Abstract
Climate change is one of the main drivers of species extinction in the twentyfirst-century. Here, we (1) quantify potential changes in species' bioclimatic area of habitat (BAH) of 135 native potential agroforestry species from the Brazilian flora, using two different climate change scenarios (SSP2-4.5 and SSP5-8.5) and dispersal scenarios, where species have no ability to disperse and reach new areas (non-dispersal) and where species can migrate within the estimated BAH (full dispersal) for 2041–2060 and 2061–2080. We then (2) assess the preliminary conservation status of each species based on IUCN criteria. Current and future potential habitats for species were predicted using MaxEnt, a machine-learning algorithm used to estimate species' probability distribution. Future climate is predicted to trigger a mean decline in BAH between 38.5–56.3% under the non-dispersal scenario and between 22.3–41.9% under the full dispersal scenario for 135 native potential agroforestry species. Additionally, we found that only 4.3% of the studied species could be threatened under the IUCN Red List criteria B1 and B2. However, when considering the predicted quantitative habitat loss due to climate change (A3c criterion) the percentages increased between 68.8–84.4% under the non-dispersal scenario and between 40.7–64.4% under the full dispersal scenario. To lessen such threats, we argue that encouraging the use of these species in rural and peri-urban agroecosystems are promising, complementary strategies for their long-term conservation.
Subject terms: Ecology, Environmental sciences
Introduction
Hundreds of species are unquestionably promising for future human welfare1, yet a large number of these species may potentially be threatened by impacts of climate change in the coming decades. Climate change is one of the important drivers affecting species survival, causing global biodiversity loss2–5. Climate change affects species in different ways, such as altering the suitability of current habitat of species, resulting in accelerated extinction rates2,6,7. The United Nations Intergovernmental Panel on Climate Change (IPCC) estimates that if Earth's average temperature rises between 2 and 3 °C, about 20 to 30% of all terrestrial biodiversity will be at high risk of extinction by the end of the century8. In the last century, land and ocean temperature showed a warming of approximately 1.0 °C 3, which may increase another 1.4 °C to 5.0 °C by 2100, if we do not reduce greenhouse gas emissions9. The latest IPCC special report reinforces the importance of keeping the temperature increase below 1.5 °C, in order to keep negative effects on natural resources, ecosystem functioning, food security and biodiversity to a minimum10. Considering the potential climate change scenarios with additional temperature increases, both widespread species and narrow-ranged endemic species will likely suffer irreparable consequences with regard to their distribution range and abundance5.
Brazil is the world’s most biodiversity-rich country, with 33,161 known species of vascular plants11, and harboring some of the largest remnants of tropical old-growth forests12. Despite the large number of native plant species, many of which with major untapped socioeconomic potential, the Brazilian agricultural industry exploits only a few, and largely, exotic crops13. Agroforestry species are those that have the function of simultaneously benefiting people's livelihoods and the ecological systems while showing great potential for multi-species intercropping14. These species are often characterized by their multiple uses, different harvest seasons and potential for market adoption15–18. Several useful native Brazilian plant species are potentially suitable for pasture production, silviculture, orchards, bioenergy, green manuring, as well as in integrated, biodiverse, multifunctional agroforestry13,19. Besides enhancing biodiversity and promoting the socio-economic development of local communities20–23, agroforestry systems can play a pivotal role in mitigating the effects of climate change: they sequester more atmospheric carbon than conventional farming24–26. Although agroforestry practices can ameliorate the impacts of climate change in Brazil, these agroecological systems are also vulnerable27. Considering the rapidly increasing human demand for plant products, native plant species from megadiverse countries undoubtedly represent a reservoir of genetic diversity, providing beneficial alleles for crop improvement and higher adaptive potential to face global changes13,28. Changes in land use may not be the main driver impacting these species as they are widely distributed among the neotropics and are easily found along streets and city squares across Brazil, some almost ruderal, regenerating in open areas of cities13,29. The future impact of climate change on species distributions should be taken into account for setting conservation priorities, as well as for promoting species conservation through their sustainable use30.
Spatial and temporal changes of species’ suitable habitat can be predicted with ecological niche models (ENMs), the most widely used tool to assess species vulnerability to changing climatic conditions36–38. Besides that, modeled habitats based on climatic variables allow us to consider the impacts of climate change on the species’ area of habitat, which is the habitat available to a particular species within its range39. Here, to consider those impacts, we modeled the species' bioclimatic area of habitat (BAH). Species dispersal is pivotal to the survival of species in the face of rapid climate change40. Thus, to better understand species responses, this central process that determines the potential spread of a population needs to be addressed in conservation assessments7. Although several studies have sought to better understand the impact of climate change on the distribution of plant species with narrow-ranged distribution or threatened with extinction, we note that no study has yet focused on species of agroforestry interest in Brazil, which generally have widespread distribution. These species are promising for conservation-by-use, an approach used by people communities for millennia in different ecosystems in Brazil41,42.
Here, we apply an ENM approach to (1) quantify potential changes in BAH of 135 native potential agroforestry species from the Brazilian flora using two climate change scenarios (SSP2-4.5 and SSP5-8.5) and two dispersal (non-dispersal and full dispersal) scenarios for 2041–2060 and 2061–2080. We then (2) assess the preliminary conservation status of each species using IUCN Red List of Threatened Species criteria43.
Results
Model performance
We evaluated the model performance through a null-model for significance testing of presence-only ENMs and retained 135 significant ENMs, corresponding to 97.1% of all species. Overall, final models showed high accuracy, indicated by AUC values ranging from 0.850 ± 0.139 to 0.985 ± 0.058, demonstrating a clear ability to distinguish suitable from unsuitable habitats. We detected no spurious correlations through inspection of species-response-curves.
Impacts on species BAH
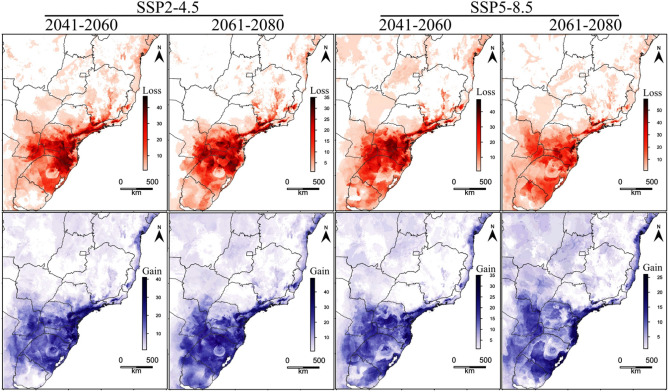
Under the non-dispersal scenario, the average decline in BAH was predicted to be between 38.5% (SSP2-4.5) and 43.5% SSP5-8.5 by 2041–2060 and between 43.4% (SSP2-4.5) and 56.3% (SSP5-8.5) by 2061–2080. For the full dispersal scenario, however, the average decline of BAH was predicted to be between 22.3% (SSP2-4.5) and 29.7% (SSP5-8.5) by 2041–2060 and between 27.4% (SSP2-4.5) and 41.9% (SSP5-8.5) by 2061–2080 (Supplementary Table S1). Although the majority of species predicted BAH losses over different scenarios, some are predicted to experience BAH gains (Fig. 1., Table 1, Supplementary Table S1). We noticed that some species were predicted to lose their entire BAH by 2041–2060 and 2061–2080, such as the medicinal species Cunila microcephala in the non-dispersal scenario and the forage species Ornithopus micranthus, in all scenarios, except for the SSP5-8.5 (2061–2080) in the non-dispersal scenario and SSP2-4.5 and SSP5-8.5 (2061–2080) in the full dispersal scenario. Species with the greatest increase in BAH were the forage species Indigofera sabulicola with an increase of 388%, the ornamental species Epidendrum fulgens (263%), and the forage species Echinochloa polystachya (259%) in the SSP5-8.5 for 2061–2080 considering the full dispersal scenario (Table 1, Supplementary Table S1).
Figure 1.
Loss (red) and gain (blue) of BAH for 135 native potential agroforestry plant species in Brazil, obtained by stacking ENM binary predictions, based on different climate scenarios and years. Legend indicates the number of species. Maps created with custom R script. Version R 4.1.1 (https://www.R-project.org/). Base map source (Brazilian states shapefile) obtained from the Brazilian Institute of Geography and Statistics (https://www.ibge.gov.br/).
Table 1.
Future range changes for 135 native potential agroforestry plant species in Brazil under two dispersal scenarios.
