Abstract
OBJECTIVES
The authors aimed to study the sensitivity and specificity of exercise treadmill testing (ETT) in the diagnosis of coronary microvascular disease (CMD), as well as the prognostic implications of ETT results in patients with CMD.
BACKGROUND
ETT is validated to evaluate for flow-limiting coronary artery disease (CAD), however, little is known about its use for evaluating CMD.
METHODS
We retrospectively studied 249 consecutive patients between 2006 and 2016 who underwent ETT and positron emission tomography within 12 months. Patients with obstructive CAD or left ventricular systolic dysfunction were excluded. CMD was defined as a coronary flow reserve <2. Patients were followed for the occurrence of a first major adverse event (composite of death or hospitalization for myocardial infarction or heart failure).
RESULTS
The sensitivity and specificity of a positive ETT to detect CMD were 34.7% (95% CI: 25.4%–45.0%) and 64.9% (95% CI: 56.7%–72.5%), respectively. The specificity of a positive ETT to detect CMD increased to 86.8% (95% CI: 80.3%–91.7%) when only classifying studies with ischemic electrocardiogram changes that lasted at least 1 minute into recovery as positive, although at a cost of lower sensitivity (15.3%; 95% CI: 8.8%–24.0%). Over a median follow-up of 6.9 years (interquartile range: 5.1 years–8.2 years), 30 (12.1%) patients met the composite endpoint, including 13 (13.3%) with CMD (n = 98). In patients with CMD, ETT result was not associated with the composite endpoint (P = 0.076).
CONCLUSIONS
Our data suggest limited sensitivity of ETT to detect CMD. However, a positive ETT with ischemic changes that persist at least 1 minute into recovery in the absence of obstructive CAD should raise suspicion for the presence of CMD given a high specificity. Further study is needed with larger patient sample sizes to assess the association between ETT results and outcomes in patients with CMD.
Keywords: coronary flow reserve, coronary microvascular disease, exercise stress testing
Coronary microvascular disease (CMD) refers to a subtype of nonobstructive coronary artery disease (CAD) that affects the structure and function of the coronary microcirculation. CMD is prevalent across a broad spectrum of cardiovascular risk factors and is associated with increased morbidity and death even in the absence of flow-limiting epicardial disease (1,2). Precise diagnosis of CMD can help direct therapy and has been associated with improved quality of life (3). Although coronary angiography is commonly used to evaluate symptomatic patients for obstructive CAD, its use to diagnose CMD is limited because the small coronary vasculature is beyond the resolution of coronary angiography. Consequently, the diagnosis of CMD requires direct interrogation (invasive or noninvasive) of coronary microvascular function (4). Because the widespread use of invasive coronary physiological assessment (including vascular function assessment with adenosine and endothelial function assessment with acetylcholine challenge (5)) has been limited, cardiac positron emission tomography (PET) imaging has emerged as an alternative approach for the evaluation of CMD (6–8).
Electrocardiogram (ECG) exercise treadmill testing (ETT) has a class I recommendation for the assessment of exercise tolerance, symptoms, arrhythmias, blood pressure response, and event risk in selected patients (9). It is also the recommended initial test in the evaluation of patients who can exercise and have an interpretable ECG at baseline (10,11). However, little is actually known about its relative utility in the evaluation of patients with suspected CMD, and current approaches for the evaluation of CMD are based only on expert consensus (4,12). In this study, we sought to evaluate both the diagnostic and prognostic value of ETT in CMD.
METHODS
POPULATION.
We retrospectively studied consecutive patients who were scheduled to undergo clinically indicated ETT (with or without imaging) at Brigham and Women’s Hospital or Massachusetts General Hospital (both in Boston, Massachusetts) and a PET myocardial perfusion study with coronary flow reserve (CFR) assessment at Brigham and Women’s Hospital for evaluation of symptoms suspected of CAD between 2006 and 2016. From 1,428 patients, we excluded those with >12 months between ETT and PET study dates, known CAD, left ventricular (LV) ejection fraction <40%, history of cardiac transplantation, left bundle branch block, ventricular pacing, or ST-segment depression ≥1 mm on baseline ECG, abnormal myocardial perfusion on PET consistent with obstructive CAD (summed stress score ≥2) (13), patients who did not undergo ETT at the time of their scheduled stress test, and patients who had a nonfatal myocardial infarction (MI) or heart failure admission between their ETT and PET (Figure 1). No patients underwent coronary revascularization between studies. Of the 249 patients in the final cohort, a majority (n = 220; 88.4%) were referred for ETT prior to (n = 184; 73.9%) or on the same day (n = 36; 14.5%) as PET. Patient demographics, clinical history, medications, and indications for testing were collected prospectively at the time of ETT and PET. Pre-test probability of obstructive CAD was assigned per recent guidelines (9,14). The Mass General Brigham Institutional Review Board approved this study.
FIGURE 1. Patient Population and ETT Results.
