Abstract
The objective of this study was to measure the performance of the Affirm Ambient Temperature Transport System (ATTS) over time and to estimate the length of time the system can preserve a vaginal specimen containing the three common organisms causing vaginitis: Trichomonas vaginalis, Candida species, and Gardnerella vaginalis (one of the causative agents of bacterial vaginosis). Women with symptoms of vaginitis presenting to one of three clinical centers were evaluated over a 4- to 8-week period. Four simultaneously obtained swabs were collected and tested by the Affirm VPIII assay at time zero with and without a preservative reagent, at 24 h with reagent, and at either 48 or 72 h with reagent. For each of the three organisms, Trichomonas, Gardnerella, and Candida, positivity at each time point was evaluated and compared to that at reference time zero with and without the ATTS. A total of 940 specimens were obtained from the three clinical sites. Eight hundred three were positive for one or more of the three organisms. Gardnerella had the highest overall positive rate (62%), followed by Candida with 18% and Trichomonas at 9%. The percent sensitivity versus control for Trichomonas ranged from 100% at time zero with and without reagent to 91% by 72 h. Gardnerella and Candida sensitivity remained at 100% for each time period. The Affirm VPIII ATTS system performed within 10% of the control swab (no transport reagent) at all four time points (0, 24, 48, and 72 h) for Trichomonas, Gardnerella, and Candida.
Vaginal discharge is among the leading causes prompting physician consultation and office visits by women in the United States (3). Proper diagnosis and treatment can be elusive if based on clinical symptoms and the character of the vaginal discharge alone. This may lead to a lack of relief from symptoms and increase cost to the patient from inadequate or improper treatment (4; American College of Obstetricians and Gynecologists technical bulletin 226, 1996).
The Affirm VPIII Microbial Identification Test detects and identifies Candida species, Gardnerella vaginalis, and Trichomonas vaginalis nucleic acid in vaginal fluid specimens from women with symptoms of vaginitis or vaginosis (Affirm VPIII package insert; Becton Dickinson and Company, Sparks, Md.). Previously, a limitation of the test was the requirement for specimens to be processed within 1 h at room temperature (RT) or 4 h with refrigeration (1). Becton Dickinson has developed a transport system that will preserve the vaginal swab specimen at RT and allow the processing of specimens for up to 3 days after collection. The Affirm Ambient Temperature Transport System (ATTS) is a pouch that includes a collection swab, a transport tube, and dropper containing the preservative transport reagent.
The purpose of this study was to measure the performance of the ATTS over time and to estimate the length of time for which the system preserves clinical vaginal specimens containing the most-common organisms, Trichomonas, Gardnerella, and/or Candida.
MATERIALS AND METHODS
Study design.
The study was conducted beginning March 1999 at three clinical centers over a period of 4 to 8 weeks. Participating institutions included the following: Wishard Health Services and St. Vincent Hospitals, Indianapolis, Ind.; Jefferson County Department of Health Sexually Transmitted Disease (STD) Clinic, Birmingham, Ala.; and Magee Womens Hospital, Pittsburgh, Pa. Institutional Review Board approval was obtained and was verified by Becton Dickinson prior to patient selection and specimen collection.
Patient selection and specimen collection.
Symptomatic women were characterized for inclusion in the study by clinical findings during examination and/or microscopic evaluation of the vaginal discharge. The symptoms and suspected diagnosis were recorded on a patient data collection sheet and submitted with each specimen.
Four simultaneously obtained swabs of the vaginal discharge were collected from women with symptoms of vaginitis or vaginosis in addition to the samples normally collected at the clinical center. All four study swabs were transported to the testing area within 1 h at RT or 4 h refrigerated and stored in the appropriate ambient temperature transport system. All specimens were tested using the BD Affirm VPIII assay on the BD MicroProbe Processor by the following methods: (i) Swab 1 (time zero; a no-reagent control) was tested within 1 h RT or 4 h refrigerated after collection. (ii) Swab 2 (time zero and ATTS) was tested at the same time as swab 1. (iii) Swab 3 (ATTS) was stored at RT for 24 h (± 4 h) after collection and then tested. (iv) Swab 4 (ATTS) was randomly alternated between the following two treatments: treatment 1, storage at RT for 48 h (± 4 h) after collection and then tested; treatment 2, storage at RT for 72 h (± 4 h) after collection and then tested. For each of the three reagent transport swabs (swabs 2, 3, and 4), the swab was immediately added to one of the ATTS tubes containing the reagent. The reagent had been dispensed into the tube immediately prior to adding the specimen swab. Transport systems containing specimen swabs 2, 3, and 4 were stored at RT (19 to 28°C) after receipt in the laboratory. The temperature of the area was recorded on a daily log sheet.
