Abstract
Background:
Atopic dermatitis (AD) is a common pediatric skin condition with significant morbidity. It is unclear what factors contribute to racial differences in disease prevalence.
Methods:
A single-site, retrospective cohort study of infants born from June 1, 2011, to April 30, 2017, was performed.
Results:
Of the 4016 infants included, 39.2% (n = 1574) were black, 38.5% (n = 1543) white (non-Hispanic), 7.1% (n = 286) Hispanic, 5.3% (n = 213) Asian, 6.5% (n = 262) “other” race, 3.4% (n = 135) multiracial, and 0.1% (n = 3) not reported. Prevalence of AD differed by race, with 37.0% (n = 583) of black, 25.8% (n = 55) of Asian, 24.1% (n = 69) of Hispanic, 23.0% (n = 31) of multiracial, 19.1% (n = 50) of “other” race, and 17.9% (n = 276) of white patients diagnosed (p < .0001). Delivery mode, NICU stay, and gestational age were all significantly associated with race. In modeling AD with logistic regression, race was significantly associated with the development of AD (p < .0001, OR black = 2.6 [2.2–3.2], OR Asian = 1.6 [1.1–2.2], OR Hispanic = 1.4 [1.0–1.9], OR multiracial 1.4 [0.91–2.2], OR “other” 0.97 [0.67–1.4], and OR white 1.0).
Conclusions:
Racial differences in rates of AD arise early in life. Diagnosis is associated with race rather than delivery mode, insurance type, and gestational age. Further investigation into these disparities and interventions to mitigate them should focus on infancy and early childhood.
Keywords: atopic dermatitis, eczema, race, infancy, pediatric
Introduction
Atopic dermatitis (AD, i.e. eczema) is a common, chronic, and relapsing skin condition characterized by xerosis, intense itch, and recurrent infection. Disease onset is typically during infancy or early childhood. Infants classically present with scaly erythematous papules and plaques on the cheeks and extensor surfaces, whereas older children usually present with eczematous plaques of the skin folds. For affected children and their families, AD leads to significant morbidity, including compromised sleep,1 behavioral issues,2 parental guilt and exhaustion,3 and impaired parental employment.3 The national financial impact of AD is staggering, with a total annual cost burden equivalent to 5.297 billion dollars in 2015.4
Though race is known to impact disease course and treatment for a wide range of dermatologic conditions, studies examining the relationship between race and AD are limited. A number of studies on AD lack racial diversity and include primarily white children.5,6 Existing studies with racial diversity are limited by bias. Some studies examined visits rather than specific patients, introducing selection bias toward children with more severe disease and thus more visits. Other studies introduced recall bias by asking parents to remember a diagnosis of eczema rather than review of the medical record.7–10
Most studies show increased prevalence of atopic dermatitis in black and Asian children compared to white children.7,9,11–18 Black and Asian children also have increased disease severity,8 with more frequent and longer hospitalizations for AD.19 Data for Hispanic children is conflicting. Some studies show that AD is less common than in white children,13,17 while others show AD is more common16 and more severe,8 with more frequent and longer hospitalizations.19
Proposed explanations for the racial disparities in AD include genetic variations in skin structure,20 bacterial colonization,21 and lesion appearance.22 Socioeconomic status (SES) appears to play a role as well, though the association is unclear. Studies show a positive correlation between SES and eczema prevalence,7,13 a negative correlation between SES and eczema severity,15,19 and persistence of racial disparities when controlling for SES.7,12–15,18
Herein, we examine the association between AD, race, and potential contributing factors in pediatric patients from a diverse population. A better understanding of racial disparities in AD will aid in developing targeted treatment and prevention strategies.
Materials and Methods
This study was approved by the University of Florida Institutional Review Board.
