Abstract
BACKGROUND
Growing evidence have demonstrated that thyroid hormones have been involved in the processes of cardiovascular metabolism. However, the causal relationship of thyroid function and cardiometabolic health remains partly unknown.
METHODS
The Mendelian randomization (MR) was used to test genetic, potentially causal relationships between instrumental variables and cardiometabolic traits. Genetic variants of free thyroxine (FT4) and thyrotropin (TSH) levels within the reference range were used as instrumental variables. Data for genetic associations with cardiometabolic diseases were acquired from the genome-wide association studies of the FinnGen, CARDIoGRAM and CARDIoGRAMplusC4D, CHARGE, and MEGASTROKE. This study was conducted using summary statistic data from large, previously described cohorts. Association between thyroid function and essential hypertension (EHTN), secondary hypertension (SHTN), hyperlipidemia (HPL), type 2 diabetes mellitus (T2DM), ischemic heart disease (IHD), myocardial infarction (MI), heart failure (HF), pulmonary heart disease (PHD), stroke, and non-rheumatic valve disease (NRVD) were examined.
RESULTS
Genetically predicted FT4 levels were associated with SHTN (odds ratio = 0.48; 95% CI = 0.04−0.82,P = 0.027), HPL (odds ratio = 0.67; 95% CI = 0.18−0.88,P = 0.023), T2DM (odds ratio = 0.80; 95% CI = 0.42−0.86,P = 0.005), IHD (odds ratio = 0.85; 95% CI = 0.49−0.98,P = 0.039), NRVD (odds ratio = 0.75; 95% CI = 0.27−0.97,P = 0.039). Additionally, genetically predicted TSH levels were associated with HF (odds ratio = 0.82; 95% CI = 0.68−0.99,P = 0.042), PHD (odds ratio = 0.75; 95% CI = 0.32−0.82,P = 0.006), stroke (odds ratio = 0.95; 95% CI = 0.81−0.97,P = 0.007). However, genetically predicted thyroid function traits were not associated with EHTN and MI.
CONCLUSIONS
Our study suggests FT4 and TSH are associated with cardiometabolic diseases, underscoring the importance of the pituitary-thyroid-cardiac axis in cardiometabolic health susceptibility.
Cardiovascular diseases (CVDs) have long been a leading cause of mortality worldwide, causing 17.8 million deaths worldwide in 2017.[1] Although the rate of CVDs is declining mostly in developed countries, the epidemic of metabolic syndrome, type 2 diabetes, stroke and other cardiometabolic diseases shows no sign of remission which, remaining major contributors to morbidity and mortality.[2]
Observational findings have shown that thyroid hormones are involved in the occurrence and development of cardiovascular diseases.[3–5] Meanwhile, increasing evidence has shown that not only overt and subclinical thyroid dysfunction but also variation in normal range thyroid functions are associated with a higher risk of CVDs.[6–9] The Rotterdam Study reported that free thyroxine levels in elderly subjects were positively associated with atherosclerosis throughout the whole disease spectrum, independent of cardiovascular risk factors.[10] The prevalence of thyroid dysfunction increases as the aging of the populations in the United States and other countries accelerates.[11] The relationship between thyroid hormones and cardiometabolic health needs to be further studied. However, the available evidence from observational studies is susceptible to confounding or reverse causation bias, and the causal relationship between thyroid function and cardiometabolic diseases remains partly unknown.
Mendelian randomization (MR) used genetic variants as proxies for the exposure of interest to mimic a randomized controlled trial (RCT) where genetic alleles are randomly assorted at conception.[12] MR analysis builds on Mendel’s second law and the fact that genetic variants are randomly distributed at conception and thus unlikely to be related to possible confounders. Thus, it can provide evidence for causality that is likely to be affected by confounding or reverse causation when certain assumptions are met. These genetic variants are robustly associated with the exposure of interest, are not associated with confounders of the exposure-outcome relationship, and do not influence the outcome through pathways other than the exposure of interest.[13]
Well-powered, genome-wide association studies (GWAS) have identified hundreds of single-nucleotide polymorphisms (SNPs) associated with thyroid hormone indices and cardiometabolic traits. This makes it possible to test genetic and potentially causal relationships between thyroid function and cardiometabolic diseases through the MR approach.
Recent MR studies have extensively examined the causal effects of thyroid dysfunction on atrial fibrillation (AF) and ischemic heart disease (IHD).[14] Nevertheless, it remains partly unknown whether thyroid dysfunction is a causal pathway of other cardiometabolic diseases, such as HF and pulmonary heart disease (PHD). Therefore, we performed a two-sample MR analysis to examine whether free thyroxine (FT4) and thyrotropin (TSH) levels have causal associations with cardiometabolic diseases.
