Abstract
Background:
Approximately 12% of routinely examined fallopian tubes of endometrial carcinoma (EC) cases have intraluminal tumor cells (ILTCs). ILTC associations with EC characteristics and outcome are understudied, and unknown in serially examined and embedded tubal fimbriae (SEE-FIM).
Methods:
Glass slides of SEE-FIM tubes for 371 EC cases were independently reviewed by two pathologists who recorded ILTC presence and characterized them as mucosal if involved and floating if not. Disagreements were reviewed by a third pathologist, and agreement between any two determined final ILTC status. Clinico-pathological associations and ILTC presence were tested for significance (p<0.05) by univariable analysis, and stage and histotype determinants were included in a multivariable analysis. Kaplan–Meier estimates and log-rank tests compared overall and EC-specific survival, and Cox proportional regression estimated hazard ratios (HRs).
Results:
ILTCs were present in 56 (15.1%) cases: 30 mucosal and 26 floating. FIGO stage 3/4, lymph-vascular space invasion, deep myometrial invasion, non-endometrioid histotype, and adjunctive chemotherapy were significantly associated with ILTC presence, and only stage was significant in the multivariable analysis. Overall, 61 women died: 30 of whom died of EC. ILTCs were non-significantly associated with higher overall and EC-specific mortality and mucosal ILTCs had the highest HRs (1.64 and 1.89 respectively).
Conclusions:
SEE-FIM tubes have a higher prevalence of ILTCs than routinely examined tubes, and high FIGO stage is an independent determinant. A prognostic effect was not found, but the higher trending HRs suggest additional study is needed to determine whether ILTCs and in particular mucosal ILTCs adversely affect prognosis.
Keywords: Endometrial carcinoma, Intraluminal tumor cells (ILTCs), SEE-FIM examined fallopian tubes, Clinico-pathological associations and outcome analyses by ILTC status
Introduction
Staging of endometrial carcinoma (EC) by the International Federation of Gynecological Oncology (FIGO) is an evolving topic as new data about prognostic factors emerge1. FIGO stage 3A represents EC spread to the outer layer of the uterus and/or to the fallopian tubes, ovaries, or ligaments of the uterus, and involvement of any part of the tube qualifies as tubal spread2,3. EC present in the lumen of the fallopian tube as intraluminal tumors cells (ILTCs) are individual or grouped cells with or without associated stroma which may be floating in the lumen and/or attached to the mucosa. ILTCs are detected in tubal washings and based on the histopathological examination of at least one full cross section of the tube, occur in 11.9% of EC cases4–6. ILTCS develop when EC sheds from the endometrial surface and migrates into the lumen of the tube or when the tube is invaded from a pelvic source. EC migration may occur before, during or after the hysterectomy procedure. Laparoscopic hysterectomy and those performed with a uterine manipulator likely promote intra-operative EC shedding as the frequency of EC material in the lumen of the tubes is significantly increased7. Viable pre- and intra-operative ILTCs may be capable of growth beyond the pelvis by shedding into the peritoneum via the open fimbriated end of the tube8. Those occurring post-operatively and secondary to the pathology examination and processing are extraneous tissue artifacts, and although they can be a diagnostic challenge for the pathologist, they are clinically insignificant9.
ILTC associations with EC clinic-pathological characteristics and prognostic role are incompletely studied. Based on data from 3 published studies, ILTCs are more likely to co-occur with aggressive tumor features such as non-endometrioid histotypes (e.g., serous), lymph-vascular space invasion (LVSI), and high tumor grade and/or stage, and may have prognostic significance in certain subgroups (stage 1 serous)6, 10, 11. The adverse prognostic effect of ILTCs was indirectly studied in cohorts of women with EC and prior histories of bilateral tubal ligation since the procedure could mitigate trans-tubal spread. Li et al found an association with lower rates of positive peritoneal cytology and lower recurrence rates in non-endometrial histotypes, and Felix et al, reported associations with lower stage disease, lower odds of peritoneal metastasis, and significant improvement in endometrial cancer-specific survival particularly for those with non-endometrioid histotypes, although these effects were mitigated when the data were adjusted for FIGO stage12, 13. Factors possibly associated with ILTC viability such as size, co-presence of stroma, and/or attachment to the epithelium of the tubal mucosa and their prognostic impact were not investigated in these studies.
The modified and full Serial Examination and Embedding of the Fimbriae (SEE-FIM) methods more comprehensively examine the fimbriated end of the fallopian tubes than the examination of one random section of the distal end14. SEE-FIM increases the detection of Serous Tubal Intraepithelial Carcinoma (STIC) and early tubal serous carcinoma15, 16. Since all clinico-pathological and outcome ILTC studies were conducted prior to the adoption of the SEE-FIM technique, it’s impact on ILTC detection in EC is unknown and has not been studied6, 10, 11. Accordingly, the current study was designed to establish the prevalence and describe the morphology of ILTCs in a cohort of women with EC whose tubes were SEE-FIM examined, and to analyze ILTC associations with EC characteristics and their impact on survival.
Materials and Methods
Study population
Data and specimens for this study were drawn from a retrospective cohort of women in the Calgary Health Zone who in the 4-year interval 2014–2017, had a unilateral or bilateral salpingectomy examined by the SEE-FIM method, and of whom approximately 900 had a diagnosis of primary EC16. All pathology examinations were conducted in a single, accredited laboratory, and the fresh hysterectomy specimens were consistently fixed, grossed, processed, cut, stained, mounted and cover-slipped using standardized up-to date procedures and automated instrumentation. Approximately 82% of ECs were endometrioid carcinomas and 18% non-endometrioid (serous, clear cell, mixed, carcinosarcoma, and undifferentiated). Since it was impractical to conduct a pathology review of all 900 EC cases, a stratified sampling according to histotype (FIGO grades 1 and 2 endometrioid carcinomas versus FIGO grade 3 endometrioid and non-endometrioid carcinomas) was conducted with a target sample size of 404 women. Random selection was used to identify 255 cases with a more aggressive histotype (160 with a non-endometrioid and 95 with a grade 3 endometrioid histotype), and 149 with a less aggressive histotype diagnosis of grades 1 or 2 endometrioid for the study. Thereafter, 16 cases were excluded which reduced the study sample to 388. The exclusions included 15 duplicate cases and 1 case of a cervical primary tumor was misclassified as an endometrial primary. The study was approved by the Health Research Ethics Board of the Alberta Cancer Committee.
Determination of ILTC characteristics
Glass slides for 388 cases were retrieved from the laboratory files and all slides of tubes and one representative slide of tumor were identified for the pathological review. Salpingectomy was bilateral in 384 (99%) and unilateral in 4 (1%). Original slides were available for all cases except one which was recut for the study. The total number of slides for review was 1,876, and per case ranged from 2 to 23 with a median of 4. Hematoxylin and Eosin (H&E) stained slides were available for each case and 13 (3.4%) cases had immunohistochemical (IHC) staining. Up to 16 antibodies ranging from 1 to 9 per case were used and included, p53, WT1, Estrogen Receptor, PAX8, p16, Progesterone Receptor, MOC31, Ki67, NapsinA, CDX2, CD10, D2–40, pooled Cytokeratin, Calretinin, CD68, and Vimentin. The selected glass slides were independently reviewed by 2 pathologists who were masked to all other clinico-pathological, and outcome information. ILTCs were diagnosed when viable tumor fragments either as tumor cells or tumor cells associated with fibrous or fibro-vascular stroma were in the same plane of focus as the tissue section of fallopian tube, and features of post-operative extraneous tissue were absent9. Three months later, both pathologists conducted a second review of a random sample of 40 cases. At each review, they recorded ILTCs by: 1) detection status categorized as present or absent, 2) tubal involvement as floating in the lumen, attached to the mucosa, and/or involving the tubal wall beyond the mucosa, 3) size categorized as less than 10, 10 to 50 or more than 50 tumor cells, and 4) co-presence of stroma with the tumor cells categorized as present or absent.
