Abstract
This Article describes a sequential Ir/Cu-mediated process for the meta-selective C─H radiofluorination of (hetero)arene substrates. In the first step, Ir-catalyzed C(sp2)─H borylation affords (hetero)aryl pinacolboronate (BPin) esters. The intermediate organoboronates are then directly subjected to copper-mediated radiofluorination with [18F]tetrabutylammonium fluoride to afford fluorine-18 labeled (hetero)arenes in high radiochemical yield and radiochemical purity. This entire process is performed on a bench-top without Schlenk or glovebox techniques and circumvents the need to isolate (hetero)aryl boronate esters. The reaction was automated on a TracerLab FXFN module with 1,3-dimethoxybenzene and a meta-tyrosine derivative. The products, [18F]1-fluoro-3,5-dimethoxybenzene and an 18F-labeled meta-tyrosine derivative, were obtained in 37 ± 5% isolated radiochemical yield and >99% radiochemical purity and 25% isolated radiochemical yield and 99% radiochemical purity, and 0.52 Ci/μmol (19.24 GBq/μmol) molar activity (Am), respectively.
Graphical Abstract
Authors are required to submit a graphic entry for the Table of Contents (TOC) that, in conjunction with the manuscript title, should give the reader a representative idea of one of the following: A key structure, reaction, equation, concept, or theorem, etc., that is discussed in the manuscript. Consult the journal’s Instructions for Authors for TOC graphic specifications.
Introduction
Positron emission tomography (PET) with 18F-labeled radiotracers is widely used for the detection, staging, and study of disease.1,2 While numerous 18F-containing molecules have been deployed in PET, those containing aromatic C─18F bonds are particularly desirable due to their resistance to metabolic defluorination. As such, there is a pressing need for synthetic methods for the late-stage radiofluorination of (hetero)arenes, particularly those that are fast (due to the short ~110 min half-life of 18F), use nucleophilic [18F]fluoride (which has high molar activity and is readily available from small medical cyclotrons), and are translatable to automated clinical production laboratories.
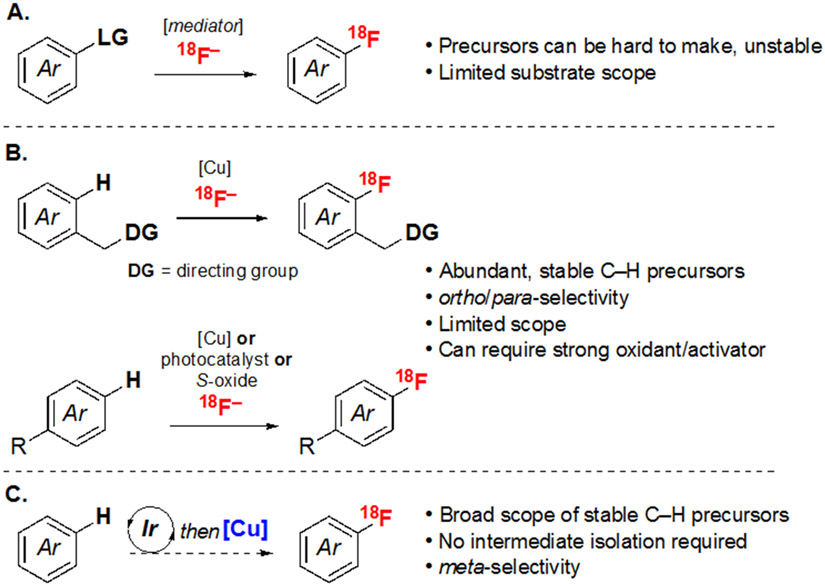
Most existing protocols for the nucleophilic radiofluorination of (hetero)arenes are limited in scope and/or utilize precursors that require multi-step syntheses (Scheme 1A). For instance, classical SNAr radiofluorination reactions require highly electron deficient (hetero)aryl halide/pseudohalide substrates.3 This electronic limitation has been overcome by moving to alternative mechanistic pathways and/or precursors, including those involving diazonium salts,4 triazenes,5 organo-nickel6 or -palladium complexes,7 phenols,8-10 hypervalent iodine derivatives,11-13 organoboron or stannane reagents,14-17 or sulfur-substituted aromatics.18-19 However, challenges with the synthesis, handling, isolation, scalability, and/or long-term storage of these precursors continue to limit widespread application of many of these methods in clinical settings.20-23 The Cu-mediated radiofluorination (CMRF) of organoboron precursors is a general (in terms of substrate scope) and practical (in terms of precursor availability and translation to automated syntheses) radiofluorination strategy that has been widely adopted for clinical use.24 Although many simple aryl boron reagents exhibit high bench-top stability, the purification, storage, and/or handling of highly functionalized (hetero)arylboron compounds (for example those derived from the late-stage borylation of bioactive scaffolds) as well as of 2-azaaryl and polyfluorinated aryl boron derivatives can be quite challenging.25
Scheme 1.
(A) Radiofluorination of prefunctionalized precursors. (B) Existing C─H radiofluorination approaches. (C) C─H radiofluorination using sequential Ir/Cu mediated processes (this work).
An attractive alternative would be to directly use C(sp2)─H substrates as precursors for nucleophilic radiofluorination. The (hetero)arene substrates of these transformations are exceptionally stable and readily available. However, there are major challenges to realizing this approach, including (1) developing strategies for the rapid activation/radiofluorination of traditionally inert C(sp2)─H bonds and (2) controlling the selectivity of 18F incorporation when there are multiple C(sp2)─H sites. Several recent reports have shown the feasibility of C(sp2)─H radiofluorination in limited contexts (Scheme 1B). For instance, aminoquinoline directing groups were used to control reactivity and selectivity in ortho-selective C(sp2)─H CMRF of (hetero)arenes.26 Additionally, para-selective electrophilic aromatic substitution (EAS) on electron rich (hetero)arenes was employed for the in situ generation of hypervalent iodine precursors for CMRF.27 A related para-selective EAS reaction was leveraged to access aryl sulfonium salts, which then undergo uncatalyzed nucleophilic radiofluorination.28 Finally, an organic photoredox approach was utilized to achieve para-selective nucleophilic radiofluorination of electron rich (hetero)arenes.29 In this report, we demonstrate a sequential Ir/Cu-mediated C(sp2)─H radiofluorination with a wide substrate scope, complementary site selectivity, and high operational simplicity compared to existing methods (Scheme 1C). This transformation merges the Ir-catalyzed C(sp2)─H borylation of (hetero)arenes30,31 with Cu-mediated radiofluorination to achieve meta-selective 18F-labeling of electronically diverse (hetero)arene substrates.