Infants born at UF Health Shands Hospital from June 1, 2011, to April 30, 2017, were included if they met the following criteria: (1) two or more well-child visits after birth and (2) at least one visit at 300 days of life or later (see Fig. 1). The electronic health record was reviewed retrospectively for information including demographic, birth, and clinical data (sex, race/ethnicity, payer, delivery mode, NICU stay, NICU length of stay, gestational age at birth, and time to diagnosis of atopic dermatitis). Race and ethnicity were self-reported by parents upon initial registration. Documentation of at least one of the following ICD-9 or 10 codes in the UF Health administrative data was used to identify children with atopic dermatitis: 691.8 (other atopic dermatitis and related conditions), L20.83 (infantile (acute) (chronic) eczema), L20.84 (intrinsic (allergic) eczema), L20.89 (other atopic dermatitis), L20.9 (atopic dermatitis, unspecified). Data were inspected for implausible values, missingness, and distributional form. Summary statistics (i.e., means, standard deviations (SD), frequencies) were computed for study variables. Bivariate analyses were conducted using chi-square tests for categorical data and t-tests or ANOVA for continuous data. We used logistic regression to simultaneously examine the association between the predictor variable of race while controlling for gestational age, insurance type, delivery mode, and time spent in the neonatal intensive care unit (NICU), with the response variable of atopic dermatitis. The level of significance was set at .05, and all hypothesis testing was two-sided. SAS version 9.4 (Cary, NC) was used for all analyses.
Figure 1:
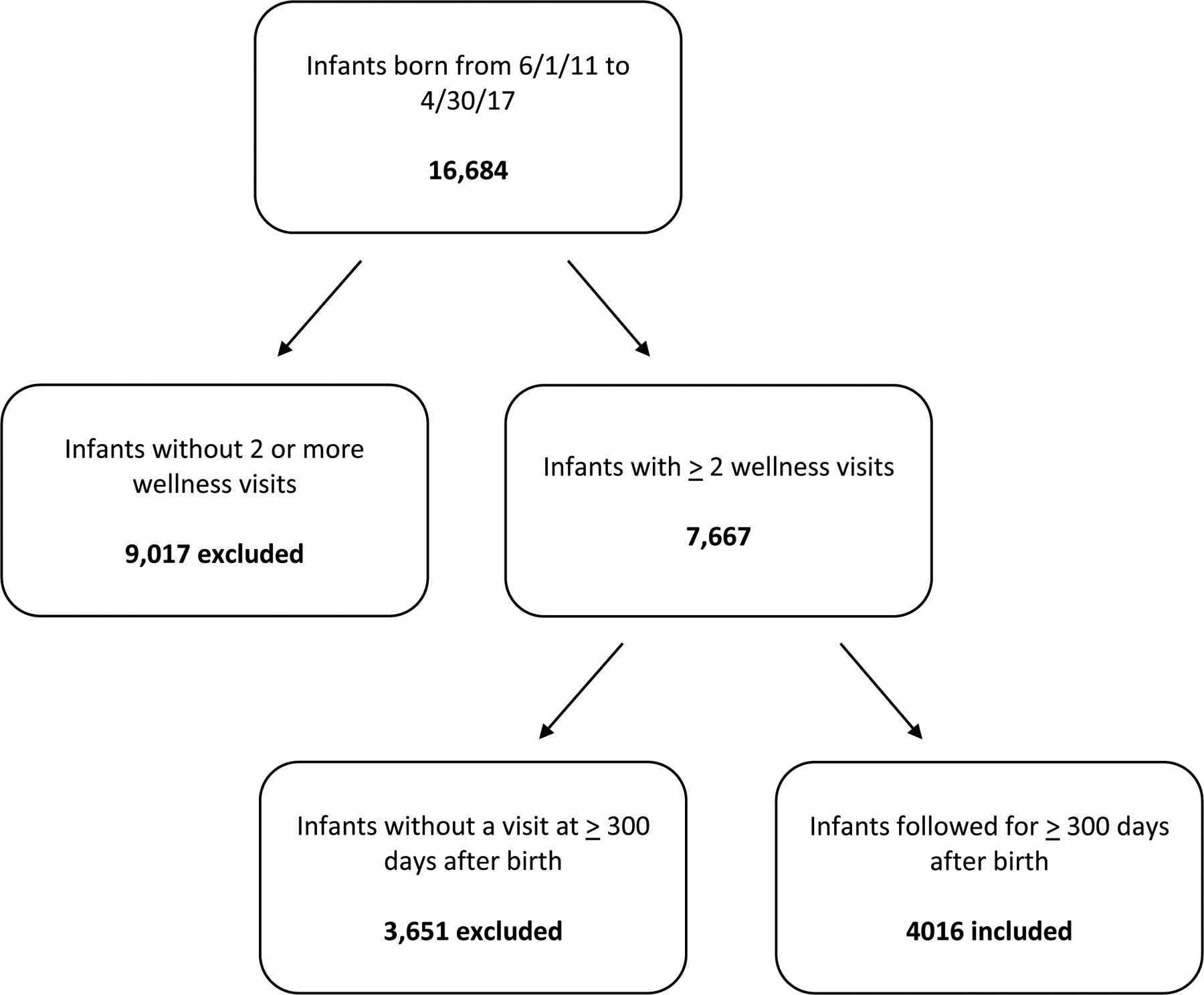
Flowchart of participants included in the study. Infants born at UF Health Shands Hospital from June 1, 2011, to April 30, 2017, were included if they met the following criteria: (1) two or more well-child visits after birth and (2) at least one visit at 300 days of life or later.