Clinical characteristics and outcomes
Clinico-pathological and outcome variables for women who reside in the province of Alberta are available in the cancer centre, electronic data base. Amongst the 388 women, data were available for 371 and missing for 17 who resided in another province. Data for the 371 were uploaded and stored in a customized Excel file. Information on age at diagnosis (<55, 55–64, ≥65), body mass index (BMI) (< 25, 25–30, ≥30 kg/m2), type of surgery (vaginal hysterectomy, abdominal hysterectomy, laparoscopic-assisted vaginal hysterectomy [LAVH], total laparoscopic hysterectomy [TLH]), peritoneal cytology (no cytology, cytology negative for malignancy, cytology positive for malignancy), FIGO stage (1, 2, 3, or 4), myometrial invasion (less than half versus half or more), LVSI (no versus yes), histotype (endometrioid, serous, carcinosarcoma, clear cell, mixed epithelial, or undifferentiated carcinomas), FIGO grade (1, 2, or 3), adjuvant radiation (none versus any), and adjuvant chemotherapy (none versus any) were collected. Women with endometrioid carcinomas were categorized as low-grade endometrioid (grades 1 or 2) or high-grade endometrioid (grade 3). TLH was performed using a uterine manipulator, and peritoneal cytology was mostly intra-operative pelvic washings and rarely ascitic fluid sampled prior to the hysterectomy.
Statistical analysis
Inter- and intra-observer reliability of the two 2 pathologists for ILTC status as present or absent was calculated using Kappa statistics. Inter-observer disagreements were identified and the glass slides were independently reviewed by a third pathologist. ILTC detection status (absent versus present) was then based on agreement between any 2 of the 3 pathologists. Characteristics of women grouped according to ILTC detection status and tubal involvement as 1) absent, 2) floating in the lumen without mucosal attachment (floating ILTCs), and 3) attached to the mucosa (mucosal ILTCs) were compared using Chi-square tests. Given the prevalence of ILTCs in our study sample, a logistic regression model including only histotype and stage as adjustment factors was implemented. Kaplan–Meier estimates and log-rank tests compared overall and EC-specific survival distributions according to ILTCs categories of absent, floating, or mucosal. Cox proportional hazards regression was used to estimate univariable hazard ratios (HRs) and 