Grade IIIb | 0 (0) | 10 (1.8) | 0.007 | 0 (0) | 8 (2.5) | 0.007 |
| Grade IVa | 0 (0) | 1 (0.2) | 0.407 | 0 (0) | 0 (0) | – |
| Grade IVb | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Grade V | 0 (0) | 2 (0.4) | 0.517 | 0 (0) | 2 (0.6) | 0.499 |
| Complication requiring major intervention (≥ grade IIIa) | 8 (2.1) | 30 (5.5) | 0.011 | 6 (1.9) | 18 (5.7) | 0.020 |
| Mortality within 30 days | 0 (0) | 2 (0.4) | 0.517 | 0 (0) | 2 (0.6) | 0.499 |
PSM propensity score matching, SD standard deviation.
The operation time was shorter in the laparoscopic group than in the open group before PSM (113.9 ± 58.6 min vs. 125.5 ± 71.3 min, P = 0.011). After PSM, the operation time of the laparoscopic group was longer than that of the open group (114.0 ± 57.0 min vs. 100.8 ± 54.5 min, P = 0.003) (Table 2).
Regardless of PSM, tumor rupture during surgery was not detected in the laparoscopic group. The laparoscopic group showed a significantly lower rate of tumor rupture during surgery than the open group, either before or after PSM (all P < 0.05). Before PSM, 13 patients experienced tumor rupture during open surgery (Table 2).
Of the 373 patients who were initially planned for laparoscopic surgery, 14 patients (3.8%) required conversion to open surgery. Detailed explanations for the reason of each case that required conversion to open surgery were described in Supplementary Table 1. The favorable or unfavorable location did not determine the conversion, regardless of PSM. However, the conversion rate was significantly higher in tumors > 5 cm than in those < 5 cm, either before or after PSM (all P < 0.05) (Supplementary Table 2).
Surgical outcomes and complications by subgroup analysis
Multivariate logistic regression analysis revealed that open resection, longer operation time, tumor size larger than 5 cm, and unfavorable locations were independent risk factors for overall complications (Supplementary Table 3). Because, the rate of overall complications can vary depending on the tumor size and locational preferences, subgroup analyses for surgical outcomes including complications were performed between laparoscopic versus open resection, according to the tumor size and locational preferences.
Regarding the subgroup analysis according to tumor size, the complication rates were not different in patients with tumors ≤ 5 cm between the two groups, but the laparoscopic group had a lower rate of overall (3.1% vs. 16.2%, P = 0.012) and major complication (1.6% vs. 12.2%, P = 0.020) than the open group, in patients with tumors > 5 cm. The hospitalization days were shorter in the laparoscopic group than in the open group, either in patients with tumors ≤ 5 cm or > 5 cm (all P < 0.05, Table 3).
Table 3.
Operative outcomes and surgical complications of patients with gastric GISTs according to tumor size.
| Variable | Tumor size ≤ 5 cm | P value | Tumor size > 5 cm | P value | ||
|---|---|---|---|---|---|---|
| Laparoscopic group (N = 254) |
Open group (N = 244) |
Laparoscopic group (N = 64) |
Open group (N = 74) |
|||
| Operation time (minute) | ||||||
| Overall | 112.5 ± 58.9 | 100.0 ± 53.3 | 0.015 | 120.1 ± 48.8 | 103.5 ± 58.6 | 0.073 |
| Conversion to open surgery | 5 (2.0) | 6 (9.4) | – | – | ||
| Tumor rupture during surgery | 0 (0) | 3 (1.2) | 0.117 | 0 (0) | 3 (4.1) | 0.248 |
| Hospital days (mean ± SD) | ||||||
| Overall | 6.5 ± 4.2 | 8.8 ± 6.7 | < 0.001 | 6.6 ± 2.7 | 9.0 ± 5.9 | 0.003 |
| Postoperative complications | ||||||
| Total number of cases with complication | 9 (3.5) | 13 (5.3) | 0.387 | 2 (3.1) | 12 (16.2) | 0.012 |
| Wound | 1 (0.4) | 5 (2.0) | 0.116 | 1 (1.6) | 5 (6.8) | 0.216 |
| Fluid collection | 1 (0.4) | 0 (0) | 0.327 | 0 (0) | 1 (1.4) | 0.351 |
| Intraabdominal bleeding | 3 (1.2) | 3 (1.2) | 0.961 | 0 (0) | 0 (0) | – |
| Luminal bleeding | 1 (0.4) | 1 (0.4) | 0.977 | 1 (1.6) | 0 (0) | 0.464 |
| Stenosis | 2 (0.9) | 1 (0.5) | 0.600 | 0 (0) | 0 (0) | – |
| Intestinal motility disorder | 0 (0) | 2 (0.8) | 0.240 | 0 (0) | 3 (4.1) | 0.248 |
| Leakage | 0 (0) | 1 (0.4) | 0.490 | 0 (0) | 0 (0) | – |
| Fistula | 0 (0) | 0 (0) | – | 0 (0) | 1 (1.4) | 0.351 |
| Pulmonary complications | 0 (0) | 2 (0.8) | 0.240 | 0 (0) | 2 (2.7) | 0.499 |
| Urinary complications | 1 (0.4) | 0 (0) | 0.327 | 0 (0) | 1 (1.4) | 0.351 |
| Clavien–Dindo classification | ||||||
| Grade I | 1 (0.4) | 3 (1.2) | 0.364 | 1 (1.6) | 1 (1.4) | 0.918 |
| Grade II | 3 (1.2) | 3 (1.2) | 1.000 | 0 (0) | 3 (4.1) | 0.248 |
| Grade IIIa | 5 (2.0) | 4 (1.6) | 0.783 | 1 (1.6) | 4 (5.4) | 0.373 |
| Grade IIIb | 0 (0) | 4 (1.6) | 0.057 | 0 (0) | 4 (5.4) | 0.123 |
| Grade IVa | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Grade IVb | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Grade V | 0 (0) | 1 (0.4) | 0.490 | 0 (0) | 1 (1.4) | 0.351 |
| Complication requiring major intervention (≥ grade IIIa) | 5 (2.0) | 9 (3.7) | 0.287 | 1 (1.6) | 9 (12.2) | 0.020 |
| Mortality within 30 days | 0 (0) | 1 (0.4) | 0.490 | 0 (0) | 1 (1.4) | 0.351 |
SD standard deviation.