Results and Discussion
Although other tandem Ir C─H borylation sequences have been reported,32 we anticipated three major challenges for combining Ir-catalyzed C(sp2)─H borylation and Cu-mediated radiofluorination of the resulting (hetero)aryl boronate esters. First, CMRF reactions are well-known to be highly sensitive to conditions (e.g., solvent, ligands, additives),33,34 thus creating potential compatibility issues with the Ir catalysis. Second, due to the sensitivity of the active Ir catalyst, the Ir-catalyzed reaction is most commonly conducted with rigorous exclusion of air and moisture, which is not feasible in standard radiochemistry labs. Third, Ir-catalyzed C(sp2)─H borylation proceeds with modest site selectivity for certain classes of substrates, which could ultimately result in mixtures of radiofluorinated products.
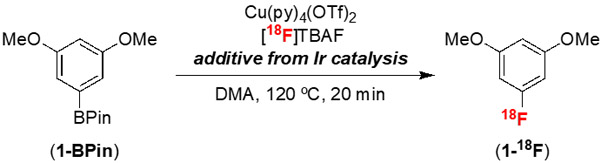
We first probed the anticipated compatibility issues by conducting the CMRF of 1-BPin in the presence of different components of the Ir-catalyzed C─H borylation reaction (Table 1). Under standard radiofluorination conditions (20 μmol 1-BPin, 0.25 equiv of Cu(py)4(OTf)2, [18F]tetrabutylammonium fluoride ([18F]TBAF) in DMA at 120 ºC for 20 min), 1-18F is formed in 80% radiochemical yield (RCY; entry 1), which was measured by multiplying radiochemical conversion (RCC) values obtained via radio-thin-layer chromatography (rTLC) analysis by radiochemical purity (RCP) values obtained via radio-high-performance liquid chromatography (rHPLC) analysis. However, as predicted, the addition of various C─H borylation reaction components significantly lowers the yield of 1-18F. Iridium sources containing chloride ligands (e.g., [Ir(COD)Cl]2, entry 2), are particularly problematic, likely due to competing reactions of the Cl− ion. Consistent with this proposal, the addition of 5 μmol of tetrabutylammonium chloride (TBACl, entry 6) completely shuts down the CMRF reaction. Moving to the halide-free Ir precursor [Ir(COD)OMe]2 restores the yield to ~80% (entry 3).
Table 1.
Impact of catalysts/ligands/reagents on Ir-catalyzed C─H borylation on CMRF of 1-BPin. Unless otherwise stated, RCYs are non-isolated and are calculated by multiplying RCC (measured via radio-TLC) by the RCP (measured via radio-HPLC).
| |||
|---|---|---|---|
| entry | [Cu] (μmol) |
additive (μmol) |
RCY (%) |
| 1 | 5 | none | 80 ± 10 |
| 2 | 5 | [Ir(COD)Cl]2 (3) | 42 ± 10 |
| 3 | 5 | [Ir(COD)OMe]2 (3) | 80 ± 6 |
| 4 | 5 | tmphen (6) | 49 ± 13 |
| 5 | 5 | dtbpy (6) | 58 ± 3 |
| 6 | 5 | TBACl (6) | 0 |
| 7 | 20 | none | 92 ± 1 |
| 8 | 20 | tmphen (6) | 88 ± 3 |
| 9 | 20 | dtbpy (6) | 91 ± 3 |
| 10 | 20 | B2Pin2 (10) | 36 ± 6 |
| 11 | 20 | HBPin (20) | 43 ± 9 |
| 12 | 20 | n-BuOH (550) | 96 ± 3 |
| 13 | 20 | B2Pin2 (10) & n-BuOH (550) |
83 ± 5 |
| 14 | 20 | HBPin (10) & n-BuOH (550) |
86 ± 2 |
Common ligands for Ir-catalyzed C─H borylation, 4,4’-di-tert-butylbipyridine (dtbpy) and 3,4,7,8-tetramethyl-1,10-phenanthroline (tmphen), also impede radiofluorination (entries 4, 5). We hypothesize that these ligate the Cu and render it less reactive. To mitigate this issue, the Cu loading was increased from 5 μmol (equimolar with the added ligands) to 20 μmol (>3-fold excess relative to the dtbpy/tmphen). This change in stoichiometry restores the radiofluorination yield to >80% (entries 7-9). Finally, B2Pin2 and HBPin inhibit the radiofluorination step (entries 10, 11). We hypothesized that this could be addressed by using an alcohol additive to quench reactive boron species.35,36 Indeed, the addition of 30 equiv of n-BuOH37,38 renders the radiofluorination reaction insensitive to boron additives (entries 13, 14).39
The C─H borylation step was next evaluated using the most compatible precatalyst and ligand, [Ir(COD)OMe]2/tmphen. Initial studies focused on identifying an operationally simple bench-top procedure, since most radiochemistry laboratories lack specialized equipment for air-free reactions. These studies revealed that the ligand, catalyst, and solvent for C─H borylation can be dispensed into a vial under ambient conditions followed by a 2 min argon sparge of the resulting solution. Subsequent addition of HBPin and 1-H followed by heating at 80 °C for 16 h results in the formation of 1-BPin in 82% NMR yield and 16 : 1 meta : ortho selectivity. This is comparable to the 92% NMR yield and identical regioselectivity obtained under rigorously dry/air-free conditions.