95% confidence intervals (CIs). Sensitivity analyses were conducted to examine the impact of ILTCs staged as FIGO3A, and to examine those that were a FIGO3A due only to tubal spread. In both analyses, cases with mucosal ILTCs present and staged as FIGO3A were recoded as not having ILTCs present. Data were analyzed using SAS/STAT software version 9.4 (SAS Institute, Cary, NC, USA). All P values were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Pathology review
There were 70 (18%) disagreements in the ILTC detection status of the 388 cases reviewed by the two pathologists following the first review, and this resulted in a moderate Kappa value of 0.42 (95% C.I., 0.31–0.53) for inter-observer reliability. Intra-observer reliabilities for the two pathologists based on a small subset of cases were perfect to moderate with Kappa values of 0.89 (95% CI., 0.69–1.00) and 0.54 (CI., 0.09–0.98). The review by the third pathologist resolved the 70 disagreements which were due to differences between the 2 pathologists in the interpretation of small groups of macrophages and strips of detached, and sometimes atypical tubal epithelium. None were due to post-operative extraneous tissue artifact9. By consensus amongst the pathologists, ILTC status was next established for the 371 cases with clinico-pathological and outcome data, and ILTCs were present in 56 (15.1%) and absent in 315 (84.9%). Amongst the 56 ILTCs, 26 (46.4%) were floating and 30 (53.6%) were mucosal groups (Figure 1a). Other tubal pathology was present in 54 (14.6%) of the 371 cases. The pathology was benign in 48 (12.9%) of the cases and comprised of lesions such as salpingitis and endometriosis, and in 6 (1.6%) was a synchronous STIC or primary, tubal serous carcinoma.
Figure 1.
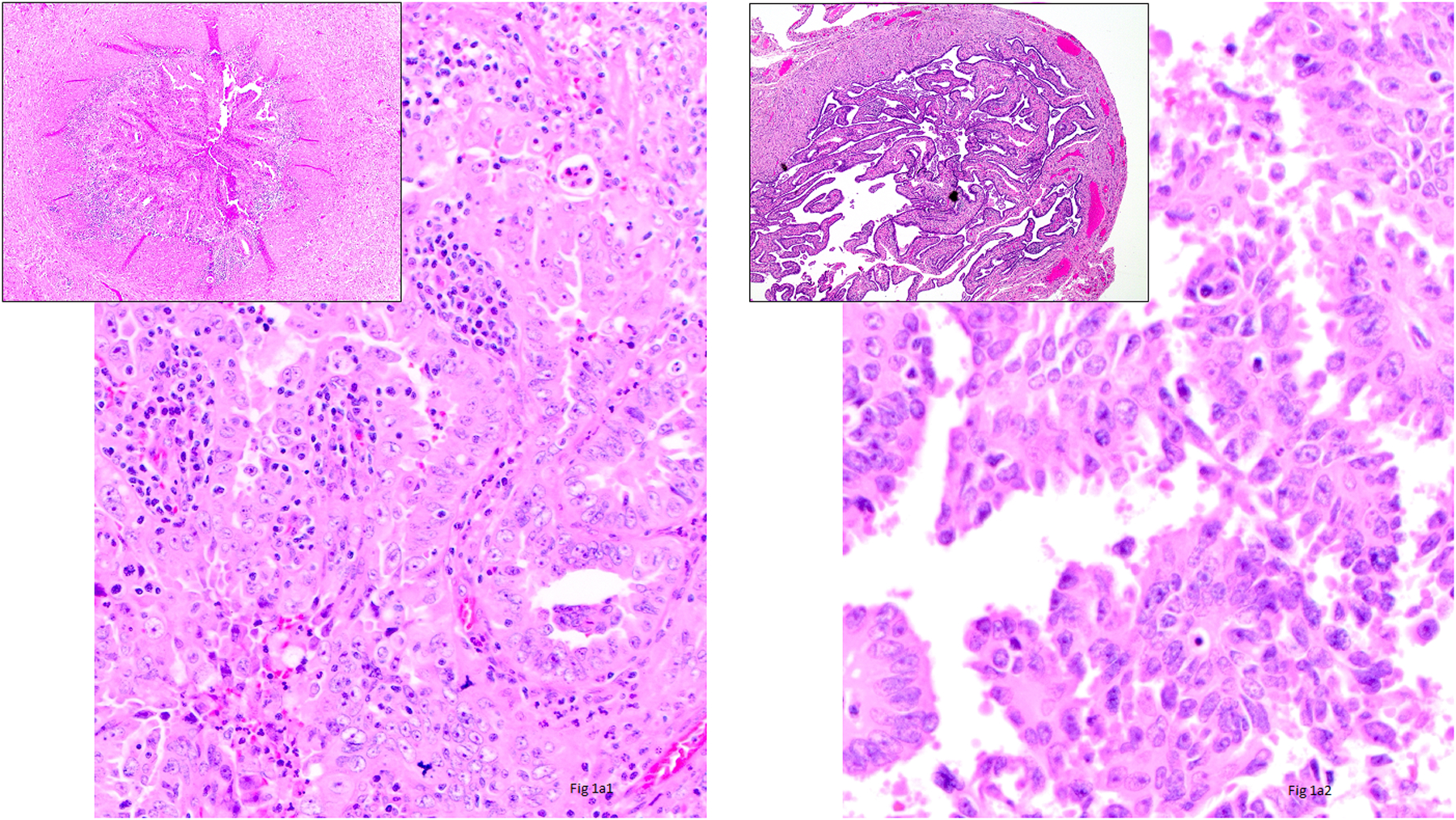
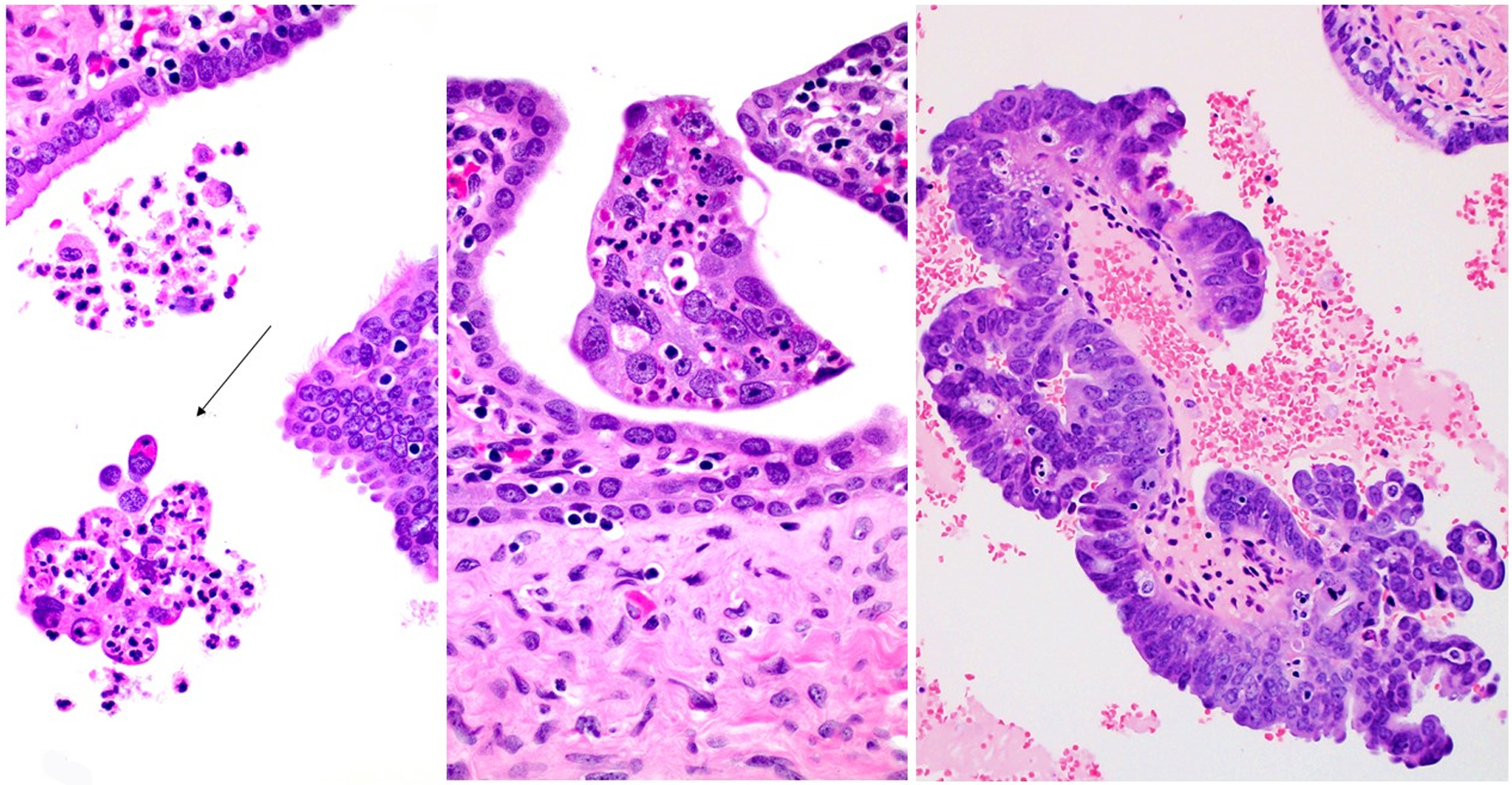
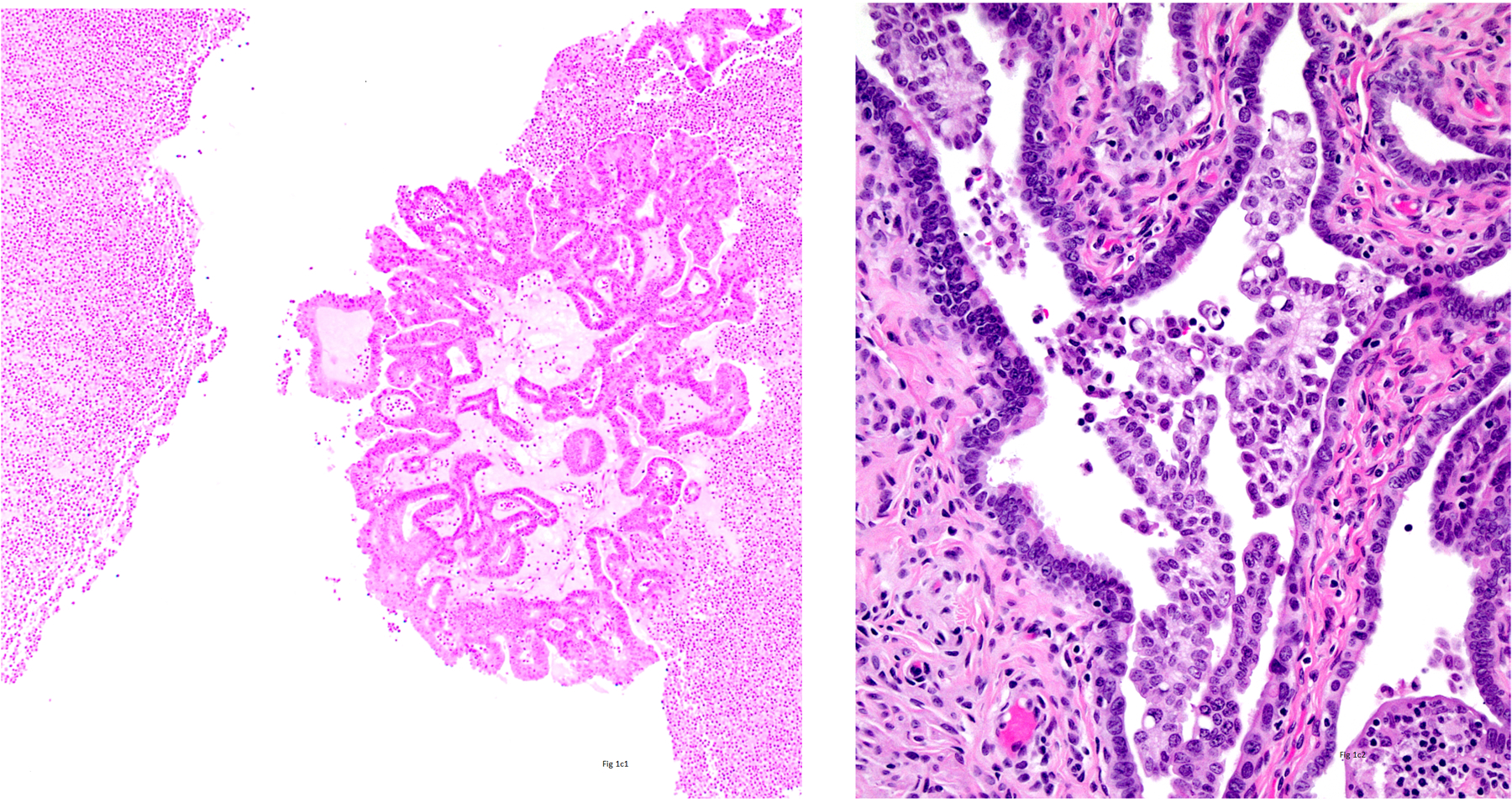
1a1) Intraluminal Tumor Cells (ILTCs) attached to the epithelium of the mucosa of the tube (Hematoxylin and Eosin ×400 and insert ×100), 1a2) ILTCs floating in the lumen (Hematoxylin and Eosin ×400 and insert ×100), 1b1) ILTCs size less than 10 cells (Hematoxylin and Eosin ×400), 1b2) ILTCs size 10–50 cells (Hematoxylin and Eosin ×400), 1b3) ILTCs size greater than 50 cells (Hematoxylin and Eosin ×400), 1c1) ILTCs associated with stroma (Hematoxylin and Eosin ×100), and 1c2) ILTCs unassociated with stroma (Hematoxylin and Eosin ×100).