Results
Our study sample included 4,016 infants. Racial distribution was diverse with 39.2% (n = 1574) black, 38.5% (n = 1543) white (non-Hispanic), 7.1% (n = 286) Hispanic, 5.3% (n = 213) Asian, 6.5% (n = 262) “other” race, and 3.4% (n = 135) multiracial patients. Approximately half (52.9%, n = 2118) of patients were covered by government insurance (Medicaid and Medicare), and 33.2% (n = 1331) held private insurance. The majority of infants (86.1%, n = 3457) did not have a NICU stay after birth (see Table 1). Delivery mode, insurance coverage, neonatal intensive care unit (NICU) length of stay, and gestational age differed between races (see Table 2).
Table 1.
Infant demographic and clinical features.a
| Characteristic | N (%) or Mean (SD) |
|---|---|
| Sex | |
| Female | 1954 (48.8%) |
| Male | 2052 (51.2%) |
| Race | |
| Asian | 213 (5.3%) |
| Black | 1574 (39.2%) |
| Hispanic | 286 (7.1%) |
| Multiracial | 135 (3.4%) |
| Other | 262 (6.5%) |
| White, non-Hispanic | 1543 (38.5%) |
| Not reported | 3 (0.1%) |
| Payer | |
| Medicaid | 2079 (51.9%) |
| Private insurance | 1331 (33.2%) |
| Managed care | 485 (12.1%) |
| Self-pay | 62 (1.6%) |
| Medicare | 39 (1%) |
| Other | 10 (< 1%) |
| Delivery mode | |
| Vaginal | 2425 (64.9%) |
| Caesarean section | 1309 (35.1%) |
| NICU stay | |
| Mean NICU length of stay (days) | 3.9 (18.1) |
| No | 3457 (86.1%) |
| Yes | 559 (13.9%) |
| Gestational age | |
| Mean Gestational age (weeks) | 38.1 (3.3) |
| Early preterm (< 28 weeks) | 119 (3.1%) |
| Very preterm (28 to < 32 weeks) | 121 (3.2%) |
| Moderate to late preterm (32 to < 37 weeks) | 448 (11.7%) |
| Early term (37 to < 39 weeks) | 1089 (28.3%) |
| Full term (39 to < 41 weeks) | 1765 (45.9%) |
| Late/post term (41 weeks or greater) | 301 (7.8%) |
Categorical responses may not sum to total sample size of 4,016 due to missing data.
Table 2.
Relationship between infant characteristics and race.