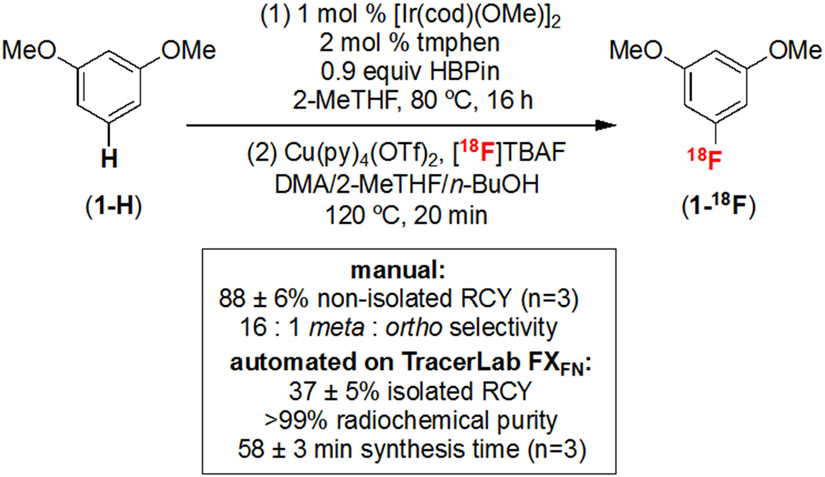
The two steps of the sequence were next combined by adding n-BuOH to the crude C─H borylation mixture and then directly subjecting this solution to radiofluorination with Cu(py)4(OTf)2 and [18F]TBAF in DMA at 120 ºC for 20 min. As shown in Scheme 2, this sequence affords 1-18F in 88 ± 6% non-isolated RCY and 16 : 1 meta : ortho selectivity, as confirmed by rHPLC. Importantly, the RCY is based on 18F as the limiting reagent.40 This sequence was directly translated to automated radiosynthesis by loading the crude C─H borylation mixture into a TracerLab FXFN synthesis module. Under automated conditions, 1-18F is produced in 37 ± 5% isolated RCY and >99% radiochemical purity (RCP, n=3), illustrating the potential for clinical translation (Scheme 2, see SI for full details).
Scheme 2.
Sequence for C─H radiofluorination of 1-H. See SI for complete experimental details. Non-isolated RCY is calculated by multiplying RCC (measured via radio-TLC) by the RCP (measured via radio-HPLC). Isolated RCY refers to the isolated recovery of the labeled product following semi-preparative HPLC purification.
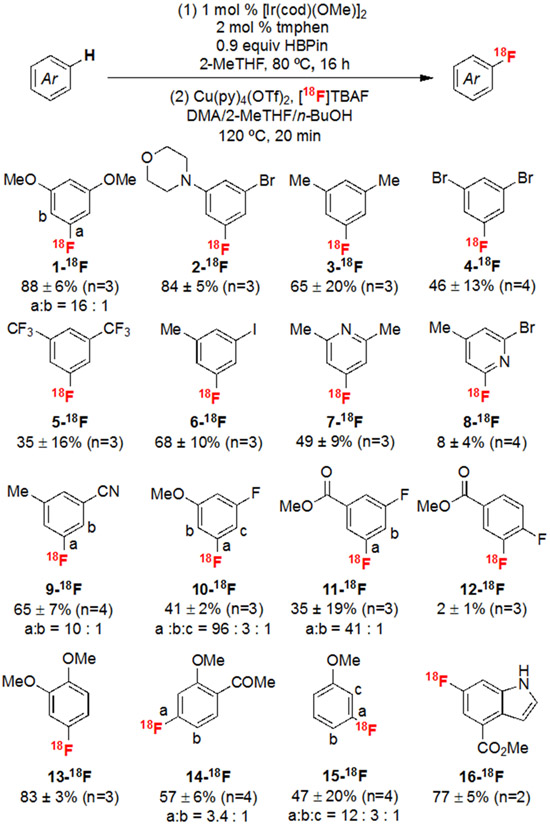
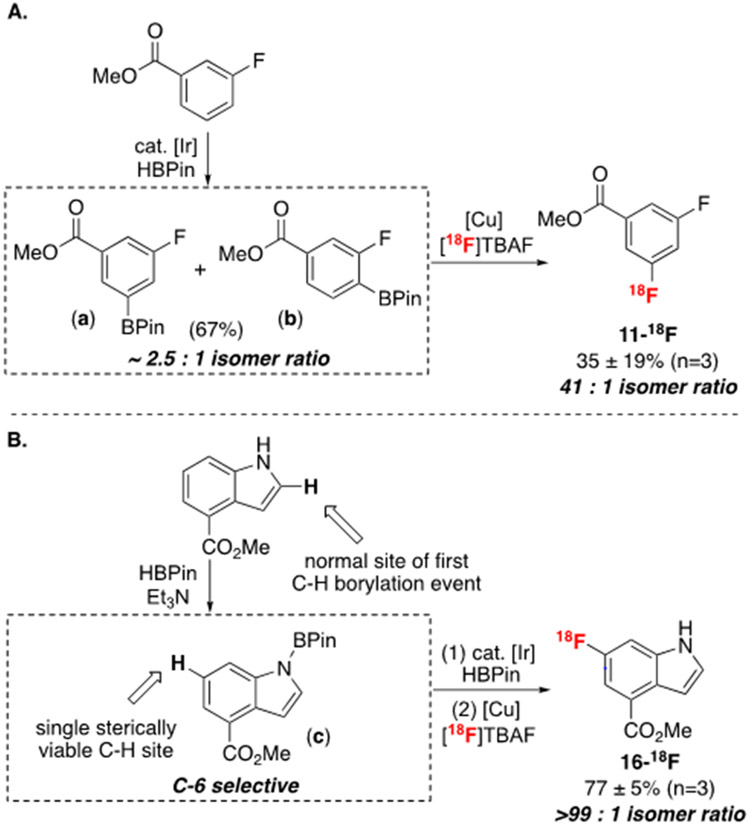
This optimized sequence is effective for the 18F-labeling of electronically diverse 1,3-disubstituted arenes, affording 1-18F to 11-18F in RCYs ranging from 8 to 88% (Scheme 3).41,42 In these examples, the 18F-labeled product is formed with high meta-selectivity, and regioisomers could be separated and quantified using analytical or semi-preparative HPLC (see SI). The C─H borylation site-selectivity is lowest for substrates bearing relatively small cyano and fluoro substituents (9-11), as expected for the sterically-controlled C─H functionalization step.43,44 However, the isomer ratio in the 18F-labeled products is typically higher than that observed in the C─H borylation step. For instance, the Ir-catalyzed C─H borylation of 9-H proceeds ortho- and meta- to the nitrile substituent with 5 : 1 selectivity favoring the less sterically congested metaboronate. However, the radiolabeling reaction affords 9-18F in 10 : 1 selectivity favoring the same position.45 Even more strikingly, C─H borylation of methyl 3-fluorobenzoate 11 affords a 2.5 : 1 mixture of isomers ArBPin a and b (Scheme 4A); however, after radiofluorination, 11-18F is formed as a 41 : 1 mixture favoring the meta-isomer b.
