Abstract
Few studies have been conducted among Asian children and adolescents with type 1 diabetes mellitus (T1DM) using do-it-yourself artificial pancreas system (DIY-APS). We evaluated real-world data of pediatric T1DM patients using DIY-APS. Data were obtained for 10 patients using a DIY-APS with algorithms. We collected sensor glucose and insulin delivery data from each participant for a period of 4 weeks. Average glycosylated hemoglobin was 6.2%±0.3%. The mean percentage of time that glucose level remained in the target range of 70 to 180 mg/dL was 82.4%±7.8%. Other parameters including time above range, time below range and mean glucose were also within the recommended level, similar to previous commercial and DIY-APS studies. However, despite meeting the target range, unadjusted gaps were still observed between the median basal setting and temporary basal insulin, which should be handled by healthcare providers.
Keywords: Algorithms; Diabetes mellitus, type 1; Insulin infusion system; Pancreas, artificial
INTRODUCTION
With the demand for strict glycemic control and advances in diabetes technology, the use of automated insulin delivery systems known as artificial pancreas system (APS) has emerged. Previous studies showed that APS improves glycosylated hemoglobin (HbA1c) level and the percentage of time blood glucose level is within 70 to 180 mg/dL (time in range [TIR]) while preventing hypoglycemia in patients with type 1 diabetes mellitus (T1DM) [1-3]. However, most APS have not been approved by the U.S. Food and Drug Administration, except for the MiniMed 670G (Medtronic Inc., Dublin, Ireland) and Control-IQ (Tandem Diabetes Care, San Diego, CA, USA) systems, which are not available in Korea. Therefore, do-it-yourself APS (DIY-APS) has been used by patients who desire to construct loop systems using open-source algorithms [4]. Few DIY-APS studies have been conducted in Asia. In addition, few studies have provided detailed graphs showing glucose and insulin profiles for individuals, with the exception of a Czech pilot study [5]. In this observational study, we investigated real-world DIY-APS data of children and adolescents with T1DM in Korea.
METHODS
Participant recruitment
We posted a recruitment notice on the website of the Korean Society of Type 1 Diabetes (http://www.ksT1DM.org/). We recruited subjects with T1DM who had been using DIY-APS for more than 1 month. The subjects voluntarily participated in the study and provided written informed consent. The study protocol was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB-No 2018-08-142) and was performed according to the guidelines of the Declaration of Helsinki.
Data acquisition
Patient blood glucose and insulin data were collected through a continuous glucose monitoring (CGM) and an insulin infusion device. Data were stored in the cloud or to a mobile phone, the same as before the study participation. The cloud was modified to transmit uploaded basal insulin data to our research server in real time. The cloud was rolled back to its pre-experimental version at the end of data acquisition. Demographic information including age, sex, height, body weight, the level of HbA1c, duration of diabetes and APS use were obtained.
Data analysis
The CGM parameters in both the daytime (6:00 AM to 12:00 AM) and nighttime (12:00 AM to 6:00 AM) were assessed, including TIR, the percentage of time blood glucose level was above 180 mg/dL (time above range [TAR]) and below 70 mg/dL (time below range [TBR]), percent coefficient of variation (%CV) and glucose management indicator (GMI). The number of hypoglycemic events, basal and bolus insulin doses, basal changes, and bolus injections were assessed. We described average blood glucose values in the form of ambulatory glucose profile reports and insulin delivery graphs of each individual. Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Among 28 screened subjects who had been using DIY-APS, a total of 11 patients were included in the study (Supplementary Fig. 1). However, since case no. 11 used an open loop as sensor-augmented pump rather than a closed loop with a DIY-APS algorithm (Fig. 1), we omitted case no. 11 from analysis.
Fig. 1.