METHODS
Study Design
The MR approach must satisfy three assumptions (Figure 1): first, the genetic variants selected as instrumental variables (IVs) must be associated with thyroid function traits; second, the genetic variants must not be associated with any confounders; third, the genetic variants must be associated with cardiometabolic-related traits and events only through thyroid function, not through alternative pathways.[13] The second and third assumptions are known as independence from pleiotropy. The study design of the present MR analysis consisted of 11 outcomes of diseases, including essential hypertension (EHTN), secondary hypertension (SHTN), hyperlipidemia (HPL), type 2 diabetes mellitus (T2DM), ischemic heart disease (IHD), myocardial infarction (MI), HF, PHD, stroke, and non-rheumatic valve disease (NRVD). All studies had been approved by a relevant ethics review board. and participants provided informed consent.
Figure 1.
Principles of MR analysis for thyroid traits and risk of cardiometabolic diseases outcomes and assumptions that need to be met to obtain unbiased estimates of causal effects.
Broken lines represent potential pleiotropic or direct causal effectsbetween variables that would violate MR assumptions. Three assumptions of MR: (1) genetic variants must be associated withthyroid traits; (2) genetic variants must not be associated with confounders; (3) genetic variants must influence the risk of cardiometabolic traits and diseases outcomes only through thyroid traits and not through any alternative pathways. MR: Mendelian randomization.
Selection and Validation of IVs
We selected the lead SNPs (P < 5 × 10 −8) for all genetic loci that have been shown to independently associate with the thyroid function, including FT4 levels within the reference range and TSH levels within the reference range as IVs from the largest GWAS among individuals of European ancestry to date. The validation of IVs can be found in the reference.[15] The r2 thresholds and p values of the IVs are reported in Table 1 and eTable 1.[15,16]
Table 1. Description of instrument variables.
| Instrument variables | Consortium or study | Sample size | Population | Year |
| ASKLEPIOS: the Asklepios study; BHS: the 1994/1995 Busselton Health Survey; CHS: cardiovascular health study; EFSOCH: Exeter Family of Childhood Health; HBCS: Helsinki Birth Cohort Study; SHIP: Study of Health in Pomerania; NBS: The Nijmegen Biomedical Study. | ||||
| FT4 | BHS, CHS, HBCS, KORA, NBS, Rotterdam Study, SardiNIA, SHIP/SHIP-Trend, TwinsUK, ASKLEPIOS, CARLA, EFSOCH, Health2006, SardiNIA2 | 26,089 | Europeans | 2018 |
| TSH | BHS, CHS, HBCS, KORA, NBS, Rotterdam Study, SardiNIA, SHIP/SHIP-Trend, TwinsUK, ValBorbera, ASKLEPIOS, CARLA, EFSOCH, Health2006, SardiNIA2 | 27,916 | Europeans | 2018 |
Cardiometabolic Diseases and Data Sources
For disease outcomes, summary-level data were extracted from the FinnGen consortium ( https://www.finngen.fi/fi) for EHTN (n =90,215), SHTN (n = 74,711), HPL (n = 92,905), T2DM (n = 131,616), PHD (n = 96,499), AF/AFL (n = 171,873), IHD (n = 96,499), and NRVD (n = 149,821) , respectivel;[17] from the Coronary ARtery DIsease Genome wide Replication and Meta-analysis (CARDIoGRAM) plus the Coronary Artery Disease (C4D) Genetics (CARDIoGRAMplusC4D) consortium for MI (n =171 873);[18] from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium for HF (n = 23,821);[19] and from 17 studies including MEGASTROKE and NINDS-SiGN (details in eTable 13) for stroke (n = 446,696).[20] The diagnosis criteria, control selection principles, and study characteristics for each cohort have been described in more detail in the original articles.[17,18,21–26] Details on GWASs from which we extracted summary-level data are presented in Table 2. The summary genetic associations datasets are reported in the Supplement (eTable 3−eTable 12).
Table 2. Description of cardiometabolic diseases.