According to locational preference, the rate of overall complications was not different between the two groups for tumors in favorable locations. However, the laparoscopic group had a significantly lower rate of complications than the open group for tumors in unfavorable locations (4.0% vs. 10.1%, P = 0.040). The hospitalization days of the laparoscopic group were shorter than those of the open group, indiscriminate of locational preference (all P < 0.05, Table 4).
Table 4.
Operative outcomes and surgical complications according to locational preference (favorable versus unfavorable).
| Variable | Favorable location | P value | Unfavorable location | P value | ||
|---|---|---|---|---|---|---|
| Laparoscopic group (N = 145) |
Open group (N = 130) |
Laparoscopic group (N = 173) |
Open group (N = 188) |
|||
| Operation time (minute) | ||||||
| Overall | 113.4 ± 59.6 | 100.5 ± 59.4 | 0.079 | 114.6 ± 54.8 | 101.1 ± 51.1 | 0.017 |
| Conversion to open surgery | 3 (2.1) | 8 (4.6) | – | – | ||
| Tumor rupture during surgery | 0 (0) | 1 (0.8) | 0.473 | 0 (0) | 5 (2.7) | 0.062 |
| Hospital days (mean ± SD) | ||||||
| Overall | 6.7 ± 5.2 | 8.6 ± 5.7 | 0.006 | 6.6 ± 4.8 | 8.9 ± 7.0 | < 0.001 |
| Postoperative complications | ||||||
| Total number of cases with complication | 4 (2.8) | 6 (4.6) | 0.525 | 7 (4.0) | 19 (10.1) | 0.040 |
| Wound | 1 (0.7) | 3 (2.3) | 0.347 | 1 (0.6) | 7 (3.7) | 0.069 |
| Fluid collection | 1 (0.7) | 0 (0) | 0.343 | 0 (0) | 1 (0.5) | 0.337 |
| Intraabdominal bleeding | 0 (0) | 1 (0.8) | 0.473 | 3 (1.7) | 2 (1.1) | 0.674 |
| Luminal bleeding | 1 (0.7) | 1 (0.8) | 0.938 | 1 (0.6) | 0 (0) | 0.479 |
| Stenosis | 0 (0) | 0 (0) | – | 2 (1.2) | 1 (0.5) | 0.609 |
| Intestinal motility disorder | 0 (0) | 0 (0) | – | 0 (0) | 5 (2.7) | 0.062 |
| Leakage | 0 (0) | 0 (0) | – | 0 (0) | 1 (0.5) | 0.337 |
| Fistula | 0 (0) | 1 (0.8) | 0.473 | 0 (0) | 0 (0) | – |
| Pulmonary complications | 0 (0) | 1 (0.8) | 0.473 | 0 (0) | 3 (1.6) | 0.249 |
| Urinary complications | 1 (0.7) | 1 (0.8) | 0.938 | 0 (0) | 0 (0) | – |
| Clavien–Dindo classification | ||||||
| Grade I | 1 (0.7) | 3 (2.3) | 0.347 | 1 (0.6) | 1 (0.5) | 0.953 |
| Grade II | 2 (1.4) | 1 (0.8) | 0.627 | 1 (0.6) | 5 (2.7) | 0.217 |
| Grade IIIa | 1 (0.7) | 3 (2.3) | 0.347 | 5 (2.9) | 6 (3.2) | 0.868 |
| Grade IIIb | 0 (0) | 0 (0) | – | 0 (0) | 7 (3.7) | 0.015 |
| Grade IVa | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Grade IVb | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Grade V | 0 (0) | 1 (0.8) | 0.473 | 0 (0) | 1 (0.5) | 0.337 |
| Complication requiring major intervention (grade ≥ IIIa) | 1 (0.7) | 4 (3.1) | 0.192 | 5 (2.9) | 14 (7.4) | 0.061 |
| Mortality within 30 days | 0 (0) | 1 (0.8) | 0.473 | 0 (0) | 1 (0.5) | 0.337 |
SD standard deviation.