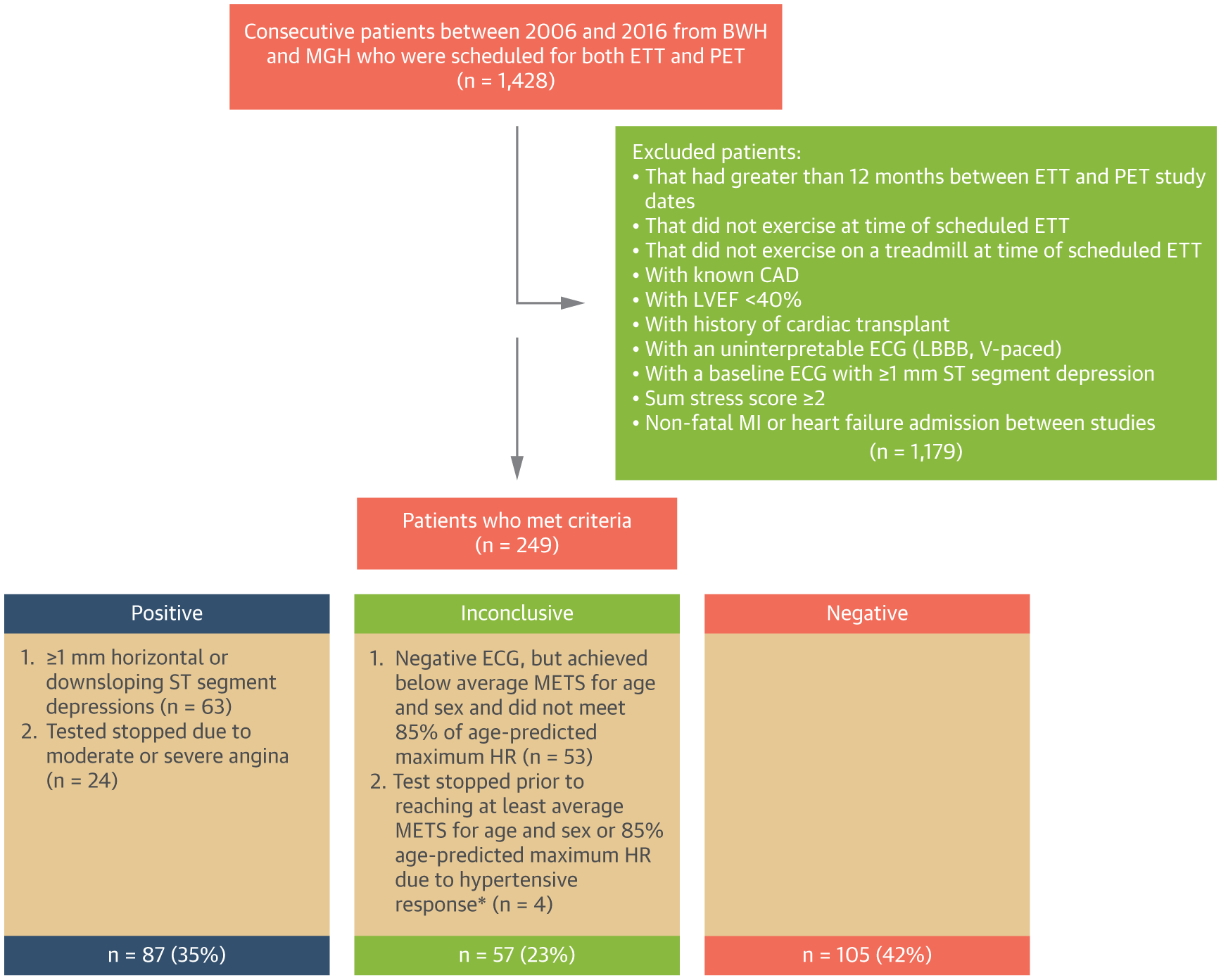
Study cohort derivation, definitions of ETT results, and frequency of ETT results. *Hypertensive response = systolic blood pressure of >250 mm Hg and/or diastolic blood pressure of >115 mm Hg. BWH = Brigham and Women’s Hospital; CAD = coronary artery disease; ECG = electrocardiogram; ETT = exercise treadmill test; HR = heart rate; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; METS = metabolic equivalents; MGH = Massachusetts General Hospital; MI = myocardial infarction; MPHR = maximum predicted heart rate; PET = positron emission tomography; SSS = sum stress score; V-paced = ventricularly paced rhythm; VT = ventricular tachycardia.
EXERCISE TREADMILL TESTING.
ETT was performed with the use of a symptom-limited Bruce protocol, modified Bruce protocol, ramp protocol, or manual protocol if clinically indicated, according to established guidelines (10). The target heart rate was determined as 85% of the maximum predicted heart rate (MPHR = 220 – age). All ST-segment measurements were performed 80 milliseconds after the J-point (15).
We categorized each test result as positive, inconclusive, or negative using conventional criteria (10,16). Positive tests were defined as: 1) horizontal or downsloping ST-segment depressions ≥1 mm; or 2) tests stopped due to moderate to severe angina (16) (substernal chest discomfort relieved with rest or nitroglycerin). Time in recovery to resolution of ST-segment depressions was recorded for all positive tests when applicable. Inconclusive tests were defined as: 1) negative ECG in the setting of sub-maximal exercise (inability to achieve at least average METS for age and sex (17,18) and did not reach ≥85% MPHR); or 2) test stopped prior to reaching at least average METS for age and sex or 85% MPHR due to hypertensive response (systolic blood pressure of >250 mm Hg and/or diastolic blood pressure of >115 mm Hg) (16). All stress ECGs were re-reviewed independently by 2 cardiologists and differences were resolved by consensus.
PET AND DIAGNOSIS OF CMD.
Patients were imaged with a standard hybrid whole-body PET-computed tomography scanner (Discovery RX or STE LightSpeed 64, GE Healthcare) with 13N-ammonia or 82Rubidium as flow tracers at rest and vasodilator-stress, as previously described (19). Summed rest, stress, and difference scores, with higher scores reflecting larger area of myocardial scar, scar plus ischemia, or ischemia, respectively, were computed (13). Rest and stress LV ejection fractions were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM, INVIA Medical Imaging Solutions). Absolute global myocardial blood flow (in mL/min/g of tissue) was quantified at rest and peak hyperemia using commercial software, as previously described (19). Per-patient global CFR was calculated as the ratio of stress to rest absolute myocardial blood flow for the entire LV. Quantitative measures of CFR were obtained from routine post-processing of PET scans at no additional clinical cost, imaging time, or radiation exposure to patients. CMD was defined as CFR <2.