| Outcomes | Consortium or study | Sample size | Population | Year |
| HF: heart failure; IHD: ischemic heart disease; MI: myocardial infarction; T2DM: type 2 diabetes mellitus; CARDIoGRAMplusC4D: the Coronary ARtery DIsease Genome wide Replication and Meta-analysis (CARDIoGRAM) plus the Coronary Artery Disease (C4D) Genetics (CARDIoGRAMplusC4D) consortium. | ||||
| Essential Hypertension | FinnGen | 15,870/74,345 | European | 2020 |
| Secondary hypertension | FinnGen | 366/74,345 | European | 2020 |
| Hyperlipidaemia | FinnGen | 1,539/91,366 | European | 2020 |
| T2DM | FinnGen | 17,616/114,000 | European | 2020 |
| IHD | FinnGen | 10,739/96,499 | European | 2020 |
| MI | CARDIoGRAM and CARDIoGRAMplusC4D | 43,676 /128,197 | Trans-Ethnics | 2018 |
| HF | CHARGE | 2,992/20,829 | Trans-Ethnics | 2010 |
| Pulmonary heart disease | FinnGen | 1,476/95,023 | European | 2020 |
| Stroke | MEGASTROKE | 40,585/40,6111 | European | 2018 |
| Non-rheumatic valve diseases | FinnGen | 3,108/75,137 | European | 2020 |
Statistical Analysis
Linkage disequilibrium assessment and pleiotropy assessments
To verify that the SNPs selected in this study met the assumptions 1 & 2, we examined the genetic association with each thyroid function trait, further measured linkage disequilibrium (LD) between all the SNPs for the same thyroid function trait, [27] and selected independent genetic variants for each thyroid function trait.[12] We chose the variants with the lowest P value for association with each thyroid function trait if genetic variants were in LD; therefore, the SNPs selected did not violate the assumption 2. All SNPs for the same thyroid function trait showed a strong association (F-statistic > 10, the strength of the instrument), thus meeting assumption 1. We used MR-Egger regression to assess the presence of pleiotropic effects on cardiometabolic outcomes. Using the MR-Egger method, the effects of SNPs upon exposure are plotted against their effects upon outcomes, and an intercept distinct from the origin provides evidence for pleiotropic effects. [14]
Mendelian randomization analysis
The estimates of the causal effects of FT4 and TSH on outcomes were analyzed using the inverse variance-weighted (IVW) method, which provides a combined estimate of the causal estimate from each SNP. IVW is equivalent to a two-stage least squares or allele score analysis using individual-level data and is hence considered here as conventional MR.[28] IVW could return an unbiased estimate in the absence of horizontal pleiotropy or when horizontal pleiotropy is balanced. To account for directional pleiotropy, we compared the results with three other MR methods: MR Egger, weighted median, and weighted mode; therefore, a consistent effect across multiple methods strengthens causal evidence. Detailed information on these MR methods has been described previously.[28–30] Finally, the MR-Egger regression test was used to evaluate the pleiotropic effects.[14] Using the MR-Egger regression method, the effect of IV on the exposure is plotted against its effect on the outcome, and an intercept distinct from the origin provides evidence for pleiotropic effects. The slope of the MR-Egger regression can provide pleiotropy-corrected causal estimates.[14] In the present MR analyses, median weighted and MR-Egger methods were considered as sensitivity analyses for MR investigations with multiple genetic variants.[28,31] Power calculations for MR were conducted based on the website mRnd ( http://cnsgenomics.com/shiny/mRnd/).
Two-sample MR analyses were conducted using “TwoSampleMR,” an R package for such analyses. It was performed using R version 4.0.3 (RStudio, PBC, USA). We used the threshold of statistical significance of P ≤ 0.003 (0.05/20) after Bonferroni correction. P ≤ 0.05 but above the Bonferroni corrected significance threshold was considered suggestive of evidence for a potential association.
RESULTS
Validation of Selected SNPs and IVs
The characteristics of the selected SNPs for thyroid function are presented in Table 1 and eTable 1. All SNPs for the thyroid function showed strong associations (F-statistic > 21.83, the strength of the instrument), thus meeting assumption 1. To examine assumptions 2 and 3, we tested whether any of the selected SNPs were influenced by LD and pleiotropy. None of the SNPs was found to be in LD with each other (identified as r2 > 0.2) in the same thyroid function trait. For associations between thyroid function and cardiometabolic diseases, intercepts from MR-Egger regression showed that the observed results were not influenced by pleiotropy (eTable 3).
Thyroid Function and Cardiometabolic Diseases
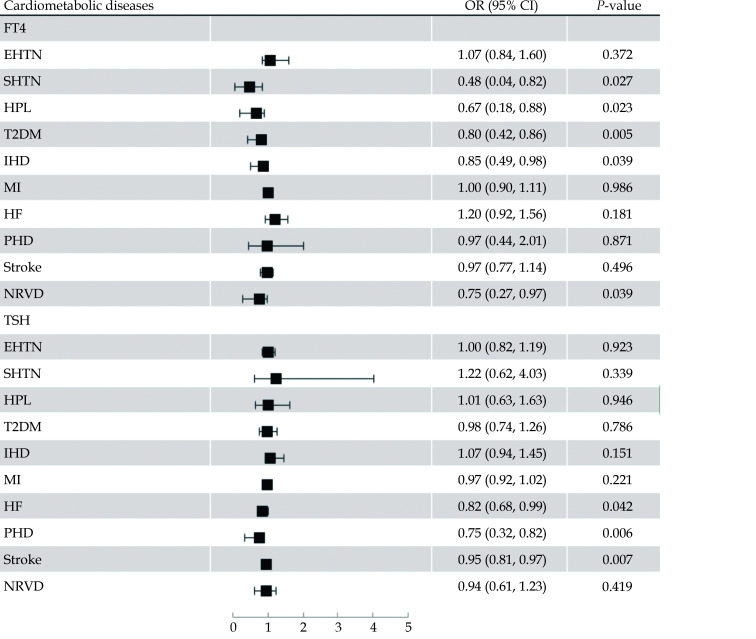
The association between genetically predicted thyroid function and cardiometabolic diseases is displayed in Table 3 and Figure 2. Per SD increased FT4 levels within the reference range were suggestively associated with lower risk of SHTN (odds ratio = 0.48, 95% CI = 0.04−0.82,P = 0.027), HPL (odds ratio = 0.67, 95% CI = 0.18−0.88,P = 0.023), T2DM (odds ratio = 0.80, 95% CI = 0.42−0.86,P = 0.005), IHD (odds ratio = 0.85, 95% CI = 0.49−0.98,P = 0.039), and NRVD (odds ratio = 0.75, 95% CI = 0.27−0.97,P = 0.039), but not with EHTN (odds ratio = 1.07, 95% CI = 0.84−1.60,P = 0.372), MI (odds ratio = 1.00, 95% CI = 0.90−1.11,P = 0.986), HF (odds ratio = 1.20, 95% CI = 0.92−1.56,P = 0.181), PHD (odds ratio = 0.97, 95% CI = 0.44−2.01,P = 0.871), and stroke (odds ratio = 0.97, 95% CI = 0.77−1.14,P = 0.496) after Bonferroni correction (Figure 2 and eFigure 1−eFigure 8).