Scheme 3.
Substrate scope. See SI for complete experimental details and minor changes to the Cu mediator structure and temperature for different substrates. Unless otherwise stated, RCYs are non-isolated and are calculated by multiplying RCC (measured via radio-TLC) by the RCP (measured via radio-HPLC).
Scheme 4.
A. Highly meta-selective CMRF preceded by non-selective C─H borylation of fluorinated 12-H. B. Highly C-6 selective CMRF of indole 16-H via BPin adduct c. Yields are non-isolated RCYs and are calculated by multiplying RCC (measured via radio-thin-layer chromatography) by the RCP (measured via radio-HPLC).
NMR studies show that the selectivity enhancement in both 9 and 11 is due to facile decomposition of the ortho-borylated intermediates under CMRF conditions. This decomposition occurs via a combination of protodeboronation and oxidation pathways (see SI for complete details).46,47 Notably, it is well documented that ortho-fluorine substituents accelerate protodeboronation in various media, supporting these conclusions.48,25a
Arenes with other substitution patterns are similarly effective substrates for this sequence. For instance, veratrole 13-H undergoes selective C─H borylation/radiofluorination to afford 13-18F in 83% RCY. The C─H borylation of 1-(2-methoxyphenyl)ethan-1-one 14-H is slow at room temperature but proceeds efficiently at 80 ºC to afford 2: 1 selectivity for the site para- to the acetyl substituent. The isomer ratio is enhanced in the CMRF step, resulting in 14-18F as a 3.4 : 1 mixture of isomers.49 Anisole 15-H undergoes C─H borylation to generate a 3.3 : 1 : trace mixture of the meta : para : ortho boronate esters. Here again, the meta-selectivity is modestly enhanced in the CMRF step (15-18F is generated in a 12 : 3 : 1 mixture). Notably, this meta-selectivity with 15-H is complementary to that obtained in C─H radiofluorination reactions involving EAS or radical cation pathways (where the para-isomer is strongly favored, Scheme 1B).27,28a,50 This protocol is also compatible with modified C─H borylation systems that override intrinsic substrate regiochemistry.51 For example, indole 16-H undergoes selective C─H borylation at C-6 through the in situ installation of a traceless BPin directing group at the N-H bond prior to the C─H borylation to form adduct c (Scheme 4B, see SI for protocol).
A final noteworthy feature of this sequence is that it does not require either (1) high conversion in the C─H borylation step or (2) the generation of isolable boronate esters. This is exemplified by the formation of product 8-18F. The Ir-catalyzed C─H borylation of 2-bromo-4-methylpyridine proceeds in low (<10%) yield as determined by 1H NMR spectroscopy. Furthermore, the intermediate 2-pyridyl-substituted boronate ester is notoriously unstable.25b,c,52 Nonetheless, this substrate was successfully functionalized in 8% RCY, thereby circumventing the need to synthesize, isolate, and store the boronate ester precursor.53
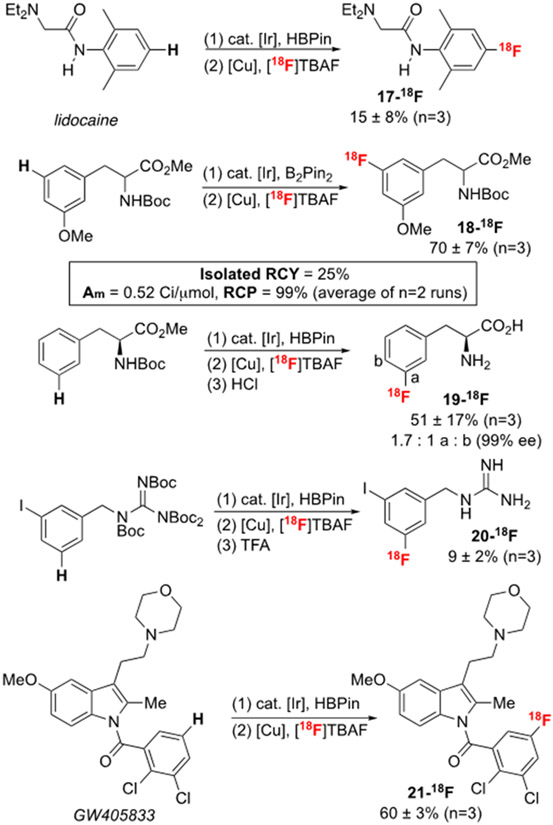
The ability to directly and selectively convert bioactive molecules into radiofluorinated analogues offers opportunities to streamline 18F-radiotracer synthesis and development. As such, it is critical to evaluate this method in the context of such scaffolds (Scheme 5). Under the standard C─H borylation/CMRF conditions, the anesthetic lidocaine reacts to furnish 17-18F in 15 ± 8% RCY. Notably, this radiolabeling approach is complementary to Hooker’s synthesis of the 18F-fluoroethyl analogue [18F]radiocaine.54
Scheme 5.
Tandem C─H radiofluorination of pharmaceutically relevant scaffolds. See SI for complete experimental details and minor changes to the Cu mediator and temperature for different substrates. Unless otherwise stated, RCYs are non-isolated and are calculated by multiplying RCC (measured via radio-thin-layer chromatography) by the RCP (measured via radio-HPLC). Isolated RCY refers to the isolated recovery of the labeled product following semi-preparative HPLC purification.