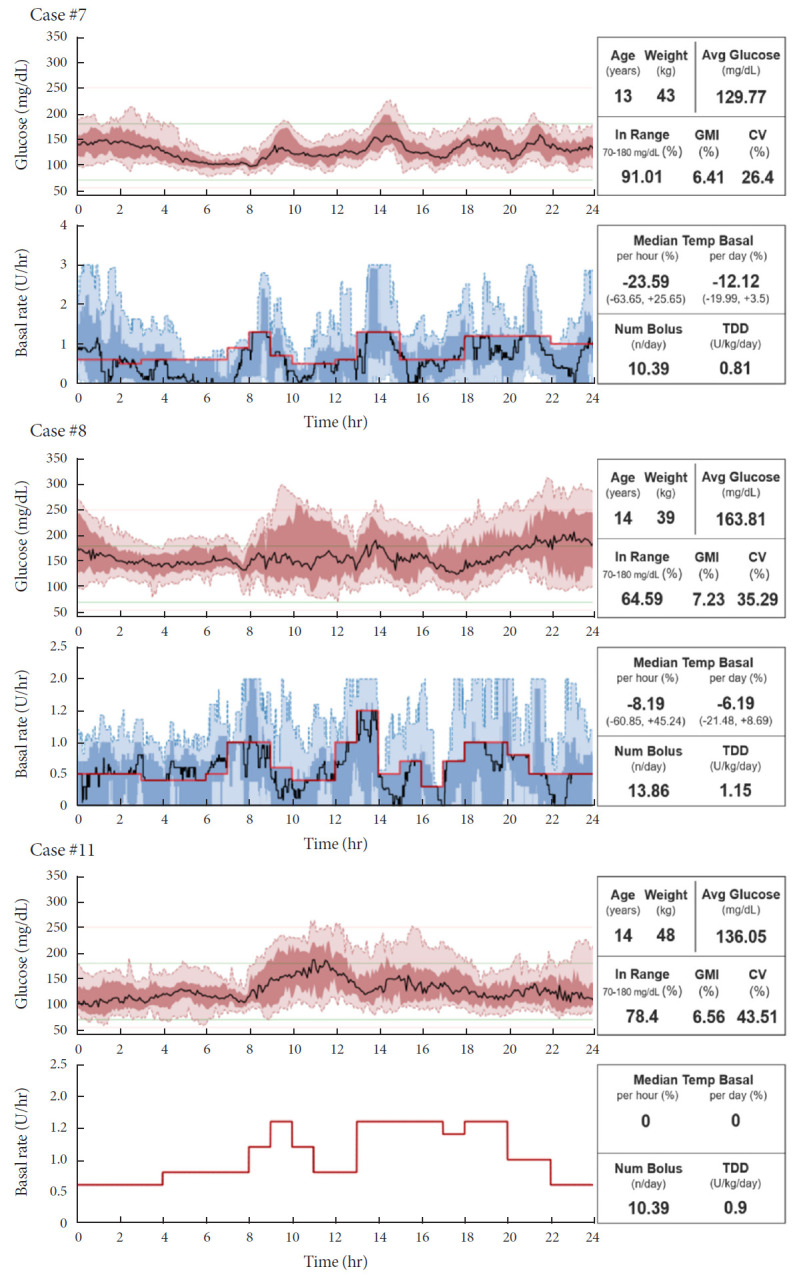
Graphs of insulin delivery and blood glucose levels for representative cases. GMI, glucose management indicator; CV, coefficient of variation; TDD, total daily dose of insulin.
The clinical characteristics and results of 10 DIY-APS users are described (Table 1). All patients were less than 19 years of age, with an average age of 10.1±3.2 years. The average HbA1c level was 6.2%±0.3%. The median duration of diabetes was 3.1 years and the median duration of DIY-APS usage was 1.9 years. Among all patients, mean TIR was 82.4%±7.8% over the total time. Nighttime TIR (84.14%±6.78%) was higher than daytime TIR (82.31%±9.0%). TBR of <70 mg/dL (2.42%) and <54 mg/dL (0.65%) were less than the recommended clinical targets [6]. During daytime, the average glucose level, frequency of basal rate change, bolus insulin injection and hypoglycemic events were significantly higher than in the nighttime.
Table 1.
Clinical characteristics and summary results of 4 weeks of DIY-APS usage
| Variable | Overall | Daytime (6:00 AM to 12:00 AM) | Nighttime (12:00 AM to 6:00 AM) | P value |
|---|---|---|---|---|
| No. of participants | 10 | - | - | - |
| Age, yr | 10.1±3.2 | - | - | - |
| Male/Female | 7 (70.0)/3 (30.0) | - | - | - |
| Weight, kg | 33.0±11.9 | - | - | - |
| BMI, kg/m2 | 17.2±2.4 | - | - | - |
| Duration of diabetes, yr | 3.1 (1.8 to 6.5) | - | - | - |
| HbA1c, % | 6.2±0.3 | - | - | - |
| Duration of DIY-APS usage, yr | 1.9±1.1 | - | - | - |
| Average glucose level, mg/dL | 135.8±11.92 | 137.78±12.48 | 130.01±13.74 | 0.017 |
| % CV of glucose | 31.57±4.43 | 31.43±4.7 | 28.46 (27.05 to 33.75) | 0.799 |
| TAR >250 mg/dL, % | 1.01 (0.65 to 2.39) | 1.18 (0.73 to 2.61) | 0.79 (0.08 to 2.49) | 0.333 |
| TAR >180 mg/dL, % | 14.77±7.85 | 14.34 (11.96 to 16.55) | 12.16±7.08 | 0.093 |
| TIR 70–180 mg/dL, % | 82.8±8.12 | 82.31±9.06 | 84.14± 6.78 | 0.799 |
| TBR <70 mg/dL, % | 2.42±1.39 | 2.0±1.27 | 3.7±3.33 | 0.074 |
| TBR <54 mg/dL, % | 0.65±0.5 | 0.45 (0.13 to 1.01) | 0.49 (0.19 to 1.66) | 0.445 |
| Hypoglycemic events | ||||
| <70 mg/dL, n/day | 0.76±0.41 | 0.53±0.32 | 0.24±0.15 | 0.011 |
| <54 mg/dL, n/day | 0.21±0.16 | 0.11 (0.08 to 0.28) | 0.04 (0.01 to 0.09) | 0.035 |
| Prolonged, n/day | 0 (0 to 0.03) | 0 | 0 | 1.000 |
| GMI, % | 6.56±0.29 | 6.61±0.3 | 6.42±0.33 | 0.017 |
| TDD, unit/kg/day | 0.79±0.16 | 0.66 (0.62 to 0.73) | 0.09±0.02 | 0.005 |
| Basal, % | 42.25±7.57 | 37.07±7.88 | 86.16±8.91 | 0.005 |
| Bolus, % | 57.75±7.57 | 62.93±7.88 | 13.84±8.91 | 0.005 |
| Median temporary basal | ||||
| Per hour, % | –21.42 (–23.25 to –17.92) | –23.44±8.98 | –10.65±25.32 | 0.386 |
| Per day, % | –10.94±11.1 | |||
| Frequency of basal changes, n/hr | 2.05 (1.97 to 2.13) | 2.26 (2.19 to 2.37) | 1.58 (1.52 to 1.72) | 0.005 |
| Bolus, n/day | 11.8±3.12 | 11.1±3.08 | 0.5 (0.43 to 0.88) | 0.005 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
DIY, do-it-yourself; APS, artificial pancreas system; BMI, body mass index; HbA1c, glycosylated hemoglobin; CV, coefficient of variation; TAR, time above range; TIR, time in range; TBR, time below range; GMI, glucose management indicator; TDD, total daily insulin dose.