| Characteristic | Race/Ethnicity | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall Cohort N (%) | Asian % [95% CI] | Black % [95% CI] | Hispanic % [95% CI] | Multiracial % [95% CI] | Other % [95% CI] | White NH % [95% CI] | p-value | |
| C-section | 1309 (35.1%) | 27.1% [21.1, 33.9] | 37.1% [34.6, 39.6] | 29.8% [24.3, 35.8] | 28.2% [20.5, 37.0] | 31.2% [25.5, 37.3] | 36.3% [33.8, 38.8] | .006 |
| Vaginal | 2425 (64.9%) | 72.9% [66.1, 78.9] | 62.9% [60.4, 65.4] | 70.2% [64.2, 75.7] | 71.8% [63.0, 79.5] | 68.8% [62.7, 74.5] | 63.7% [61.2, 66.2] | |
| Private | 1331 (33.2%) | 85.9% [80.5, 90.3] | 26.4% [24.2, 28.7] | 45.5% [39.6, 51.4] | 54.1% [45.3, 62.8] | 59.5% [53.3, 65.5] | 60.1% [57.6, 62.6] | <.001 |
| Public | 2118 (52.9%) | 14.1% [9.7, 19.5] | 73.6% [71.3, 75.8] | 54.6% [48.6, 60.4] | 45.9% [37.2, 54.7] | 40.5% [34.5, 46.7] | 39.9% [37.4, 42.4] | |
| NICU Stay | 559 (13.9%) | 6.6% [3.6, 10.8] | 14.9% [13.2, 16.7] | 9.8% [6.6, 13.8] | 8.9% [4.7, 15.0] | 11.8% [8.2, 16.4] | 15.5% [13.7, 17.4] | <.001 |
| Early preterm (< 28 wks) | 119 (3.1%) | 1.0% [0.1, 3.5] | 3.5% [2.6, 4.6] | 3.6% [1.7, 6.5] | 1.6% [0.2, 5.6] | < 1.0% [0.1, 2.9] | 3.4% [2.5, 4.4] | <.001 |
| Very preterm (28 to < 32 wks) | 121 (3.2%) | 2.0% [0.5, 5.0] | 3.6% [2.2, 4.7] | 1.1% [0.2, 3.1] | 3.2% [0.9, 7.9] | 2.4% [0.9, 5.2] | 3.3% [2.4, 4.3] | |
| Moderate to late preterm (32 to < 37 wks) | 448 (11.7%) | 5.4% [2.7, 9.5] | 14.0% [12.6, 15.8] | 10.4% [7.1, 14.6] | 11.1% [6.2, 17.9] | 10.6% [7.0, 15.1] | 10.6% [9.1, 12.3] | |
| Early term (37 to < 39 wks) | 1089 (28.3%) | 26.1%) [20.2, 32.7] | 31.6% [29.3, 34.1 | 23.7% [18.9, 29.2] | 31.8% [23.7, 40.6] | 25.6% [20.3, 31.5] | 26.3% [24.0, 28.6] | |
| Full term (39 to < 41 wks) | 1765 (45.9%) | 58.6% [51.5, 65.5] | 42.1% [39.6, 44.6] | 52.2% [46.1, 58.2] | 45.2% [36.4, 54.4] | 49.2% [42.8, 55.6] | 46.5% [43.9, 49.1] | |
| Late/post term (≥ 41 wks) | 301 (7.8%) | 6.9% [3.8, 11.3] | 5.2% [4.1, 6.4] | 9.0% [5.9, 3.0] | 7.1% [3.3, 13.1] | 11.4% [7.7, 16.0] | 10.0% [8.5, 11.6] | |
During the study period, 26.6% (n = 1064) of the sample was diagnosed with atopic dermatitis (95% CI = [25.2%, 27.9%]). Prevalence of atopic dermatitis differed by race, with AD present in 37.0% (n = 583) of black, 25.8% (n = 55) of Asian, 24.1% (n = 69) of Hispanic, 23.0% (n = 31) of multiracial, 19.1% (n = 50) of “other” race, and 17.9% (n = 276) of white patients during the study period (p < .0001, see Table 3). Mean time to diagnosis of AD significantly differed between groups (see Table 3) and ranged from 284 days (Asian infants) to 377 days (white infants).
Table 3.
Relationship between infant characteristics and atopic dermatitis.