Table 3. Mendelian randomization of thyroid function and cardiometabolic diseases.
| Simple | Weighted | IVW | ||||||
| OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |||
| AF: atrial fibrillation; AFL: atrial flutter; EHTN: essential hypertension; FT4: free thyroxine; HF: heart failure; HOCM: hypertrophic obstructive cardiomyopathy; HPL: hyperlipidemia; IHD: ischemic heart disease; IVW: inverse-variance weighted; MI: myocardial infarction; NRVD: non-rheumatic valve disease; PHD: pulmonary heart disease; SHTN: secondary hypertension; TSH: thyrotropin; T2DM: type 2 diabetes mellitus. | ||||||||
| FT4 | ||||||||
| EHTN | 1.09 (0.79,1.86) | 0.384 | 1.04 (0.73,1.64) | 0.662 | 1.07 (0.84,1.60) | 0.372 | ||
| SHTN | 0.45 (0.02,1.21) | 0.075 | 0.47 (0.02,1.24) | 0.081 | 0.48 (0.04,0.82) | 0.027 | ||
| HPL | 0.73 (0.15,1.59) | 0.231 | 0.67 (0.13,1.18) | 0.097 | 0.67 (0.18,0.88) | 0.023 | ||
| T2DM | 0.79 (0.34,0.98) | 0.044 | 0.77 (0.34,0.91) | 0.019 | 0.80 (0.42,0.86) | 0.005 | ||
| IHD | 0.83 (0.40,1.10) | 0.108 | 0.82 (0.39,1.02) | 0.058 | 0.85 (0.49,0.98) | 0.039 | ||
| MI | 0.99 (0.87,1.12) | 0.871 | 1.02 (0.92,1.14) | 0.647 | 1.00 (0.90,1.11) | 0.986 | ||
| HF | 1.18 (0.78,1.79) | 0.435 | 1.17 (0.81,1.68) | 0.401 | 1.20 (0.92,1.56) | 0.181 | ||
| PHD | 0.99 (0.32,2.95) | 0.956 | 0.84 (0.23,1.96) | 0.469 | 0.97 (0.44,2.01) | 0.871 | ||
| Stroke | 0.99 (0.78,1.24) | 0.883 | 0.93 (0.68,1.03) | 0.086 | 0.97 (0.77,1.14) | 0.496 | ||
| NRVD | 0.72 (0.19,1.16) | 0.1 | 0.95 (0.39,2.00) | 0.756 | 0.75 (0.27,0.97) | 0.039 | ||
| TSH | ||||||||
| EHTN | 1.03 (0.82,1.40) | 0.629 | 1.00 (0.77,1.30) | 0.987 | 1.00 (0.82,1.19) | 0.923 | ||
| SHTN | 1.13 (0.36,4.81) | 0.677 | 1.25 (0.45,6.27) | 0.445 | 1.22 (0.62,4.03) | 0.339 | ||
| HPL | 1.04 (0.55,2.17) | 0.805 | 0.95 (0.46,1.72) | 0.718 | 1.01 (0.63,1.63) | 0.946 | ||
| T2DM | 0.97 (0.70,1.21) | 0.572 | 0.97 (0.70,1.22) | 0.578 | 0.98 (0.74,1.26) | 0.786 | ||
| IHD | 1.06 (0.82,1.60) | 0.42 | 1.01 (0.74,1.40) | 0.901 | 1.07 (0.94,1.45) | 0.151 | ||
| MI | 0.98 (0.90,1.05) | 0.534 | 0.97 (0.90,1.05) | 0.506 | 0.97 (0.92,1.02) | 0.221 | ||
| HF | 0.88 (0.66,1.17) | 0.39 | 0.89 (0.68,1.17) | 0.421 | 0.82 (0.68,0.99) | 0.042 | ||
| PHD | 0.84 (0.33,1.33) | 0.245 | 0.77 (0.29,1.04) | 0.065 | 0.75 (0.32,0.82) | 0.006 | ||
| Stroke | 0.94 (0.76,1.00) | 0.043 | 0.95 (0.78,1.02) | 0.085 | 0.95 (0.81,0.97) | 0.007 | ||
| NRVD | 0.97 (0.56,1.54) | 0.781 | 0.99 (0.58,1.61) | 0.897 | 0.94 (0.61,1.23) | 0.419 | ||
Figure 2.