Oncologic outcomes
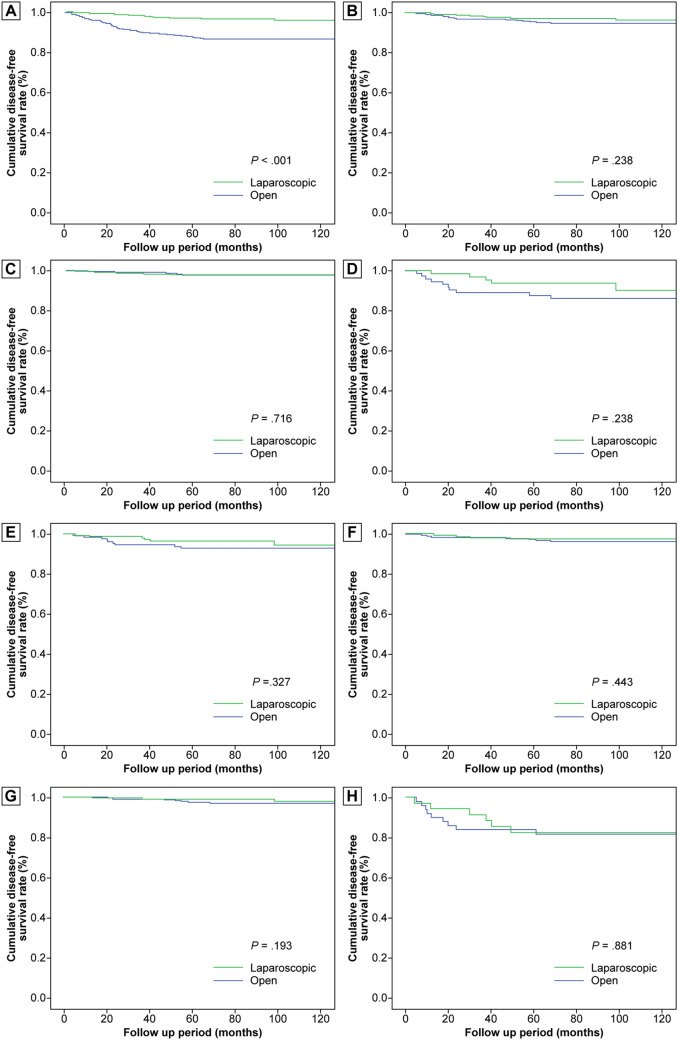
Before PSM, the open group had more patients with adjuvant chemotherapy, longer follow-up duration, recurrence, and death. The recurrence-free survival rates of the laparoscopic group were superior to those of the open groups (all P < 0.05). After PSM, the parameters related to oncologic outcomes were not different between the two groups. The recurrence-free survival rates were not different between the two groups, regardless of tumor size, locational preference, and NIH risk classification (all P > 0.05) (Table 5, Fig. 1).
Table 5.
Oncologic outcomes for laparoscopic versus open resection before and after propensity score matching.
| Variables | Before PSM | P value | After PSM | P value | ||
|---|---|---|---|---|---|---|
| Laparoscopic group (N = 373) |
Open group (N = 542) |
Laparoscopic group (N = 318) |
Open group (N = 318) |
|||
| Chemotherapy | ||||||
| Preoperative | 1 (0.3) | 6 (1.1) | 0.251 | 1 (0.3) | 2 (0.6) | 0.563 |
| Adjuvant | 7 (1.9) | 52 (9.6) | < 0.001 | 7 (2.2) | 7 (2.2) | 0.000 |
| Duration of follow-up | ||||||
| Mean | 93.6 | 103.5 | < 0.001 | 95.1 | 98.8 | 0.114 |
| Median (range) | 88.0 (11.0–164.7) | 106.4 (0.1–167.9) | 88.5 (25.2–164.7) | 100.7 (0.1–144.2) | ||
| Recurrence | ||||||
| Total number | 13 (3.5) | 72(13.3) | < 0.001 | 10 (3.1) | 16 (5.0) | 0.317 |
| Stomach | 5 (1.3) | 16 (3.0) | 0.121 | 5 (1.6) | 3 (0.9) | 0.725 |
| Small bowel | 0 (0) | 1 (0.2) | 0.407 | 0 (0) | 1 (0.3) | 0.317 |
| Liver | 2 (0.5) | 36 (6.6) | < 0.001 | 1 (0.3) | 7 (2.2) | 0.069 |
| Peritoneum | 5 (1.3) | 20 (3.7) | 0.038 | 4 (1.3) | 2 (0.6) | 0.686 |
| Bone | 0 (0) | 3 (0.6) | 0.275 | 0 (0) | 0 (0) | – |
| Wound | 0 (0) | 1 (0.3) | 0.408 | 0 (0) | 1 (0.3) | 0.317 |
| Lung | 0 (0) | 1 (0.2) | 0.407 | 0 (0) | 1 (0.3) | 0.317 |
| Axilla | 1 (0.3) | 3 (0.6) | 0.649 | 1 (0.3) | 2 (0.6) | 0.563 |
| Alive | ||||||
| Total number | 365 (97.9) | 499 (92.1) | < 0.001 | 311 (97.8) | 307 (96.5) | 0.474 |
| with recurrence free | 355 (95.2) | 448 (82.7) | < 0.001 | 303 (95.3) | 296 (93.1) | 0.173 |
| with recurrence | 10 (2.7) | 51 (9.4) | < 0.001 | 8 (2.5) | 11 (3.5) | 0.643 |
| Death | ||||||
| Total number | 8 (2.1) | 43 (7.9) | < 0.001 | 7 (2.2) | 11 (3.5) | 0.474 |
| Died due to GIST | 3 (0.8) | 21 (3.9) | 0.005 | 2 (0.6) | 5 (1.6) | 0.451 |
| Died of other causes | 5 (1.3) | 22 (4.1) | 0.017 | 5 (1.6) | 6 (1.9) | 0.761 |
GIST gastrointestinal stromal tumor, PSM propensity score matching.