| Aromatic species | NON-DISPERSAL | FULL DISPERSAL | ||||||
|---|---|---|---|---|---|---|---|---|
| 2041–2060 | 2061–2080 | 2041–2060 | 2061–2080 | |||||
| SSP2-4.5 | SSP5-8.5 | SSP2-4.5 | SSP5-8.5 | SSP2-4.5 | SSP5-8.5 | SSP2-4.5 | SSP5-8.5 | |
| Capsicum flexuosum | − 36.1 | − 47.7 | − 39.2 | − 60.1 | − 33.7 | − 35.8 | − 38.0 | − 57.1 |
| Pimenta pseudocaryophyllus | − 45.3 | − 51.7 | − 52.9 | − 77.6 | − 38.9 | − 47.6 | − 44.8 | − 76.5 |
| Schinus terebinthifolia | − 28.6 | − 33.6 | − 30.6 | − 47.0 | − 12.7 | − 14.7 | − 16.6 | − 31.3 |
| Tropaeolum pentaphyllum | − 30.9 | − 29.9 | − 34.1 | − 39.2 | − 2.9 | 3.1 | 0.1 | − 14.1 |
| Fibrous species | ||||||||
| Coleataenia prionitis | − 17.9 | − 38.5 | − 33.6 | − 52.1 | 50.6 | 26.1 | 54.4 | 32.7 |
| Geonoma gamiova | − 6.1 | − 6.6 | − 17.5 | − 35.2 | 56.1 | 86.1 | 25.0 | 28.8 |
| Gynerium sagittatum | − 43.5 | − 47.8 | − 48.1 | − 58.8 | − 32.0 | − 34.2 | − 34.8 | − 42.0 |
| Philodendron corcovadense | − 35.4 | − 38.1 | − 40.9 | − 45.1 | − 7.6 | 1.1 | − 12.5 | 14.5 |
| Schoenoplectus californicus | − 27.5 | − 32.8 | − 32.4 | − 51.0 | − 22.8 | − 28.4 | − 25.9 | − 47.9 |
| Food species | ||||||||
| Acca sellowiana | − 22.6 | − 28.7 | − 34.6 | − 39.4 | − 7.1 | − 20.1 | − 10.3 | − 13.4 |
| Annona crassiflora | − 68.2 | − 82.9 | − 76.3 | − 91.8 | − 61.0 | − 77.9 | − 71.5 | − 89.1 |
| Araucaria angustifolia | − 46.2 | − 52.9 | − 52.7 | − 66.6 | − 46.1 | − 52.9 | − 52.6 | − 66.5 |
| Butia eriospatha | − 67.7 | − 77.3 | − 67.8 | − 87.4 | − 45.2 | − 50.4 | − 52.7 | − 64.3 |
| Campomanesia xanthocarpa | − 38.9 | − 32.1 | − 36.6 | − 47.8 | − 35.5 | − 25.8 | − 35.2 | − 43.3 |
| Eugenia involucrata | − 42.4 | − 44.5 | − 45.6 | − 61.8 | − 39.9 | − 40.4 | − 43.6 | − 59.7 |
| Eugenia pyriformis | − 34.2 | − 60.1 | − 53.4 | − 60.9 | − 17.6 | − 38.9 | − 42.1 | − 48.8 |
| Eugenia uniflora | − 39.4 | − 43.8 | − 42.8 | − 56.2 | − 9.6 | − 8.0 | − 16.6 | − 33.8 |
| Euterpe edulis | − 50.1 | − 52.7 | − 58.0 | − 69.7 | − 19.9 | − 19.9 | − 20.3 | − 33.7 |
| Opuntia elata | − 12.0 | − 58.6 | − 57.8 | − 47.9 | 45.8 | − 28.7 | − 26.2 | 12.3 |
| Passiflora actinia | − 46.5 | − 44.3 | − 41.6 | − 49.4 | − 37.3 | − 21.6 | − 33.6 | − 26.2 |
| Physalis pubescens | − 43.2 | − 48.1 | − 47.1 | − 61.9 | − 41.4 | − 46.0 | − 45.5 | − 59.2 |
| Plinia peruviana | − 79.6 | − 87.3 | − 76.0 | − 88.3 | − 56.9 | − 68.0 | − 60.2 | − 64.2 |
| Psidium cattleianum | − 26.5 | − 31.9 | − 37.4 | − 46.1 | − 10.2 | − 20.7 | − 26.6 | − 34.0 |
| Vasconcellea quercifolia | − 42.1 | − 51.5 | − 52.8 | − 50.3 | − 26.3 | − 43.0 | − 44.5 | − 34.8 |
| Forage species (Fabaceae) | ||||||||
| Adesmia bicolor | − 64.5 | − 69.0 | − 74.1 | − 80.1 | − 48.2 | − 58.5 | − 48.6 | − 76.0 |
| Adesmia latifolia | − 68.3 | − 47.2 | − 51.8 | − 52.9 | 40.8 | 39.3 | 13.8 | 23.5 |
| Adesmia tristis | − 94.4 | − 97.0 | − 98.0 | − 99.2 | − 94.4 | − 96.9 | − 98.0 | − 99.2 |
| Desmodium adscendens | − 15.7 | − 14.4 | − 18.5 | − 16.3 | 76.6 | 122.9 | 91.9 | 190.9 |
| Desmodium barbatum | − 17.0 | − 17.5 | − 19.8 | − 24.1 | 25.0 | 29.0 | 30.8 | 29.0 |
| Desmodium incanum | − 27.7 | − 29.9 | − 31.9 | − 41.9 | − 21.5 | − 24.5 | − 27.6 | − 37.7 |
| Desmodium subsericeum | − 59.1 | − 70.8 | − 65.1 | − 77.5 | − 44.4 | − 63.7 | − 55.7 | − 68.7 |
| Indigofera sabulicola | 0.3 | 0.3 | 0.5 | 0.0 | 183.7 | 218.4 | 191.0 | 387.7 |
| Leptospron adenanthum | − 10.5 | − 11.8 | − 12.6 | − 10.3 | 62.7 | 79.6 | 74.6 | 119.2 |
| Macroptilium psammodes | − 20.9 | − 30.3 | − 32.3 | − 12.9 | 115.3 | 123.1 | 115.5 | 227.9 |
| Ornithopus micranthus | − 100.0 | − 100.0 | − 100.0 | − 95.1 | − 100.0 | − 100.0 | − 95.1 | − 95.1 |
| Stylosanthes leiocarpa | − 37.6 | − 36.2 | − 37.2 | − 30.7 | − 21.8 | − 2.5 | − 14.8 | 19.4 |
| Trifolium polymorphum | − 47.1 | − 48.8 | − 67.6 | − 81.8 | 2.6 | 2.3 | − 5.0 | − 48.1 |
| Trifolium riograndense | − 91.1 | − 75.5 | − 95.3 | − 86.4 | − 91.1 | − 75.2 | − 95.3 | − 86.4 |
| Vigna luteola | − 16.9 | − 23.1 | − 21.7 | − 26.2 | 0.5 | − 4.7 | − 5.8 | − 11.8 |
| Forage species (Poaceae) | ||||||||
| Axonopus compressus | − 36.4 | − 34.8 | − 37.5 | − 55.2 | − 23.8 | − 19.7 | − 22.6 | − 42.0 |
| Axonopus fissifolius | − 24.6 | − 26.5 | − 28.0 | − 34.0 | 98.5 | 143.3 | 121.8 | 182.7 |
| Axonopus obtusifolius | − 53.4 | − 57.9 | − 59.1 | − 76.0 | 0.2 | − 3.6 | − 7.1 | − 34.3 |
| Bothriochloa laguroides | − 54.8 | − 50.9 | − 60.6 | − 77.3 | − 38.6 | − 23.8 | − 37.6 | − 65.5 |
| Bromus auleticus | − 81.0 | − 82.3 | − 69.7 | − 88.5 | − 79.2 | − 80.3 | − 52.3 | − 83.6 |
| Bromus catharticus | − 35.8 | − 44.3 | − 40.7 | − 49.6 | − 33.2 | − 41.5 | − 36.2 | − 45.8 |
| Dichanthelium sabulorum | − 35.4 | − 38.2 | − 45.1 | − 62.9 | − 34.4 | − 33.9 | − 44.0 | − 60.3 |
| Echinochloa polystachya | − 7.6 | − 8.3 | − 9.8 | − 10.8 | 174.3 | 210.0 | 187.5 | 259.4 |
| Hemarthria altissima | − 11.4 | − 7.0 | − 4.6 | − 8.4 | 60.2 | 47.2 | 73.2 | 116.0 |
| Ischaemum minus | − 29.9 | − 40.9 | − 47.1 | − 6.0 | 42.2 | 36.8 | 22.6 | 141.5 |
| Mnesithea selloana | − 41.1 | − 49.9 | − 64.4 | − 72.4 | 36.3 | 28.6 | 30.0 | 23.3 |
| Nassella neesiana | − 30.7 | − 31.9 | − 34.4 | − 46.3 | − 26.3 | − 29.4 | − 21.2 | − 45.4 |
| Paspalum almum | − 2.0 | − 18.8 | − 14.4 | − 16.9 | 93.1 | 48.4 | 54.3 | 63.5 |
| Paspalum denticulatum | − 22.9 | − 31.1 | − 31.2 | − 43.5 | − 1.8 | 0.6 | 7.8 | − 17.9 |
| Paspalum dilatatum | − 26.1 | − 26.3 | − 27.8 | − 45.8 | − 25.6 | − 26.0 | − 27.2 | − 45.7 |
| Paspalum glaucescens | − 59.0 | − 42.0 | − 55.2 | − 61.0 | − 36.7 | 5.7 | − 37.8 | − 41.9 |
| Paspalum guenoarum | − 24.1 | − 28.3 | − 31.4 | − 42.3 | − 11.9 | − 14.6 | − 17.8 | − 28.1 |
| Paspalum jesuiticum | − 69.4 | − 74.1 | − 81.2 | − 95.9 | − 69.4 | − 74.1 | − 81.2 | − 95.9 |
| Paspalum lepton | − 5.6 | − 6.6 | − 12.3 | − 5.4 | 117.6 | 111.5 | 137.9 | 127.1 |
| Paspalum modestum | 0.3 | − 4.1 | − 1.6 | − 4.2 | 110.6 | 99.8 | 102.6 | 143.0 |
| Paspalum notatum | − 26.0 | − 27.1 | − 28.0 | − 42.0 | − 25.2 | − 26.0 | − 26.9 | − 40.6 |
| Paspalum pumilum | − 41.8 | − 39.3 | − 39.7 | − 50.2 | − 41.0 | − 34.7 | − 38.5 | − 47.9 |
| Paspalum regnellii | − 16.8 | − 37.4 | − 29.0 | − 40.4 | 40.4 | 17.0 | 7.5 | 20.1 |
| Poa lanigera | − 30.2 | − 28.6 | − 30.3 | − 46.7 | − 3.2 | − 9.1 | 4.1 | − 25.3 |
| Schizachyrium tenerum | − 49.8 | − 51.2 | − 53.4 | − 68.9 | − 46.3 | − 46.2 | − 50.0 | − 64.3 |
| Medicinal species | ||||||||
| Achyrocline satureioides | − 45.7 | − 48.0 | − 47.8 | − 59.1 | − 45.0 | − 47.0 | − 47.3 | − 57.4 |
| Baccharis articulata | − 48.8 | − 53.2 | − 50.9 | − 58.7 | − 44.2 | − 43.7 | − 45.1 | − 53.3 |
| Baccharis crispa | − 39.2 | − 45.4 | − 44.7 | − 57.8 | − 38.2 | − 42.7 | − 43.1 | − 56.6 |
| Baccharis dracunculifolia | − 42.9 | − 52.8 | − 51.6 | − 64.5 | − 37.4 | − 44.7 | − 45.6 | − 58.4 |
| Bauhinia forficata | − 42.5 | − 44.9 | − 43.4 | − 55.3 | − 33.0 | − 32.4 | − 33.1 | − 45.1 |
| Bromelia antiacantha | − 28.2 | − 29.1 | − 26.0 | − 23.4 | 26.5 | 34.6 | 26.9 | 27.0 |
| Casearia sylvestris | − 37.1 | − 38.3 | − 39.0 | − 50.4 | − 7.3 | − 7.5 | − 6.8 | 1.7 |
| Cecropia glaziovii | − 42.6 | − 51.8 | − 55.4 | − 69.1 | − 21.7 | − 41.0 | − 40.3 | − 54.2 |