Protected aromatic amino acid derivatives undergo high yielding radiofluorination to afford products such as 18-18F and 19-18F. These have potential applications for imaging dopaminergic metabolism and tumor proliferation.55 Automated labeling was followed by semi-preparative HPLC purification to afford 18-18F in 25% isolated RCY, 99% RCP, and 0.52 Ci/μmol (19.24 GBq/μmol) Am (n=2). ICP-MS analysis of 18-18F obtained from this procedure indicated an Ir content of 13.46 ng, which is below the exposure limits (e.g. parenteral = 10 μg/day) stipulated for human use.56 This analysis further emphasizes the suitability of this radiolabeling method for use in conjunction with human PET imaging studies. Furthermore, manual labeling of 19-H to afford phenylalanine derivatives was achieved following acidic deprotection in HCl, and the meta- and para-regiosomers were separated using analytical rHPLC.57 Over the C─H radiofluorination protocol and the subsequent deprotection 19-18F was obtained in >99% ee as determined via chiral HPLC analysis.
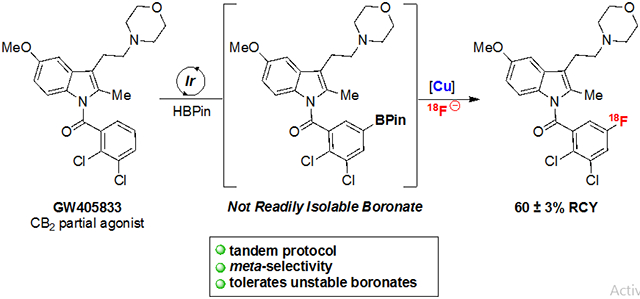
This method is also effective for the meta-selective radiofluorination of a protected guanidine. Deprotection of the crude product with trifluoroacetic acid delivered 20-18F. Notably, previous access to related imaging agents required the multi-step synthesis of iodonium precursors.58 Finally, the densely functionalized cannabinoid receptor 2 partial agonist GW405833 undergoes C─H radiofluorination to afford 21-18F in 60 ± 3% RCY. Multiple attempts to chromatographically isolate the boronate ester intermediate of this transformation led to the recovery of protodeboronated GW405833 substrate. Our approach enables high-yielding radiofluorination by circumventing the requirement to isolate/store this boronate. Once again, the incorporation of 18F onto the aromatic ring complements existing radiolabeling strategies for this molecule, which involve the multi-step installation of a [18F]fluoroethyl group.59 Overall, these examples highlight the broad functional group compatibility of the method, including tolerance of esters, amines, indoles, amides, and protected guanidines.
Conclusions
In summary, this report describes the development of a method for the meta-selective borylation/CMRF of aromatic C─H bonds. This approach enables the rapid and selective 18F-labeling of lead compounds for the development of imaging agents. Execution of the tandem procedure is operationally simple and readily translated to automated synthesis on a TracerLab FXFN module. As such, we anticipate that it can be adopted for both exploratory and clinical radiosyntheses of 18F PET imaging agents.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH [Award Number R01EB021155 (J.S.W., M.S.S. and P.J.H.S.) and Award Number F32GM136022 (L.S.S.)]. Our gratitude is extended to Angela Dial (University of Michigan Earth and Environmental Science Department) for performing ICP-MS analysis.
Footnotes
Supporting Information
- Materials and methods; preparation of precursors and reference standards; radiofluorination details; screening information; NMR spectra; HPLC traces.
REFERENCES
- (1).Brooks AF; Topczewski JJ; Ichiishi N; Sanford MS; Scott PJH; Late-Stage [18F]Fluorination: New Solutions to Old Problems. Chem. Sci, 2014, 5, 4545–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Brooks AF; Makaravage KJ; Wright JS; Sanford MS; Scott PJH Fluorine-18 Radiochemistry. In Handbook of Radiopharmaceuticals: Methodology and Applications 2nd Ed; Kilbourn MR, Scott PJH, Eds.; John Wiley & Sons Ltd., 2021; pp. 250–289. [Google Scholar]
- (3).Preshlock S; Tredwell M; Gouverneur V 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev 2016, 116, 719–766. [DOI] [PubMed] [Google Scholar]
- (4).Nozaki T; Tanaka Y The Preparation of F18-Labelled Aryl Fluorides. Int. J. Appl. Radiat. Isot 1967, 18, 111–119. [Google Scholar]
- (5).Tewson TJ; Welch MJ Preparation of Fluorine-18 Aryl Fluorides: Piperidyl Triazenes as a Source of Diazonium Salts. J. Chem. Soc. Chem. Commun 1979, 1149–1150. [Google Scholar]
- (6).Hoover AJ; Lazari M; Ren H; Narayanam MK; Murphy JM; Van Dam RM; Hooker JM; Ritter T A Transmetalation Reaction Enables the Synthesis of [18F]5-Fluorouracil from [18F]Fluoride for Human PET Imaging. Organometallics 2016, 35, 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lee E; Kamlet AS; Powers DC; Neumann CN; Boursalian GB; Furuya T; Choi DC; Hooker JM; Ritter T A Fluoride-Derived Electrophilic Late-Stage Fluorination Reagent for PET Imaging. Science 2011, 334, 639–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Neumann CN; Hooker JM; Ritter T Concerted Nucleophilic Aromatic Substitution with 19F− and 18F−. Nature 2016, 534, 369–373. [DOI] [PubMed] [Google Scholar]
- (9).Beyzavi MH; Mandal D; Strebl MG; Neumann CN; D’Amato EM; Chen J; Hooker JM; Ritter T 18F-Deoxyfluorination of Phenols via Ru π-Complexes. ACS Cent. Sci 2017, 3, 944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Strebl MG; Campbell AJ; Zhao W-N; Schroeder FA; Riley MM; Chindavong PS; Morin TM; Haggarty SJ; Wagner FF; Ritter T; Hooker JM HDAC6 Brain Mapping with [18F]Bavarostat Enabled by a Ru-Mediated Deoxyfluorination. ACS Cent. Sci 2017, 3, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Rotstein BH; Stephenson NA; Vasdev N; Liang SH Spirocyclic Hypervalent Iodine(III)-Mediated Radiofluorination of Non-Activated and Hindered Aromatics. Nat. Commun 2014, 5, 4365. [DOI] [PubMed] [Google Scholar]