Figure 1.
Comparison of disease-free survival between the laparoscopic and open groups. (a) Disease-free survival in all patients, before 1:1 propensity score matching. (b) Disease-free survival in all patients, after 1:1 propensity score matching. (c) Disease-free survival in patients with tumor ≤ 5 cm, after 1:1 propensity score matching. (d) Disease-free survival in patients with tumor > 5 cm, after 1:1 propensity score matching. (e) Disease-free survival in patients with tumor at favorable location, after 1:1 propensity score matching. (f) Disease-free survival in patients with tumor at unfavorable location, after 1:1 propensity score matching. (g) Disease-free survival in patients with very low to intermediate risk by National Institutes of Health classification, after 1:1 propensity score matching. (h) Disease-free survival in patients with high risk by National Institutes of Health classification, after 1:1 propensity score matching.
Multivariate Cox regression analysis revealed the following independent risk factors: R1 (vs. R0) resection (hazard ratio [HR] = 6.327, 95% confidence interval [CI] = 1.826–21.919, P = 0.004), tumor size > 5 cm (vs. ≤ 5 cm) (HR = 3.080, 95% CI = 1.368–6.934, P = 0.007), mitotic count > 5, ≤ 10 (vs. ≤ 5) (HR = 8.090, 95% CI = 2.651–24.685, P < 0.001), mitotic count > 10 (vs. ≤ 5) (HR = 32.666, 95% CI = 10.586–100.798, P < 0.001), and tumor rupture during surgery (HR = 63.479, 95% CI = 17.407–231.488, P < 0.001) (Table 6).
Table 6.
Univariate and multivariate Cox regression analysis for variables to predict the recurrence.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||||
|---|---|---|---|---|---|---|
| B | Hazard ratio (95% CI) | P value | B | Hazard ratio (95% CI) | P value | |
| Age, years per increase | 0.006 | 1.006 (0.972–1.041) | 0.740 | |||
| Male (vs. female) | 0.461 | 1.586 (0.728–3.453) | 0.245 | |||
| Body mass index (kg/m2, per increase) | − 0.069 | 0.934 (0.821–1.062) | 0.296 | |||
| Laparoscopy (vs. open) | − 0.472 | 0.624 (0.283–1.375) | 0.242 | |||
| Extent of resection | ||||||
| Gastrectomy versus wedge or enucleation | 1.401 | 4.059 (1.629–10.116) | 0.003 | |||
| Radicality, R1 (vs. R0 resection) | 2.146 | 8.550 (2.567–28.484) | < 0.001 | 1.845 | 6.327 (1.826–21.919) | 0.004 |
| Adjuvant therapy | 2.238 | 9.377 (3.226–27.259) | < 0.001 | |||
| Tumor size | ||||||
| > 5 cm (vs. ≤ 5 cm) | 1.626 | 5.082 (2.334–11.064) | < 0.001 | 1.125 | 3.080 (1.368–6.934) | 0.007 |
| Mitotic rate (per 50 HPF) | ||||||
| > 5, ≤ 10 (vs. ≤ 5) | 1.913 | 6.771 (2.348–19.524) | < 0.001 | 2.091 | 8.090 (2.651–24.685) | < 0.001 |
| > 10 (vs. ≤ 5) | 3.135 | 22.983 (8.617–61.298) | < 0.001 | 3.486 | 32.666 (10.586–100.798) | < 0.001 |
| NIH risk classification | ||||||
| High (vs. very low to intermediate) | 2.457 | 11.664 (5.290–25.721) | < 0.001 | |||
| Tumor rupture during surgery | 3.208 | 24.724 (8.467–72.190) | < 0.001 | 4.151 | 63.479 (17.407–231.488) | < 0.001 |
Covariates were age, sex, body mass index (kg/m2), laparoscopic approach, extent of resection, radicality, adjuvant therapy, tumor size, mitotic rate, NIH risk classification, tumor rupture during surgery.
CI confidence interval, HPF high power field, NIH National institutes of health.
Discussion
This study reviewed the multicentric database from 13 institutions in Korea and 2 in Japan, with more than 1019 patients who underwent laparoscopic and open resection for primary gastric GIST. Long-term surgical and oncologic outcomes for the treatment of primary gastric GIST have been reported based on this retrospective data19, and this is a subsequent, collateral study comparing the outcomes between laparoscopic and open surgery. Since resection of gastric GISTs is simple and does not require extensive lymphadenectomy with wide tumor-free margins20,21, the benefits of laparoscopic surgery for gastric GIST may not be as obvious as in gastric cancer surgery. Moreover, while Korean Laparo-endoscopic Gastrointestinal Surgery Study Group (KLASS) RCTs proved superiority of laparoscopic over open gastrectomy, no other RCTs have demonstrated the beneficial effect of laparoscopic resection for gastric GIST. Therefore, it seems impatient to generalize that a laparoscopic approach is beneficial for GIST surgery. Even, Xiong et al.18 enrolled more patients than current study, they could only compare 128 cases in each group for analysis after PSM. However, the current study remained the largest number of cases after PSM, with more than 300 patients in each group16,18. The PSM was carried out in the direction of preserving the sample size as many as possible, by including only four essential variables that may affect the preference of laparoscopic approach and the patient's clinical course. After eliminating the preferential selection bias with PSM, the clinicopathologic characteristics were balanced between the two groups. The distribution of the propensity score was more concentrated, and the absolute SMDs of matching covariates decreased near 0.1. All these efforts contributed to overcoming the bias of a retrospective approach and provided robustness to the current study.