ILTC characteristics
Table 1 shows the distribution of size and stromal association amongst all 56 ILTCs and separately by the floating and mucosal ILTC groups. Overall and amongst both groups, most ILTCs were composed of more than 50 cells (Figure 1b), and in most stroma was present (Figure 1c). No significant difference in size of ILTC clusters was noted between the two groups although mucosal ILTCs tended to be larger than floating ILTCs. Differences in stromal association were significant with 80% of mucosal ILTCs showing the co-presence of stroma compared with 53.9% of floating ILTCs (p=0.04).
Table 1.
Intraluminal Tumor Cell size and stromal status in 56 endometrial carcinoma cases and compared by group of tubal involvement
| All ILTCs n=56 |
||||
|---|---|---|---|---|
| Floating ILTCsa n=26 |
Mucosal LTCsb n=30 |
p-value | ||
| Size, n (%) c | 0.18d | |||
| < 10 cells | 4 (7.1) | 2 (7.7) | 2 (6.7) | |
| 10–50 cells | 13 (23.2) | 9 (34.6) | 4 (13.3) | |
| >50 cells | 39 (69.6) | 15 (57.7) | 24 (80.0) | |
| Stroma, n (%) c | 0.04e | |||
| Absent | 18 (32.1) | 12 (46.2) | 6 (20.0) | |
| Present | 38 (67.9) | 14 (53.9) | 24 (80.0) |
ILTC=intraluminal tumor cell
Floating ILTCs; Floating in the lumen of the fallopian tube and without mucosal attachment
Mucosal ILTCs: Attached to the mucosa of the fallopian tube and with or without floating ILTCs
Column percentage
Fisher exact p-value comparing mucosal and floating ILTCs
Chi-square p-value comparing mucosal and floating ILTCs
EC characteristics by ILTC detection status
Characteristics of the overall study population and according to ILTC detection status are shown in Table 2, along with univariable and multivariable ORs. Data was available for all characteristics for all 371 cases with the exception of body mass index (BMI) which was missing in 39 (10.5%), and peritoneal cytology which was missing in 1 (0.3%). Most of all 371 women and the 315 with ILTCs absent were 65 years of age or older, had a BMI greater than 30, had an abdominal hysterectomy, and did not undergo adjuvant radiation and/or chemotherapy of any type. Most did not have peritoneal cytology, and of those that did, most results were negative for malignancy which included benign and atypical results. Most ECs were a FIGO stage 1, invaded less than half of the myometrium, were negative for LVSI, and were a low-grade endometrioid histotype.
Table 2.
Odds ratios and 95% confidence intervals for associations between clinical and tumor characteristics and status of Intraluminal Tumor Cell involvement of the fallopian tube in 371 endometrial carcinoma cases
| All (n=371) | ILTC status | ||||||
|---|---|---|---|---|---|---|---|
| Absent (n=315) | Present (n=56) | ||||||
| n (%) | n (%)a | n (%)a | OR (95% CI)b | p-valuec | OR (95% CI)d | p-valuec | |
| Age | 0.48 | ---- | |||||
| <55 | 55 (14.8) | 49 (89.1) | 6 (10.9) | 1.00 | |||
| 55–64 | 136 (36.7) | 112 (82.4) | 24 (17.7) | 1.75 (0.67, 4.55) | |||
| ≥65 | 180 (48.5) | 154 (85.6) | 26 (14.4) | 1.38 (0.54, 3.54) | |||
| BMI (kg/m2)e | 0.16 | ---- | |||||
| Normal weight (<25) | 84 (22.6) | 72 (85.7) | 12 (14.3) | 1.00 | |||
| Overweight (25–29.9) | 78 (21.0) | 60 (76.9) | 18 (23.1) | 0.80 (0.37, 1.73) | |||
| Obese (≥30) |
170 (45.8) | 150 (88.2) | 20 (11.8) | 1.80 (0.80, 4.03) | |||
| Surgery | 0.50 | ---- | |||||
| Abdominal H | 277 (74.7) | 237 (85.6) | 40 (14.4) | 1.00 | |||
| LAVH | 76 (20.5) | 64 (84.2) | 12 (15.8) | 1.11 (0.55, 2.24) | |||
| Vaginal H | 9 (2.4) | 6 (66.7) | 3 (33.3) | 2.96 (0.71, 12.33) | |||
| TLH | 9 (2.4) | 8 (88.9) | 1 (11.1) | 0.74 (0.09, 6.08) | |||
| Peritoneal cytology f | 0.13 | ---- | |||||
| None | 334 (90.3) | 284 (85.0) | 50 (15.0) | 1.00 | |||
| Negative for malignancy | 29 (7.8) | 26 (89.7) | 3 (10.3) | 0.66 (0.19, 2.25) | |||
| Positive for malignancy | 7 (1.9) | 4 (57.1) | 3 (42.9) | 4.26 (0.93, 19.61) | |||
| FIGO Stage | <0.0001 | <0.0001 | |||||
| 1 | 231 (62.3) | 216 (93.5) | 15 (6.5) | 1.00 | 1.00 | ||
| 2 | 19 (5.1) | 15 (79.0) | 4 (21.1) | 1.28 (1.17, 1.39) | 4.22 (1.23, 14.53) | ||
| 3 | 83 (22.4) | 63 (75.9) | 20 (24.1) | 4.57 (2.21, 9.45) | 4.65 (2.10, 10.26) | ||
| 4 | 38 (10.2) | 21 (55.3) | 17 (44.7) | 11.66 (5.10, 26.64) | 11.21 (4.18, 30.04) | ||
| Myometrial invasion | 0.01 | ---- | |||||
| Less than half | 197 (53.1) | 176 (89.3) | 21 (10.7) | 1.00 | |||
| Half or more | 174 (46.9) | 139 (79.9) | 35 (20.1) | 2.11 (1.18, 3.79) | |||