| Copaifera trapezifolia | − 19.3 | − 22.3 | − 25.1 | − 39.6 | 20.1 | 9.5 | 15.0 | − 17.5 |
| Croton celtidifolius | − 35.0 | − 54.2 | − 44.0 | − 81.6 | − 32.8 | − 53.3 | − 40.6 | − 81.4 |
| Cunila microcephala | − 100.0 | − 100.0 | − 100.0 | − 100.0 | − 42.4 | − 33.1 | − 48.3 | − 88.1 |
| Drimys brasiliensis | − 73.8 | − 76.9 | − 77.9 | − 91.7 | − 73.8 | − 76.9 | − 77.9 | − 91.7 |
| Echinodorus grandiflorus | − 20.0 | − 28.5 | − 26.8 | − 36.5 | − 17.4 | − 23.9 | − 23.3 | − 31.4 |
| Equisetum giganteum | − 36.2 | − 39.8 | − 38.1 | − 48.7 | − 31.0 | − 34.8 | − 31.8 | − 40.7 |
| Hypericum caprifoliatum | − 5.5 | − 11.2 | − 12.8 | − 19.9 | 29.6 | 7.2 | 2.6 | 5.1 |
| Ilex paraguariensis | − 40.0 | − 41.2 | − 46.3 | − 57.8 | − 38.8 | − 37.3 | − 44.9 | − 57.6 |
| Jodina rhombifolia | − 57.5 | − 58.4 | − 59.5 | − 62.2 | − 51.5 | − 52.5 | − 53.0 | − 50.9 |
| Mikania glomerata | − 41.7 | − 37.1 | − 47.6 | − 56.9 | − 12.9 | − 6.0 | − 18.8 | − 24.4 |
| Mikania laevigata | − 27.4 | − 23.0 | − 26.5 | − 32.8 | − 12.8 | 3.2 | − 14.4 | − 4.5 |
| Monteverdia ilicifolia | − 23.7 | − 36.8 | − 37.5 | − 41.5 | − 18.9 | − 30.5 | − 33.2 | − 36.5 |
| Ocimum carnosum | − 17.3 | − 41.1 | − 36.1 | − 35.7 | − 10.3 | − 34.4 | − 30.0 | − 29.4 |
| Piper umbellatum | − 43.7 | − 50.4 | − 48.9 | − 63.7 | − 37.6 | − 42.2 | − 45.0 | − 54.4 |
| Plantago australis | − 38.5 | − 42.0 | − 40.6 | − 53.3 | − 34.0 | − 37.3 | − 35.1 | − 46.1 |
| Sambucus australis | − 32.5 | − 37.8 | − 38.0 | − 39.5 | − 26.3 | − 34.1 | − 29.6 | − 32.7 |
| Smilax campestris | − 44.7 | − 45.7 | − 47.0 | − 62.2 | − 37.6 | − 36.2 | − 41.4 | − 54.9 |
| Solanum mauritianum | − 43.5 | − 45.2 | − 48.8 | − 61.0 | − 39.1 | − 39.2 | − 45.9 | − 58.3 |
| Solanum paniculatum | − 42.6 | − 54.0 | − 46.9 | − 62.0 | − 26.4 | − 38.2 | − 30.5 | − 30.1 |
| Sorocea bonplandii | − 43.8 | − 56.4 | − 52.3 | − 68.1 | − 21.1 | − 39.5 | − 21.9 | − 58.3 |
| Trichilia catigua | − 38.5 | − 66.0 | − 56.5 | − 79.4 | − 8.5 | − 34.9 | − 23.5 | − 51.8 |
| Varronia curassavica | − 29.1 | − 33.2 | − 32.5 | − 36.5 | 65.3 | 122.4 | 83.6 | 229.5 |
| Wilbrandia ebracteata | − 36.3 | − 28.6 | − 23.2 | − 60.5 | 28.2 | 20.5 | 42.3 | − 33.6 |
| Zollernia ilicifolia | − 38.8 | − 42.5 | − 42.4 | − 57.4 | − 14.1 | − 6.6 | − 15.0 | − 36.1 |
| Ornamental species | ||||||||
| Ananas bracteatus | − 44.6 | − 46.6 | − 46.0 | − 60.2 | − 37.3 | − 37.7 | − 39.5 | − 52.7 |
| Aspilia montevidensis | − 27.5 | − 28.9 | − 35.6 | − 27.6 | − 2.3 | 9.9 | − 10.8 | 13.4 |
| Calliandra tweedii | − 32.7 | − 42.9 | − 42.4 | − 52.5 | − 27.8 | − 33.9 | − 38.1 | − 44.5 |
| Cortaderia selloana | − 35.0 | − 44.0 | − 39.2 | − 57.7 | − 30.1 | − 39.4 | − 31.6 | − 52.6 |
| Dyckia distachya | − 44.7 | − 99.6 | − 71.8 | − 91.7 | 159.0 | − 36.8 | 44.4 | 21.4 |
| Epidendrum fulgens | − 18.1 | − 19.0 | − 37.5 | − 3.7 | 30.3 | 123.1 | 14.2 | 264.0 |
| Fuchsia regia | − 51.2 | − 56.4 | − 56.9 | − 79.9 | − 49.7 | − 54.9 | − 55.6 | − 79.3 |
| Gomesa flexuosa | − 38.1 | − 40.6 | − 39.2 | − 60.5 | − 23.0 | − 31.4 | − 24.6 | − 52.4 |
| Handroanthus chrysotrichus | − 38.5 | − 43.2 | − 45.3 | − 60.5 | − 14.9 | − 25.0 | − 21.0 | − 40.7 |
| Heliconia farinosa | − 13.7 | − 15.5 | − 12.6 | − 20.6 | 27.7 | 64.1 | 71.9 | 29.1 |
| Jacaranda puberula | − 40.2 | − 49.4 | − 50.1 | − 63.7 | − 27.0 | − 36.9 | − 36.2 | − 52.5 |
| Parodia ottonis | − 63.9 | − 56.0 | − 72.6 | − 82.5 | − 51.8 | − 42.4 | − 68.5 | − 76.6 |
| Petunia integrifolia | − 4.2 | − 5.6 | − 12.7 | − 12.2 | 7.3 | 1.2 | − 10.1 | − 4.9 |
| Pyrostegia venusta | − 49.3 | − 46.6 | − 46.3 | − 48.9 | − 12.0 | 22.2 | 8.4 | 70.5 |
| Rumohra adiantiformis | − 34.9 | − 38.3 | − 41.6 | − 52.2 | − 34.6 | − 36.6 | − 41.5 | − 51.8 |
| Syagrus romanzoffiana | − 61.0 | − 62.4 | − 61.8 | − 75.5 | − 27.3 | − 30.0 | − 21.7 | − 56.0 |
| Tibouchina sellowiana | − 41.8 | − 53.9 | − 50.3 | − 74.5 | − 9.1 | − 39.6 | − 35.0 | − 69.8 |
| Trichocline catharinensis | − 54.1 | − 85.6 | − 83.3 | − 89.0 | − 53.0 | − 71.2 | − 83.3 | − 86.7 |
| Verbena rigida | − 47.5 | − 68.4 | − 67.0 | − 69.3 | − 32.6 | − 50.8 | − 49.8 | − 66.2 |
| Timber species | ||||||||
| Apuleia leiocarpa | − 56.0 | − 61.0 | − 60.6 | − 75.3 | − 52.8 | − 57.0 | − 57.8 | − 71.1 |
| Aspidosperma polyneuron | − 49.7 | − 57.1 | − 47.7 | − 63.6 | 34.7 | 41.4 | 29.4 | 155.9 |
| Ateleia glazioveana | − 28.3 | − 15.3 | − 36.7 | − 17.0 | 17.3 | 39.2 | − 11.5 | 27.4 |
| Balfourodendron riedelianum | − 29.0 | − 52.7 | − 37.7 | − 51.6 | − 24.2 | − 47.3 | − 31.1 | − 46.0 |
| Cabralea canjerana | − 42.4 | − 52.0 | − 50.9 | − 61.6 | − 40.4 | − 48.1 | − 48.5 | − 57.1 |
| Calophyllum brasiliense | − 39.8 | − 41.2 | − 43.3 | − 40.7 | 104.5 | 137.5 | 125.3 | 183.0 |
| Cedrela fissilis | − 48.4 | − 54.1 | − 52.8 | − 67.1 | − 47.8 | − 52.6 | − 52.5 | − 66.3 |
| Colubrina glandulosa | − 60.7 | − 59.6 | − 54.9 | − 74.7 | − 52.3 | − 49.9 | − 45.3 | − 69.8 |
| Cordia trichotoma | − 54.0 | − 59.9 | − 58.4 | − 72.6 | − 41.5 | − 45.2 | − 44.8 | − 57.6 |
| Enterolobium contortisiliquum | − 46.8 | − 63.3 | − 55.7 | − 74.8 | − 23.9 | − 44.9 | − 32.0 | − 49.3 |
| Handroanthus heptaphyllus | − 26.4 | − 30.7 | − 38.3 | − 58.2 | 28.0 | 25.7 | 7.3 | − 12.4 |
| Hyeronima alchorneoides | − 34.8 | − 38.2 | − 36.3 | − 45.4 | − 1.1 | 13.7 | 9.7 | 32.6 |
| Miconia cinnamomifolia | − 8.9 | − 15.5 | − 14.6 | − 27.0 | 27.9 | 12.4 | 15.0 | − 13.0 |
| Mimosa scabrella | − 44.8 | − 61.5 | − 61.2 | − 83.5 | − 44.8 | − 61.4 | − 61.2 | − 83.5 |
| Nectandra lanceolata | − 40.2 | − 52.1 | − 49.7 | − 60.8 | − 34.6 | − 47.3 | − 44.9 | − 56.9 |
| Ocotea puberula | − 38.2 | − 39.7 | − 39.7 | − 55.4 | − 34.0 | − 33.3 | − 35.4 | − 50.8 |
| Parapiptadenia rigida | − 38.4 | − 48.3 | − 44.5 | − 56.5 | − 20.9 | − 17.1 | − 26.8 | − 33.5 |
| Peltophorum dubium | − 76.5 | − 79.4 | − 74.8 | − 88.4 | − 58.3 | − 61.5 | − 49.8 | − 68.9 |
| Piptocarpha angustifolia | − 41.7 | − 65.7 | − 69.9 | − 72.2 | − 9.2 | − 47.3 | − 59.5 | − 56.3 |
| Schizolobium parahyba | − 38.6 | − 36.0 | − 36.7 | − 52.2 | − 24.1 | − 11.1 | − 15.9 | − 39.1 |
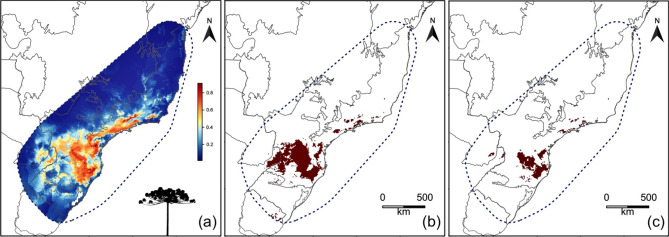
Looking at specific groups by their main use, we estimate loss of BAH ranging from 2,9% (Tropaeolum pentaphyllum) to 76.5% (Pimenta pseudocaryophyllus) for aromatic species; 6.1% (Geonoma gamiova) to 58.8% (Gynerium sagittatum) for fibrous species; 7.1% (Acca sellowiana) to 91.8% (Annona crassiflora) for food species; 2.5% (Stylosanthes leiocarpa) to 100% (O. micranthus) for forage species from Fabaceae family; 1.6% (Paspalum modestum) to 95.9% (Paspalum jesuiticum) for forage species from Poaceae family; 4.5% (Mikania laevigata) to 100% (C. microcephala) for medicinal species; 2,3% (Aspilia montevidensis) to 99.6% (Dyckia distachya) for ornamental species; and 1.1% (Hyeronima alchorneoides) 88,4% (Peltophorum dubium) for medicinal species (Table 1, Supplementary Table S1). The species Araucaria angustifolia, which has already been traditionally combined in agroecological practices in southern Brazil is predicted to reduce up to 66% of its BAH under SSP5-8.5 by 2061–2080 in both dispersal scenarios (Fig. 2, Supplementary Table S1).