We demonstrated that laparoscopic resection provided superior outcomes, such as shorter hospitalization days, lower rate of tumor rupture, and overall and wound complications than open surgery for the treatment of gastric GIST, even in the early era of the laparoscopic surgery. The patients with tumors larger than 5 cm and in unfavorable locations were also provided with advantages of laparoscopic surgery. Intestinal motility disorder and tumor rupture were not identified in laparoscopic surgery. While the beneficial effects of laparoscopic distal gastrectomy in the KLASS 01 and 02 trials were obtained through the surgeons’ strict quality control9,10, the results of the current study are more realistic, as they were based on the data generated by the surgeons’ unrefined experience for laparoscopic surgery. The superior surgical outcome of the laparoscopic approach can be explained by the following reasons. First, the highly magnified view through the laparoscopic camera might enable the surgeon to perform more meticulous dissection, which could avoid unexpected bleeding or tumor rupture9. Second, less manipulation of the bowel and smaller wound, might enable patients to have early ambulation and return to eating, with less pain and better peristalsis22,23. Third, although the annual experience of laparoscopic surgery differed from one person to another (0–100 cases) in the mid to late 1990s, all surgeons participating in current study had at least 5 years of experiences performing 50–150 cases of open gastrectomy per year prior to initiating laparoscopic gastric surgery. From the year of 2003, even surgeons without any experience in laparoscopic procedures began performing at least 30 cases of laparoscopic surgeries for gastric cancer or GIST every year. Besides, resection of gastric GISTs is simple and does not require extensive lymphadenectomy with wide tumor-free margins as in gastric cancer surgery. These various factors might have allowed to provide an opportunity for better realization of minimal invasiveness, which ultimately resulted in faster recovery and better postoperative outcomes, even in the early era of laparoscopic surgery without surgeon’s quality control.
Nevertheless, laparoscopic approaches have traditionally been recommended for patients with GISTs less than 5 cm, or in favorable locations, according to the NCCN guidelines24,25. Huang et al. reported that the unfavorable group had longer hospital stays and more postoperative complications than the favorable group26. We also revealed that tumor size larger (vs. less) than 5 cm and unfavorable (vs. favorable) location were independent risk factors for postoperative complications. For these reasons, it is meaningful to investigate whether laparoscopic surgery can lower the rate of surgical complications under these circumstances. Fortunately, successful laparoscopic resection for gastric GISTs larger than 5 cm has been steadily reported with the largest diameters up to 10.5 cm and 11.5 cm27,28. Indeed, the postoperative outcomes of laparoscopic surgery were reported to be comparable or even better with tumors larger than 5 cm29,30. Regarding the unfavorable locations, the incidence of grade III–IV postoperative complications from laparoscopic surgery was reported to be significantly less than that of open surgery (2.3% vs. 29.0%, P = 0.001)26. This study also demonstrated that laparoscopic resection provided shorter hospital stays and lower complication rates in patients with tumors larger than 5 cm and at unfavorable locations.
Despite the superior surgical outcome of laparoscopic surgery, surgeons face skepticism that laparoscopic resection also requires laparotomy at least as large as the size of the tumor to retrieve the specimen. In the open surgery, laparotomy larger than tumor size is often necessary to obtain a better view of the invisible side beyond tumor for safe resection. However, laparoscopic camera can reach and visualize the marginal area beyond the large tumor even in unfavorable locations. Laparoscopic surgery only requires a length of incision that fits the tumor size, with the aid of laparoscopic pouch bag. As the laparoscopic procedures become more minimally invasive, the wound length can be shortened to the minimum size, required only for specimen retrieval4,31. Although analysis of the incision length was not possible due to the retrospective nature of the current study, we expect that the shorter wound length would have contributed to the lower rate of wound complication in laparoscopic resection than in open surgery, as in previous studies32.
Of the 373 cases with laparoscopic resection, 14 cases (3.8%) required conversion to open surgery, lower than that in most reported series (7% [range 0–25])16,29. Our study showed that the conversion rate was not related to locational preference. Instead, the conversion rate was higher in patients with tumors larger than 5 cm (Supplementary Table 2). Considering that Kitano et al.33 first introduced the laparoscopic technique in the field of gastric cancer surgery in 1994, laparoscopic resection for gastric GIST would not have been actively performed in the early 2000s, which is similar to our results (Supplementary Fig. 2). Moreover, the gap in operation time between the laparoscopic and open groups narrowed and improved from 2006 (108.53 ± 63.43 min. vs. 97.62 ± 46.36 min, P = 0.177), compared to the year before 2006 (119.87 ± 48.68 min. vs. 101.94 ± 57.05 min., P = 0.001). Recent studies reported a faster operation time in laparoscopic resection than in open surgery for gastric GISTs larger than 5 cm29. With advances in laparoscopic techniques, the rate of conversion to open surgery may reduce further.
A superior RFS in laparoscopic resection than in open surgery, can be explained by the differences in the clinical and pathological characteristics, which exert a great impact on the oncologic outcome of GIST34. Following our elimination of confounding variables using 1:1 PSM, we showed no significant difference in the recurrence-free survival between the laparoscopic and open groups, even in patients with tumors larger than 5 cm, at unfavorable locations, and in high-risk groups. Several studies including our previous research reported that male sex and non-gastric location were also risk factors for recurrence after resection of gastric GIST19,20,35. In the current study, multivariate Cox regression analysis revealed that tumor size > 5 (vs. ≤ 5) cm, higher mitotic count, R1 (vs. R0) resection, and tumor rupture during surgery were independent risk factors for the recurrence of gastric GIST, after eliminating confounding effects by PSM. Laparoscopic or open approaches were not associated with recurrence. The patients with laparoscopic resection and open surgery had similar recurrence and survival rates, by balancing the baseline characteristics of patients after PSM.