OUTCOMES.
Patients were followed for the occurrence of a first major adverse event, defined as a composite of death or hospitalization for nonfatal MI or heart failure. Day 0 was defined as the date of the first study performed (ETT or PET). Ascertainment of clinical endpoints (death, MI hospitalization, and heart failure hospitalization) was determined by blinded adjudication of the longitudinal medical record, Mass General Brigham Research Patient Data Registry, and the National Death Index. For an event to be classified as admission for nonfatal MI or heart failure, discharge with a primary hospitalization diagnosis of MI or heart failure, respectively, was required. In addition, only events meeting the 2018 Fourth Universal Definition of MI or defined clinical criteria for the presence of symptoms, signs, and escalation of therapy for heart failure were classified as such (20).
STATISTICAL ANALYSIS.
Categorical variables are reported as frequencies with percentage. Continuous variables are expressed as mean ± SD. We used chi-square and one-way analysis of variance to evaluate for differences in categorical and continuous baseline characteristics, respectively. The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio along with exact (Clopper-Pearson) 95% CIs, were calculated for all ETT studies using CFR <2 by PET as the gold standard in the entire cohort and in women only. We also performed 2 sensitivity analyses by narrowing the criteria for a positive ETT to: 1) horizontal or downsloping ST-segment depressions ≥1 mm, and 2) horizontal or downsloping ST-segment depressions ≥1 mm that persisted at least 1 minute into recovery because previous data have shown that patients who have normalization of ischemic ECG changes quickly in recovery often have normal subsequent study findings (21).
To study the prognostic use of ETT in CMD, we constructed Kaplan-Meier curves to illustrate survival free from first major adverse event. Differences between ETT subgroups were tested using the log-rank test. Cox proportional hazards models were used to examine the association between ETT result in patients with CMD (CFR <2). Cox proportional hazards assumptions tests based on graphical methods and Schoenfeld residuals were used to verify that proportional hazards assumptions were met. All tests were 2-sided, and a value of P < 0.05 was considered statistically significant. Statistical analysis was performed with the use of Stata version 15.0 (Statacorp).
RESULTS
STUDY POPULATION.
The baseline characteristics of the study population stratified by ETT examination results are presented in Table 1. The mean age of the overall cohort was 58.9 ± 11.5 years, 64% were women, and 54% were white. ETT was negative for ischemia in 105 (42%), inconclusive in 57 (23%), and positive for ischemia in 87 (35%) patients (Table 1). The baseline age, body mass index, sex, and race did not differ across the ETT groups. Patients with inconclusive ETTs were more likely to have diabetes and to be current smokers, but the frequency of smoking in the study population was low (8%). The prevalence of hypertension and dyslipidemia were similar across the 3 ETT groups. Among the 249 ETT examinations performed, 195 (78.3%) were performed without imaging. The median time between ETT and PET was 18 days (interquartile range [IQR]: 2 days-123 days). The mean stress and rest global myocardial blood flow were 2.4 ± 0.8 mL/min/g and 1.1 ± 0.4 mL/min/g, respectively, with a mean CFR of 2.3 ± 0.7. The median CFR was similar (2.2; IQR: 1.7–2.8).
TABLE 1.
Baseline Characteristics Stratified by ETT Results
| All (N = 249) | Negative ETT (n = 105) | Inconclusive ETT (n = 57) | Positive ETT (n = 87) | P Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y) | 58.9 ± 11.5 | 59.3 ± 11.8 | 57.4 ± 11.7 | 58.7 ± 11.0 | 0.429 |
| Female | 158 (63.5) | 65 (61.9) | 37 (64.9) | 56 (64.4) | 0.908 |
| White | 135 (54.2) | 62 (59.1) | 23 (40.4) | 50 (57.5) | 0.056 |
| Black | 56 (22.5) | 25 (23.8) | 15 (26.3) | 16 (18.4) | 0.491 |
| BMI (kg/m2) | 30.1 ± 6.7 | 30.1 ± 6.9 | 31.5 ± 6.9 | 29.2 ± 6.4 | 0.129 |
| Pre-test probability of obstructive CAD (%) | 12.9 ± 10.1 | 12.7 ± 10.2 | 13.1 ± 10.6 | 12.8 ± 9.8 | 0.975 |
| Medical history | |||||
| Hypertension | 168 (67.5) | 70 (66.7) | 44 (77.2) | 54 (62.1) | 0.162 |
| Diabetes | 59 (23.7) | 18 (17.1) | 20 (35.1) | 21 (24.1) | 0.037 |
| Dyslipidemia | 147 (59.0) | 68 (64.8) | 28 (49.1) | 51 (58.6) | 0.154 |
| COPD | 12 (4.8) | 1 (1.0) | 6 (10.5) | 5 (5.8) | 0.022 |
| Tobacco use | 20 (8.0) | 7 (6.7) | 10 (17.5) | 3 (3.5) | 0.008 |
| Medications | |||||
| Aspirin | 126 (50.6) | 50 (47.6) | 27 (47.4) | 49 (56.3) | 0.417 |
| Beta-blocker | 112 (45.0) | 43 (41.0) | 31 (54.4) | 38 (43.7) | 0.248 |
| RAS-blocker | 70 (28.1) | 27 (25.7) | 17 (29.8) | 26 (29.9) | 0.772 |
| Statin | 116 (46.6) | 49 (46.7) | 22 (38.6) | 45 (51.7) | 0.303 |
| Insulin | 21 (8.4) | 7 (6.7) | 8 (14.0) | 6 (6.9) | 0.222 |
| Nitrate | 15 (6.0) | 4 (3.8) | 2 (3.5) | 9 (10.3) | 0.110 |
| PET imaging parameters | |||||
| Change in LVEF (%) (stress-rest) | 6.5 ± 5.5 | 6.0 ± 5.6 | 5.8 ± 5.2 | 7.5 ± 5.6 | 0.102 |
| Change in EDV (mL) (stress-rest) | 7.4 ± 12.7 | 9.2 ± 11.0 | 6.3 ± 12.2 | 6.0 ± 14.6 | 0.164 |
| Change in ESV (mL) (stress-rest) | −3.4 ± 6.7 | −2.5 ± 6.6 | −3.4 ± 6.2 | −4.5 ± 7.2 | 0.126 |
| Rest MBF (mL/min/g) | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.2 | 1.1 ± 0.4 | 0.233 |
| Stress MBF (mL/min/g) | 2.4 ± 0.8 | 2.5 ± 0.9 | 2.3 ± 0.8 | 2.4 ± 0.8 | 0.307 |
| CFR | 2.3 ± 0.7 | 2.3 ± 0.7 | 2.3 ± 0.8 | 2.3 ± 0.6 | 0.831 |
| CFR <2 | 98 (39.4) | 39 (37.1) | 25 (43.9) | 34 (39.1) | 0.704 |
Values are mean ± SD or n (%). Baseline demographics, medical history, medications, and noninvasive imaging parameters for the study cohort stratified by ETT results.