Figure 2.
Decline of BAH by climate change for A. angustifolia, a species traditionally combined with other agricultural crops from Atlantic Forest. a) Current habitat suitability. Blue to red indicates the increase of suitability b-c) Future suitable habitats based on SSP2-4.5 and SSP5-8.5 scenarios for 2061–2080. Wine red colour indicates the remaining BAH. Estimated BAH is surrounded by dotted lines in royal blue colour. Maps created with custom R script. Version R 4.1.1 (https://www.R-project.org/). Base map source (Terrestrial biomes shapefile) obtained from the Brazilian Ministry of the Environment (https://www.gov.br/mma/).
IUCN Red list preliminary assessment
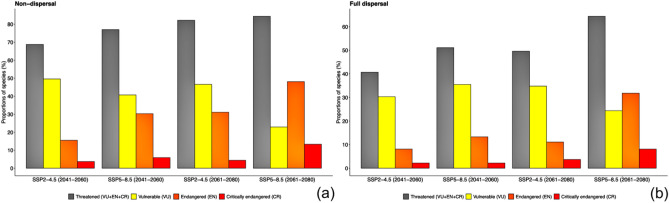
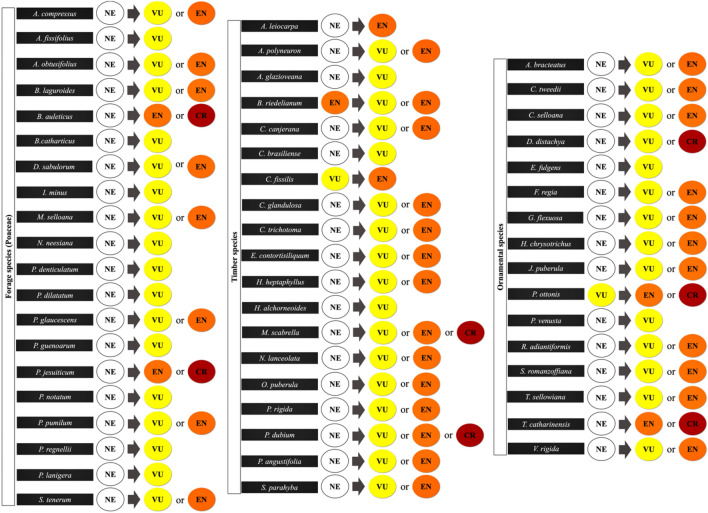
Assessing the geographic range (B1a + B2a criteria), we found that only 4.3% of the native species was qualified for a Threat category (Supplementary Table S2). However, when considering the predicted quantitative habitat loss due to climate change (A3c criterion) the percentages increased. We observed that 68.8% (SSP2-4.5/2041–2060) to 84.4% (SSP5-8.5/2061-2080) under the non-dispersal scenario (Fig. 3a, Supplementary Table S1) and 40.7% (SSP2-4.5/2041-2060) to 64.4% (SSP5-8.5/2061-2080) under the full dispersal scenario of the species could be qualified as threatened according to IUCN Red List criteria (Fig. 3b, Supplementary Table S1). The highest proportions of species were qualified as vulnerable with 49.6% (SSP2-4.5/2041-2060), 40.7% (SSP5-8.5/2041-2060) and 46.6% (SSP2-4.5/2061-2080) under the non-dispersal scenario and 30.3% (SSP2-4.5/2041-2060), 35.5% (SSP5-8.5/2041-2060) and 34.8% (SSP2-4.5/2061-2080) under the full dispersal scenario. The exception was the SSP5-8.5 (2061-2080) scenario, where the largest proportion was qualified as endangered in both the non-dispersal scenario (48.1%) and full dispersal scenario (31.8%) (Fig. 3a,b). Approximately 96% of the species have not yet been accessed by the IUCN and are currently Not Evaluated (NE). Here, we show that 86% of these species are predicted to change from Not Evaluated to a threat category, Vulnerable (VU), Endangered (EN) and Critically Endangered (CR), in all major uses and based on different climate change scenarios (Fig. 4, Table 2, Supplementary Fig. S1). All species groups changed from 50 to 100% of their species to the Vulnerable category. Timber species had the highest percentage of their species (84.2%) changing to the Endangered category and, the forage species belonging to the Fabaceae family had the highest (55.6%) changing to the Critically Endangered category.
Figure 3.
Percentage of Brazilian agroforestry plant species potentially qualified in a threat categories VU + EN + CR, and in separate categories Vulnerable (VU), Endangered (EN) and Critically Endangered (CR), under two different climate change scenarios (SSP2-4.5 and SSP5-8.5) for the following time periods: 2041–2060 and 2061–2080, assuming two dispersal scenarios: (a) non-dispersal and (b) full dispersal.
Figure 4.
Native potential agroforestry plant species changing from a current assessed IUCN category or Not Evaluated (NE) to a threat category, Vulnerable (VU), Endangered (EN) and Critically Endangered (CR), based on different major uses due to climate change.
Table 2.
Number and percentage of species changing from Not Evaluated to a threat category, Vulnerable (VU), Endangered (EN) and Critically Endangered (CR), in all major uses and in at least one climate change scenario.
| Main use | VU | EN | CR | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Aromatic species | 4 | 100.0 | 2 | 50.0 | – | – |
| Fibrous species | 4 | 100.0 | 2 | 50.0 | – | – |
| Food species | 12 | 80.0 | 11 | 73.3 | 3 | 20.0 |
| Forage species (Fabaceae) | 6 | 66.7 | 5 | 55.6 | 5 | 55.6 |
| Forage species (Poaceae) | 10 | 50.0 | 9 | 45.0 | 2 | 10.0 |
| Medicinal species | 27 | 93.1 | 20 | 69.0 | 3 | 10.3 |
| Ornamental species | 14 | 87.5 | 12 | 75.0 | 3 | 18.8 |
| Timber species | 17 | 89.5 | 16 | 84.2 | 2 | 10.5 |
Discussion
Climate change is an important driver of species extinction4,25. We found that future climate change was predicted to cause a decline in BAH between 38.5–56.3% under the non-dispersal scenario and between 22.3–41.9% under the full dispersal scenario in 135 Brazilian native species. Several studies forecasted the impacts of climate change on species distribution by using ENMs worldwide44–47, and consequently on ecosystem functionality4,48. The worst-case scenario (SSP5-8.5) showed the highest average decline in BAH in both non-dispersal (56.3%) and full dispersal (41.9%), when compared to the stabilization scenario (SSP2-4.5) and in 2061–2080, showing that species tend to be more threatened in this scenario and year, as demonstrated in other studies conducted with Brazilian species49,50. Our study evaluated widespread plant species, such as Axonopus fissifolius, occurring in all Brazilian phytogeographic domains and species with a narrow-ranged distribution, such as Adesmia bicolor from South Brazilian grasslands (Pampa). We noticed that both narrow-ranged and widespread species may be impacted by climate change. For instance, the narrow-ranged species O. micranthus, used in annual forage crops, is predicted to lose up to 100% of its suitable habitat in most climate change scenarios. Our models also suggested that the widespread Brazilian peppertree (Schinus terebinthifolia) may lose up to 47% of its original habitats. These results need to be interpreted with caution, since all species that were predicted to lose 100% of BAH have small sample sizes (n < 50, Supplementary Table S1), which is one of the main determinants of model accuracy51. Some species tend to be favored by certain climate change scenarios under full dispersal scenario52,53, such as here the medicinal species Varronia curassavica, with an increase of BAH up to 230%; the ornamental species Epidendrum fulgens up to 264% and the forage species Indigofera sabulicola up to 387%. Although some species were predicted to expand their range, this does not guarantee the survival of these species, since other drivers, such as the capability of poor dispersal species to cope with climate change54, deforestation44,55 and other land-use changes threaten Brazilian ecosystems and the survival of their associated species56.