Graphs of insulin delivery and blood glucose levels of each individual are shown (Fig. 1, Supplementary Fig. 2). The red line in the graph represents the basal setting for each patient. Case no. 7 achieved the lowest CV (26.4%) and highest TIR (91.01) among the participants. Case no. 8 showed skewed patterns of median temporary basal values and high CV. Unadjusted gaps were seen between the median basal setting and temporary basal setting, despite the appropriate mean glucose or GMI.
Results for TIR, TAR, TBR and mean glucose satisfied the recommended level [6] set in previous studies using commercial APS such as the MiniMed 670G or Control-IQ system [2,7].
DISCUSSION
To the best of our knowledge, this is the first study reporting CGM data along with a graph of delivered insulin dose for each individual with T1DM. We observed that overall CGM variables were within the recommended target ranges throughout the daytime and nighttime [6,8]. Furthermore, we found that some patients used DIY-APS without correcting the basal insulin preset, even though the preset was outside the 10 to 90 percentile of the temporary basal range. Unadjusted settings existed even when TIR, GMI and CV were appropriate. This finding reinforces the role of healthcare providers in helping patients adjust their DIY-APS settings [9].
Higher TIR and less frequent hypoglycemic events were observed in the nighttime than in daytime, while the temporary basal setting was often a negative value. These findings suggest that the basal insulin settings were too strict, especially during the daytime, probably with the intention of strict glucose control.
We compared the data with those of previous studies of pediatric patients using APS. Braune et al. [10] reported that DIY-APS improved HbA1c levels and TIR in patients with well-controlled glycemic status before use of DIY-APS. Breton et al. [7] showed that pediatric T1DM patients using the Tandem Control-IQ system achieved better glycemic control than sensor-augmented insulin pump users. In line with previous studies, our study reconfirmed that appropriate glycemic control could be achieved in pediatric DIY-APS users without increasing hypoglycemia. However, since the participants were highly motivated, promising TIR after using DIY-APS could be associated with a well-controlled TIR before using DIY-APS [11]. Therefore, head-to-head studies comparing APS algorithms are needed. A recent multinational randomized controlled trial reported an investigational advanced hybrid closed-loop system reduced hyperglycemia without increasing hypoglycemia in adolescents and young adults with T1DM compared with MiniMed 670G [12].
Many patients have experienced improved quality of life after using DIY-APS [4,13]. Reduction of HbA1c, increased TIR and less hypoglycemia gave them confidence in managing their diabetes and led to increased feelings of safety while sleeping or engaging in physical activities.