This study has some limitations. First, the patients were enrolled from the year 2000, when surgical methods, laparoscopic indications, and pre and postoperative treatment guidelines for gastric GIST were not standardized. Since the worldwide incidence of gastric GIST is rare, it took about 8 years to recruit more than 1000 patients. Additionally, imatinib mesylate (Gleevec) was covered by the Korean health insurance system from June 2001. Therefore, the trends in treatment for gastric GIST may have changed during our study period. Nevertheless, we tried to further highlight the advantages of laparoscopic surgery by demonstrating that laparoscopic approach was superior to open surgery in the treatment of gastric GISTs even in the early 2000s, when laparoscopic techniques were not fully advanced. Second, since we were unable to review the detailed pathologic information of patients prior to the introduction of electrical medical records, the staining information for CD117 and CD34 was not available in some patients; we only included patients with clear medical records who were diagnosed with gastric GIST after surgical resection. In each institution, the diagnosis of gastric GIST was confirmed by reviewing the patient's pathologic findings, which included both typical morphological features and information from IHC staining. We inevitably included patients without information on IHC staining for CD117 and CD34 to preserve as many cases as possible. All patients negative for CD117 were positive for CD34, and the remaining patients with available data on IHC also showed a similar rate of distribution of C-kit positivity as in previous studies26,36. Third, detailed information on accidental intraoperative injury to adjacent organs could not be analyzed for similar reasons. Fourth, although the concept of favorable and unfavorable locations had not been established during the period 2000–2007, only patients with clear information about both longitudinal and circumferential locations on the endoscopic and surgical records could be included in this study. Therefore, we were able to classify patients with favorable and unfavorable locations by retrospective reviewing the longitudinal and circumferential locations24,26. Fifth, although PSM is a good method to reduce selection bias, it cannot eliminate this bias completely as RCT would be able to. We excluded only the cases with distant metastasis or invasion to adjacent organs, and then matched the variables determining the preferences for the laparoscopic approach, to preserve as many cases as possible. Therefore, similar recurrence and survival rates between laparoscopic resection and open surgery were obtained by balancing baseline characteristics, not due to the washing out effect of PSM. Finally, PSM analysis inevitably excluded the most of cases requiring combined resection and patients with tumors larger than 15 cm, the superiority of laparoscopic surgery could not be analyzed in these population (Table 1). Future studies are expected to provide answer for these issues.
Conclusions
Even in the early era of laparoscopic surgery with unrefined experiences, laparoscopic resection could provide a lower rate of complications and shorter hospitalization for patients with gastric GIST than open surgery, without compromising oncological safety. Since its first introduction in the early 1990s, laparoscopic gastrectomy has technologically revolutionized the treatment of gastric cancer and almost 30 years have passed. Considering the development of laparoscopic technology over decades, and the current study having large number of cases with more than 300 in each group after 1:1 matching, wider adoption of laparoscopic resection is expected in clinical practice, even in patients with tumor sizes larger than 5 cm and in unfavorable locations.
Materials and methods
Study design and cohorts
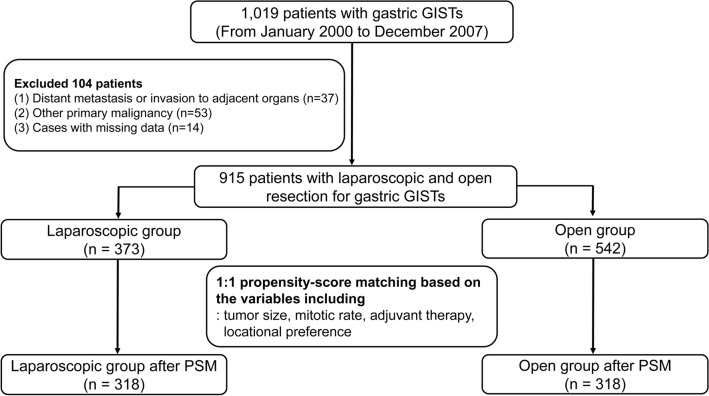
From 13 institutions in Korea and 2 institutions in Japan, we retrospectively reviewed the prospectively collected database of 1019 patients who were diagnosed with gastric GIST after surgical resection between January 2000 and December 2007. In each institution, the diagnosis of gastric GIST was confirmed by reviewing the patient's pathologic findings, which included typical morphological features and staining for immunohistochemistry (IHC). The exclusion criteria were as follows: (1) distant metastasis or invasion of the adjacent organs; (2) concomitant other primary malignancy; and (3) cases with missing data (Fig. 2).
Figure 2.
The flow chart for the patient selection model.