BMI = body mass index; CAD = coronary artery disease; CFR = coronary flow reserve; COPD = chronic obstructive pulmonary disease; EDV = end-diastolic volume; ESV = end-systolic volume; ETT = exercise treadmill testing; LVEF = left ventricular ejection fraction; MBF = myocardial blood flow; PET = positron emission tomography; RAS = renin-angiotensin system.
The baseline characteristics of patients with and without CMD are presented in Supplemental Table 1. CFR was reduced (<2) in 98 (39.4%) patients and preserved in 151 (60.6%) patients. The mean ages of patients with and without CMD were 61.0 ± 11.5 and 57.5 ± 11.4 years, respectively (P = 0.022). The baseline body mass index, sex, race, comorbidities, and medications did not differ significantly between the 2 groups.
OTHER TESTING AFTER A POSITIVE ETT.
In addition to PET, 15 of the 87 patients who had a positive ETT (17.2%) underwent coronary computed tomography angiography or invasive coronary angiography within 12 months of their PET study (9 on the same day, 4 after the PET, and 2 before the PET). None of these 15 studies showed obstructive coronary disease (defined as a luminal stenosis of ≥70% in any major epicardial vessel). An additional 52 patients (59.8%) underwent formal coronary artery calcium (CAC) scoring at the time of PET. Among these 52 studies, 32 (61.5%) had no CAC (CAC score 0), 5 (9.6%) had mild CAC (CAC score 1–100), 7 (13.5%) had moderate CAC (CAC score 101–500), and 8 (15.4%) had extensive CAC (CAC score >500).
RELATIONSHIP BETWEEN ETT RESULT AND CFR.
Overall, 98 patients (39.4%) of the study cohort had a CFR <2, consistent with CMD. There were no statistical differences in the prevalence of CMD across the negative, inconclusive, or positive ETT groups (Table 1). Baseline characteristics of the study cohort stratified by CFR <2 and CFR ≥2 are listed in Supplemental Table 1 and baseline characteristics of the CMD subgroup stratified by ETT result are listed in Supplemental Table 2.
The sensitivity and specificity of a positive ETT to detect CMD in the entire cohort were 34.7% (95% CI: 25.4%–45.0%) and 64.9% (95% CI: 56.7%–72.5%), respectively (Table 2). When the criteria for a positive ETT were narrowed to only ischemic ECG changes, the sensitivity and specificity to detect CMD were 22.5% (95% CI: 14.6%–32.0%) and 76.2% (95% CI: 68.6%–82.7%), respectively (Table 2). Finally, when the criteria for a positive ETT were further narrowed to only ischemic ECG changes that persisted at least 1 minute into recovery, the sensitivity and specificity to detect CMD were 15.3% (95% CI: 8.8%–24.0%) and 86.8% (80.3%–91.7%), respectively (Table 2). The positive and negative likelihood ratios were close to 1 for each of the criteria cutoffs. When including only women (n = 158), the positive likelihood ratio was higher across the positive ETT criteria (Table 3).
TABLE 2.
Test Characteristics: ETT for CMD (Entire Cohort)
| All Patients (N = 249) | ||||||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI), % | Specificity (95% CI), % | Positive Predictive Value (95% CI), % | Negative Predictive Value (95% CI), % | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) | |
| Positive ETT | 34.7 (25.4–45.0) | 64.9 (56.7–72.5) | 39.1 (28.8–50.5) | 60.5 (52.5–68.1) | 0.99 (0.70–1.40) | 1.01 (0.84–1.21) |
| Positive ETT with ischemic ECG changes | 22.5 (14.6–32.0) | 76.2 (68.6–82.7) | 37.9 (25.5–51.6) | 60.2 (52.9–67.2) | 0.94 (0.59–1.50) | 1.02 (0.89–1.17) |
| Positive ETT with ischemic ECG changes that persist at least 1 min into recovery | 15.3 (8.8–24.0) | 86.8 (80.3–91.7) | 42.9 (26.3–60.7) | 61.2 (54.3–67.8) | 1.16 (0.62–2.15) | 0.98 (0.88–1.08) |
Gold standard for coronary microvascular disease was CFR <2 via stress positron emission tomography.
CMD = coronary microvascular disease; ECG = electrocardiogram; other abbreviations as in Table 1.
TABLE 3.