Odds ratios for associations of genetically predicted higher standardized FT4 and TSH levels within the reference range with cardiometabolic diseases.
EHTN: essential hypertension; HF: heart failure; HPL: hyperlipidemia; LHD: ischemic heart disease; MI: myocardial infarction; NRVD: non-rheumatic valve diseases; PHD: pulmonary heart disease; SHTN: secondary hypertension; TSH: thyrotropin; T2DM: type2 diabetes mellitus.
Regarding TSH, we observed that genetically predicted increased TSH levels were suggestively associated with lower risks of HF (odds ratio = 0.82, 95% CI = 0.68−0.99,P = 0.042), PHD (odds ratio = 0.75, 95% CI = 0.32−0.82,P = 0.006), and stroke (odds ratio = 0.95, 95% CI = 0.81−0.97,P = 0.007) after Bonferroni correction, but not with other cardiometabolic traits (Figure 2 and eFigure 1−eFigure 8).
MR power calculation ( http://cnsgenomics.com/shiny/mRnd) assuming a statistical confidence level of 0.05, an R2 value equaling genus heritability, and an effect size shown in eTable 1 suggested statistical power less than 80% for the associations of FT4 with SHTN but power greater than 80% for other significant associations. In addition, the associations were consistent in sensitivity analyses that used the simple median and weighted median methods (Table 3). The MR-Egger intercepts from MR-Egger regression for each outcome were centered at the origin with a confidence interval including the null, suggesting no strong unbalanced horizontal pleiotropy (eTable 2). In leave-one-out analyses, we found that no single genetic variants had an influential influence on the results for cardiometabolic diseases.
DISCUSSION
This study provides novel evidence that variations in thyroid function in the normal range are associated with cardiometabolic diseases. There was no significant causal relationship found between thyroid function and EHTN and MI.
To the best of our knowledge, this is the first MR study to examine the causal relationship of genetically predicted thyroid function with SHTN, PHD, HF and NRVD. Previous studies have found that thyroid metabolism is closely related to glucolipid metabolism.[32] However, an MR study observed significant associations between TSH levels and T2DM risk. there was also controversy in another MR study.[33] In our study, we found a suggestive association of genetically increased FT4 levels and a lower T2DM risk. The MR results for thyrotropin in this report are consistent with a previous study finding that lower normal FT4 levels were associated with a higher risk of T2DM.[34]
Of further note, it has been found that thyroid hormones have a direct effect on HMG-CoA expression and therefore might affect the functions of physiologic, metabolic, molecular, and transcriptional levels in the liver.[35] It has also been found that cholesterol and low-density lipoprotein can be reduced by L-thyroxine supplementation in euthyroid subjects,[36] although the effects of exogeneous thyroxine may not necessarily be the same as the genetically determined lifelong exposure of increased thyroxine levels, which indirectly indicates the association between thyroxine and lipid metabolism.
As a cardiometabolic disease, it has been revealed that hypertension was related to serum thyroid hormone levels in both overt hypothyroidism and subclinical hypothyroidism patients.[4,37] Nevertheless, there have been conflicting results regarding the correlation between thyroid function and hypertension in euthyroid adults.[38,39] An observational study reported that serum TSH levels within the upper reference range were associated with a higher risk of hypertension.[38] In our study, we found that genetically predicted FT4 levels were associated with secondary hypertension but not with essential hypertension, indicating that serum-free thyroxine is a potentially important but overlooked cause of hypertension.
Since hypertension, HPL and T2DM are common risk factors for coronary artery disease, in previous studies, low-normal thyroid function has been proven to be associated with increased coronary atherosclerosis, increased carotid intima media thickness and increased severity of carotid plaque burden.[40,41] Evidence from population studies on the association between thyroid dysfunction and CHD morbidity and mortality is conflicting, and not all studies have shown a positive association.[5,42,43] The inconsistency in the results of observational studies may be due to differences in the populations as well as the durations of follow-up in the various studies. In our MR analysis, the results support the possibility that lower FT4 levels within the normal range were associated with a higher risk of IHD but not MI. In a cohort of euthyroid men and women (n = 2,173), a low baseline FT4 level was associated with a high risk of coronary artery calcium (CAC) score progression over 4 years.[44] Although the mechanism remains unclear, variations in thyroid hormone status might have impacts on lipid metabolism, vascular remodeling, and endothelial function.[45–47]
Apart from IHD, it is generally known that thyroid function has interrelationships with the development of heart failure on many facets. In this study, genetically increased TSH levels showed a suggestive association with a lower risk of heart failure. Although excessive release of thyroxine could cause increased left ventricular mass, impaired diastolic function and blood volume from activation of the renin-angiotensin-aldosterone axis and tachycardia,[48] our study results reflect a trend that increased TSH levels within the reference range may have relevant impacts on HF. The mechanism may be related to the dynamic balance of thyroid hormone in euthyroid subjects.