| Characteristic | Rate of atopic dermatitis N (%) or Mean (SD) |
P Value | OR [95% CI] |
|---|---|---|---|
| Sex | 0.62 | ||
| Female | 512 (26.2%) | ||
| Male | 552 (26.9%) | ||
| Race/ethnicity | <.001 | ||
| Asian | 55 (25.8%) | 1.6 [1.1, 2.2] | |
| Black | 583 (37.0%) | 2.6 [2.2, 3.2] | |
| Hispanic | 69 (24.1%) | 1.4 [1.0, 1.9] | |
| Multiracial | 31 (23.0%) | 1.4 [0.91, 2.2] | |
| Other | 50 (19.1%) | 0.97 [0.67, 1.4] | |
| White, non-Hispanic | 276 (17.9%) | 1.0 | |
| Payer | <.001 | 1.1 [0.91, 1.3] | |
| Private | 438 (23.3%) | ||
| Public | 622 (29.4%) | ||
| Delivery mode | .01 | 0.86 [0.73, 1.0] | |
| Caesarean section | 315 (24.1%) | ||
| Vaginal | 675 (27.8%) | ||
| NICU stay | <.001 | 0.99 [0.98, 1.0] | |
| No | 960 (27.8%) | ||
| Yes | 105 (18.8%) | ||
| NICU length of stay (days) | <.001 | ||
| No atopic dermatitis | 4.6 (20.1) | ||
| Atopic dermatitis | 2.0 (10.9) | ||
| Gestational age (weeks) | < .001 | 1.0 [0.99, 1.1] | |
| Early preterm (< 28 weeks) | 13 (10.9%) | ||
| Very preterm (28 to < 32 weeks) | 23 (19.0%) | ||
| Moderate to late preterm (32 to < 37 weeks) | 129 (28.8%) | ||
| Early term (37 to < 39 weeks) | 295 (27.1%) | ||
| Full term (39 to < 41 weeks) | 486 (27.5%) | ||
| Late/post term (41 weeks or greater) | 72 (23.9%) | ||
| Time to AD diagnosis (days) | .009 | ||
| Asian | 284 (268) | ||
| Black | 305 (317) | ||
| Hispanic | 358 (328) | ||
| Multiracial | 317 (325) | ||
| Other | 347 (371) | ||
| White | 377 (339) |
Payer was significantly associated with the development of atopic dermatitis; 29.4% (n = 622) of children with public and 23.3% (n = 438) of children with private insurance were diagnosed with AD (p < .0001). Delivery mode, neonatal intensive care unit (NICU) stay, and gestational age were all also significantly associated with atopic dermatitis (see Table 3). In modeling AD with logistic regression including these predictor variables, only NICU stay (p =0.039, OR = 0.99 [0.98–1.0]) and race (p < .0001, OR black = 2.6 [2.2–3.2], OR Asian = 1.6 [1.1–2.2], OR Hispanic = 1.4 [1.0–1.9], OR multiracial = 1.4 [0.91–2.2], OR “other” = 0.97 [0.67–1.4], OR white = 1.0) were significantly associated with the development of AD (see Table 4).
Table 4:
Logistic Regression: Odds ratio estimates with outcome of atopic dermatitis.
| Odds Ratio Estimates | Logistic Regression | |||
|---|---|---|---|---|
| Race (compared with white infants) | OR | 95% CI | p value | |
| Asian | 1.6 | 1.1 | 2.2 | 0.016 |
| Black | 2.6 | 2.2 | 3.2 | <.001 |
| Hispanic | 1.4 | 1.0 | 1.9 | 0.038 |
| Multiracial | 1.4 | 0.91 | 2.2 | 0.127 |
| Other | 0.97 | 0.67 | 1.4 | 0.852 |
| Delivery mode (c-section vs vaginal) | 0.86 | 0.73 | 1.0 | 0.076 |
| NICU stay | 0.99 | 0.98 | 1.0 | 0.039 |
| Gestational age | 1.0 | 0.99 | 1.1 | 0.177 |
| Insurance type (public vs private) | 1.1 | 0.91 | 1.3 | 0.434 |
Discussion
Our results show striking racial differences in rates of AD arising early in life. Racial differences in insurance type, delivery mode, and gestational age did not explain the association.
In this sample, black infants were diagnosed with AD significantly more often than white infants (37.0% vs 17.9 %) with an odds ratio for the development of atopic dermatitis of 2.6 compared to white infants. Nearly a quarter of Asian and Hispanic children were diagnosed with AD during the study period (25.8% and 24.1%, respectively), with odds ratios for the development of atopic dermatitis of 1.6 and 1.4 compared to white children, respectively. This is consistent with prior studies showing a higher prevalence of AD in black, Hispanic, and Asian children compared to white children, though as previously mentioned, data for Hispanic children is conflicting.7,9,11–18 Our study, however, observed more pronounced racial differences and higher overall AD prevalence than in prior studies (8.7–16.1% for white children and 15.9–19.3% for black children7,12,13,17). These findings may be driven by several factors. First, we specifically examined the early childhood period. AD is more common in infants and young children,7,11,13 and 80% of children outgrow the condition by eight years old.23 Inclusion of older children and teens in our analysis would dilute AD prevalence, as was seen in prior studies examining kids from infancy into adulthood.7,11,13,17 Wegienka et al.,18 who reported findings most similar to ours (AD prevalence of 13.5% for white and 27% for black children), examined the prevalence of atopic dermatitis at age two. Second, our data included atopic dermatitis diagnosed by a healthcare provider, as indicated by an ICD-9 or 10 code. These methodological measures were employed to minimize the recall and selection biases present in prior studies which may have affected disease prevalence. Third, our study drew from the diverse population served by our institution, including Asian, Hispanic, black, and white children, while many prior studies had limited racial diversity. Specifically, the high percentage of black babies in our sample (39.2%) makes our study more inclusive and relevant to this population, providing a more accurate representation of true disease prevalence.