| LVSI | 0.002 | ---- | |||||
| Absent | 204 (55.0) | 184 (90.2) | 20 (9.8) | 1.00 | |||
| Present | 167 (45.0) | 131 (78.4) | 36 (21.6) | 2.53 (1.40, 4.56) | |||
| Histotype | 0.02 | 0.63 | |||||
| Low-grade endometrioid | 146 (39.3) | 132 (90.4) | 14 (10.0) | 1.00 | 1.00 | ||
| High-grade endometrioid | 75 (20.2) | 65 (86.7) | 10 (13.3) | 1.45 (0.61, 3.44) | 0.79 (0.31, 2.03) | ||
| Serous | 73 (19.7) | 52 (71.2) | 21 (28.8) | 3.81 (1.80, 8.05) | 1.35 (0.54, 3.37) | ||
| Carcinosarcoma | 34 (9.2) | 29 (85.3) | 5 (14.7) | 1.63 (0.54, 4.87) | 0.64 (0.19, 2.14) | ||
| Clear cell | 20 (5.4) | 19 (95.0) | 1 (5.0) | 0.50 (0.06, 3.99) | 0.24 (0.03, 2.12) | ||
| Mixed epithelial | 20 (5.4) | 16 (80.0) | 4 (20.0) | 2.36 (0.69, 8.04) | 1.07 (0.28, 4.13) | ||
| Undifferentiated | 3 (0.8) | 2 (66.7) | 1 (33.3) | 4.71 (0.40, 55.34) | 0.60 (0.04, 8.31) | ||
| Radiation | 0.76 | ---- | |||||
| None | 306 (82.5) | 259 (84.6) | 47 (15.4) | 1.00 | |||
| Any | 65 (17.5) | 56 (86.2) | 9 (13.9) | 0.89 (0.41, 1.91) | |||
| Chemotherapy | <0.0001 | ---- | |||||
| None | 218 (58.8) | 202 (92.7) | 16 (7.3) | 1.00 | |||
| Any | 153 (41.2) | 113 (73.9) | 40 (26.1) | 4.47 (2.40, 8.34) | |||
Abbreviations and symbols: ILTCs=intraluminal tumor cells, BMIe=body mass index and data missing for n=39 cases, H=hysterectomy, LAVH=laparoscopic assisted vaginal hysterectomy, TLH=total laparoscopic hysterectomy, LVSI= Lymph-vascular space invasion, Peritoneal cytologyf=data missing for n=1case, CI=Confidence intervals,
Row percentage,
Unadjusted Odds Ratios (ORs) and 95% Confidence Intervals (CIs),
Wald chi-square p-value,
ORs and 95% CIs adjusted for age (<55, 55–64, ≥65), FIGO 2009 stage (1, 2, 3, 4), LVSI (no vs. yes), and histotype (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, clear cell, mixed epithelial, undifferentiated)
In contrast to the overall and negative for ILTCs study populations, most of the 56 cases with ILTCs present had adjuvant radiation and/or chemotherapy of any type. Most of their ECs were a FIGO stage 3, invaded half or more of the myometrium, were positive for LVSI, and were a non-endometrioid histotype. Amongst the 6 that had peritoneal cytology, half were positive for malignancy which included suspicious and malignant results. Amongst the 20 cases with ILTCs present and a FIGO 3 staging, 7 were a FIGO stage 3A: 4 due to ovarian spread and 3 due to tubal spread. In univariable models, higher odds of ILTC present were associated with increasing stage (in comparison to stage 1; stage 2 OR: 1.28, 95% CI=1.17–1.39; stage 3 OR: 4.57, 95% CI=2.21–9.45; stage 4 OR: 11.66, 95% CI=5.10–26.64), LVSI present (OR: 2.53, 95% CI=1.40–4.56), invasion of half or more of the myometrium (OR=2.11, 95% CI=1.18–3.79), histotype (serous versus. low-grade endometrioid OR: 3.81, 95% CI=1.80–8.05), and chemotherapy (any versus none OR: 4.47, 95% CI=2.40–8.34). No associations between the presence of ILTCs and age, BMI, type of surgery, peritoneal cytology, and adjuvant radiation were observed. In a multivariable model including stage and histotype determinants, stage remained significantly associated with the presence of ILTCs (p<0.0001), while histotype was not (p=0.63). Sensitivity analyses reclassifying FIGO stage 3A cases due to tubal spread did not result in any appreciable differences in the regression analyses.
EC characteristics by ILTC absence, and mucosal and floating groups
Table 3 presents distributions of clinical and tumor characteristics according to ILTC absence and to presence subdivided into floating and mucosal groups. Most of the floating ILTC group were aged 55–64, were obese, had an abdominal hysterectomy and did not have peritoneal cytology or adjunctive radiation, but did have adjunctive chemotherapy. Most had low-grade endometrioid histotype tumors which were FIGO stage 1, invaded half or more of the myometrium, and had LVSI present. The result of the one case with peritoneal cytology was negative for malignancy. Distributions were similar amongst the mucosal ILTCs except half were aged 65 or older and were overweight, and most had FIGO stage 3 tumors and a serous histotype. Amongst the 5 with peritoneal cytology, most (60%) results were positive for malignancy. Similar to the associations noted in Table 2, significant differences in stage, myometrial invasion, LVSI, histotype and adjuvant chemotherapy according to ILTCs were observed, but unlike Table 2, peritoneal cytology was significantly associated. For each of these significant factors, presence was greater among cases with mucosal ILTCs compared with floating ILTCs. For example, among cases with stage 4 disease, 31.6% had mucosal ILTCs in comparison with 13.2% of floating ILTCs, and among cases with peritoneal cytology results of positive for malignancy 42.9% had mucosal ILTCs compared to none of the floating ILTCs.