The study evaluated the performance of the ATTS by comparing simultaneously collected vaginal swabs stored in a sample collection tube containing ATTS reagent. Swabs were tested after 0, 24, 48, and 72 h of storage at ambient temperature and compared against a control swab from the same patient stored in the sample collection tube without ATTS reagent. The study did not attempt to correlate the Affirm Microprobe results with patient diagnosis or any method of diagnosis.
Statistical analysis.
For each of the three organisms, Trichomonas vaginalis, Gardnerella vaginalis, and Candida, positivity at each point was evaluated by tabulating the outcome for each sample at the reference point (time zero with or without reagent) and the outcome at the evaluated time point. Samples that were noncompliant at either the reference point or the evaluated time point were excluded from consideration for the evaluation of that time point, but not for other time points. This would include specimens that were improperly collected or specimens that were collected properly but not tested within the designated timed intervals.
RESULTS
A total of 940 patients were enrolled in the study and 803 specimens were positive for at least one of these organisms. A total of 425 specimens were from Wishard Health Services Women's Visit Center and St. Vincent obstetrics-gynecology Clinic; 350 specimens were from the Jefferson County Health Department's Sexually Transmitted Disease (STD) Clinic located in Birmingham, Ala.; and 165 specimens were from Magee Womens Hospital and associated sites. Table 1 summarizes the specimen totals by site, the number of positive four-swab specimens, where one or more swabs were positive for a patient, and positivity rates for each of the three pathogens (Trichomonas, Gardnerella, and Candida). For each institution, the highest positivity rate was for Gardnerella, followed by Candida and Trichomonas.
TABLE 1.
Specimen totals and ATTS organism positivity rates for the different sites
| Hospital | No. of specimens (%)
|
|||
|---|---|---|---|---|
| Total | Positive for Trichomonas | Positive for Gardnerella | Positive for Candida | |
| Wishard and St. Vincent | 425 | 30 (7) | 195 (46) | 58 (14) |
| Alabama | 350 | 31 (9) | 270 (77) | 69 (20) |
| Magee | 165 | 23 (14) | 113 (69) | 44 (28) |
| All | 940 | 84 (9) | 578 (62) | 171 (18) |
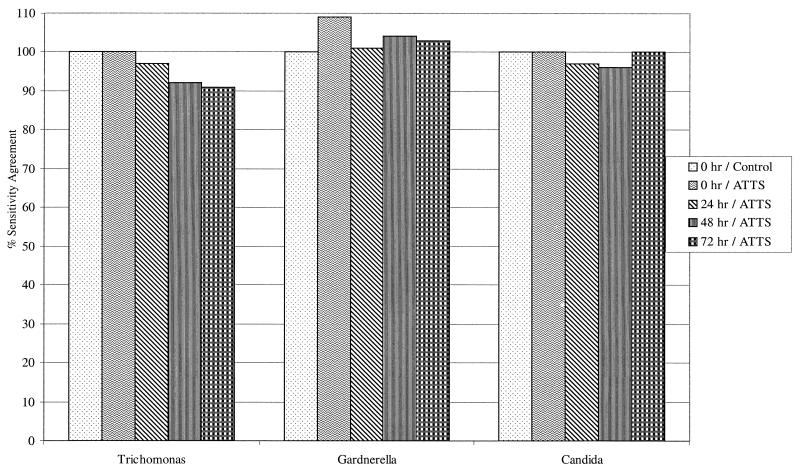
Each of the three organisms (Trichomonas, Gardnerella, and Candida) was evaluated for positivity at each time point by tabulating the outcome for each sample at the reference point (time zero without reagent always equals 100%) versus the outcome at the evaluated time. Figure 1 illustrates the percent sensitivity agreement for each organism at each time point with reagent compared to that of the control without reagent. The total percent positive for Trichomonas ranged from 90 at time zero with and without reagent to 87, 82, and 81 at 24, 48, and 72 h, respectively. For Gardnerella, the total percent positive improved from 81 to 90 with reagent at time zero and was approximately 83 at 24, 48, and 72 h. The percent positive for Candida remained essentially unchanged at 81 from time zero with and without reagent and at 24, 48, and 72 h. Gardnerella exhibited the greatest increase in sensitivity versus the control followed by Candida and Trichomonas.