Test Characteristics: ETT For CMD (Women Only)
| Women Only (n = 158) | ||||||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI), % | Specificity (95% CI), % | Positive Predictive Value (95% CI), % | Negative Predictive Value (95% CI), % | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) | |
| Positive ETT | 38.3 (26.1–51.8) | 66.3 (56.1–75.6) | 41.1 (28.1–55.0) | 63.7 (53.6–73.0) | 1.14 (0.74–1.74) | 0.93 (0.73 to 1.19) |
| Positive ETT with ischemic ECG changes | 26.7 (16.1–39.7) | 78.6 (69.1–86.2) | 43.2 (27.1–60.5) | 63.6 (54.4–72.2) | 1.24 (0.71–2.19) | 0.93 (0.78–1.12) |
| Positive ETT with ischemic ECG changes that persist at least 1 min into recovery | 18.3 (8.8–24.0) | 87.8 (80.3–91.7) | 47.8 (26.8–69.4) | 63.7 (55.0–71.8) | 1.50 (0.71–3.18) | 0.93 (0.81–1.07) |
RELATIONSHIP BETWEEN SYMPTOMS AND WORKLOAD DURING ETT AND CFR.
A total of 201 (80.7%) patients were referred to undergo an ETT for the evaluation of chest pain (22% typical, 39% atypical, and 39% non-anginal) or dyspnea. The remaining patients were referred for risk factors for CAD or abnormal ECG (n = 19; 7.7%), perioperative risk assessment (n = 13; 5.2%), palpitations/arrhythmia (n = 12; 4.8%), or presyncope/syncope (n = 4; 1.6%). During the ETT, 73 (29.3%) patients reported chest pain and 66 (26.5%) patients reported dyspnea (47 [18.9%] had dyspnea alone). As shown in Table 4, there was no significant relationship seen between ETT symptoms (chest pain or dyspnea) and CFR (2.0 cutoff). The mean Duke Treadmill Score and the proportion of patients with less than average METS for age and sex were similar in both groups.
TABLE 4.
Symptoms And Exercise Characteristics Stratified By CFR
| CFR ≥2 (n = 151) | CFR <2 (n = 98) |
P Value | |
|---|---|---|---|
| Dyspnea | 34 (22.5) | 32 (32.7) | 0.077 |
| Chest pain | 49 (32.5) | 24 (24.5) | 0.178 |
| METS less than average for age and sex | 51 (34.7) | 38 (40.0) | 0.403 |
| Duke Treadmill Score | 4.1 ± 0.4 | 3.7 ± 0.5 | 0.438 |
Values are n (%) or mean ± SD. Frequency of symptoms during ETT, functional capacity, and Duke Treadmill Score. Patients could report dyspnea and chest pain.
Abbreviations as in Table 1.
CLINICAL OUTCOMES.
Over a median follow-up of 6.9 years (IQR: 5.1–8.2), a total of 30 (12.1%) patients met the primary composite endpoint of all-cause death (n = 17), hospitalization for nonfatal MI (n = 4), or hospitalization for heart failure (n = 9). Thirteen of the 98 patients with CMD (13.3%) met the primary composite endpoint of all-cause death (n = 8), hospitalization for nonfatal MI (n = 1), or hospitalization for heart failure (n = 4). Among patients with CMD, ETT result was not significantly associated with adverse event-free survival (log-rank test P = 0.076) (Figure 2).
FIGURE 2. Kaplan-Meier Curves Examining Outcomes According to ETT Result Among Patients With CMD.
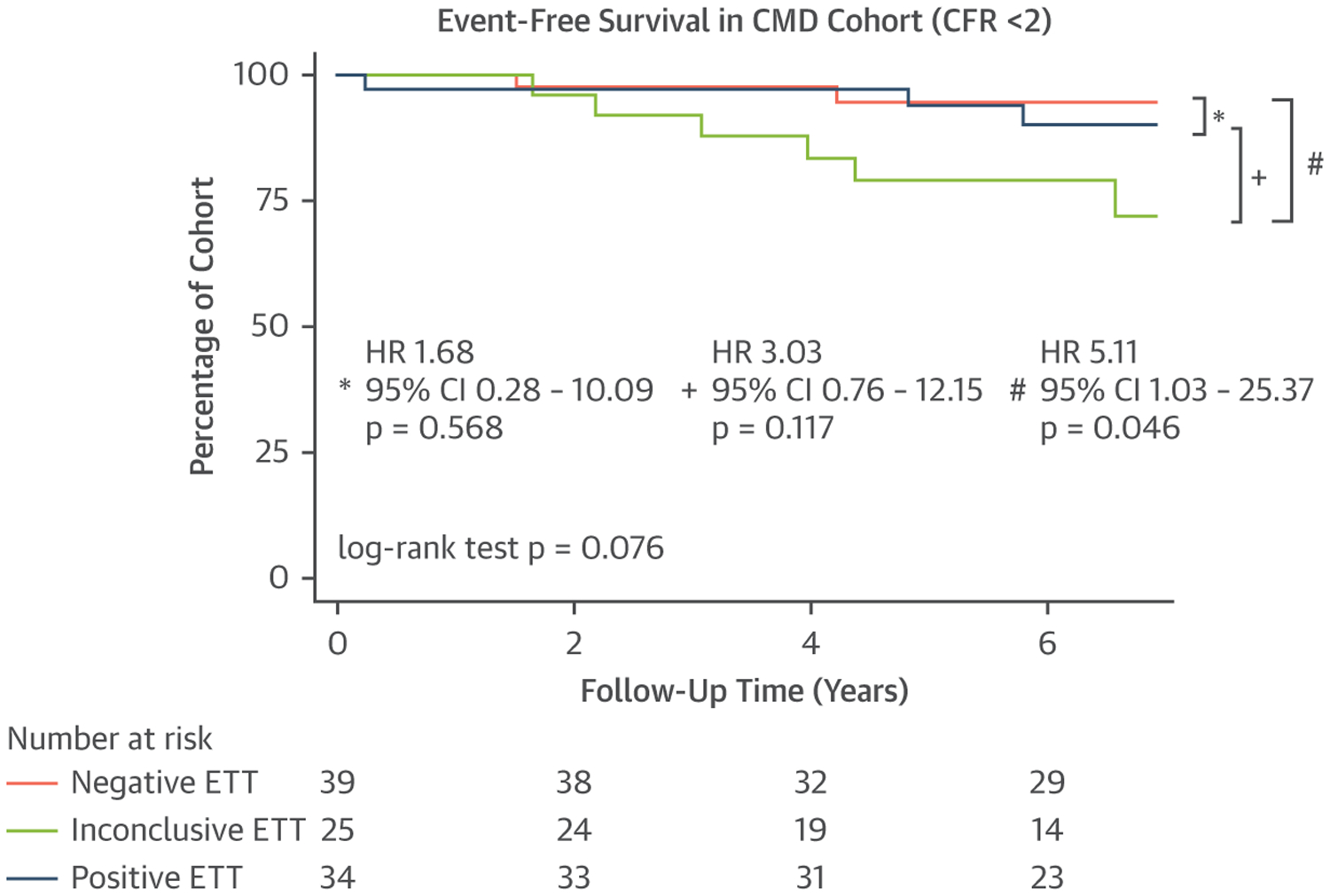
Event-free survival for the CFR <2 subgroup stratified by ETT result. CFR = coronary flow reserve; CMD = coronary microvascular disease; other abbreviations as in Figure 1.