Reasons for the racial differences in AD are unclear. Socioeconomic status (SES) is a commonly proposed explanation. In the current literature, the relationship between SES and eczema prevalence is complex, with seemingly conflicting relationships described. Eczema prevalence is greater in children of higher SES,7,13 yet eczema severity and hospital admissions are associated with lower household income, lower parental education level, public insurance, and non-white race/ethnicity.15,19 Institutional racism likely contributes to the aforementioned effect, with limited access to care causing later presentation when disease is more severe. We found that public insurance (used as a marker of SES) was significantly associated with AD prevalence in the present study but did not explain the relationship between race and eczema (p = 0.434), consistent with a number of prior studies.7,12–15,18.
Underlying genetic variations in skin structure20 and bacterial colonization21 may play a role. Atopic dermatitis in African Americans and Asians has distinct a molecular profile compared to that of European Americans.24,25 Ceramide/cholesterol ratios also differ beetween races,20 impacting skin barrier function, an important factor in the pathogenesis of atopic dermatitis. Filaggrin is a structural protein imperative for epidermal barrier function. Filaggrin loss of function mutations are known to vary by race in children and may also play a role in the racial differences seen.26
Cultural and environmental factors also likely contribute. Attending daycare and shorter duration of breastfeeding are risk factors for atopic dermatitis that may differ between races.9 Skin care regimens and interactions with the skin microbiome may also contribute. Larger studies are needed to evaluate the complex interplay of these factors
Another possible explanation is that the racial differences in AD lesional morphology complicate or expedite diagnosis. For example, the subtle distinction between infantile atopic and seborrheic dermatitis may be more apparent in lighter skin compared to darker skin. In a recent review of images in two major dermatology textbooks, only 22 to 32% of images were of individuals with skin of color.27 Thus, inadequate training in skin of color may lead rashes unrelated to AD to be labeled as AD, thus increasing apparent disease prevenance.
Limitations of this study include duration of follow-up, which differed for each patient based on birthdate during the study period. Differences between duration of follow-up for groups of participants may have biased the “opportunity” for some patients to develop eczema. Additionally, medical providers with differing clinical specialties diagnosed AD; diagnosis was not confirmed by a dermatologist or other subspecialist trained in atopic dermatitis (unless the child was seen in a subspecialty clinic during the study period) and instead was based on ICD diagnostic codes. We examined a single, tertiary care institution, so results may not be applicable to smaller, community hospital settings.
In our cohort, racial differences in rates of AD were apparent early in life. Diagnosis is associated with race rather than the examined confounders. Further investigation into these disparities and subsequent interventions to mitigate them should focus on infancy and early childhood.
Acknowledgements
We acknowledge the University of Florida Integrated Data Repository (IDR) and the UF Health Office of the Chief Data Officer for providing the analytic data set for this project. Additionally, the Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards UL1TR000064 and UL1TR001427.
Funding:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards UL1TR000064 and UL1TR001427
Footnotes
Conflict of interest: None.