FIG. 1.
Sensitivity agreement for Trichomonas, Gardnerella, and Candida by ATTS. Each organism at each time point with reagent is compared to the control without reagent.
DISCUSSION
The Affirm VPIII test detects the presence of clinically significant levels of Trichomonas, Gardnerella, and Candida RNA in a vaginal specimen. When the transport and testing of a specimen is delayed, RNA (unlike DNA) is very unstable and rapidly degrades once the organism becomes nonviable. This rapid degradation is especially apparent with Trichomonas. Conversely, if the organisms were to stay viable and multiply during transport, there is a greater possibility of the test being falsely positive. This is especially true for Gardnerella and Candida, where clinically insignificant levels of organisms might multiply to clinically significant levels.
Our findings confirm that the BD Affirm VPIII Ambient Temperature Transport collected swab results were within the manufacturer-specified 10% range of the control swabs without transport reagent at all four time points (0, 24, 48, and 72 h) for all three organisms. Additional findings include the 9% improvement in sensitivity for Gardnerella with the addition of transport reagent at time zero. There is speculation that the reagent immediately preserves the Gardnerella nucleic acid, while in the zero-hour control with no reagent, the nucleic acid may degrade during the transport window of 1 h at RT or 4 h refrigerated.
Site differences for each organism were also noted. In general, specimens collected from Wishard and St. Vincent Hospitals and Magee Womens Hospital showed improved performance when the delayed swabs were compared to the zero-hour control for Gardnerella and Candida; however, specimens from Alabama showed a decrease in sensitivity when the delayed swabs were compared to the zero-hour control. One explanation for this difference in performance may be related to the timing or testing of the zero-hour controls. For Wishard and St. Vincent Hospital and Magee Womens Hospital, specimen swabs were transported batched, refrigerated, and rarely processed before the 4-h maximum time. In contrast, in Alabama, nurse practitioners at the clinics performed the Affirm VPIII assay on the time-zero controls on each individual specimen at the clinical collection site, and the 24-, 48-, and 72-h tests were transported to the research laboratory where research technicians performed the test. As such, the Alabama zero-hour testing of control swabs occurred earlier than at Wishard and Magee Womens. Thus, the time delay from testing the zero-hour control swabs to testing of the 24-, 48-, and 72-h swabs was the longest in Alabama. All sites exhibited a slight decrease in sensitivity for detection of Trichomonas over time, although it was still within the desired specifications of ±10%.
The increase in time from specimen collection to testing is especially needed for laboratories that perform reference testing. With the ATTS, clinicians can collect specimens, send them to a central location for testing, and have the transport and test time delayed up to 72 h with little to no decrease in sensitivity. The Affirm test is more sensitive than conventional wet mounts (2) and is easier to perform.
We conclude that the BD Affirm VPIII Ambient Room Temperature Transport System allows vaginal specimens to be processed up to 68 h later than conventionally collected swabs without significant decrease in sensitivity or specificity. This will allow sites that cannot transport specimens within 1 h at RT or 4 h refrigerated to utilize the Affirm system for diagnosis of vaginitis.
REFERENCES
- 1.Briselden A N, Hillier S. Evaluation of Affirm VP microbial identification test for Gardnerella vaginalis and Trichomonas vaginalis. J Clin Microbiol. 1994;32:148–152. doi: 10.1128/jcm.32.1.148-152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferris D G, Hendrich J, Payne P M, Getts A, Rassekh R, Mathis D, Litaker M S. Office laboratory diagnosis of vaginitis: clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract. 1995;41:575–581. [PubMed] [Google Scholar]
- 3.Kent H L. Epidemiology of vaginitis. Am J Obstet Gynecol. 1991;165:1168–1176. doi: 10.1016/s0002-9378(12)90722-x. [DOI] [PubMed] [Google Scholar]
- 4.Sobel J D. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152:924–935. doi: 10.1016/s0002-9378(85)80003-x. [DOI] [PubMed] [Google Scholar]