The primary outcomes were surgical complications. Secondary outcomes included disease-free survival, rate of recurrence, and survival. Patients were divided into two groups: those who underwent laparoscopic gastric resection (laparoscopic group) and those who underwent open surgery (open group). The prospectively collected clinicopathologic data and operative parameters from each institution were retrospectively reviewed. Mitotic rate was defined as the number of mitoses per 50 high-power fields. Risk stratification was performed according to the modified National Institutes of Health (NIH) risk classification. Although, the concept of favorable and unfavorable locations had not been established during the period 2000–2007, we were able to classify patients according to unfavorable (the gastroesophageal junction, the lesser curvature, the posterior wall, the antrum, and the pylorus) and favorable locations (all locations except the unfavorable locations, which included the greater curvature and anterior wall of the stomach), by retrospectively reviewing endoscopic and operative records with longitudinal and circumferential locations24,26. Therefore, only patients with clear information about both longitudinal and circumferential locations on the endoscopic and surgical records were included in this study. Patients were followed up every 6 months or annually, and the follow-up information included adjuvant treatment, survival or death, and recurrences.
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB number: H2006-176-1135) and each participating institution, conducted in accordance with the 1964 Declaration of Helsinki and newer amendments.
Propensity score matching
Each case from the laparoscopic group was 1:1 propensity score matched to control cases in the open group. The matching variables included tumor size, mitotic rate, locational preference (favorable, or unfavorable), and adjuvant chemotherapy. The propensity score of each patient was estimated by logistic regression (SPSS version 25; IBM Corp., Armonk, NY) and matched nearest-neighbor value within a caliper 0.02 times the standard deviation of the estimated score. After 1:1 PSM, the balance of covariates between the laparoscopic and open groups was measured by calculating the standardized mean difference. A standardized mean difference (SMD) of less than 0.1 was considered to achieve balance and SMD ranging from 0.1 to 0.25 were considered as acceptable disparity between groups37.
Surgical and oncologic outcome
The operative outcomes including surgical complications, operation time, tumor rupture during surgery and hospitalization days were compared between the laparoscopic and open groups, both before and after 1:1 PSM. After performing multivariate logistic regression analysis to reveal the independent factors for surgical complications, we separately collected the patients with such risk factors to determine the presence of complications. We then compared the operative outcomes including surgical complications between the laparoscopic and open groups, for these subgroups. Complication categories were based on the categories used in previous phase III multi-center randomized controlled trials evaluating the complications of laparoscopic gastrectomy. Intestinal motility disorder refers to the peristaltic problem in intestinal tract including mechanical or paralytic ileus, intestinal obstructions38,39.
Survival periods were defined as the time from operation to death associated with the GIST or not, which were censored at the final follow-up visit. The recurrence-free survival was calculated as the time from operation to the event of recurrent disease. Recurrence-free survival was compared between the laparoscopic and open groups, either before or after 1:1 PSM. Subgroup analysis for recurrence-free survival was performed according to tumor size, locational preference, and NIH risk classification. Multivariate analysis with Cox proportional hazard regression was used to determine the independent prognostic factors to predict the tumor recurrence.
Statistical analysis
The chi-square test was used for categorical variables, and Student’ s t-test was used to compare continuous variables between the two groups. All tests were two-sided, and a P value of < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 25. The recurrence-free survival was assessed by Kaplan–Meier analysis and the log-rank test.
Compliance with ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB number: H2006-176-1135) and each participating institution. The requirement for informed consent was waived by the IRB of Seoul National University Hospital (IRB number: H2006-176-1135) and each participating institution, because of the retrospective nature of this study.
Supplementary Information
Author contributions
Conception and design: S-H.P., H-J.L. Analysis and interpretation of the data: S-H.P., H-J.L., M-C.K., J-H.Y., T-S.S., W-J.H., S-W.R., Y.K., Y-W.K., S-U.H., H-H.K., D-J.P., W.K., S-I.L., H.C., G-S.C., J-J.K., K-H.K., M-W.Y., H-K.Y. Drafting of the article: S-H.P. Critical revision of the article for important intellectual content: S-H.P., H-J.L. Final approval of the article: S-H.P., H-J.L., M-C.K., J-H.Y., T-S.S., W-J.H., S-W.R., Y.K., Y-W.K., S-U.H., H-H.K., D-J.P., W.K., S-I.L., H.C., G-S.C., J-J.K., K-H.K., M-W.Y., H-K.Y.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-05044-x.