Table 3.
Distributions of clinical and tumor characteristics in 371 endometrial carcinomas by Intraluminal Tumor Cell absence and 2 groups of tubal involvement
| Characteristics, n (%)a | ILTCs | |||
|---|---|---|---|---|
| Absent n=315 (row%) |
Floating ILTCsb n=26 (row%) |
Mucosal ILTCsc n=30 (row%) |
p-valued | |
| Age | 0.37 | |||
| <55 | 49 (89.1) | 4 (7.3) | 2 (3.6) | |
| 55–64 | 112 (82.4) | 13 (9.6) | 11 (8.1) | |
| ≥65 | 154 (85.6) | 9 (5.0) | 17 (9.4) | |
| BMI (kg/m2)e | 0.18 | |||
| Normal weight (<25) | 72 (85.7) | 5 (6.0) | 7 (8.3) | |
| Overweight (25–29.9) | 60 (76.9) | 6 (7.7) | 12 (15.4) | |
| Obese (≥30) | 150 (88.2) | 11 (6.5) | 9 (5.3) | |
| Surgery | 0.16 | |||
| Abdominal H | 237 (85.6) | 25 (9.0) | 15 (5.4) | |
| LAVH | 64 (84.2) | 3 (4.0) | 9 (11.8) | |
| Vaginal H | 6 (66.7) | 2 (22.2) | 1 (11.1) | |
| TLH | 8 (88.9) | 0 (0.0) | 1 (11.1) | |
| Peritoneal cytology f | 0.004 | |||
| None | 284 (85.0) | 29 (8.7) | 21 (6.3) | |
| Negative for malignancy | 26 (89.7) | 1 (3.4) | 2 (6.9) | |
| Positive for malignancy | 4 (57.1) | 0 (0.0) | 3 (42.9) | |
| FIGO Stage | <0.0001 | |||
| 1 | 216 (93.5) | 11 (4.8) | 4 (1.7) | |
| 2 | 15 (79.0) | 4 (21.1) | 0 (0.0) | |
| 3 | 63 (75.9) | 6 (7.2) | 14 (16.9) | |
| 4 | 21 (55.3) | 5 (13.2) | 12 (31.6) | |
| Myometrial invasion | 0.02 | |||
| Less than half | 176 (89.3) | 12 (6.1) | 9 (4.6) | |
| Half or more | 139 (79.9) | 14 (8.1) | 21 (12.1) | |
| LVSI | 0.002 | |||
| Absent | 184 (90.2) | 12 (5.9) | 8 (3.9) | |
| Present | 131 (78.4) | 14 (8.4) | 22 (13.2) | |
| Histotype | 0.02 | |||
| Low-grade endometrioid | 132 (90.4) | 9 (6.2) | 5 (3.4) | |
| High-grade endometrioid | 65 (86.7) | 6 (8.0) | 4 (5.3) | |
| Serous | 52 (71.2) | 8 (11.0) | 13 (17.8) | |
| Carcinosarcoma | 29 (85.3) | 1 (2.9) | 4 (11.8) | |
| Clear cell | 19 (95.0) | 1 (5.0) | 0 (0.0) | |
| Mixed epithelial | 16 (80.0) | 1 (5.0) | 3 (15.0) | |
| Undifferentiated | 2 (66.7) | 0 (0.0) | 1 (33.3) | |
| Radiation | 0.81 | |||
| None | 259 (84.6) | 21 (6.9) | 26 (8.5) | |
| Any | 56 (86.2) | 5 (7.7) | 4 (6.2) | |
| Chemotherapy | <0.0001 | |||
| None | 202 (92.7) | 12 (5.5) | 4 (1.8) | |
| Any | 113 (73.9) | 14 (9.2) | 26 (17.0) | |
Abbreviations and symbols: ILTCs=intraluminal tumor cells,,BMIe=body mass index and data missing for n=39 cases, H=hysterectomy, LAVH=laparoscopic assisted vaginal hysterectomy, TLH=total laparoscopic hysterectomy, LVSI=lymph-vascular space involvement, Peritoneal cytologyf=data missing for n=1case,
Row percentages,
Floating ILTCS; Floating in the lumen of the fallopian tube and without mucosal attachment,
Mucosal ILTCs: Attached to the mucosa of the fallopian tube and with or without floating ILTCs,
Chi-square p-value
EC outcome by ILTC
Outcome was assessed for all 371 women in the study. During a median 1.9 years of follow-up (range: 34 days-5.1 years), 61 women (16.4%) died, among whom 30 (49.2%) died of EC. More than a quarter (26.8%, n=16) of women with ILTCs present died from any cause compared to those without ILTCs (14.6%, n=46), and 12.5% (n=7) compared to 7.3% (n=23) died of EC for non significantly higher univariable HRs of 1.59 (95% C.I., 0.89, 2.86) and 1.55 (95% CI, 0.67, 3.62) respectively. Proportions of all-cause deaths were similar between those with floating ILTCs (26.9%) versus those with mucosal ILTCs (26.7%) and non-significantly higher univariable HRs of 1.55 (95% CI., 0.73–3.30) and 1.64 (95% CI., 0.74, 3.64) were observed (Figure 2). Proportions of deaths were somewhat similar and HRs higher but non-significant when EC-specific survival amongst the 2 groups was examined (Figure 3). Based on these insignificant results, multivariable analyses were not conducted. Sensitivity analyses reclassifying FIGO stage 3A cases with tubal spread did not result in any appreciable differences in the survival analyses.
Figure 2.
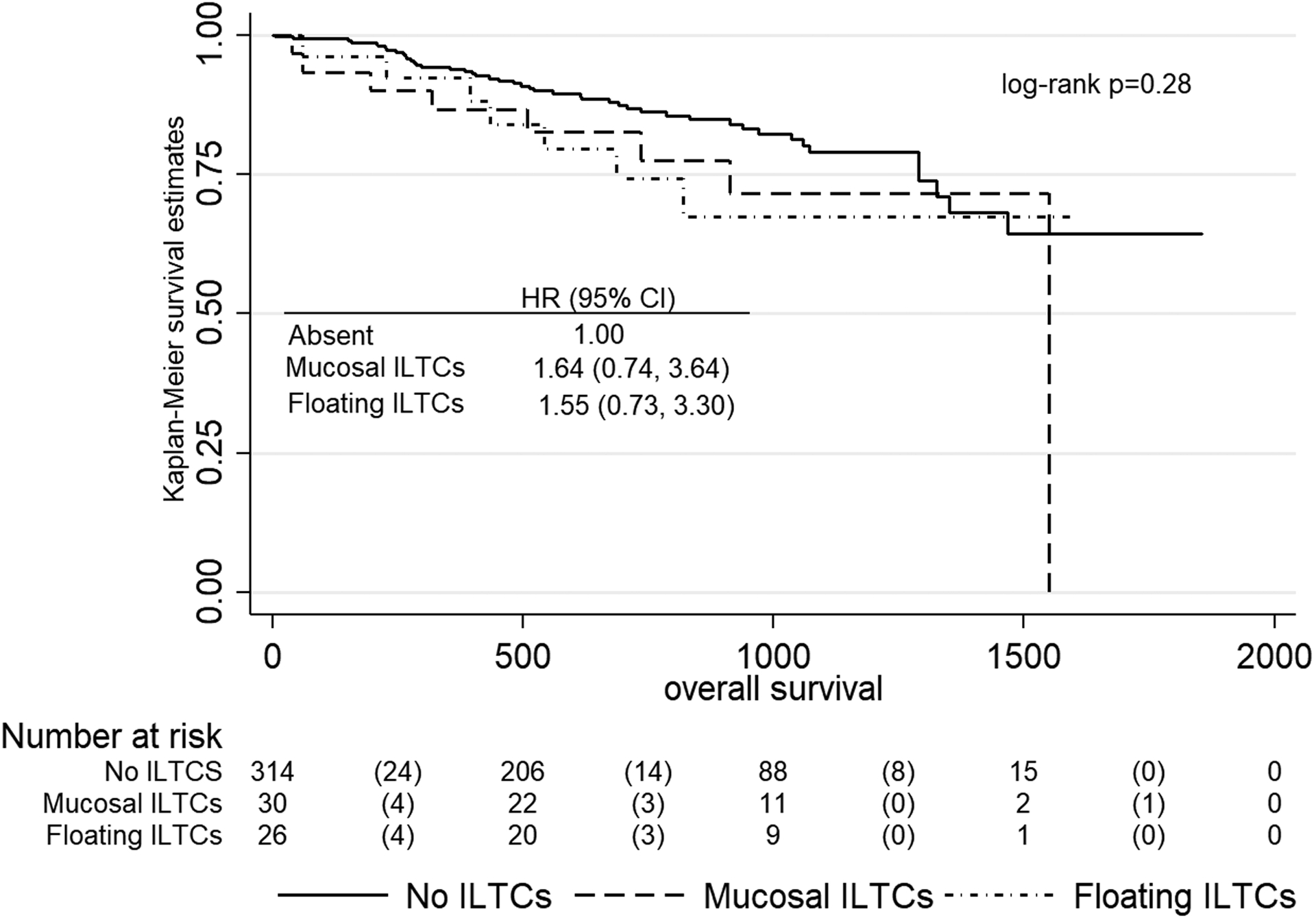
Overall survival by absence, and floating and mucosal groups of Intraluminal Tumor Cells involving the fallopian tube
Figure 3.