Rapid range changes of species may, in turn, impact material, non-material and regulating contributions of nature to people2,48,57,58. The global productivity of farms may be negatively affected by climate change throughout most of the tropics59. Smallholders and traditional Brazilian communities such as the caiçaras, quilombolas and indigenous peoples make use of these species in agroforestry practices13, a land use management system that increases carbon sequestration in biomass and soils24,60. These systems improve people’s livelihoods by simultaneously providing income, food security, fuel, medicine, forage, and/or other goods and services. Moreover, increased tree cover due to agroforestry may help to mitigate climate change23,57,61. Here we present evidence of how currently suitable areas for cultivation of these species may become unsuitable in the future. This may lead to a severe decline in people's livelihoods and regional food security23,62. Moreover, under climate shifts species survival is likely to be threatened by biogeographic barriers such as an agricultural or otherwise ecologically degraded landscape matrix which would prevent species migration to climatically more suitable areas63.
The outcomes of climate change predicted by biogeographical and ecological studies have been neglected and have barely been integrated into conservation planning30,64. Prioritization of conservation efforts is often based on a species’ extinction risk65. Determining whether a taxon is threatened with extinction depends on biological indicators, such as rapid population decline, and qualifying species in a threat category may assist in decision making43. Our analyses highlight that 68.8% to 84.4% (non-dispersal) and 40.7% to 64.4% (full dispersal) of our species of interest may become threatened. Additionally, species already threatened according to the IUCN red list; A. angustifolia (CR), Butia eriospatha (VU), Balfourodendron riedelianum (EN), Cedrela fissilis (VU) and Parodia ottonis (VU) will remain listed as such as a result of climate change. In assessing Amazonian tree species, Gomes et al. (2019) found that 43–46% of trees species should be listed as threatened according to IUCN A2, A4, B1 and D2 criteria for RCP 2.6 and RCP 8.5 respectively. Similarly, Zizka et al. (2020), analyzing the conservation status of species of Bromeliaceae based on the geographic range in the Americas, showed that a total of 81% of bromeliad species are possibly threatened according to IUCN red list criteria. These authors observed that the medicinal species Bromelia antiacantha is possibly not threatened (LC or NT) in the current scenario, which agrees with our full dispersal scenario results that show that the BAH of this species may remain stable under all future climate change scenarios as well. Elias et al.66 assessed the conservation status of eleven palm species through A2, A4 and B2 criteria in the state of Santa Catarina (Atlantic Forest) and qualified Euterpe edulis and G. gamiova as Vulnerable and Butia catarinensis and Butia eriospatha as Endangered. According to our findings, E. edulis may be categorized as Endangered for showing a decline in BAH of over 50% in all climate change scenarios when assuming species have no dispersal. However, when we considered the full dispersal scenario, we noted that although there are declines in BAH in all climate change scenarios, only in the SSP5-8.5 for 2061–2080 this species might be qualified as Vulnerable. For G. gamiova we observed a similar result (Vulnerable) exclusively in the SSP5-8.5 for 2061–2080 assuming no dispersal. On the other hand, assuming the full dispersal scenario, we noted an increase in BAH of up to 86% in the SSP5-8.5 for 2041–2060 for all climate change scenarios. Our results are equally consistent with the assessment of these authors for B. catarinensis and B. eriospatha. We still recorded an alarming scenario for B. eriospatha, qualifying the species as Critically endangered because of a decline of BAH declines over 87% (non-dispersal) under the worst-case scenario SSP5-8.5 for 2061–2080. Thus, given the large number of agroforestry species at risk of extinction, and the low number of species assessed by the IUCN Red List, we emphasize an urgent need for updates of the official list of threatened species, to provide a more precise indicator for threatened plant species conservation planning in Brazil. Despite all legal implications for threatened species in Brazil, agroforestry systems can act as an alternative to overcome part of the conflicts between conventional agricultural production and the conservation of natural resources, as can be seen in the new forest code (Law 12.651/2012), which provides explicit provisions for sustainable agroforestry. Additionally, a specific Law (12.854/2013) promotes forest recovery activities and the implementation of agroforestry systems34.
It is well known that deforestation is a primary driver leading species to extinction67. However, climate change is expected to overtake this driver in a few decades44. The majority of the species prioritized in the “Plants for the future initiative”, found mainly in the Atlantic Forest have great potential to be conserved through sustainable practices, particularly by smallholders and traditional communities13. The Atlantic Forest remains endangered as a result of continued deforestation and the future of this forest relies on well-structured conservation plans based on reliable information55,68,69. The Atlantic Forest cover has been reduced to less than 20% of its original size70,71, distributed mainly in small and disturbed fragments of less than 50 hectares56,72. Thus, most of the endemic species in the Atlantic Forest biome could already be qualified as critically endangered according to IUCN criteria43. On the other hand, despite our predicted catastrophic scenarios for native Atlantic Forest species, we observe that species such as Bracatinga (M. scabrella), Brazilian peppertree (Schinus terebinthifolia) and Peruvian groundcherry (Physalis peruviana), are distributed across the neotropics and easily found along streets and city squares all over Brazil. Many of these are pioneers, some almost ruderal, regenerating easily in open city areas13,29. Many of these forest species have endured over 500 years of deforestation, and still remain abundant in the Atlantic Forest even after losing approximately 80% of their natural habitat29,56,71. Hence, we note the need for studies that address species response to global changes to better understand the resilience potential of these species.
Protecting people’s livelihoods in a rapidly changing climate may be one of the great challenges of the twenty-first century. Although it is not shared by all of the scientific community, as discussed by Loreau73, species with economic value seem to have advantages for conservation over those with non-economic value as can be seen in long-term human activities such as protection, transport and planting of useful species and removal of non-useful species by local communities31,42,74. Indeed, socioeconomic underutilization of plant resources may in some cases even jeopardize socioecological synergies of tropical forest resilience58. All species analyzed here have a great potential to be conserved through a conservation-by-use approach, because of their different uses that do not necessarily jeopardize reproduction and persistence. They play an important role among local communities41,48,50,75,76 and farmers’ livelihoods31,42. Searching for evidence of conservation among species with economic and cultural values, Reis et al.42 noticed that the species Ilex paraguariensis, A. angustifolia and B. antiacantha were intentionally favored through protection, transplantation and selection by farmers. Furthermore, Donazzolo et al.32 noted that management of Acca sellowiana populations, retained high level of genetic diversity and tended to increase the species genetic variability. We argue that adopting measures, such as the establishment of new agroforestry systems to increase carbon sequestration, the selection of varieties capable of withstanding new climates and the improvement of habitat connectivity to facilitate species migration/dispersal should be a strategy for short-term and long-term conservation and people’s livelihoods.
ENMs are widely used to forecast the distribution of species across geographic space and time. Building meaningful models to estimate the future distribution of species for an uncertain future requires very specific decisions and interpretations with extreme caution51,77,78. Several uncertainties and complexities are related to our study. Modeling a large number of species can make the species-specific selection of predictors methodologically and practically complex77,79. To mitigate this, we selected the most suitable environmental predictors for different plant growth forms. Model performance evaluation is a key step for ENM studies and probably the most problematic one owing to its complexity79. The random cross-validation approach is the most common practice, adopted by modelers to evaluate model performance, where datasets are split into k folds, using one part to test the model and the remaining (k-1 folds) to calibrate the model80,81. To reduce the over-optimistic nature of cross-validation, we applied a null-model for significance testing of presence-only ENMs82. Binarization of continuous probabilities output is commonly employed by modelers to quantify species range changes and build species richness over time83. Nevertheless, Santini et al.51 recently concluded that this practice reduces the predictive probability of models. Although we binarized ENMs outputs to quantify the climate change impacts, we applied a threshold highly indicated for conservation purposes for showing high performance in the identification of suitable areas and commonly used50,84–86. We assumed that the species are at equilibrium with the environment87 and occurrence records were sampled randomly88. Furthermore, we included no biotic interactions40,89, adaptations and evolution90 into our modeling approach. For dispersal37,40, we considered two scenarios (non-dispersal and full dispersal), but we limited the full dispersal scenario to species BAH, since we understand that plant dispersal rates over 0.1 km/year might not occur for vascular and non-vascular plants5,64,91 or over 100 km for Brazilian tree species as result of climate change44,92. Although humans fundamentally affect dispersal and alter landscapes by transporting individuals93–95, we did not include human-mediated dispersal data in our models due to the lack of information related to human migrations as well as for each specific species. Another limitation of our study is to restrict our analysis to the estimated BAH of species, which may mask some macroecological patterns, yet adopting this conservative approach allows us to observe more concise species responses and diminish model overfitting36,44,96.
In summary, we showed that future climate will likely trigger a decline in BAH between 38.5–56.3% under the non-dispersal scenario and between 22.3–41.9% under the full dispersal scenario of several native potential agroforestry species from the Brazilian flora. Additionally, we found that only 4.3% of the studied species could be threatened under the IUCN criteria B1 and B2. However, when considering the IUCN criterion A3, 68.8–84.4% (non-dispersal) and 40.7–64.4% (full dispersal) of our species of interest could be qualified as threatened. Although accessing genetic material with quality for native species might be difficult and the scenarios used here estimate considerable losses for 2041–2060 and 2061–2080, we argue that actions such as the promotion of these species in agroecosystems are promising alternatives to increase their population sizes. We urge that public policies involving farmers and local communities be adopted, as practices and management systems implemented by them have proven to maintain landscapes with productive forest fragments, and consequently favors species and forest conservation. Lastly, we highly recommend the development of scientific research towards biotechnological applications to select promising genotypes for a changing global climate.