- (12).Pike VW; Aigbirhio FI Reactions of Cyclotron-Produced [18F]Fluoride with Diaryliodonium Salts—A Novel Single-Step Route to No-Carrier-Added [18F]Fluoroarenes. J. Chem. Soc., Chem. Commun 1995, 2215–2216. [Google Scholar]
- (13).Ichiishi N; Brooks AF; Topczewski JJ; Rodnick ME; Sanford MS; Scott PJH Copper-Catalyzed [18F]Fluorination of (Mesityl)(aryl)iodonium Salts. Org. Lett 2014, 16, 3224–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Makaravage KJ; Brooks AF; Mossine AV; Sanford MS; Scott PJH Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF. Org. Lett 2016, 18, 5440–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Mossine AV; Brooks AF; Makaravage KJ; Miller JM; Ichiishi N; Sanford MS; Scott PJH Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett 2015, 17, 5780–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Tredwell M; Preshlock SM; Taylor NJ; Gruber S; Huiban M; Passchier J; Mercier J; Génicot C; Gouverneur V A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes. Angew. Chem. Int. Ed 2014, 53, 7751–7755. [DOI] [PubMed] [Google Scholar]
- (17).Taylor NJ; Emer E; Preshlock S; Schedler M; Tredwell M; Verhoog S; Mercier J; Genicot C; Gouverneur V Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc 2017, 139, 8267–8276. [DOI] [PubMed] [Google Scholar]
- (18).Chun JH; Morse CL; Chin FT; Pike VW No-Carrier-Added [18F]Fluoroarenes from the Radiofluorination of Diaryl Sulfoxides. Chem. Commun 2013, 49, 2151–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Sander K; Gendron T; Yiannaki E; Cybulska K; Kalber TL; Lythgoe MF; Årstad E Sulfonium Salts as Leaving Groups for Aromatic Labelling of Drug-like Small Molecules with Fluorine-18. Sci. Rep 2015, 5, 9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ren H; Wey H-Y; Strebl M; Neelamegam R; Ritter T; Hooker JM Synthesis and Imaging Validation of [18F]MDL100907 Enabled by Ni-Mediated Fluorination. ACS Chem. Neurosci 2014, 5, 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sanford MS; Scott PJH Moving Metal-Mediated 18F-Fluorination from Concept to Clinic. ACS Cent. Sci 2016, 2, 128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Argentini M; Wiese C; Weinreicht R Syntheses of 5-Fluoro-D/L-Dopa and [18F]5-Fluoro-L-Dopa, J. Fluor. Chem 1994, 68, 141–144. [Google Scholar]
- (23).Riss PJ; Kuschel S; Aigbirhio FI No Carrier-Added Nucleophilic Aromatic Radiofluorination Using Solid Phase Supported Arenediazonium Sulfonates and 1-(Aryldiazenyl)Piperazines. Tetrahedron Lett. 2012, 53, 1717–1719. [Google Scholar]
- (24).(a) Mossine A; Tanzey S; Brooks AF; Makaravage KJ; Ichiishi N; Miller JM; Henderson BD; Erhard T; Bruetting C; Skaddan MB; Sanford MS; Scott PJH Synthesis of High Molar Activity [18F]6-Fluoro-L-DOPA Suitable for Human Use by Cu-Mediated Fluorination of a BPin Precursor. Nat. Protoc 2020, 15, 1742–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wright JS; Kaur T; Preshlock S; Tanzey SS; Winton WP; Sharninghausen LS; Brooks AF; Sanford MS; Scott PJH Copper-Mediated Late-Stage Radiofluorination: Five Years of Impact on Pre-Clinical and Clinical PET Imaging. Clin. Transl. Imaging 2020, 8, 167–206. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Preshlock S; Calderwood S; Verhoog S; Tredwell M; Huiban M; Hienzsch A; Gruber S; Wilson TC; Taylor NJ; Cailly T; Schedler M; Collier TL; Passchier J; Smits R; Mollitor J; Hoepping A; Mueller M; Genicot C; Mercier J; Gouverneur V Enhanced Copper-Mediated 18F-Fluorination of Aryl Boronic Esters Provides Eight Radiotracers for PET Applications. Chem. Commun 2016, 52, 8361–8364. [DOI] [PubMed] [Google Scholar]
- (25).(a) Budiman YP; Westcott SA; Radius U; Marder TB Fluorinated Aryl Boronates as Building Blocks in Organic Synthesis. Adv. Synth. Catal, 2021, DOI: 10.1002/adsc.202001291. [DOI] [Google Scholar]; (b) Sadler SA; Tajuddin H; Mkhalid IAI; Batsanov AS; Albesa-Jove D; Cheung MS; Maxwell AC; Shukla L; Roberts B; Blakemore DC; Lin Z; Marder TB; Steel PG Iridium-Catalysed C─H Borylation of Pyridines. Org. Biomol. Chem, 2014, 12, 7318–7327. [DOI] [PubMed] [Google Scholar]; (c) Mkhalid IAI; Coventry DN; Albesa-Jové D; Batsanov AS; Howard JAK; Perutz RN; Marder TB Iridium-Catalysed Borylation of C─H Bonds in Nitrogen-Containing Heterocycles: Regioselectivity in the Synthesis of Heteroarylboronate Esters,” Angew. Chem. Int. Ed, 2006, 46, 489–491. [DOI] [PubMed] [Google Scholar]