Furthermore, the evidence of the relationship between thyroid function and the risk of stroke in euthyroid subjects is rare in observational studies. However, there were findings suggesting that stroke is significantly associated with low serum thyrotropin in patients with diabetes.[49] There was no causal association between genetically predicted thyroid function and ischemic stroke.[39] In our study, we found a suggestive association of lower TSH levels within the normal range with a higher risk of stroke.
Moreover, we also analyzed associations between thyroid function and PHD. Previously, pulmonary artery systolic pressure levels were shown to be directly related to the TSH value.[50] In our MR study, genetically predicted increased TSH levels were associated with a lower risk of PHD. Since thyroid hormones can enhance the alteration of the cardiovascular system by modulating circulation and peripheral vascular beds, they might mediate the occurrence and development of PHD.[50] Combined with our findings, evaluation of thyroid function in PHD patients may be warranted. In addition, we also found that genetically predicted increased FT4 levels were suggestively associated with a lower risk of NRVD. Evidence that focuses on the association of thyroid hormone levels and NRVD is rare. An association between mitral valve prolapse (MVP) and hyperthyroidism has been described in adults.[51] However, there was also evidence showing that no increase in MVP was present in the prevalence of euthyroid or hypothyroid disorders.[52] Future studies are needed to clarify the mechanisms underlying these associations.
Our MR study supports that the previous association of thyroid function and cardiometabolic diseases in observationnal studies may be partly due to residual confounding and reverse causality. However, genetic variation has an important influence on different thyroid function setpoints for each individual, resulting in bias in observational studies. Therefore, a better selection of subjects, such as standardized (regarding time of day) or multiple thyroid function measurements to define an individual’s setpoint, would increase the power of future research and help in the decision making of whether euthyroid outliers should be treated according to individual patients' normal values.
The present study has several strengths. First, we systematically assessed the causal role of thyroid function in the development of EHTN, SHTN, HPL, T2DM, IHD, MI, HF, PHD, stroke, and NRVD. The common limitation in previous observation studies is the heterogeneity among individual studies that used different thyroid function cutoffs, different confounding factors for adjustment, and varying disease definitions. Second, we used the data from large genome-wide association studies and the MR design, which might avoid bias from reverse causation and reduce confounding.
LIMITATIONS
Nevertheless, there are several limitations meriting consideration. First, MR has stringent core assumptions. Although we selected SNPs at the level of genome-wide significance and F statistics suggested a strong genetic association with thyroid function, our findings might be affected by weak instrument bias. Second, the influence of genetic determinants might be damped or buffered by compensatory developmental processes, i.e., canalization. Third, cardiometabolic diseases selected as outcomes in our study may have overlap or interaction on pathophysiological processes, so the associations between them may be further explored with other statistical analyses, i.e., mediating effects analysis. Meanwhile, a series of cardiometabolic diseases simultaneously included in our MR approach may inevitably affect the power of analysis. Thus, we cannot rule out that the lack of association with those outcomes is due to insufficient power. Fourth, the GWAS for all exposures was conducted in people with normal thyroid function. Finally, it is challenging to completely rule out an alternative direct causal pathway for all MR analyses, particularly for the thyroid function index determined by both thyroid function and multiple genetic variants.
CONCLUSIONS
In summary, we demonstrate that variations in thyroid function within the normal range were causally associated with cardiometabolic diseases, including SHTN, T2DM, IHD, HF, PHD, stroke, and NRVD, underscoring the importance of the pituitary-thyroid-cardiac axis in heart failure and stroke susceptibility.
ARTICLE INFORMATION
Author Contributions
YT, CS and TH designed the research. JW, ZZ and CY wrote the paper and performed the data analysis and interpretation. ZZ accomplished the figures of the article. WW, KZ, XM, JG, JT, WW, JY and JZ collected and organized data. All authors contributed to the statistical analysis, critically reviewed the manuscript during the writing process, and approved the final version to be published.
Conflicts of Interest
None.
Funding/Support
This work is supported by National Key R&D Program of China (2020YFC2004700), National Natural Science Foundation of China (No. 81825003, 91957123, 81800327, 81900272), the Peking University Start-up Grant (BMU2018YJ002), High-performance Computing Platform of Peking University and Beijing Technology and Business University Grant (No.88442Y0033), Fundamental Research Funds for the Central Universities (3332019044). The funding sources did not have any influence of the study design, analyses, interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication.
Data Availability Statement
The original contributions presented in the study are publicly available. The data can be found at GWAS Catalog ( https://www.ebi.ac.uk/gwas/), FinnGen ( https://finngen.gitbook.io/documentation/), CARDIoGRAM and CARDIoGRAMplusC4D ( http://cardiogramplusc4d.org/), MEGASTROKE ( http://www.megastroke.org/), and CHARGE ( http://www.chargeconsortium.com/).