References
- 1.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet (Lond. Engl.) 2013;382:973–983. doi: 10.1016/s0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 2.Dematteo RP, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 3.Joensuu H, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA. 2012;307:1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 4.Yang HK, et al. Clinicopathologic characteristics of gastrointestinal stromal tumor of the stomach. Hepatogastroenterology. 2008;55:1925–1930. [PubMed] [Google Scholar]
- 5.Bischof DA, et al. Open versus minimally invasive resection of gastric GIST: A multi-institutional analysis of short- and long-term outcomes. Ann. Surg. Oncol. 2014;21:2941–2948. doi: 10.1245/s10434-014-3733-3. [DOI] [PubMed] [Google Scholar]
- 6.De Vogelaere K, Hoorens A, Haentjens P, Delvaux G. Laparoscopic versus open resection of gastrointestinal stromal tumors of the stomach. Surg. Endosc. 2013;27:1546–1554. doi: 10.1007/s00464-012-2622-8. [DOI] [PubMed] [Google Scholar]
- 7.Blackstein ME, et al. Gastrointestinal stromal tumours: Consensus statement on diagnosis and treatment. J. Can. Gastroenterol. 2006;20:157–163. doi: 10.1155/2006/434761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson B, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—A population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT) Ann. Surg. 2019;270:983–991. doi: 10.1097/sla.0000000000003217. [DOI] [PubMed] [Google Scholar]
- 10.Kim HH, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: The KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–513. doi: 10.1001/jamaoncol.2018.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen QL, et al. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: An updated systematic review and meta-analysis. World J. Surg. Oncol. 2014;12:206. doi: 10.1186/1477-7819-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong H, et al. Laparoscopic surgery versus open resection in patients with gastrointestinal stromal tumors: An updated systematic review and meta-analysis. Am. J. Surg. 2017;214:538–546. doi: 10.1016/j.amjsurg.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, et al. Meta-analysis on the efficacy and safety of laparoscopic surgery for large gastric gastrointestinal stromal tumors. Am. Surg. 2021;87:450–457. doi: 10.1177/0003134820951482. [DOI] [PubMed] [Google Scholar]
- 14.Zheng L, Ding W, Zhou D, Lu L, Yao L. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: A meta-analysis. Am. Surg. 2014;80:48–56. doi: 10.1177/000313481408000124. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao CY, Yang CY, Lai IR, Chen CN, Lin MT. Laparoscopic resection for large gastric gastrointestinal stromal tumor (GIST): Intermediate follow-up results. Surg. Endosc. 2015;29:868–873. doi: 10.1007/s00464-014-3742-0. [DOI] [PubMed] [Google Scholar]
- 16.Piessen G, et al. Laparoscopic versus open surgery for gastric gastrointestinal stromal tumors: What is the impact on postoperative outcome and oncologic results? Ann. Surg. 2015;262:831–839. doi: 10.1097/sla.0000000000001488. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, et al. Retrospective study of laparoscopic versus open gastric resection for gastric gastrointestinal stromal tumors based on the propensity score matching method. Surg. Endosc. 2017;31:374–381. doi: 10.1007/s00464-016-4983-x. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Z, et al. Laparoscopic versus open surgery for gastric gastrointestinal stromal tumors: A propensity score matching analysis. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment Tract. 2020;24:1785–1794. doi: 10.1007/s11605-019-04318-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim MC, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: Multicenter observational study from Korea and Japan. Medicine. 2015;94:e1526. doi: 10.1097/md.0000000000001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMatteo RP, et al. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann. Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silberhumer GR, et al. Surgery for gastrointestinal stromal tumors of the stomach. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2009;13:1213–1219. doi: 10.1007/s11605-009-0872-0. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, et al. Long-term quality of life after laparoscopy-assisted distal gastrectomy for gastric cancer. Surg. Endosc. 2007;21:2150–2153. doi: 10.1007/s00464-007-9322-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim YW, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: Results of a prospective randomized clinical trial. Ann. Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 24.von Mehren M, et al. Gastrointestinal stromal tumors, version 2.2014. J. Natl. Compr. Cancer Netw. JNCCN. 2014;12:853–862. doi: 10.6004/jnccn.2014.0080. [DOI] [PubMed] [Google Scholar]
- 25.von Mehren M, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN. 2018;16:536–563. doi: 10.6004/jnccn.2018.0025. [DOI] [PubMed] [Google Scholar]
- 26.Huang CM, et al. Can laparoscopic surgery be applied in gastric gastrointestinal stromal tumors located in unfavorable sites? A study based on the NCCN guidelines. Medicine. 2017;96:e6535. doi: 10.1097/md.0000000000006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huguet KL, et al. Laparoscopic gastric gastrointestinal stromal tumor resection: the mayo clinic experience. Arch. Surg. (Chicago, Ill : 1960) 2008;143:587–590. doi: 10.1001/archsurg.143.6.587. [DOI] [PubMed] [Google Scholar]
- 28.Ma JJ, et al. Laparoscopic gastric resection approaches for gastrointestinal stromal tumors of stomach. Surg. Laparosc. Endosc. percutaneous Tech. 2011;21:101–105. doi: 10.1097/SLE.0b013e3182139546. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, et al. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): A size-matched comparison. Surg. Endosc. 2014;28:2577–2583. doi: 10.1007/s00464-014-3506-x. [DOI] [PubMed] [Google Scholar]
- 30.Kim KH, et al. Long term survival results for gastric GIST: Is laparoscopic surgery for large gastric GIST feasible? World J. Surg. Oncol. 2012;10:230. doi: 10.1186/1477-7819-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH, Jun KH, Chin HM. Short-term surgical outcomes of laparoscopy-assisted versus totally laparoscopic Billroth-II gastrectomy for gastric cancer: A matched-cohort study. BMC Surg. 2017;17:45. doi: 10.1186/s12893-017-0245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi JL, Xu M, Zhang MR, Li Y, Zhou ZG. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors (GISTs): A Size-location-matched case-control study. World J. Surg. 2017;41:2345–2352. doi: 10.1007/s00268-017-4005-8. [DOI] [PubMed] [Google Scholar]
- 33.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg. Laparosc. Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 34.von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:136–143. doi: 10.1200/jco.2017.74.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joensuu H, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/s1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 36.Khoo CY, et al. Laparoscopic wedge resection for suspected large (≥5 cm) gastric gastrointestinal stromal tumors. Surg. Endosc. 2017;31:2271–2279. doi: 10.1007/s00464-016-5229-7. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Little TD. A practical guide to propensity score analysis for applied clinical research. Behav. Res. Ther. 2017;98:76–90. doi: 10.1016/j.brat.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Kim W, et al. Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: Short-term outcomes from a multicenter randomized controlled trial (KLASS-01) Ann. Surg. 2016;263:28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, et al. Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT) Ann. Surg. 2019;270:983–991. doi: 10.1097/SLA.0000000000003217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.