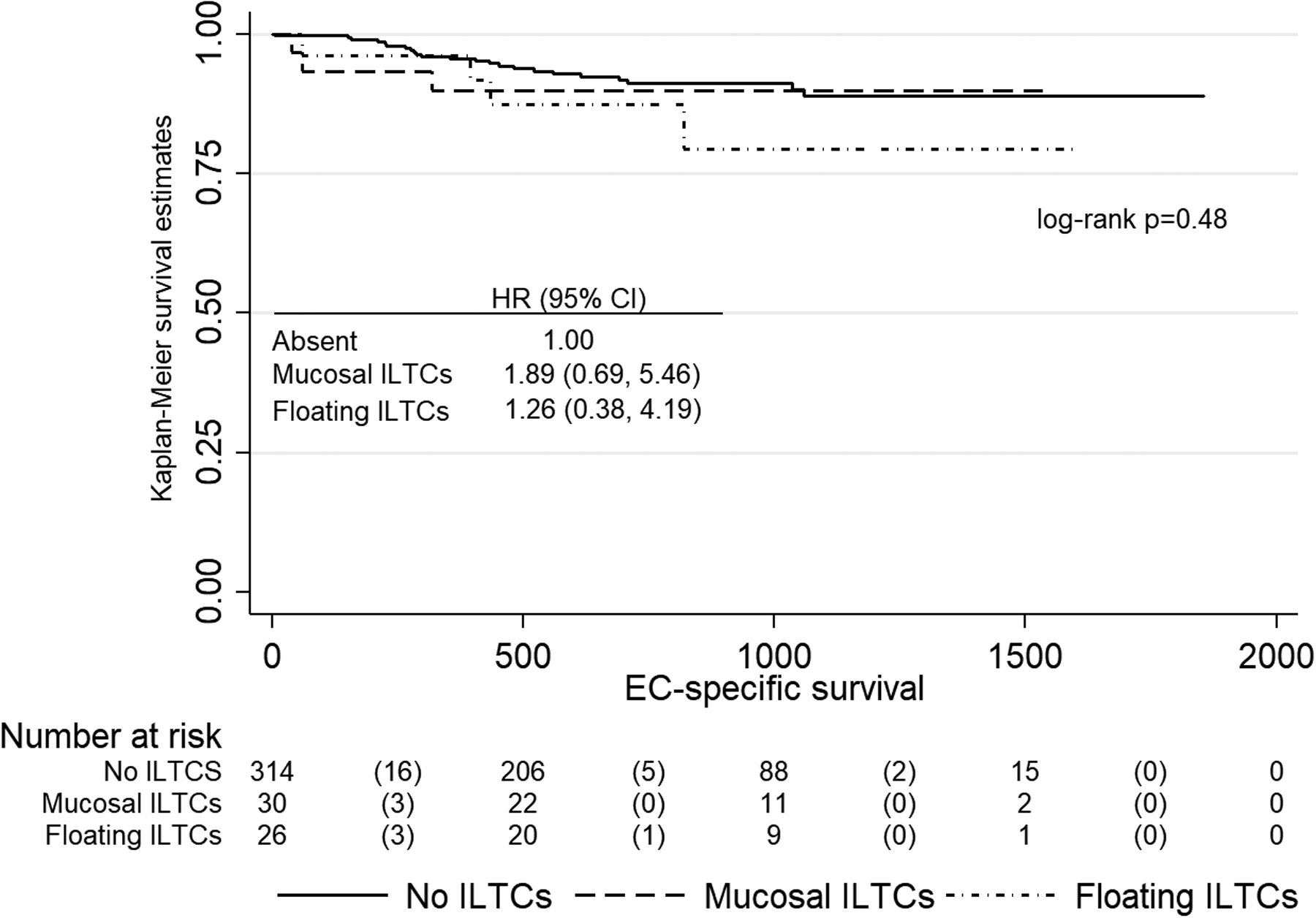
Endometrial carcinoma specific survival by absence, and floating and mucosal groups of Intraluminal Tumor Cells involving the fallopian tube
Discussion
In this single institution-based study of 371 women with EC whose tubes were SEE-FIM examined, 56 (15.1%) had ILTCs present. Since this is higher than the 11.9% of routinely examined tubes, it suggests SEE-FIM sectioning can marginally increase ILTC detection. High FIGO stage namely, stage 3 and 4 was an independent determinant of ILTC presence although univariable analyses showed non-endometrioid histotypes, LVSI, deep myometrial invasion, and adjunctive chemotherapy were significant co-variables. In grouping the ILTCs as absent, mucosal or floating, significant associations between mucosal ILTCs and these same five aggressive tumor variables with the addition of positive peritoneal cytology occurred. A non-significant increase in mortality was associated with the presence of ILTCs, and was seen for floating ILTC and for mucosal ILTC groups. The highest HRs for overall and EC-specific survival occurred with the mucosal ILTCs (1.64 and 1.89 respectively). Reclassifying FIGO stage 3A cases with tubal spread did not result in any appreciable differences in either the logistic regression or survival analyses.
This is the first documentation of the prevalence of ILTCs in SEE-FIM examined tubes of EC cases. Since the 15.1% prevalence is marginally higher than the 11.9% reported in routinely examined tubes of comparable EC populations of EC, SEE-FIM examination may increase the detection of ILTCs6. SEE-FIM examination involves pathological examination of the entire fallopian tubes or as a modified version, examination of the entire fimbriae and one random section of the middle and uterine end of the tube14. Routine examination of grossly normal fallopian tubes of EC cases is variable and can range from one random section to three sections which randomly sample the proximal, middle and distal parts of the tubes3. Examination of the entire fimbriae in the SEE-FIM method increases the detection of STIC and early tubal serous carcinoma and is routine practice for tubal samples of women with familial ovarian cancer syndrome15. Since it can also increase the detection of tubal serous malignancies in women who have no familial risk, in recent years a full or modified SEE-FIM has become standard pathology practice for the examination of tubes in this studies laboratory, and in a number of United States laboratories14, 16. A modified SEE-FIM was performed on all surgically removed tubes in this study except for the 20% with a serous histotype who had a full SEE-FIM performed. Thus 15.1% represents the prevalence of ILTC detection in tubes processed in a modified to complete SEE-FIM ratio of 4:1. Prevalence might even be higher if all tubes had a full SEE-FIM, but was not investigated further due to the overall low ILTC prevalence in the study population.
The pathological diagnosis of ILTCs can be challenging as indicated by the moderate inter-observer reliability kappa of 0.42 and variable intra-observer kappa values of 0.89 and 0.54. Based on H&E-stained glass slides of the tube, diagnostic pitfalls amongst the 70 disagreements included intraluminal macrophages occurring singly or in aggregates and/or detached tubal epithelium with reactive or degenerative changes. Some ILTCs were possibly post-operative and examples of extraneous tissue9. This is unlikely as none of the disagreements were due to extraneous tissue, and the extraneous tissue rate for the laboratory in the study interval as monitored by the provincial Anatomical Pathology Quality Assurance plan was very low at 0.4%17. Some ILTCs were possibly intra-operative events secondary to laparoscopic surgery and/or use of a uterine manipulator. This is also unlikely given that laparoscopic surgery was infrequent (22.9%) and only 2.4% involved the use of a manipulator. Thus, the ILTCs included in the study are mostly pre-operative events and represent the limits of diagnostic accuracy which can be achieved on H&E-stained tissue sections using a median number of 4 slides per case and the infrequent use of IHC. Deeper H&E-stained levels of the paraffin block and IHC with select antibodies such as CD68 for discriminating macrophages versus EC cells and WT1 for tubal epithelium versus endometrioid carcinoma cells could improve diagnostic accuracy and reliability18, 19. The possibility remains therefore, that more ancillary testing could have increased ILTC detection and resulted in an even higher ILTC prevalence in the SEE-FIM examined tubes.