We sincerely acknowledge the original GWASs and the related consortiums (FinnGen, CARDIoGRAM, CARDIoGRAMplusC4DDIAGRAM, CHARGE, MEGASTROKE) for the collection and management of the large-scale data resources. We also want to acknowledge the participants and investigators of the studies in our research.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
We sincerely acknowledge the original GWASs and the related consortiums (FinnGen, CARDIoGRAM, CARDIoGRAMplusC4DDIAGRAM, CHARGE, MEGASTROKE) for the collection and management of the large-scale data resources. We also want to acknowledge the participants and investigators of the studies in our research.
Contributor Information
Chun-Li SHAO, Email: chunlishao@126.com.
Yi-Da TANG, Email: drtangyida@126.com.
References
- 1.Powell KL, Stephens SR, Stephens AS Cardiovascular risk factor mediation of the effects of education and Genetic Risk Score on cardiovascular disease: a prospective observational cohort study of the Framingham Heart Study. BMJ Open. 2021;11:e045210. doi: 10.1136/bmjopen-2020-045210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Blaha MJ Cardiometabolic Medicine: A call for a new subspeciality training track in internal medicine. Am J Med. 2019;132:788–790. doi: 10.1016/j.amjmed.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Bianco AC, Salvatore D, Gereben B, et al Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 4.Grais IM, Sowers JR Thyroid and the heart. Am J Med. 2014;127:691–698. doi: 10.1016/j.amjmed.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selmer C, Olesen JB, Hansen ML, et al Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab. 2014;99:2372–2382. doi: 10.1210/jc.2013-4184. [DOI] [PubMed] [Google Scholar]
- 6.Chaker L, Baumgartner C, den Elzen WP, et al Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2015;100:2181–2191. doi: 10.1210/jc.2015-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collet TH, Gussekloo J, Bauer DC, et al Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172:799–809. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekkers OM, Horvath-Puho E, Cannegieter SC, et al Acute cardiovascular events and all-cause mortality in patients with hyperthyroidism: a population-based cohort study. Eur J Endocrinol. 2017;176:1–9. doi: 10.1530/EJE-16-0576. [DOI] [PubMed] [Google Scholar]
- 9.Rodondi N, den Elzen WP, Bauer DC, et al Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bano A, Chaker L, Mattace-Raso FU, et al Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the Rotterdam study. Circ Res. 2017;121:1392–1400. doi: 10.1161/CIRCRESAHA.117.311603. [DOI] [PubMed] [Google Scholar]
- 11.Hollowell JG, Staehling NW, Flanders WD, et al Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor DA, Harbord RM, Sterne JA, et al Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 13.Bowden J, Davey Smith G, Burgess S Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellervik C, Roselli C, Christophersen IE, et al Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: A Mendelian Randomization Study. JAMA Cardiol. 2019;4:144–152. doi: 10.1001/jamacardio.2018.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teumer A, Chaker L, Groeneweg S, et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun. 2018; 9: 4455.
- 16.Schultheiss UT, Teumer A, Medici M, et al A genetic risk score for thyroid peroxidase antibodies associates with clinical thyroid disease in community-based populations. J Clin Endocrinol Metab. 2015;100:E799–E807. doi: 10.1210/jc.2014-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The FinnGen consortium. FinnGen documentation of R3 release, 2020. https://finngen.gitbook.io/documentation/ (accessed on July 28, 2020).
- 18.Nikpay M, Goel A, Won HH, et al A comprehensive 1, 000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith NL, Felix JF, Morrison AC, et al Association of genome-wide variation with the risk of incident heart failure in adults of European and African ancestry: a prospective meta-analysis from the cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Circ Cardiovasc Genet. 2010;3:256–266. doi: 10.1161/CIRCGENETICS.109.895763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik R, Chauhan G, Traylor M, et al Multiancestry genome-wide association study of 520, 000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loehr LR, Rosamond WD, Chang PP, et al Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 22.White AD, Folsom AR, Chambless LE, et al Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 23.Ives DG, Fitzpatrick AL, Bild DE, et al Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 24.McKee PA, Castelli WP, McNamara PM, et al The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 25.Bleumink GS, Knetsch AM, Sturkenboom MC, et al Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Remme WJ, Swedberg K Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–1560. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 27.Genomes Project C, Abecasis GR, Auton A, et al An integrated map of genetic variation from 1, 092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess S, Butterworth A, Thompson SG Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartwig FP, Davey Smith G, Bowden J Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yavorska OO, Burgess S Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–39. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden J, Davey Smith G, Haycock PC, et al Consistent Estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kus A, Marouli E, Del Greco MF, et al Variation in normal range thyroid function affects serum cholesterol levels, blood pressure, and type 2 diabetes risk: a Mendelian randomization study. Thyroid. 2021;31:721–731. doi: 10.1089/thy.2020.0393. [DOI] [PubMed] [Google Scholar]