- (26).Lee SJ; Makaravage KJ; Brooks AF; Scott PJH; Sanford MS Copper-Mediated Aminoquinoline-Directed Radiofluorination of Aromatic C─H Bonds with K18F. Angew. Chem. Int. Ed 2019, 131, 3151–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).McCammant MS; Thompson S; Brooks AF; Krska SW; Scott PJH; Sanford MS Cu-Mediated C─H 18F-Fluorination of Electron-Rich (Hetero)arenes. Org. Lett 2017, 19, 3939–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).(a) Xu P; Zhao D; Berger F; Hamad A; Rickmeier J; Petzold R; Kondratiuk M; Bohdan K; Ritter T Site-Selective Late-Stage Aromatic [18F]Fluorination via Aryl Sulfonium Salts. Angew. Chem. Int. Ed 2020, 59, 1956–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]; For radiofluorination reactions of aryl sulfonium salts, see: (b) Mu L; Fischer CR; Holland JP; Becaud J; Schubiger PA; Schibli R; Ametamey SR; Graham K; Stellfeld T; Dinkelborg LM; Lehmann L 18F-Radiolabeling of Aromatic Compounds Using Triarylsulfonium Salts. Chem. Eur. J 2012, 889–892. [Google Scholar]; (c) Gendron T; Sander K; Cybulska K; Benhamou L; Sin PKB; Khan A; Wood M; Porter MJ; Årstad E Ring-Closing Synthesis of Dibenzothiophene Sulfonium Salts and Their Use as Leaving Groups for Aromatic 18F-Fluorination. J. Am. Chem. Soc, 140, 11125–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).(a) Chen W; Huang Z; Tay NES; Giglio B; Wang M; Wang H; Wu Z; Nicewicz DA; Li Z Direct Arene C─H Fluorination with 18F– via Organic Photoredox Catalysis. Science 2019, 364, 1170–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang L; White AR; Chen W; Wu Z; Nicewicz DA; Li Z Direct Radiofluorination of Arene C─H Bonds via Photoredox Catalysis Using a Peroxide as the Terminal Oxidant. Org. Lett 2020, 22, 7971–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ishiyama T; Takagi J; Ishida K; Miyaura N; Anastasi NR; Hartwig JF Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc 2002, 124, 390–391. [DOI] [PubMed] [Google Scholar]
- (31).Cho J-Y; Tse MK; Holmes D; Maleczka RE; Smith MR Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C─H Bonds. Science 2002, 295, 305–308. [DOI] [PubMed] [Google Scholar]
- (32).(a) Some examples of tandem Ir C─H Borylation-functionalization sequences include Murphy JM; Liao X; Hartwig JF J. Am. Chem. Soc 2007, 129, 15434–15435. [DOI] [PubMed] [Google Scholar]; (b) Liskey CW; Liao X; Hartwig JF; Cyanation of arenes via iridium-catalyzed borylation. J. Am. Chem. Soc 2010, 132, 11389–11391. [DOI] [PubMed] [Google Scholar]; (c) Harrisson P; Morris J; Marder TB; Steel PG Microwave-accelerated iridium-catalyzed borylation of aromatic C─H bonds. Org. Lett, 2009, 11, 3586–3589. [DOI] [PubMed] [Google Scholar]; (d) Harrison P; Morris J; Steel PG; Marder TB A One-Pot, Single Solvent Process for Tandem, Catalyzed C─H Borylation/Suzuki-Miyaura Cross-Coupling Sequences” Synlett, 2009, 147–150. [Google Scholar]; (e) Tajuddin H; Shukla L; Maxwell AC; Marder TB; Steel PG “One-Pot” Tandem C─H Borylation/1,4-Conjugate Addition/Reduction Sequence. Org. Lett, 2010, 12, 5700–5703. [DOI] [PubMed] [Google Scholar]
- (33).Mossine AV; Brooks AF; Bernard-Gauthier V; Bailey JJ; Ichiishi N; Schirrmacher R; Sanford MS; Scott PJH Automated Synthesis of PET Radiotracers by Copper-Mediated 18F-Fluorination of Organoborons: Importance of the Order of Addition and Competing Protodeborylation. J. Label. Compd. Radiopharm 2018, 61, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Mossine AV; Brooks AF; Ichiishi N; Makaravage KJ Sanford MS; Scott PJH Development of Customized [18F] Fluoride Elution Techniques for the Enhancement of Copper-Mediated Late-Stage Radiofluorination. Sci. Rep 2017, 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Romero EA; Peltier JL; Jazzar R; Bertrand G Catalyst-Free Dehydrocoupling of Amines, Alcohols, and Thiols with Pinacol Borane and 9-Borabicyclononane (9-BBN). Chem. Commun 2016, 52, 10563–10565. [DOI] [PubMed] [Google Scholar]
- (36).Kuehn L; Jammal DG; Lubitz K; Marder TB; Radius U Stoichiometric and Catalytic Aryl–Cl Activation and Borylation Using NHC-Stabilized Nickel(0) Complexes. Chem. Eur. J 2019, 25, 9514–9521. [DOI] [PubMed] [Google Scholar]
- (37).Zischler J; Kolks N; Modemann D; Neumaier B; Zlatopolskiy BD Alcohol-Enhanced Cu-Mediated Radiofluorination. Chem. Eur. J 2017, 23, 3251–3256. [DOI] [PubMed] [Google Scholar]
- (38).Orlovskaya VV; Modemann DJ; Kuznetsova OF; Fedorova OS; Urusova EA; Kolks N; Neumaier B; Krasikova RN; Zlatopolskiy BD Alcohol-Supported Cu-Mediated 18F-Fluorination of Iodonium Salts under “Minimalist” Conditions. Molecules 2019, 24, 3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).HBPin was selected as the boron source owing to operational simplicity and since it more efficiently forms the resting-state of trisboryl Ir(III) catalyst (see: Boller TM; Murphy JM; Hapke M; Ishiyama T; Miyaura N; Hartwig JF Mechanism of the mild functionalization of arenes by diboron reagents catalyzed by iridium complexes. Intermediacy and chemistry of bipyridine-ligated iridium trisboryl complexes. J. Am. Chem. Soc 2005, 127, 14263–14278). However, tandem labeling sequences were also effective with B2Pin2 (see 18-H). [DOI] [PubMed] [Google Scholar]
- (40).This explains why the yield for the two step C─H borylation/radiofluorination sequence (88%; determined based on 18F as limiting reagent) is higher than that of the C–H borylation step (82%; determined based on 1-BPin as the limiting reagent).
- (41).Electron deficient substrates afford better yields using Cu(impy)4(OTf)2 (impy=imidazo[1,2-b]pyridazine) in place of Cu(py)4(OTf)2 (see ref 17).
- (42).A modified C─H borylation procedure was developed to address the inhibitory effect of some functional groups, such as the C─I bond in 6-H and the basic nitrogen in pyridine 7-H. This involved heating the solution of [Ir], ligand, and HBPin in order to more efficiently generate the active catalyst prior to substrate addition (see SI for full details).