Methods
Study area and target species
The study area includes the Atlantic Forest and Pampa grasslands in Eastern South America. We modelled 139 native potential agroforestry plant species (i.e. aromatic, fibre, food, forage, medicinal, ornamental and timber species) prioritized by the Brazilian Ministry of the Environment initiative “Native species of the Brazilian flora of current and potential economic value—Plants for the Future—Southern Region” (Espécies Nativas da Flora Brasileira de Valor Econômico Atual e Potencial – Plantas para o futuro – Região Sul). This initiative seeks to promote the sustainable use of Brazilian native plant species often used in different regions of the country13. In addition to contributing to the country's commitments under the Convention on Biological Diversity (CBD) and International Treaty on Plant Genetic Resources for Food and Agriculture, particularly with regard to promoting the sustainable use of biodiversity components13, these species provide food security for local communities and have commercial value in national and foreign markets. In spite of the fact that not all species have already been found in current agricultural systems or been managed by farmers, they all have one or multiple uses and can be combined in mixed cropping. Some species such as A. angustifolia31, A. sellowiana32, E. edulis33, Ilex paraguariensis34, and Mimosa scabrella35 have already traditionally been combined with other agricultural crops in managed landscapes in southern Brazil. All taxonomic authorities and species common names are listed in Supplementary Table S3.
Species occurrence data
Occurrence data for the 139 species evaluated here was downloaded from the Global Biodiversity Information Facility (https://doi.org/10.15468/dl.vjezvb) 97. We collected a total of 28,860 unique records. The sample size for species ranged from 12 (Ornithopus micrantus) to 5464 (Casearia sylvestris). We standardized botanical names using the R package ‘flora’98, which uses the nomenclature accepted by the Brazilian Flora 2020 project (http://floradobrasil.jbrj.gov.br/). To avoid modeling truncated niches, we extracted all records from an extent, defined by latitudes 60°S-15°N and longitudes 90°-30°W99. We checked the geographical consistencies of all records using the cleaning pipeline proposed by Gomes et al.36. Firstly, we removed all occurrences outside the Neotropics. Then, we removed all records with missing latitude and longitude, using the function ‘cleancoordinates’ from the R package ‘CoordinateCleaner’100. Finally, we estimated the kernel density for each species in order to remove spatial outliers using the density function from the R package ‘stats’101. As geographic sampling biases are common among biological collections102,103, which can lead to over-representation of environmental conditions, we spatially filtered the species occurrence data over a distance of 20 km using the R package ‘spThin’ in order to diminish spatial autocorrelation104. We did not model species for which there were less than ten records, as models fit with few data may not be reliable105,106.
Environmental predictors
We obtained 19 bioclimatic variables from the Worldclim version 2.1. (http://worldclim.org) at a resolution of 5 arc-minutes (roughly 10 km at the equator), to characterize the species climatic requirements107. These environmental variables represent the time period of 1970–2000. Predictors were selected (1) a priori based on their biological significance for different plant growth forms108 (Supplementary Table S4). These predictors are critical in determining the distribution limits of a wide range of plant growth forms and are highly related to plant physiological responses109,110. We then (2) checked for multicollinearity by examining the correlation structure of the predictor variables through the variance inflation factor (VIF) for epiphyte, fern, graminoid, herb, hydrophyte, lithophyte, shrub, tree and vine species111. This measure evaluates how much the variance of an estimated regression coefficient increases if their predictors are correlated112. We kept only predictors with VIF values below 5113. The VIFs were checked using the function ‘vifstep’ in the R package ‘usdm’114. The retained predictors are shown in Supplementary Table S5.
Modeling approach
We used bioclimatic habitat suitability to assess the potential impacts of climate change on species’ BAH and inform IUCN Red List assessments115. To delimit BAH, we incorporated a 100 km buffer around species extent of occurrence (EOO), as we understand that plant dispersal rates over 0.1 km/year might not occur for vascular and non-vascular plants5,64,91, and adopting a conservative approach reduces model overfitting36,44,96. EOOs were quantified by drawing a minimum convex polygon (MCP) around known species records as recommend by IUCN43. Current and future potential habitats for species were predicted using MaxEnt v.3.4.1 k, a machine-learning algorithm used to estimate species' probability distribution116. MaxEnt is the presence-based method widely used for having high performance when compared to other available algorithms36,105,117–119. ENMs were fitted using the following parameters in the MaxEnt: bootstrap method with 100 replicates, 500 maximum iterations, 10,000 points of background, and Cloglog output format. We only kept linear and quadratic features to avoid overfitting of the models and as recommended by Merow et al. because of the absence of a biological justification with the variables used120,121. Furthermore, we inspected species-response-curves to avoid spurious calibrations, following the evaluation strip method proposed by Elith et al.122. This method investigates the effect of one variable at a time, keeping the others constant at their mean values122. To assess robustness and alert policy-makers for the uncertainties typically associated with these methods, each ENM was tested against a bias corrected null-model as proposed by Raes and ter Steege82. The AUC values of the ENMs built with n occurrence records were tested against the upper AUC values of the lower quantile of 95% of the AUC values obtained from 100 × n points drawn and predicted randomly. Only significant ENMs were projected to future climatic conditions.
Future projections
The climate projections were carried out according to the Sixth Assessment Report (AR6) of the IPCC, using two Shared Socioeconomic Pathways (SSPs) as reference (SSP2-4.5 and SSP5-8.5). SSPs are projections of future climates, based on different socioeconomic assumptions such as population, technological, and economic growth. The SSP2-4.5 (2041–2060) is a stabilization scenario, assuming global temperature increases ranging from 2.1 to 4.3 °C and mean warming of 3.0 °C. The SSP5-8.5 (2061–2080) represents the worst-case scenario, assuming absence of climate change policies, with global temperatures continue to rise throughout the twenty-first century, with estimates ranging from 3.8 to 7.4 °C and mean warming of 5.0 °C9. We averaged eight different global climate models: BCC-CSM2-MR, CNRM-CM6-1, CNRM-ESM2-1, CanESM5, IPSL-CM6A-LR, MIROC-ES2L, MIROC6 and MRI-ESM2-0 to take into account the uncertainties related to future climate conditions81. The fitted consensus ENMs were projected to these two datasets to obtain predicted future maps of habitat suitability for each species.
To map changes in future ranges of species, we converted the continuous habitat suitability into binaries using the maximum training sensitivity plus specificity threshold85,123. This threshold is indicated for conservation purposes for its high performance in the identification of suitable areas50,84–86. To assess whether species will face a decline or expansion in BAH under future climate conditions, we quantified the difference between the relative number of pixels occupied in current and future BAHs. We assumed that species have no dispersal capacity and full dispersal capacity for 2041–2060 and 2061–2080 timeframes. In the first scenario, species do not have the ability to disperse and reach new areas (pixels) in future climate scenarios. In the second, species can migrate within the estimated BAH of each species. Here, to ensure transparency and reproducibility for reporting ENMs, we adhered to the ODMAP (Overview, Data, Model, Assessment, Prediction) protocol v1.0. (Supplementary Table S6), as proposed by Zurell et al.78. All analyses were conducted within R environment version R 4.1.1101.
IUCN Red list preliminary assessment
The IUCN Red List assessments provide important information related to species status, trends and threats for the establishment of conservation planning and improvement of decision-making43,124,125. Criterion B is linked to the geographic range and has two sub-criteria (B1 and B2), which are based on the extent of occurrence (EOO) and B2 on the area of occupancy (AOO), respectively43. Further, three other conditions (a, b, and c) describe aspects of the biology and potential decline of the taxon in response to threats43. At least one sub-criterion and two conditions must be met to qualify a given species as threatened43. Following the guidelines for using the IUCN Red List Categories and Criteria version 14, we calculated the geographic range (B1a + B2a criteria) using the R package ‘ConR’126. Additionally, we evaluated predicted quantitative habitat loss due to climate change by assessing the decline in habitat quality (A3c criterion), suspected to be met in the future, to qualify whether a particular species would be in a threat category43. The classification of threat includes: Vulnerable (VU), Endangered (EN) and Critically Endangered (CR). To qualify a species as Vulnerable, qualitative habitat loss must be ≥ 30%, Endangered ≥ 50% and Critically Endangered ≥ 80%. These categories are related to the risk of extinction of species in the wild43.
Supplementary Information
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. R.A.F.L. was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 795114.
Author contributions
V.P.L., H.S., I.S. conceived the study; V.P.L., R.A.F.L., F.J., N.R., H.S. analyzed the data and V.P.L led the writing with contributions from all the authors.
Accession codes
Datasets generated and codes are available on GBIF https://doi.org/10.15468/dl.vjezvb and on GitHub repository https://github.com/vplima/ENM.git.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06234-3.
References
- 1.Antonelli A, Smith RJ, Simmonds MSJ. Unlocking the properties of plants and fungi for sustainable development. Nat. Plants. 2019;5:1100–1102. doi: 10.1038/s41477-019-0554-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen I-C, Hill JK, Ohlemuller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 3.IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 10.5281/zenodo.3553579 (2019).
- 4.Pecl GT, et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science. 2017;355:eaai9214. doi: 10.1126/science.aai9214. [DOI] [PubMed] [Google Scholar]
- 5.Warren R, et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Chang. 2013;3:678–682. [Google Scholar]
- 6.Destro GFG, Fernandes V, Andrade AFA, De Marco P, Terribile LC. Back home? Uncertainties for returning seized animals to the source-areas under climate change. Glob. Change Biol. 2019;25:3242–3253. doi: 10.1111/gcb.14760. [DOI] [PubMed] [Google Scholar]
- 7.Travis JMJ, et al. Dispersal and species’ responses to climate change. Oikos. 2013;122:1532–1540. [Google Scholar]
- 8.IPCC. Summary for policymakers. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (2014).
- 9.IPCC. Summary for policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems (eds Shukla, P.R. & J. Skea, E. C.) (2019).
- 10.IPCC. Special Report on 1.5 degrees: Summary for Policymakers. In Global Warming of 1.5°C. An IPCC Special Report on the Impacts of global Warming of 1.5°C Above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Cha (2018).