This study has several limitations. First, selection bias existed due to the study design. It was impossible to confirm whether the use of DIY-APS improved glycemic control compared to before DIY-APS initiation. Socioeconomic status might also have effects on accessibility to DIY-APS. Second, due to the small sample size, it is difficult to apply the outcomes to other children/adult patients with T1DM using DIY-APS. Large-scale randomized studies are needed, including more children/adult patients with T1DM using DIY-APS [14]. Last, the latest APS features such as Autosens, advanced meal assist, super microbolus (SMB), and meal announcements were not available to most of participants. Some caregivers avoided using SMB due to safety concerns and performed manual bolus injections instead of using a bolus calculator. For frequent manual bolus users, the recently released Control-IQ algorithm, which has an automated correction bolus, might be helpful to reduce manual bolus injections.
In conclusion, it was possible to achieve appropriate glycemic control in pediatric T1DM patients in real-world use of DIY-APS. Healthcare providers should monitor the DIY-APS settings of each patient even when CGM parameters are within target glucose ranges.
Acknowledgments
We would like to thank the DIY-APS users in the Korean Society of Type 1 Diabetes (SugarTree, http://www.ksT1DM.org/) who shared their DIY-APS records with us for the current study. This research did not receive any support or funding from the public or commercial sectors.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: M.S.C., S.L., S.M.P., J.H.K.
Acquisition, analysis, or interpretation of data: M.S.C., S.L., J. K., G.K., S.M.P., J.H.K.
Drafting the work or revising: M.S.C., S.L., S.M.P., J.H.K.
Final approval of the manuscript: M.S.C., S.L., J.K., G.K., S.M.P., J.H.K.
FUNDING
None
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0011.
Patient disposition. APS, artificial pancreas system.
Graphs of insulin delivery and blood glucose levels for residual cases. GMI, glucose management indicator; CV, coefficient of variation; TDD, total daily dose of insulin.
REFERENCES
- 1.Sherr JL, Cengiz E, Palerm CC, Clark B, Kurtz N, Roy A, et al. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care. 2013;36:2909–14. doi: 10.2337/dc13-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:155–63. doi: 10.1089/dia.2016.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melmer A, Zuger T, Lewis DM, Leibrand S, Stettler C, Laimer M. Glycaemic control in individuals with type 1 diabetes using an open source artificial pancreas system (OpenAPS) Diabetes Obes Metab. 2019;21:2333–7. doi: 10.1111/dom.13810. [DOI] [PubMed] [Google Scholar]
- 4.Lewis D, LeibrandS S, #OpenAPS Community Real-world use of open source artificial pancreas systems. J Diabetes Sci Technol. 2016;10:1411. doi: 10.1177/1932296816665635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petruzelkova L, Soupal J, Plasova V, Jiranova P, Neuman V, Plachy L, et al. Excellent glycemic control maintained by open-source hybrid closed-loop androidAPS during and after sustained physical activity. Diabetes Technol Ther. 2018;20:744–50. doi: 10.1089/dia.2018.0214. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S73–84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 7.Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383:836–45. doi: 10.1056/NEJMoa2004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleppo G, Webb K. Continuous glucose monitoring integration in clinical practice: a stepped guide to data review and interpretation. J Diabetes Sci Technol. 2019;13:664–73. doi: 10.1177/1932296818813581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S77–88. doi: 10.2337/dc20-S007. [DOI] [PubMed] [Google Scholar]
- 10.Braune K, O’Donnell S, Cleal B, Lewis D, Tappe A, Willaing I, et al. Real-world use of do-it-yourself artificial pancreas systems in children and adolescents with type 1 diabetes: online survey and analysis of self-reported clinical outcomes. JMIR Mhealth Uhealth. 2019;7:e14087. doi: 10.2196/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoelwer MJ, Kanapka LG, Wadwa RP, Breton MD, Ruedy KJ, Ekhlaspour L, et al. Predictors of time-in-range (70-180mg/dL) achieved using a closed-loop control system. Diabetes Technol Ther. 2021;23:475–81. doi: 10.1089/dia.2020.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397:208–19. doi: 10.1016/S0140-6736(20)32514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed SH, Ewins DL, Bridges J, Timmis A, Payne N, Mooney C, et al. Do-it-yourself (DIY) artificial pancreas systems for type 1 diabetes: perspectives of two adult users, parent of a user and healthcare professionals. Adv Ther. 2020;37:3929–41. doi: 10.1007/s12325-020-01431-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnside M, Lewis D, Crocket H, Wilson R, Williman J, Jefferies C, et al. CREATE (Community deRivEd AutomaTEd insulin delivery) trial: randomised parallel arm open label clinical trial comparing automated insulin delivery using a mobile controller (AnyDANA-loop) with an open-source algorithm with sensor augmented pump therapy in type 1 diabetes. J Diabetes Metab Disord. 2020;19:1–15. doi: 10.1007/s40200-020-00547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient disposition. APS, artificial pancreas system.
Graphs of insulin delivery and blood glucose levels for residual cases. GMI, glucose management indicator; CV, coefficient of variation; TDD, total daily dose of insulin.