- (43).Chotana GA; Rak MA; Smith MR Sterically Directed Functionalization of Aromatic C─H Bonds: Selective Borylation Ortho to Cyano Groups in Arenes and Heterocycles. J. Am. Chem. Soc 2005, 127, 10539–10544. [DOI] [PubMed] [Google Scholar]
- (44).Miller SL; Chotana GA; Fritz JA; Chattopadhyay B; Maleczka RE; Smith MR C─H Borylation Catalysts that Distinguish Between Similarly Sized Substituents Like Fluorine and Hydrogen. Org. Lett, 2019, 21, 6388–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Since Ir is known to induce selective protodeboronation, we considered the possibility that residual C─H borylation materials may be responsible for this selectivity enhancement. However, comparable regioselectivities were obtained in the CMRF of purified boronates derived from 14-H in the presence/absence of the C─H borylation components (see SI). See: Kallepalli VA; Gore KA; Shi F; Sanchez L,; Chotana GA; Miller SL; Maleczka RE; Smith MR Harnessing C─H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization. Journal of Organic Chemistry, 2015, 80, 8341–8353. [Google Scholar]
- (46).(a) Similar selectivity enhancement was observed for 9-18F and 10-18F. There was some run-to-run variability in the C─H borylation selectivity. However, only traces of ortho-18F-labeled isomers were detected in all runs.; (b) Subjecting a 2.5 : 1 mixture of a : b to the Cu radiofluorination conditions without added [18F]TBAF led to quantitative consumption of b. In contrast, isomer a remained after 20 min (see SI for further details).
- (47).We thank a reviewer for offering alternative explanations for the regioselectivity enhancements observed in the fluoroarene substrates. These have been examined using meta- and ortho-substituted BPin precursors 11-BPin, which do not afford appreciable quantities of the ortho-labeled radiofluorine under the CMRF conditions, ruling out other pathways. These controls demonstrate that isomerization of the radiofluorine products via a benzyne mediated by an IrH species does not operate (see SI for details).
- (48).(a) Kuivila HG; Reuwer JF Jr.; Mangravite JA Electrophilic Displacement Reactions: XV. Kinetics and Mechanism of the Base-Catalyzed Protodeboronation of Areneboronic Acids. Can. J. Chem 1963, 41, 3081–3090. [Google Scholar]; (b) Cox PA; Reid M; Leach AG; Campbell AD; King EJ; Lloyd-Jones GC Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion. J. Am. Chem. Soc 2017, 139, 13156–13165. [DOI] [PubMed] [Google Scholar]
- (49).CMRF of the independently synthesized boronate regioisomers corresponding to 14-H employed as mixtures in various ratios gave regiochemical outcomes consistent with regioselectivity observed in the tandem protocol, see the SI for details. For a prior investigation of this substrate under a different Ir C─H borylation system, see: Tajuddin H; Harrisson P; Bitterlich B; Collings JC; Sim N; Batsanov AS; Cheung MS; Kawamorita S; Maxwell AC; Shukla L; Morris J; Lin Z.; Marder TB; Steel PG Iridium-Catalyzed C─H Borylation of Quinolines and Unsymmetrical 1,2-Disubstituted Benzenes: Insights into Steric and Electronic Effects on Selectivity Chem. Sci 2012, 3, 3505–3515. [Google Scholar]
- (50).Coenen HH; Franken K; Kling P; Stöcklin KG Direct Electrophilic Radiofluorination of Phenylalanine, Tyrosine and Dopa. Int. J. Radiat. Appl. Instrumentation 1988, 39, 1243–1250. [Google Scholar]
- (51).(a) Preshlock SM; Plattner DL; Maligres PE; Krska SW; Maleczka RE; Smith MR A Traceless Directing Group for C─H Borylation. Angew. Chem. Int. Ed 2013, 52, 12915–12919. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wright JS; Scott PJH; Steel PG Iridium-Catalysed C─H Borylation of Heteroarenes: Balancing Steric and Electronic Regiocontrol. Angew. Chem. Int. Ed 2021, 60, 2796–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Campeau L-C; Rousseaux S; Fagnou, K. A Solution to the 2-Pyridyl Organometallic Cross-Coupling Problem: Regioselective Catalytic Direct Arylation of Pyridine N-Oxides. J. Am. Chem. Soc 2005, 127, 18020–18021. [DOI] [PubMed] [Google Scholar]
- (53).No competing SNAr labeling was observed during this CMRF reaction, which was confirmed by subjecting 8-H to the labeling conditions.
- (54).Hooker JM; Strebl MG; Schroeder FA; Wey H-Y; Ambardekar AV; McKinsey TA; Schoenberger M Imaging Cardiac SCN5A using the Novel F-18 Radiotracer Radiocaine. Sci. Rep 2017, 7, 42136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Muoio B; Giovanella L; Treglia G Recent Developments of 18F-FET PET in Neuro-oncology. Curr. Med. Chem 2017, 25, 3061–3073. [DOI] [PubMed] [Google Scholar]
- (56).(a) Teasdale A; Thompson S ICH Q3D Elemental Impurities. In ICH Quality Guidelines: An Implementation Guide; Teasdale A, Elder D, Nims RW, Eds; John Wiley & Sons, Inc. 2018; pp 233–280. [Google Scholar]; (b) ICH Official Website. https://www.ich.org/page/quality-guidelines (accessed 2021-04-21).
- (57).In the case of monosubstituted 19-H, the isomeric mixture could be resolved with HPLC following acidic deprotection in HCl. This analysis showed a 1.7:1 regioisomeric ratio favoring the meta-functionalized isomer.
- (58).Yamaguchi A; Hanaoka H; Higuchi T; Tsushima Y Radiolabeled (4-Fluoro-3-Iodobenzyl)Guanidine Improves Imaging and Targeted Radionuclide Therapy of Norepinephrine Transporter-Expressing Tumors. J. Nucl. Med 2018, 59, 815–821. [DOI] [PubMed] [Google Scholar]
- (59).Evens N; Vandeputte C; Muccioli GG; Lambert DM; Baekelandt V; Verbruggen AM; Debyser Z; Van Laere K; Bormans GM Synthesis, in vitro and in vivo Evaluation of Fluorine-18 Labelled FE-GW405833 as a PET Tracer for Type 2 Cannabinoid Receptor Imaging. Bioorg. Med. Chem 2011, 19, 4499–4505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.