DISCUSSION
In this study, we found that the presence of ECG abnormalities on ETT has a low sensitivity for the diagnosis of CMD in symptomatic patients without LV dysfunction or obstructive CAD on PET myocardial perfusion imaging. A positive ETT, particularly caused by ischemic ECG changes that persisted at least 1 minute into recovery, had a high specificity for the diagnosis of CMD. Importantly, ETT result has a limited effect on post-test probability of CMD given positive and negative likelihood ratio values close to 1, except for in women who had ischemic ECG changes that persisted 1 minute into recovery (positive likelihood ratio: 1.50).
Although exercise-induced ischemic ECG changes during angina-like chest pain have been traditionally considered an important feature in the diagnosis of CMD (22,23), our data would suggest that this notion is outdated. The high specificity of a positive ETT caused by ischemic ECG changes that persist at least 1 minute into recovery in this population for the diagnosis of CMD is an important new finding from our study and parallels data from previous studies linking CMD and abnormalities on myocardial perfusion imaging (24–28) and exercise testing (29). Additionally, undiagnosed CMD may be the reason why patients with abnormal exercise ECG findings but normal stress echocardiography findings were recently found to be at increased risk of adverse clinical events (30). An abnormal exercise ECG may be a marker of microvascular disease that is undetectable using traditional imaging methods, and should prompt further evaluation in the right clinical context.
Although prior studies have shown that approximately 30%–60% of patients with CMD present with angina (31–35), atypical angina is also common (36), and patients can also present with reduced exercise tolerance and/or exertional dyspnea (4,31–35,37,38). This wide spectrum of clinical presentations of CMD may explain why a low CFR was not associated with a specific ETT symptom profile in previous reports (33) or in our study cohort.
The potential association between ETT results and outcomes in patients with CMD should be studied with larger patient sizes. One of the current limitations of PET is that myocardial stress blood flows are obtained using pharmacological stress agents, and, therefore, helpful and important exercise data are not captured routinely for patients at the time of PET. The exploratory data from our study suggest that exercise testing may be of incremental prognostic value beyond PET in patients with CMD.
Based on the data from our study, the Central Illustration presents how sensitive and specific a positive ETT result is for CMD based on ETT findings. In patients with normal myocardial perfusion and/or no obstructive CAD via noninvasive or invasive coronary angiography, CMD is likely to be present in a patient who has a positive ETT caused by ischemic ECG changes that last at least 1 minute into recovery. A positive ETT result is less specific, but more sensitive, for CMD if positive criteria are expanded to ischemic ECG changes that resolve quickly in recovery and moderate/severe angina requiring termination of the ETT.
CENTRAL ILLUSTRATION. Sensitivity and Specificity of Positive ETT in Suspected Symptomatic CMD.