References
- 1.Bender BG, Leung SB, Leung DYM. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: An objective life quality measure. J Allergy Clin Immunol 2003; 111: 598–602. [DOI] [PubMed] [Google Scholar]
- 2.Daud LR, Garralda ME, David TJ. Psychosocial adjustment in preschool children with atopic eczema. Arch Dis Child 1993; 69: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapidus CS, Kerr PE. Social impact of atopic dermatitis. Med Health R I 2001; 84: 294–295. [PubMed] [Google Scholar]
- 4.Drucker AM, Wang AR, Li W-Q, Sevetson E, Block JK, Qureshi AA. The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J Invest Dermatol 2017; 137: 26–30. [DOI] [PubMed] [Google Scholar]
- 5.Egeberg A, Andersen YMF, Gislason G, Skov L, Thyssen JP. Neonatal risk factors of atopic dermatitis in Denmark - Results from a nationwide register-based study. Pediatr Allergy Immunol 2016; 27: 368–374. [DOI] [PubMed] [Google Scholar]
- 6.Mallen CD, Mottram S, Wynne-Jones G, Thomas E. Birth-related exposures and asthma and allergy in adulthood: a population-based cross-sectional study of young adults in North Staffordshire. J Asthma 2008; 45: 309–312. [DOI] [PubMed] [Google Scholar]
- 7.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol 2011; 131: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis 2014; 25: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Sundell J. Life style and home environment are associated with racial disparities of asthma and allergy in Northeast Texas children. Sci Total Environ 2011; 409: 4229–4234. [DOI] [PubMed] [Google Scholar]
- 10.Fischer AH, Shin DB, Margolis DJ, Takeshita J. Racial and ethnic differences in health care utilization for childhood eczema: An analysis of the 2001–2013 Medical Expenditure Panel Surveys. J Am Acad Dermatol 2017; 77: 1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horii KA, Simon SD, Liu DY, Sharma V. Atopic dermatitis in children in the United States, 1997–2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics 2007; 120: e527–e534. [DOI] [PubMed] [Google Scholar]
- 12.Williams HC, Pembroke AC, Forsdyke H, Boodoo G, Hay RJ, Burney PG. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol 1995; 32: 212–217. [DOI] [PubMed] [Google Scholar]
- 13.Fu T, Keiser E, Linos E, Rotatori RM, Sainani K, Lingala B et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol 2014; 31: 21–26. [DOI] [PubMed] [Google Scholar]
- 14.Moore MM, Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Camargo CA, Gold DR et al. Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics 2004; 113: 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mar A, Tam M, Jolley D, Marks R. The cumulative incidence of atopic dermatitis in the first 12 months among Chinese, Vietnamese, and Caucasian infants born in Melbourne, Australia. J Am Acad Dermatol 1999; 40: 597–602. [DOI] [PubMed] [Google Scholar]
- 16.Mahdavinia M, Fox SR, Smith BM, James C, Palmisano EL, Mohammed A et al. Racial Differences in Food Allergy Phenotype and Health Care Utilization among US Children. J Allergy Clin Immunol Pract 2017; 5: 352–357.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief 2013; : 1–8. [PubMed] [Google Scholar]
- 18.Wegienka G, Havstad S, Joseph CL, Zoratti E, Ownby D, Woodcroft K et al. Racial Disparities in Allergic Outcomes in African Americans Emerge as Early as Age 2 Years. Clin Exp Allergy 2012; 42: 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narla S, Hsu DY, Thyssen JP, Silverberg JI. Predictors of Hospitalization, Length of Stay, and Costs of Care Among Adult and Pediatric Inpatients With Atopic Dermatitis in the United States. Dermatitis 2018; 29: 22–31. [DOI] [PubMed] [Google Scholar]
- 20.Jungersted JM, Høgh JK, Hellgren LI, Jemec GBE, Agner T. Ethnicity and stratum corneum ceramides. Br J Dermatol 2010; 163: 1169–1173. [DOI] [PubMed] [Google Scholar]
- 21.Merriman JA, Mueller EA, Cahill MP, Beck LA, Paller AS, Hanifin JM et al. Temporal and Racial Differences Associated with Atopic Dermatitis Staphylococcus aureus and Encoded Virulence Factors. mSphere 2016; 1: e00295–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vachiramon V, Tey HL, Thompson AE, Yosipovitch G. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol 2012; 29: 395–402. [DOI] [PubMed] [Google Scholar]
- 23.Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): A systematic review and meta-analysis. J Am Acad Dermatol 2016; 75: 681–687.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. Journal of Allergy and Clinical Immunology 2015; 136: 1254–1264. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal RD, Pavel AB, Glickman J, Chan TC, Zheng X, Zhang N et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol 2019; 122: 99–110.e6. [DOI] [PubMed] [Google Scholar]
- 26.Margolis DJ, Mitra N, Wubbenhorst B, D’Andrea K, Kraya AA, Hoffstad O et al. Association of Filaggrin Loss-of-Function Variants With Race in Children With Atopic Dermatitis. JAMA Dermatol 2019. doi: 10.1001/jamadermatol.2019.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lester JC, Taylor SC, Chren M-M. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol 2019; 180: 1521–1522. [DOI] [PubMed] [Google Scholar]