- 11.Ulloa Ulloa C, et al. An integrated assessment of the vascular plant species of the Americas. Science. 2017;358:1614–1617. doi: 10.1126/science.aao0398. [DOI] [PubMed] [Google Scholar]
- 12.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 13.Coradin, L., Siminski, A. & Reis, A. Espécies Nativas da Flora Brasileira de Valor Econômico Atual e Potencial – Plantas para o futuro – Região Sul. (Ministério do Meio Ambiente, 2011).
- 14.Nair PKR. An introduction to agroforestry. Springer; 1993. [Google Scholar]
- 15.Sinclair FL. A general classification of agroforestry practice. Agrofor. Syst. 1999;46:161–180. [Google Scholar]
- 16.Somarriba E. Revisiting the past: An essay on agroforestry definition. Agrofor. Syst. 1992;19:233–240. [Google Scholar]
- 17.Cerda R, et al. Contribution of cocoa agroforestry systems to family income and domestic consumption: Looking toward intensification. Agrofor. Syst. 2014;88:957–981. [Google Scholar]
- 18.Montagnini F. Integrating Landscapes: Agroforestry for Biodiversity Conservation and Food Sovereignty. New York: Springer; 2017. [Google Scholar]
- 19.Siddique, I., Dionísio, A. C. & Simões-Ramos, G. A. Construindo Conhecimentos Sobre Agroflorestas em Rede. (UFSC, 2017).
- 20.Jose S. Agroforestry for conserving and enhancing biodiversity. Agrofor. Syst. 2012;85:1–8. [Google Scholar]
- 21.Sistla SA, et al. Agroforestry Practices Promote Biodiversity and Natural Resource Diversity in Atlantic Nicaragua. PLoS One. 2016;11:e0162529. doi: 10.1371/journal.pone.0162529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos PZF, Crouzeilles R, Sansevero JBB. Can agroforestry systems enhance biodiversity and ecosystem service provision in agricultural landscapes? A meta-analysis for the Brazilian Atlantic Forest. For. Ecol. Manage. 2019;433:140–145. [Google Scholar]
- 23.Reppin S, Kuyah S, de Neergaard A, Oelofse M, Rosenstock TS. Contribution of agroforestry to climate change mitigation and livelihoods in Western Kenya. Agrofor. Syst. 2020;94:203–220. [Google Scholar]
- 24.Marconi L, Armengot L. Complex agroforestry systems against biotic homogenization: The case of plants in the herbaceous stratum of cocoa production systems. Agric. Ecosyst. Environ. 2020;287:106664. [Google Scholar]
- 25.Somarriba E, et al. Carbon stocks and cocoa yields in agroforestry systems of Central America. Agric. Ecosyst. Environ. 2013;173:46–57. [Google Scholar]
- 26.De Stefano A, Jacobson MG. Soil carbon sequestration in agroforestry systems: A meta-analysis. Agrofor. Syst. 2017;92:285–299. [Google Scholar]
- 27.Gomes LC, et al. Agroforestry systems can mitigate the impacts of climate change on coffee production: A spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 2020;294:106858. [Google Scholar]
- 28.Kofsky J, Zhang H, Song B-H. The Untapped Genetic Reservoir: The Past, Current, and Future Applications of the Wild Soybean (Glycine soja) Front. Plant Sci. 2018;9:285–299. doi: 10.3389/fpls.2018.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzi, H. Arvores Brasileiras. (Plantarum, 2016).
- 30.Zwiener VP, et al. Planning for conservation and restoration under climate and land use change in the Brazilian Atlantic Forest. Divers. Distrib. 2017;23:955–966. [Google Scholar]
- 31.Zechini AA, et al. Genetic conservation of Brazilian Pine (Araucaria angustifolia) through traditional land use. Econ. Bot. 2018;72:166–179. [Google Scholar]
- 32.Donazzolo J, Stefenon VM, Guerra MP, Nodari RO. On farm management of Acca sellowiana (Myrtaceae) as a strategy for conservation of species genetic diversity. Sci. Hortic. (Amsterdam) 2020;259:108826. [Google Scholar]
- 33.Favreto R, Mello RSP, de Moura Baptista LR. Growth of Euterpe edulis Mart (Arecaceae) under forest and agroforestry in southern Brazil. Agrofor. Syst. 2010 doi: 10.1007/s10457-010-9321-z. [DOI] [Google Scholar]
- 34.Siminski A, dos Santos KL, Wendt JGN. Rescuing agroforestry as strategy for agriculture in Southern Brazil. J. For. Res. 2016;27:739–746. [Google Scholar]
- 35.da Silva LCR, Machado SA, Galvão F, Filho AF. Floristic evolution in an agroforestry system cultivation in Southern Brazil. An. Acad. Bras. Cienc. 2016 doi: 10.1590/0001-3765201620150026. [DOI] [PubMed] [Google Scholar]
- 36.Gomes VHF, et al. Species distribution modelling: Contrasting presence-only models with plot abundance data. Sci. Rep. 2018;8:1003. doi: 10.1038/s41598-017-18927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guisan A, Thuiller W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 38.Raes, N. & Aguirre-Gutiérrez, J. A Modeling Framework to Estimate and Project Species Distributions in Space and Time Pontocaspian biodiversity RIse and DEmise View project Current and Future Biodiversity Patterns in Mainland Southeast Asia View project. (2018).
- 39.Brooks TM, et al. Measuring terrestrial area of habitat (AOH) and its utility for the IUCN red list. Trends Ecol. Evol. 2019;34:977–986. doi: 10.1016/j.tree.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Soberon J, Peterson AT. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005;2:1–10. [Google Scholar]
- 41.Levis C, et al. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science. 2017;355:925–931. doi: 10.1126/science.aal0157. [DOI] [PubMed] [Google Scholar]
- 42.Reis MS, et al. Domesticated landscapes in Araucaria Forests, Southern Brazil: A multispecies local conservation-by-use system. Front. Ecol. Evol. 2018;6:1–14. [Google Scholar]
- 43.IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prep. by Stand. Petitions Comm. (2019).
- 44.Gomes VHF, Vieira ICG, Salomão RP, ter Steege H. Amazonian tree species threatened by deforestation and climate change. Nat. Clim. Chang. 2019;9:547–553. [Google Scholar]
- 45.Guo Y, et al. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Sci. Rep. 2017;7:46221. doi: 10.1038/srep46221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues P, Silva J, Eisenlohr P, Schaefer C. Climate change effects on the geographic distribution of specialist tree species of the Brazilian tropical dry forests. Braz. J. Biol. 2015;75:679–684. doi: 10.1590/1519-6984.20913. [DOI] [PubMed] [Google Scholar]
- 47.Wilson OJ, Walters RJ, Mayle FE, Lingner DV, Vibrans AC. Cold spot microrefugia hold the key to survival for Brazil’s critically endangered araucaria tree. Glob. Chang. Biol. 2019;25:4339–4351. doi: 10.1111/gcb.14755. [DOI] [PubMed] [Google Scholar]
- 48.Cámara-Leret R, et al. Climate change threatens New Guinea’s biocultural heritage. Sci. Adv. 2019;5:eaaz1455. doi: 10.1126/sciadv.aaz1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esser LF, Saraiva DD, Jarenkow JA. Future uncertainties for the distribution and conservation of Paubrasilia echinata under climate change. Acta Bot. Brasilica. 2019;33:770–776. [Google Scholar]
- 50.Lima VP, Marchioro CA, Joner F, ter Steege H, Siddique I. Extinction threat to neglected Plinia edulis exacerbated by climate change, yet likely mitigated by conservation through sustainable use. Austral Ecol. 2020;45:376–383. [Google Scholar]
- 51.Santini L, Benítez-López A, Maiorano L, Čengić M, Huijbregts MAJ. Assessing the reliability of species distribution projections in climate change research. Divers. Distrib. 2021;27:1–16. [Google Scholar]
- 52.Raes N, et al. Historical distribution of Sundaland’s Dipterocarp rainforests at Quaternary glacial maxima. Proc. Natl. Acad. Sci. 2014;111:16790–16795. doi: 10.1073/pnas.1403053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaz ÚL, Nabout JC. Using ecological niche models to predict the impact of global climate change on the geographical distribution and productivity of Euterpe oleracea Mart. (Arecaceae) in the Amazon. Acta Bot. Brasilica. 2016;30:290–295. [Google Scholar]
- 54.Sánchez-Fernández D, et al. Thermal niche estimators and the capability of poor dispersal species to cope with climate change. Sci. Rep. 2016;6:23381. doi: 10.1038/srep23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Lima RAF, et al. How much do we know about the endangered Atlantic Forest? Reviewing nearly 70 years of information on tree community surveys. Biodivers. Conserv. 2015;24:2135–2148. [Google Scholar]
- 56.Ribeiro MC, et al. The Brazilian Atlantic Forest: A Shrinking Biodiversity Hotspot. New York: Springer; 2011. [Google Scholar]
- 57.Díaz S, et al. Assessing nature’s contributions to people. Science. 2018;359:270–272. doi: 10.1126/science.aap8826. [DOI] [PubMed] [Google Scholar]
- 58.Siddique I, et al. Woody species richness drives synergistic recovery of socio-ecological multifunctionality along early tropical dry forest regeneration. For. Ecol. Manag. 2021;482:118848. [Google Scholar]
- 59.Harvey CA, et al. Climate-smart landscapes: Opportunities and challenges for integrating adaptation and mitigation in tropical agriculture. Conserv. Lett. 2014;7:77–90. [Google Scholar]
- 60.Schneidewind U, et al. Carbon stocks, litterfall and pruning residues in monoculture and agroforestry cacao production systems. Exp. Agric. 2019;55:452–470. [Google Scholar]
- 61.Dinesh, D., Campbell, B. M., Bonilla-findji, O. & Richards, M. 10 Best Bet Innovations for Adaptation in Agriculture: A supplement to the UNFCCC NAP Technical Guidelines. Working paper 215 (2017).
- 62.Lin BB, Perfecto I, Vandermeer J. Synergies between agricultural intensification and climate change could create surprising vulnerabilities for crops. Bioscience. 2008;58:847–854. [Google Scholar]
- 63.Perfecto, I., John Vandermeer & Angus Wright. 2019 Nature’s Matrix: Linking Agriculture, Biodiversity Conservation and Food Sovereignty. (Routledge, 2019).