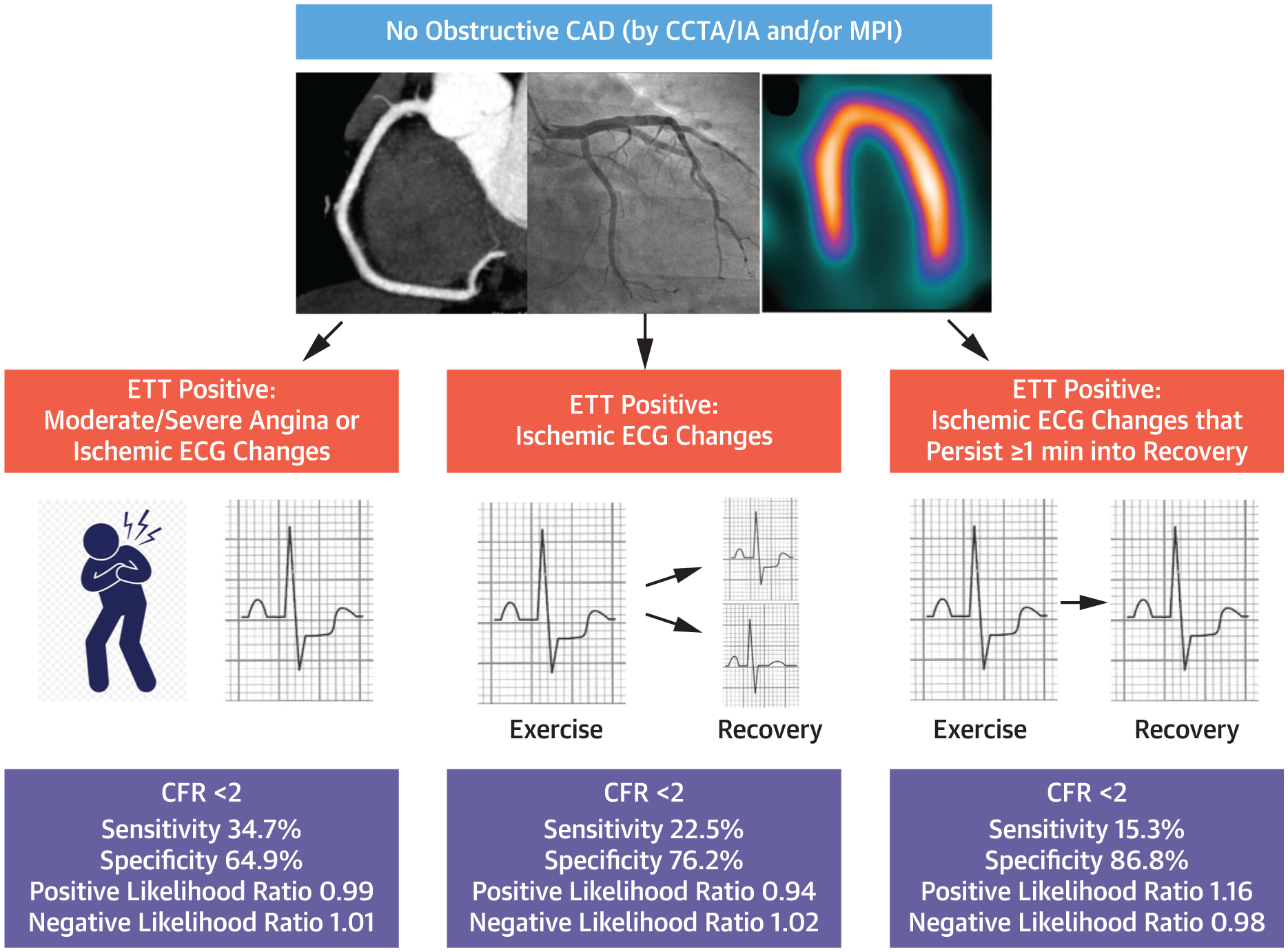
If anatomic testing (CCTA or IA) and/or perfusion imaging reveal no obstructive CAD and ETT is positive (moderate/severe angina or ischemic ECG changes), the diagnosis of CMD should be considered given a specificity of 64.9%. If criteria for a positive ETT are further narrowed to ischemic ECG changes that persist at least 1 minute into recovery, CMD is likely based on our data because specificity increases to 86.8%. Of note, the positive and negative likelihood ratios close to 1 indicate that ETT result only marginally affects post-test probability of disease. CAD = coronary artery disease; CCTA = coronary computed tomography angiography; CFR = coronary flow reserve; CMD = coronary microvascular disease; ECG = electrocardiogram; ETT = exercise treadmill testing; IA = invasive angiography; MPI = myocardial perfusion imaging.
STUDY LIMITATIONS.
It is a 2-center, retrospective observational study in which the population consisted of patients clinically referred for PET myocardial perfusion imaging at 1 center and for ETT at 1 of 2 centers within 12 months. Therefore, patients who were unable to exercise on a treadmill were not included. Additionally, it is possible that patient-specific characteristics changed between ETT and PET studies. However, the median time between studies was 18 days. To focus on the diagnostic and prognostic value of ETT in CMD, we excluded from our analysis patients with known CAD or LV systolic dysfunction. Although it is possible that the cohort contained some patients with multivessel, obstructive CAD without perfusion abnormalities on PET, our clinical experience has previously suggested that this is unlikely (39). The modest sample size and very small number of outcome events limit the rigor of the prognostic analysis. Finally, it is possible that a normal CFR may have underestimated the possibility of epicardial or microvascular vasospasm as a principal mechanism for angina and abnormal ECG response to exercise in some patients (40,41). Understanding these important limitations, these results may have clinical implications for the use of ETT in the diagnosis of CMD and risk stratification of patients with CMD based on ETT results.
CONCLUSIONS
In conclusion, although ETT is an accepted risk assessment tool for the evaluation of myocardial ischemia, our data suggest limited sensitivity of ETT to detect CMD. Therefore, clinicians should consider alternative noninvasive or invasive testing when CMD is suspected. However, exercise-induced horizontal or downsloping ST-segment depressions that persist at least 1 minute into recovery in the absence of obstructive CAD should raise suspicion for the presence of CMD given a high specificity. Further studies are needed to examine the association between ETT results and risk of adverse events in patients with CMD, and if existing and novel risk factor modification strategies should be considered for these patients.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
In patients without CAD or abnormal LV systolic function who underwent both ETT and PET within 12 months, a positive ETT with ischemic ECG changes that persisted at least 1 minute into recovery had low sensitivity but high specificity for the diagnosis of CMD (CFR <2).