Published literature on ILTC associations with EC characteristics is limited. Three prior studies of EC patients evaluated routinely examined fallopian tube specimens for ILTC presence and correlated this feature with other tumor characteristics6, 10, 11. In their analysis of 262 EC cases, over-sampled for women with high-grade EC (226 high-grade and 36 low-grade), Stewart and colleagues reported a significantly higher prevalence of ILTC prevalence among women with high-grade compared to low-grade EC (26.1% vs. 2.8%, respectively)11. Moreover, in the subgroup of 226 women with high-grade disease, ILTC presence was significantly more likely to co-occur with other aggressive tumor features, including positive peritoneal cytology, positive peritoneal spread, and positive lymph node involvement. Snyder and colleagues examined trans tubal spread among 87 women with serous EC and reported an ILTC prevalence of 18%10. All women with ILTCs present had evidence of peritoneal spread compared with only 13% of women without ILTCs. Felix et al, in a study of 295 patients with ILTCs and EC of different histotypes observed associations between ILTC presence and higher stage, LVSI, and non-endometrioid histotypes in univariable models, with stage being an independent predictor in multivariable analysis6. Collectively, these analyses are consistent with the hypothesis that ILTC presence co-occurs with other adverse tumor characteristics and these relationships may be particularly important for women diagnosed with certain EC histotypes.
Clinical and pathological associations of ILTCs detected in SEE-FIM examined tubes of EC cohorts have not been examined. Similar to the studies of ILTCs in routinely examined tubes, significant associations with ILTC presence occurred for aggressive tumor variables of non endometrioid histotype and high stage, and additionally for LVSI, deep myometrial invasion, and adjunctive chemotherapy6, 10, 11. The frequency of tubal material consistent with floating ILTCs and positive peritoneal cytology is significantly increased when the hysterectomy is performed with the use of a uterine manipulator7. In this study, no such associations occurred. This draws attention to the clinical importance of pre-operative ILTCS, although the absence of such associations may relate to the infrequency of manipulator use and peritoneal cytology sampling. High FIGO stage was the only independent variable as the others were co-variates of higher stage, and this was unaffected by the exclusion of ILTC cases that were staged as a FIGO3A due to tubal spread. Unlike prior studies, the presence or absence of mucosal attachment of the ILTC to the tube was examined. Significant associations with the same 5 aggressive tumor variables plus peritoneal cytology were observed amongst mucosal ILTCs in comparison to floating ILTCs. These variables together with their larger size and supportive stroma may provide mucosal ILTCs a survival advantage over floating ILTCs, and enable their growth within the peritoneum after exiting the tube.
Published literature on the prognostic role of ILTCs is also limited. Felix et al, found decreased survival amongst those with stage I serous carcinoma but not for those with higher stages in a study of 295 patients with ILTCs detected in routinely examined tubes and EC of different histotypes6. The potentially adverse ILTC effect was indirectly studied in women with EC and who had a prior tubal ligation, a surgery that could block trans tubal transmission. Li et al in a study of 562 women found tubal ligation was associated with lower rates of positive peritoneal cytology amongst all histotypes, and in those with non-endometrial histotypes with lower stage and recurrence rates resulting in improved prognosis12. In Cox proportional regression analysis, tubal ligation was inversely associated with lower endometrial carcinoma-specific mortality (HR 0.47). Felix et al reported lower stage disease, lower odds of peritoneal metastasis, and a statistically significant 26% improvement in EC-specific survival.in a cohort of 4,489 EC cases13. These results were even more striking among women diagnosed with non-endometrioid histotypes, which were comprised of serous, carcinosarcoma, or clear cell carcinomas. In the current study, the prognostic effect of ILTCs was non-significant although the HRs for overall and EC-specific survival with floating ILTCs and also with mucosal ILTCs were increased even when the cases of FIGO stage 3A due to tubal spread were excluded. Although multivariate analysis was not conducted because of the non-significant results, the study highlighted possible adverse prognostic roles for both floating and mucosal ILTCs, and more so for the latter since they had the highest HRs for overall and EC-specific survival (1.64 and 1.89 respectively). Future larger studies are needed to more definitively assess whether ILTCs are a significant independent prognostic factor, mucosal ILTCs are more adverse than the floating type, and amongst the floating ILTCs if those which occur intraoperatively and secondary to the surgical procedure are prognostic.
Funding sources:
The project and participation of ASF was supported by the National Cancer Institute (R03CA230673).
Footnotes
Conflict of interest disclosure: None to declare
References
- 1.Haltia UM, Bützow R, Leminen A, et al. FIGO 1988 versus 2009 staging for endometrial carcinoma: A comparative study on prediction of survival and stage distribution according to histologic subtype. J Gynecol Oncol. 2014; 25: 30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCluggage WG Pathologic Staging of Endometrial Carcinomas: Selected Areas of Difficulty. Adv Anat Pathol. 2018; 25: 71–84. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Hirschowitz L, Zaino R, et al. Pathologic Prognostic Factors in Endometrial Carcinoma (Other Than Tumor Type and Grade). Int J Gynecol Pathol. 2019; 38 Suppl 1: S93–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arikan G, Reich O, Weiss U, et al. Are endometrial carcinoma cells disseminated at hysteroscopy functionally viable? Gynecol Oncol. 2001; 83: 221–6. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felix AS, Sinnott JA, Vetter MH, et al. Detection of endometrial cancer cells in the fallopian tube lumen is associated with adverse prognostic factors and reduced survival. Gynecol Oncol. 2018; 150: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krizova A, Clarke BA, Bernardini MQ et al. Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: A blinded, retrospective review. Am J Surg Pathol 2011; 35: 115–26. [DOI] [PubMed] [Google Scholar]
- 8.Jia L, Yuan Z, Wang Y, et al. Primary sources of pelvic serous cancer in patients with endometrial intraepithelial carcinoma. Mod Pathol. 2015; 28: 118–27. [DOI] [PubMed] [Google Scholar]
- 9.Layfield LJ, Witt BL, Metzger KG et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011; 136: 767–72. [DOI] [PubMed] [Google Scholar]
- 10.Snyder MJ, Bentley R, Robboy SJ. Transtubal spread of serous adenocarcinoma of the endometrium: an underrecognized mechanism of metastasis. Int J Gynecol Pathol. 2006; 25: 155–60. [DOI] [PubMed] [Google Scholar]
- 11.Stewart CJ, Doherty DA, Havlat M, et al. Transtubal spread of endometrial carcinoma: correlation of intra-luminal tumour cells with tumour grade, peritoneal fluid cytology, and extra-uterine metastasis. Pathology. 2013; 45: 382–7. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Li M, Zhao L, et al. Prior Tubal Ligation Might Influence Metastatic Spread of Nonendometrioid Endometrial Carcinoma. Int J Gynecol Cancer. 2016; 26: 1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felix AS, Brinton LA, McMeekin DS, et al. Relationships of Tubal Ligation to Endometrial Carcinoma Stage and Mortality in the NRG Oncology/Gynecologic Oncology Group 210 Trial. J Natl Cancer Inst. 2015; 107: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samimi G, Trabert B, Duggan MA, Robinson JL, Coa KI, Waibel E, Garcia E, Minasian LM, Sherman ME. Processing of fallopian tube, ovary, and endometrial surgical pathology specimens: A survey of U.S. laboratory practices. Gynecol Oncol. 2018; 148: 515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006; 30: 230–6. [DOI] [PubMed] [Google Scholar]
- 16.Samimi G, Trabert B, Geczik AM, et al. Population Frequency of Serous Tubal Intraepithelial Carcinoma (STIC) in Clinical Practice Using SEE-Fim Protocol. JNCI Cancer Spectr. 2018; 2: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duggan MA and Trotter T. Alberta Health Service: Anatomical Pathology Quality Assurance Plan. CJP. 2016; 8: 10–22. [Google Scholar]
- 18.Gottfried E, Kunz-Schughart LA, Weber A, et al. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008: 67: 453–63. [DOI] [PubMed] [Google Scholar]
- 19.Bárcena C, Oliva E. WT1 expression in the female genital tract. Adv Anat Pathol. 2011; 18: 454–65. [DOI] [PubMed] [Google Scholar]