- 33.Bos MM, Smit RAJ, Trompet S, et al Thyroid Signaling, Insulin Resistance, and 2 Diabetes Mellitus: A Mendelian Randomization Study. J Clin Endocrinol Metab. 2017;102:1960–1970. doi: 10.1210/jc.2016-2816. [DOI] [PubMed] [Google Scholar]
- 34.Kong X, Wang J, Gao G, et al Association between free thyroxine levels and diabetic retinopathy in euthyroid patients with type 2 diabetes mellitus. Endocr Res. 2020;45:111–118. doi: 10.1080/07435800.2019.1690504. [DOI] [PubMed] [Google Scholar]
- 35.Beukhof CM, Massolt ET, Visser TJ, et al Effects of thyrotropin on peripheral thyroid hormone metabolism and serum lipids. Thyroid. 2018;28:168–174. doi: 10.1089/thy.2017.0330. [DOI] [PubMed] [Google Scholar]
- 36.Michalopoulou G, Alevizaki M, Piperingos G, et al High serum cholesterol levels in persons with ‘high-normal’ TSH levels: should one extend the definition of subclinical hypothyroidism? Eur J Endocrinol. 1998;138:141–145. doi: 10.1530/eje.0.1380141. [DOI] [PubMed] [Google Scholar]
- 37.Martin SS, Daya N, Lutsey PL, et al Thyroid function, cardiovascular risk factors, and incident atherosclerotic cardiovascular disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Clin Endocrinol Metab. 2017;102:3306–3315. doi: 10.1210/jc.2017-00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He W, Li S, Wang B, et al Dose-response relationship between thyroid stimulating hormone and hypertension risk in euthyroid individuals. J Hypertens. 2019;37:144–153. doi: 10.1097/HJH.0000000000001826. [DOI] [PubMed] [Google Scholar]
- 39.Larsson SC, Allara E, Mason AM, et al Thyroid function and dysfunction in relation to 16 cardiovascular diseases. Circ Genom Precis Med. 2019;12:e002468. doi: 10.1161/CIRCGEN.118.002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iervasi G, Molinaro S, Landi P, et al Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526–1532. doi: 10.1001/archinte.167.14.1526. [DOI] [PubMed] [Google Scholar]
- 41.Takamura N, Akilzhanova A, Hayashida N, et al Thyroid function is associated with carotid intima-media thickness in euthyroid subjects. Atherosclerosis. 2009;204:e77–e81. doi: 10.1016/j.atherosclerosis.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Boekholdt SM, Titan SM, Wiersinga WM, et al Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010;72:404–410. doi: 10.1111/j.1365-2265.2009.03640.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhao JV, Schooling CM Thyroid function and ischemic heart disease: a Mendelian randomization study. Sci Rep. 2017;7:8515. doi: 10.1038/s41598-017-07592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park HJ, Kim J, Han EJ, et al Association of low baseline free thyroxin levels with progression of coronary artery calcification over 4 years in euthyroid subjects: the Kangbuk Samsung Health Study. Clin Endocrinol (Oxf) 2016;84:889–895. doi: 10.1111/cen.12946. [DOI] [PubMed] [Google Scholar]
- 45.Fukuyama K, Ichiki T, Imayama I, et al Thyroid hormone inhibits vascular remodeling through suppression of cAMP response element binding protein activity. Arterioscler Thromb Vasc Biol. 2006;26:2049–2055. doi: 10.1161/01.ATV.0000233358.87583.01. [DOI] [PubMed] [Google Scholar]
- 46.Xiang GD, He YS, Zhao LS, et al Impairment of endothelium-dependent arterial dilation in Hashimoto’s thyroiditis patients with euthyroidism. Clin Endocrinol (Oxf) 2006;64:698–702. doi: 10.1111/j.1365-2265.2006.02531.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee JJ, Pedley A, Marqusee E, et al Thyroid function and cardiovascular disease risk factors in euthyroid adults: a cross-sectional and longitudinal study. Clin Endocrinol (Oxf) 2016;85:932–941. doi: 10.1111/cen.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozkurt B, Colvin M, Cook J, et al Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the american heart association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 49.Seghieri G, Bardini G, Fascetti S, et al Stroke is related to lower serum thyrotropin levels in patients with diabetes mellitus. Diabetes Res Clin Pract. 2003;62:203–209. doi: 10.1016/j.diabres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Scicchitano P, Dentamaro I, Tunzi F, et al Pulmonary hypertension in thyroid diseases. Endocrine. 2016;54:578–587. doi: 10.1007/s12020-016-0923-8. [DOI] [PubMed] [Google Scholar]
- 51.Channick BJ, Adlin EV, Marks AD, et al Hyperthyroidism and mitral-valve prolapse. N Engl J Med. 1981;305:497–500. doi: 10.1056/NEJM198108273050906. [DOI] [PubMed] [Google Scholar]
- 52.Zullo MA, Devereux RB, Kramer-Fox R, et al Mitral valve prolapse and hyperthyroidism: effect of patient selection. Am Heart J. 1985;110:977–980. doi: 10.1016/0002-8703(85)90195-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The original contributions presented in the study are publicly available. The data can be found at GWAS Catalog ( https://www.ebi.ac.uk/gwas/), FinnGen ( https://finngen.gitbook.io/documentation/), CARDIoGRAM and CARDIoGRAMplusC4D ( http://cardiogramplusc4d.org/), MEGASTROKE ( http://www.megastroke.org/), and CHARGE ( http://www.chargeconsortium.com/).