TRANSLATIONAL OUTLOOK:
Future studies are needed to further determine the prognostic value of exercise testing in patients with CMD and its potential role in risk stratification.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr Divakaran has received a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL094301) and a joint KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst and the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679-10). Dr Zhou has received a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL094301). Dr Osborne has received a KL2/Catalyst Medical Research Investigator Training award (an appointed KL2 award) from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR002542). Dr Taqueti has received grant number K23 HL135438 from the National Heart, Lung, and Blood Institute. Dr Dorbala has received grant number R01 HL130563 from the National Heart, Lung, and Blood Institute. Dr Di Carli has received grant number R01 HL132021 from the National Heart, Lung, and Blood Institute. This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health. Dr Osborne has served as a consultant for Intrinsic Imaging, LLC. Dr Dorbala is a member of an advisory board for General Electric Health Care. Dr Di Carli has received research grant support from Spectrum Dynamics and consulting fees from Sanofi and General Electric. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CFR
coronary flow reserve
- CMD
coronary microvascular disease
- ECG
electrocardiogram
- ETT
exercise treadmill test
- IQR
interquartile range
- LV
left ventricle
- MI
myocardial infarction
- MPHR
maximum predicted heart rate
- PET
positron emission tomography
Footnotes
APPENDIX For supplemental tables, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 2.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312: 1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 4.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 5.Ford TJ, Ong P, Sechtem U, et al. Assessment of vascular dysfunction in patients without obstructive coronary artery disease: why, how, and when. J Am Coll Cardiol Intv. 2020;13:1847–1864. [Google Scholar]
- 6.Schindler TH, Bateman TM, Berman DS, et al. Appropriate use criteria for PET myocardial perfusion imaging. J Nucl Med. 2020;61:1221–1265. [DOI] [PubMed] [Google Scholar]
- 7.Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–1653. [DOI] [PubMed] [Google Scholar]
- 8.Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. J Am Coll Cardiol Img. 2012;5: 430–440. [DOI] [PubMed] [Google Scholar]
- 9.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41: 407–477. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation. 2002;106:1883–1892. [DOI] [PubMed] [Google Scholar]
- 11.Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei JCS, Merz CNB. Coronary microvascular dysfunction causing cardiac ischemia in women. JAMA. 2019;322:2334–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 14.Juarez-Orozco LE, Saraste A, Capodanno D, et al. Impact of a decreasing pre-test probability on the performance of diagnostic tests for coronary artery disease. Eur Heart J Cardiovasc Imaging. 2019;20:1198–1207. [DOI] [PubMed] [Google Scholar]
- 15.Shaw LJ, Peterson ED, Shaw LK, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98: 1622–1630. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 1997;30:260–311. [DOI] [PubMed] [Google Scholar]
- 17.Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol. 1993;22:175–182. [DOI] [PubMed] [Google Scholar]
- 18.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. [DOI] [PubMed] [Google Scholar]
- 19.El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med. 2009;50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. [DOI] [PubMed] [Google Scholar]
- 21.Christman MP, Bittencourt MS, Hulten E, et al. Yield of downstream tests after exercise treadmill testing: a prospective cohort study. J Am Coll Cardiol. 2014;63:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon RO, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation. 1992;85:883–892. [DOI] [PubMed] [Google Scholar]
- 23.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo S, Nakamura Y, Matsumoto T, Takahashi M, Kinoshita M. Detection of coronary microvascular disease by means of cardiac scintigraphy. Can J Cardiol. 2002;18:183–186. [PubMed] [Google Scholar]
- 25.van de Wiele C, Rimbu A, Belhocine T, de Spiegeleer B, Sathekge M, Maes A. Reversible myocardial perfusion defects in patients not suffering from obstructive epicardial coronary artery disease as assessed by coronary angiography. Q J Nucl Med Mol Img. 2018;62:325–335. [DOI] [PubMed] [Google Scholar]
- 26.Cavusoglu Y, Entok E, Timuralp B, et al. Regional distribution and extent of perfusion abnormalities, and the lung to heart uptake ratios during exercise thallium-201 SPECT imaging in patients with cardiac syndrome X. Can J Cardiol. 2005;21:57–62. [PubMed] [Google Scholar]
- 27.Iosseliani D, Kluchnikov I, Koval A, Smirnov M, Bhattacharya P. X-syndrome: is there impairment of myocardial perfusion during stress? Int J Card Imaging. 1994;10:149–153. [DOI] [PubMed] [Google Scholar]
- 28.Rahman H, Ryan M, Lumley M, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019;140: 1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong P, Athanasiadis A, Hill S, Schäufele T, Mahrholdt H, Sechtem U. Coronary microvascular dysfunction assessed by intracoronary acetylcholine provocation testing is a frequent cause of ischemia and angina in patients with exercise-induced electrocardiographic changes and unobstructed coronary arteries. Clin Cardiol. 2014;37: 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daubert MA, Sivak J, Dunning A, et al. Implications of abnormal exercise electrocardiography with normal stress echocardiography. JAMA Intern Med. 2020;180(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mygind ND, Michelsen MM, Pena A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Am Heart Assoc. 2016;5: e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. J Am Coll Cardiol Intv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 35.Shah NR, Cheezum MK, Veeranna V, et al. Ranolazine in symptomatic diabetic patients without obstructive coronary artery disease: impact on microvascular and diastolic function. J Am Heart Assoc. 2017;6(5):e005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bechsgaard DF, Hove JD, Suhrs HE, et al. Women with coronary microvascular dysfunction and no obstructive coronary artery disease have reduced exercise capacity. Int J Cardiol. 2019;293: 1–9. [DOI] [PubMed] [Google Scholar]
- 39.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanza GA, Manzoli A, Pasceri V, et al. Ischemic-like ST-segment changes during Holter monitoring in patients with angina pectoris and normal coronary arteries but negative exercise testing. Am J Cardiol. 1997;79:1–6. [DOI] [PubMed] [Google Scholar]
- 41.el-Tamimi H Mansour M, Pepine CJ, Wargovich TJ, Chen H. Circadian variation in coronary tone in patients with stable angina. Protective role of the endothelium. Circulation. 1995;92:3201–3205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