- 64.Hannah L, et al. 30% land conservation and climate action reduces tropical extinction risk by more than 50% Ecography. 2020;43:943–953. [Google Scholar]
- 65.Zizka A, et al. Biogeography and conservation status of the pineapple family (Bromeliaceae) Divers. Distrib. 2020;26:183–195. [Google Scholar]
- 66.Elias GA, Lima JMT, dos Santos R. Threatened flora from the State of Santa Catarina, Brazil: Arecaceae. Hoehnea. 2019;46:e322018. [Google Scholar]
- 67.Pimm SL, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344:1246752–1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 68.Brancalion PHS, et al. What makes ecosystem restoration expensive? A systematic cost assessment of projects in Brazil. Biol. Conserv. 2019;240:108274. [Google Scholar]
- 69.Crouzeilles R, et al. There is hope for achieving ambitious Atlantic Forest restoration commitments. Perspect. Ecol. Conserv. 2019;17:80–83. [Google Scholar]
- 70.Magnago LFS, et al. Would protecting tropical forest fragments provide carbon and biodiversity cobenefits under REDD+? Glob. Chang. Biol. 2015;21:3455–3468. doi: 10.1111/gcb.12937. [DOI] [PubMed] [Google Scholar]
- 71.Rodrigues AC, Villa PM, Neri AV. Fine-scale topography shape richness, community composition, stem and biomass hyperdominant species in Brazilian Atlantic forest. Ecol. Indic. 2019;102:208–217. [Google Scholar]
- 72.de Lima RAF, et al. The erosion of biodiversity and biomass in the Atlantic Forest biodiversity hotspot. Nat. Commun. 2020;11:6347. doi: 10.1038/s41467-020-20217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loreau M. Reconciling utilitarian and non-utilitarian approaches to biodiversity conservation. Ethics Sci. Environ. Polit. 2014;14:27–32. [Google Scholar]
- 74.Berkes, F. & Folke, C. Linking social and ecological resilience and sustainability. In Linking Social and Ecological Systems. Management Practices and Social Mechanisms for Building Resilience (Cambridge University Press, Cambridge, 2000).
- 75.Fernandes RC, Piovezana L. The Kaingang perspectives on land and environmental rights in the south of Brazil. Ambient. Soc. 2015;18:111–128. [Google Scholar]
- 76.Machado Mello AJ, Peroni N. Cultural landscapes of the Araucaria Forests in the northern plateau of Santa Catarina, Brazil. J. Ethnobiol. Ethnomed. 2015;11:51. doi: 10.1186/s13002-015-0039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thuiller W, Guéguen M, Renaud J, Karger DN, Zimmermann NE. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019;10:1446. doi: 10.1038/s41467-019-09519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zurell D, et al. A standard protocol for reporting species distribution models. Ecography. 2020;43:1261–1277. [Google Scholar]
- 79.Warren DL, Matzke NJ, Iglesias TL. Evaluating presence-only species distribution models with discrimination accuracy is uninformative for many applications. J. Biogeogr. 2020;47:167–180. [Google Scholar]
- 80.Leroy B, et al. Without quality presence-absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018;45:1994–2002. [Google Scholar]
- 81.Araujo M, New M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 82.Raes N, ter Steege H. A null-model for significance testing of presence-only species distribution models. Ecography. 2007;30:727–736. [Google Scholar]
- 83.Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B Biol. Sci.285, 20180792 (2018). [DOI] [PMC free article] [PubMed]
- 84.Loiselle, B. A. et al. Avoiding pitfalls of using species distribution models in conservation planning. Conserv. Biol. 17, 1591–1600 (2003).
- 85.Bean WT, Stafford R, Brashares JS. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography. 2012;35:250–258. [Google Scholar]
- 86.Meyer ALS, Pie MR, Passos FC. Assessing the exposure of lion tamarins (Leontopithecus spp.) to future climate change. Am. J. Primatol. 2014;76:551–562. doi: 10.1002/ajp.22247. [DOI] [PubMed] [Google Scholar]
- 87.Araújo MB, Pearson RG. Equilibrium of species’ distributions with climate. Ecography. 2005;28:693–695. [Google Scholar]
- 88.Guillera-Arroita G, et al. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015;24:276–292. [Google Scholar]
- 89.Bascompte J, García MB, Ortega R, Rezende EL, Pironon S. Mutualistic interactions reshuffle the effects of climate change on plants across the tree of life. Sci. Adv. 2019;5:eaav2539. doi: 10.1126/sciadv.aav2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- 91.Thuiller W, et al. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 2008;9:137–152. [Google Scholar]
- 92.Mayle FE. Millennial-scale dynamics of southern Amazonian rain forests. Science. 2000;290:2291–2294. doi: 10.1126/science.290.5500.2291. [DOI] [PubMed] [Google Scholar]
- 93.Bullock JM, et al. Human-mediated dispersal and the rewiring of spatial networks. Trends Ecol. Evol. 2018;33:958–970. doi: 10.1016/j.tree.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Ordonez, J. C. Constraints and opportunities for tree diversity management along the forest transition curve to achieve multifunctional agriculture. Curr. Opin. Environ. Sustain.6, 54–60 (2014).
- 95.Levis, C. et al. How People Domesticated Amazonian Forests. Front. Ecol. Evol.5, 171 (2018).
- 96.Mendes P, Velazco SJE, de Andrade AFA, De Marco P. Dealing with overprediction in species distribution models: How adding distance constraints can improve model accuracy. Ecol. Modell. 2020;431:109180. [Google Scholar]
- 97.GBIF. GBIF Occurrence. https://www.gbif.org, 10.15468/dl.vjezvb (2019)
- 98.Carvalho, G. flora: Tools for Interacting with the Brazilian Flora 2020. R package version 0.3.0. (2017).
- 99.Raes N. Partial versus full species distribution models. Nat. Conserv. 2012;10:127–138. [Google Scholar]
- 100.Zizka A, et al. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019;10:744–751. [Google Scholar]
- 101.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna (2020).
- 102.Oliveira U, et al. The strong influence of collection bias on biodiversity knowledge shortfalls of Brazilian terrestrial biodiversity. Divers. Distrib. 2016;22:1232–1244. [Google Scholar]
- 103.Daru BH, et al. Widespread sampling biases in herbaria revealed from large-scale digitization. New Phytol. 2018;217:939–955. doi: 10.1111/nph.14855. [DOI] [PubMed] [Google Scholar]
- 104.Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography. 2015;38:541–545. [Google Scholar]
- 105.Proosdij ASJ, Sosef MSM, Wieringa JJ, Raes N. Minimum required number of specimen records to develop accurate species distribution models. Ecography. 2016;39:542–552. [Google Scholar]
- 106.Beaumont LJ, et al. Which species distribution models are more (or less) likely to project broad-scale, climate-induced shifts in species ranges? Ecol. Modell. 2016;342:135–146. [Google Scholar]
- 107.Fick SE, Hijmans RJ. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. [Google Scholar]
- 108.Fourcade Y, Besnard AG, Secondi J. Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Glob. Ecol. Biogeogr. 2018;27:245–256. [Google Scholar]
- 109.Austin MP, Van Niel KP. Improving species distribution models for climate change studies: Variable selection and scale. J. Biogeogr. 2011;38:1–8. [Google Scholar]
- 110.Woodward, F. I. Climate and Plant Distribution. (Cambridge Univ. Press., 1987).
- 111.IUCN. Plant Growth Forms Classification Scheme. Version: 1.0. https://www.iucnredlist.org/resources/classification-schemes (2020).
- 112.Dormann CF, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46. [Google Scholar]
- 113.Fremout T, et al. Mapping tree species vulnerability to multiple threats as a guide to restoration and conservation of tropical dry forests. Glob. Chang. Biol. 2020;26:3552–3568. doi: 10.1111/gcb.15028. [DOI] [PubMed] [Google Scholar]
- 114.Naimi, B. Package ‘ usdm ’. R Topics Document (2015).
- 115.Syfert MM, et al. Using species distribution models to inform IUCN Red List assessments. Biol. Conserv. 2014;177:174–184. [Google Scholar]
- 116.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006;190:231–259. [Google Scholar]
- 117.Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010;1:330–342. [Google Scholar]
- 118.Muñoz-Pajares AJ, et al. Niche differences may explain the geographic distribution of cytotypes in Erysimum mediohispanicum. Plant Biol. 2018;20:139–147. doi: 10.1111/plb.12605. [DOI] [PubMed] [Google Scholar]
- 119.Peng L-P, et al. Modelling environmentally suitable areas for the potential introduction and cultivation of the emerging oil crop Paeonia ostii in China. Sci. Rep. 2019;9:3213. doi: 10.1038/s41598-019-39449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Merow C, Smith MJ, Silander JA. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography. 2013;36:1058–1069. [Google Scholar]
- 121.Boucher-Lalonde V, Morin A, Currie DJ. How are tree species distributed in climatic space? A simple and general pattern. Glob. Ecol. Biogeogr. 2012;21:1157–1166. [Google Scholar]
- 122.Elith J, Ferrier S, Huettmann F, Leathwick J. The evaluation strip: A new and robust method for plotting predicted responses from species distribution models. Ecol. Modell. 2005;186:280–289. [Google Scholar]
- 123.Jiménez-Valverde A, Lobo JM. Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecologica. 2007;31:361–369. [Google Scholar]
- 124.Betts J, et al. A framework for evaluating the impact of the IUCN Red List of threatened species. Conserv. Biol. 2020;34:632–643. doi: 10.1111/cobi.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.ter Steege H, et al. Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci. Adv. 2015;1:e1500936. doi: 10.1126/sciadv.1500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dauby G, et al. ConR : An R package to assist large-scale multispecies preliminary conservation assessments using distribution data. Ecol. Evol. 2017;7:11292–11303. doi: 10.1002/ece3.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated and codes are available on GBIF https://doi.org/10.15468/dl.vjezvb and on GitHub repository https://github.com/vplima/ENM.git.